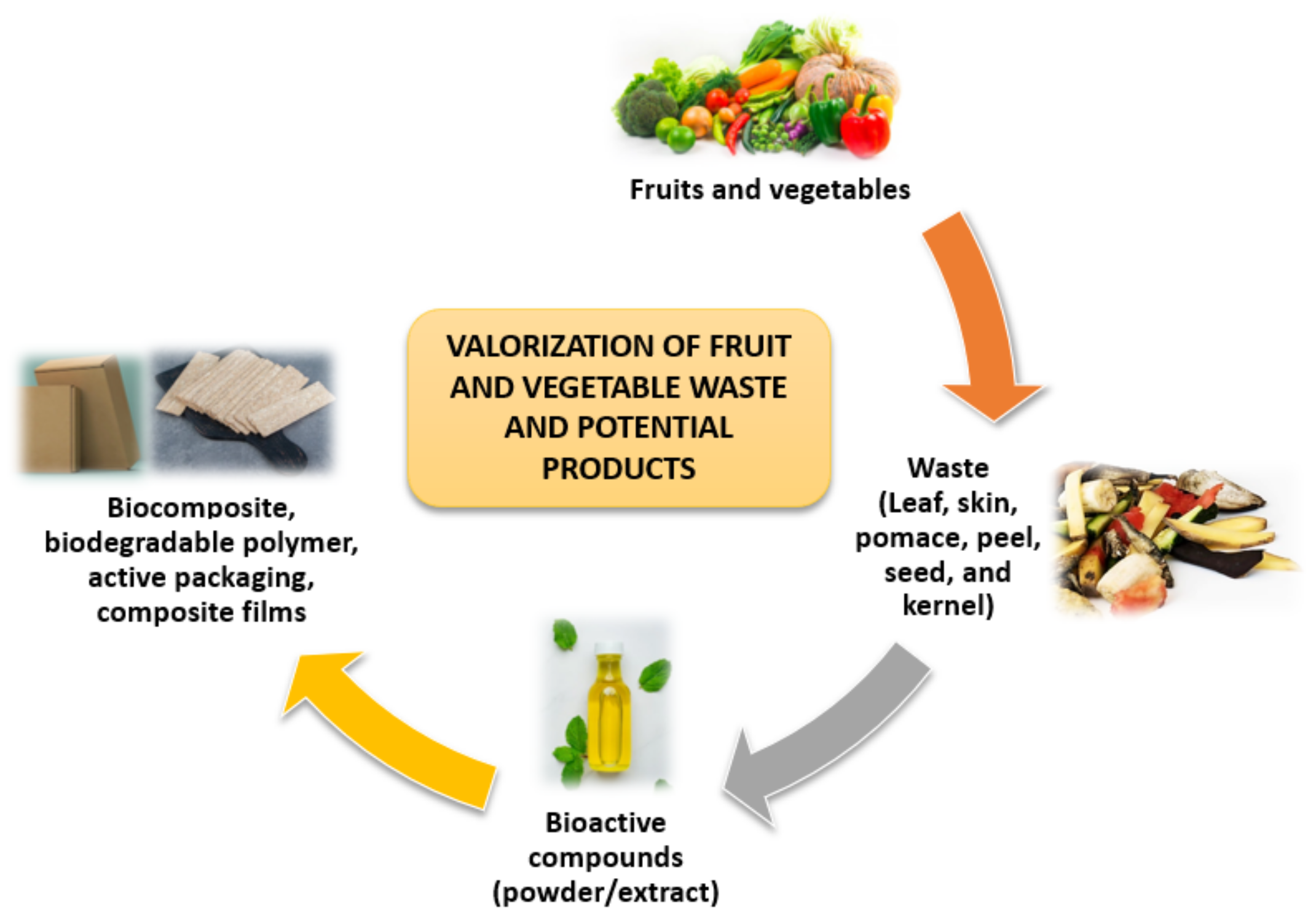
Progress in the Valorization of Fruit and Vegetable Wastes: Active Packaging, Biocomposites, By-Products, and Innovative Technologies Used for Bioactive Compound Extraction
Abstract
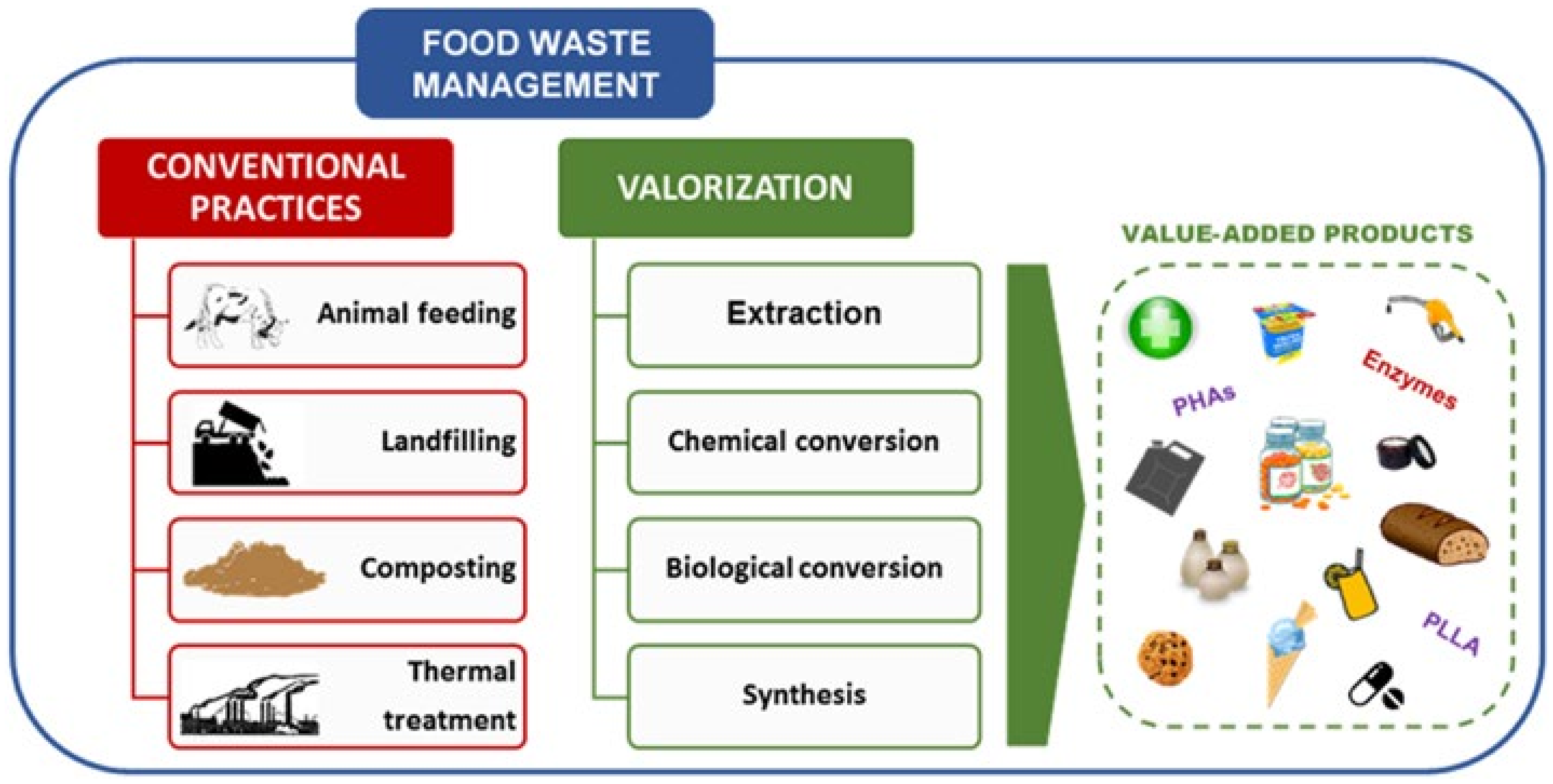
1. Introduction
2. Active Packaging and pH Indicator Film
2.1. Active Packaging
2.2. pH Indicator Films
3. Biocomposites
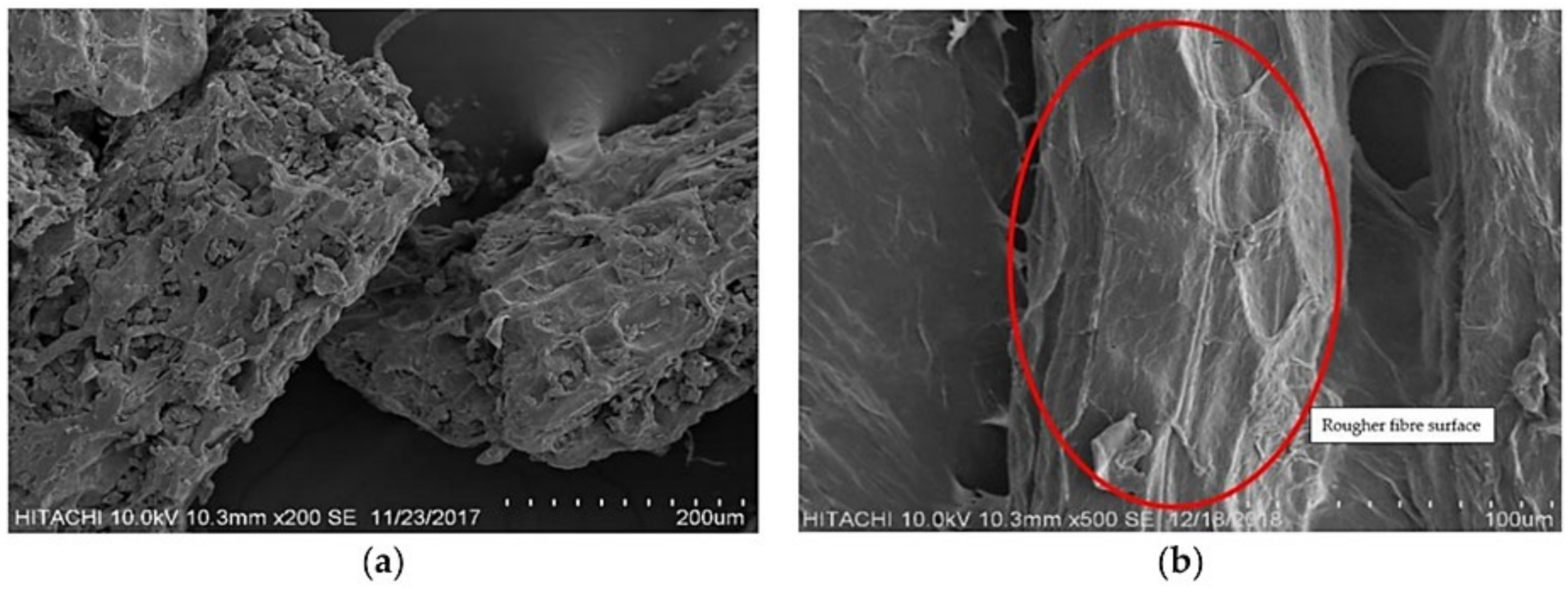
3.1. Lignocellulosic Fiber
3.2. Extract
3.3. Powder and Husk
3.4. Isolation of Fiber from FVW
4. By-Products
5. Innovative Technologies Used for Bioactive Compound Extraction
5.1. Thermal Extraction
5.1.1. Conventional Heat Extraction
5.1.2. Soxhlet Extraction
5.1.3. Microwave-Assisted Extraction
5.1.4. Pressurized Liquid Extraction
5.1.5. Subcritical Water Extraction
5.2. Non-Thermal Extraction
5.2.1. High-Pressure Processing
5.2.2. Supercritical Fluid Extraction (SFE)
5.2.3. Pulsed Electric Field (PEF)
5.2.4. Ultrasound-Assisted Extraction (UAE)
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tonini, D.; Albizzati, P.F.; Astrup, T.F. Environmental impacts of food waste: Learnings and challenges from a case study on UK. Waste Manag. 2018, 76, 744–766. [Google Scholar] [CrossRef]
- Stenmarck, Â.; Jensen, C.; Quested, T.; Moates, G.; Buksti, M.; Cseh, B.; Juul, S.; Parry, A.; Politano, A.; Redlingshofer, B. Estimates of European Food Waste Levels; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016. [Google Scholar]
- FAO. The State of Food and Agriculture 2019: Moving forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019; pp. 2–13. [Google Scholar]
- International Food Policy Research Institute. 2019 Global Food Policy Report; International Food Policy Research Institute: Washington, DC, USA, 2019. [Google Scholar]
- Fiore, M.; Pellegrini, G.; Sala, P.L.; Conte, A.; Liu, B. Attitude toward food waste reduction: The case of Italian consumers. Int. J. Glob. Small Bus. 2017, 9, 185–201. [Google Scholar] [CrossRef]
- Mc Carthy, U.; Uysal, I.; Badia-Melis, R.; Mercier, S.; O’Donnell, C.; Ktenioudaki, A. Global food security–Issues, challenges and technological solutions. Trends Food Sci. Technol. 2018, 77, 11–20. [Google Scholar] [CrossRef]
- FAO. Tracking Progress on Food and Agriculture-Related SDG Indicators 2020—A Report on the Indicators under FAO Custodianship; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Edwiges, T.; Frare, L.; Mayer, B.; Lins, L.; Triolo, J.M.; Flotats, X.; de Mendonça Costa, M.S.S. Influence of chemical composition on biochemical methane potential of fruit and vegetable waste. Waste Manag. 2018, 71, 618–625. [Google Scholar] [CrossRef]
- Negri, C.; Ricci, M.; Zilio, M.; D’Imporzano, G.; Qiao, W.; Dong, R.; Adani, F. Anaerobic digestion of food waste for bio-energy production in China and Southeast Asia: A review. Renew. Sustain. Energy Rev. 2020, 133, 110138. [Google Scholar] [CrossRef]
- Yang, J.; Cai, W.; Ma, M.; Li, L.; Liu, C.; Ma, X.; Li, L.; Chen, X. Driving forces of China’s CO2 emissions from energy consumption based on Kaya-LMDI methods. Sci. Total. Environ. 2020, 711, 134569. [Google Scholar] [CrossRef]
- Bong, C.P.C.; Ho, W.S.; Hashim, H.; Lim, J.S.; Ho, C.S.; Tan, W.S.P.; Lee, C.T. Review on the renewable energy and solid waste management policies towards biogas development in Malaysia. Renew. Sustain. Energy Rev. 2017, 70, 988–998. [Google Scholar] [CrossRef]
- De Laurentiis, V.; Corrado, S.; Sala, S. Quantifying household waste of fresh fruit and vegetables in the EU. Waste Manag. 2018, 77, 238–251. [Google Scholar] [CrossRef]
- Barrera, E.L.; Hertel, T. Global food waste across the income spectrum: Implications for food prices, production and resource use. Food Policy 2021, 98, 101874. [Google Scholar] [CrossRef]
- Brizi, A.; Biraglia, A. “Do I have enough food”? How need for cognitive closure and gender impact stockpiling and food waste during the COVID-19 pandemic: A cross-national study in India and the United States of America. Personal. Individ. Differ. 2021, 168, 110396. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.-E.; Liu, G.; Cheng, S. Rural household food waste characteristics and driving factors in China. Resour. Conserv. Recycl. 2021, 164, 105209. [Google Scholar] [CrossRef]
- McCarthy, B.; Kapetanaki, A.B.; Wang, P. Completing the food waste management loop: Is there market potential for value-added surplus products (VASP)? J. Clean. Prod. 2020, 256, 120435. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef] [PubMed]
- Fabi, C.; Cachia, F.; Conforti, P.; English, A.; Moncayo, J.R. Improving data on food losses and waste: From theory to practice. Food Policy 2021, 98, 101934. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Álvarez, C.; Mullen, A.M.; Pojić, M.; Hadnađev, T.D.; Papageorgiou, M. Classification and target compounds. In Food Waste Recovery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 21–49. [Google Scholar]
- Li, W.; Bhat, S.A.; Li, J.; Cui, G.; Wei, Y.; Yamada, T.; Li, F. Effect of excess activated sludge on vermicomposting of fruit and vegetable waste by using novel vermireactor. Bioresour. Technol. 2020, 302, 122816. [Google Scholar] [CrossRef]
- Chen, D.M.-C.; Bodirsky, B.L.; Krueger, T.; Mishra, A.; Popp, A. The world’s growing municipal solid waste: Trends and impacts. Environ. Res. Lett. 2020, 15, 074021. [Google Scholar] [CrossRef]
- Liakou, V.; Pateraki, C.; Palaiogeorgou, A.-M.; Kopsahelis, N.; de Castro, A.M.; Freire, D.M.G.; Nychas, G.-J.E.; Papanikolaou, S.; Koutinas, A. Valorisation of fruit and vegetable waste from open markets for the production of 2, 3-butanediol. Food Bioprod. Process. 2018, 108, 27–36. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and vegetable waste management and the challenge of fresh-cut salad. Trends Food Sci. Technol. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Chatterjee, B.; Mazumder, D. New approach of characterizing fruit and vegetable waste (FVW) to ascertain its biological stabilization via two-stage anaerobic digestion (AD). Biomass Bioenergy 2020, 139, 105594. [Google Scholar] [CrossRef]
- Bayram, B.; Ozkan, G.; Kostka, T.; Capanoglu, E.; Esatbeyoglu, T. Valorization and Application of Fruit and Vegetable Wastes and By-Products for Food Packaging Materials. Molecules 2021, 26, 4031. [Google Scholar] [CrossRef]
- Kadzińska, J.; Janowicz, M.; Kalisz, S.; Bryś, J.; Lenart, A. An overview of fruit and vegetable edible packaging materials. Packag. Technol. Sci. 2019, 32, 483–495. [Google Scholar] [CrossRef]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Figueroa, K.H.N.; García, N.V.M.; Vega, R.C. Cocoa By-Products. In Food Wastes and By-Products: Nutraceutical and Health Potential; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 373–411. [Google Scholar]
- Trigo, J.P.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products–Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 1388–1416. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 91, pp. 157–225. [Google Scholar]
- Calderón-Oliver, M.; López-Hernández, L.H. Food vegetable and fruit waste used in meat products. Food Rev. Int. 2020, 1–27. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, S.J.; Choi, D.S.; Hur, S.J. Current topics in active and intelligent food packaging for preservation of fresh foods. J. Sci. Food Agric. 2015, 95, 2799–2810. [Google Scholar] [CrossRef]
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1126. [Google Scholar] [CrossRef]
- Dash, K.K.; Ali, N.A.; Das, D.; Mohanta, D. Thorough evaluation of sweet potato starch and lemon-waste pectin based-edible films with nano-titania inclusions for food packaging applications. Int. J. Biol. Macromol. 2019, 139, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Walkiewicz, A.; Pertile, G.; Frąc, M.; Cieślak, K.J.; Zdunek, A. Evaluation of nanocomposite made of polylactic acid and nanocellulose from carrot pomace modified with silver nanoparticles. Polymers 2020, 12, 812. [Google Scholar] [CrossRef] [PubMed]
- Montero, Y.; Souza, A.G.; Oliveira, É.R.; dos Santos Rosa, D. Nanocellulose functionalized with cinnamon essential oil: A potential application in active biodegradable packaging for strawberry. Sustain. Mater. Technol. 2021, 29, e00289. [Google Scholar]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Melo-Banda, J.A.; Páramo-García, U.; Paraguay-Delgado, F.; García-Alamilla, R.; Martínez-Hernández, A.L.; Zapién-Castillo, S. Chitosan-starch films with natural extracts: Physical, chemical, morphological and thermal properties. Materials 2018, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Luchese, C.L.; Garrido, T.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Development and characterization of cassava starch films incorporated with blueberry pomace. Int. J. Biol. Macromol. 2018, 106, 834–839. [Google Scholar] [CrossRef]
- De Moraes Crizel, T.; Costa, T.M.H.; de Oliveira Rios, A.; Flôres, S.H. Valorization of food-grade industrial waste in the obtaining active biodegradable films for packaging. Ind. Crop. Prod. 2016, 87, 218–228. [Google Scholar] [CrossRef]
- Shukor, U.; Nordin, N.; Tawakkal, I.; Talib, R.; Othman, S. Utilization of jackfruit peel waste for the production of biodegradable and active antimicrobial packaging films. In Biopolymers and Biocomposites from Agro-Waste for Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 171–192. [Google Scholar]
- Priyadarshi, R.; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Othman, S.H.; Tarmiti, N.A.N.; Shapi’i, R.A.; Zahiruddin, S.M.M.; Tawakkal, I.S.M.A.; Basha, R.K. Starch/banana pseudostem biocomposite films for potential food packaging applications. BioResources 2020, 15, 3984–3998. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Lee, J.Y.; Lee, Y.S. Development of polyvinyl alcohol and apple pomace bio-composite film with antioxidant properties for active food packaging application. J. Food Sci. Technol. 2016, 53, 1608–1619. [Google Scholar] [CrossRef]
- Lin, D.; Zheng, Y.; Wang, X.; Huang, Y.; Ni, L.; Chen, X.; Wu, Z.; Huang, C.; Yi, Q.; Li, J. Study on physicochemical properties, antioxidant and antimicrobial activity of okara soluble dietary fiber/sodium carboxymethyl cellulose/thyme essential oil active edible composite films incorporated with pectin. Int. J. Biol. Macromol. 2020, 165, 1241–1249. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Ziaee, E.; Eskandari, M.H.; Hosseini, S.M.H. Characterization of basil seed gum-based edible films incorporated with Zataria multiflora essential oil nanoemulsion. Carbohydr. Polym. 2017, 166, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Caetano, K.; Lopes, N.A.; Costa, T.M.H.; Brandelli, A.; Rodrigues, E.; Flôres, S.H.; Cladera-Olivera, F. Characterization of active biodegradable films based on cassava starch and natural compounds. Food Packag. Shelf Life 2018, 16, 138–147. [Google Scholar] [CrossRef]
- Emam-Djomeh, Z.; Moghaddam, A.; Yasini Ardakani, S.A. Antimicrobial activity of pomegranate (Punica granatum L.) peel extract, physical, mechanical, barrier and antimicrobial properties of pomegranate peel extract-incorporated sodium caseinate film and application in packaging for ground beef. Packag. Technol. Sci. 2015, 28, 869–881. [Google Scholar] [CrossRef]
- Madhusudan, P.; Chellukuri, N.; Shivakumar, N. Smart packaging of food for the 21st century–A review with futuristic trends, their feasibility and economics. Mater. Today Proc. 2018, 5, 21018–21022. [Google Scholar] [CrossRef]
- Luchese, C.L.; Abdalla, V.F.; Spada, J.C.; Tessaro, I.C. Evaluation of blueberry residue incorporated cassava starch film as pH indicator in different simulants and foodstuffs. Food Hydrocoll. 2018, 82, 209–218. [Google Scholar] [CrossRef]
- Luchese, C.L.; Sperotto, N.; Spada, J.C.; Tessaro, I.C. Effect of blueberry agro-industrial waste addition to corn starch-based films for the production of a pH-indicator film. Int. J. Biol. Macromol. 2017, 104, 11–18. [Google Scholar] [CrossRef]
- Sohany, M.; Tawakkal, I.S.M.A.; Ariffin, S.H.; Shah, N.N.A.K.; Yusof, Y.A. Characterization of Anthocyanin Associated Purple Sweet Potato Starch and Peel-Based pH Indicator Films. Foods 2021, 10, 2005. [Google Scholar] [CrossRef]
- Chen, S.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S. Development of pH indicator and antimicrobial cellulose nanofibre packaging film based on purple sweet potato anthocyanin and oregano essential oil. Int. J. Biol. Macromol. 2020, 149, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Z.; Zhang, M.; Bhandari, B.; Yang, C.-H. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 2020, 100, 105438. [Google Scholar] [CrossRef]
- Ezati, P.; Tajik, H.; Moradi, M.; Molaei, R. Intelligent pH-sensitive indicator based on starch-cellulose and alizarin dye to track freshness of rainbow trout fillet. Int. J. Biol. Macromol. 2019, 132, 157–165. [Google Scholar] [CrossRef]
- Andretta, R.; Luchese, C.L.; Tessaro, I.C.; Spada, J.C. Development and characterization of pH-indicator films based on cassava starch and blueberry residue by thermocompression. Food Hydrocoll. 2019, 93, 317–324. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, X.; Qin, Y.-R.; Zhang, Y.-H.; Wang, X.-P.; Wang, J.-Y.; Ning, Z.-X.; Ruan, Q.-J.; Zhang, Y.-S. Preparation and characterization of a novel colorimetric indicator film based on gelatin/polyvinyl alcohol incorporating mulberry anthocyanin extracts for monitoring fish freshness. Food Res. Int. 2019, 126, 108604. [Google Scholar] [CrossRef]
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf Life 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Ventorino, V.; Robertiello, A.; Viscardi, S.; Ambrosanio, A.; Faraco, V.; Pepe, O. Bio-based chemical production from Arundo donax feedstock fermentation using Cosenzaea myxofaciens BPM1. BioResources 2016, 11, 6566–6581. [Google Scholar] [CrossRef]
- Kringel, D.H.; Dias, A.R.G.; Zavareze, E.d.R.; Gandra, E.A. Fruit wastes as promising sources of starch: Extraction, properties, and applications. Starch-Stärke 2020, 72, 1900200. [Google Scholar] [CrossRef]
- Hassan, M.A.; Yee, L.-N.; Yee, P.L.; Ariffin, H.; Raha, A.R.; Shirai, Y.; Sudesh, K. Sustainable production of polyhydroxyalkanoates from renewable oil-palm biomass. Biomass Bioenergy 2013, 50, 1–9. [Google Scholar] [CrossRef]
- Sulaiman, A.; Othman, N.; Baharuddin, A.S.; Mokhtar, M.N.; Tabatabaei, M. Enhancing the halal food industry by utilizing food wastes to produce value-added bioproducts. Procedia-Soc. Behav. Sci. 2014, 121, 35–43. [Google Scholar] [CrossRef]
- Reis, M.; Albuquerque, M.; Villano, M.; Majone, M. Mixed Culture Processes for Polyhydroxyalkanoate Production from Agro-Industrial Surplus/Wastes as Feedstocks; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Chumee, J.; Khemmakama, P. Carboxymethyl cellulose from pineapple peel: Useful green bioplastic. Adv. Mater. Res. 2014, 979, 366–369. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef]
- Kim, H.; Moon, J.Y.; Kim, H.; Lee, D.-S.; Cho, M.; Choi, H.-K.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010, 121, 429–436. [Google Scholar] [CrossRef]
- Wang, S.; Marcone, M.F.; Barbut, S.; Lim, L.-T. Fortification of dietary biopolymers-based packaging material with bioactive plant extracts. Food Res. Int. 2012, 49, 80–91. [Google Scholar] [CrossRef]
- Mohd Nordin, N.; Anuar, H.; Buys, Y.F.; Ali, F.; Thomas, S.; Mohd Nasir, N.A. Effect of freeze-dried durian skin nanofiber on the physical properties of poly(lactic acid) biocomposites. Polym. Compos. 2021, 42, 842–848. [Google Scholar] [CrossRef]
- Singh, V.K.; Mukhopadhyay, S. Studies on the effect of hybridization on sound insulation of coir-banana-polypropylene hybrid biocomposites. J. Nat. Fibers 2020, 1–10. [Google Scholar] [CrossRef]
- Anuar, H.; Abd Rashid, S.M.S.; Nordin, N.M.; Ali, F.; Buys, Y.F.; Thomas, S.; Nasir, N.A.M.; Asri, S.E.A.M. Potential of fabrication of durian skin fiber biocomposites for food packaging application through the electricity impact analysis. IIUM Eng. J. 2021, 22, 294–305. [Google Scholar] [CrossRef]
- Gisan, K.; Chan, M.; Koay, S. Solvent-cast Biofilm from Poly(lactic) Acid and Durian Husk Fiber: Tensile, Water Absorption, and Biodegradation Behaviors. J. Nat. Fibers 2020, 1–12. [Google Scholar] [CrossRef]
- Ong, H.; Kam, K.; Islam, A.; Bautista-Patacsil, L.; Villagracia, A. Flexural and thermal properties of biocomposites from Cerbera manghas L. and polypropylene. In Proceedings of the 1st International Conference on Biomass Utilization and Sustainable Energy, Virtual Conference, Malaysia, 15–16 December 2020; p. 012050. [Google Scholar]
- Żelaziński, T. Properties of biocomposites from rapeseed meal, fruit pomace and microcrystalline cellulose made by press pressing: Mechanical and physicochemical characteristics. Materials 2021, 14, 890. [Google Scholar] [CrossRef]
- Tanwar, R.; Gupta, V.; Kumar, P.; Kumar, A.; Singh, S.; Gaikwad, K.K. Development and characterization of PVA-starch incorporated with coconut shell extract and sepiolite clay as an antioxidant film for active food packaging applications. Int. J. Biol. Macromol. 2021, 185, 451–461. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Effect of date fruit waste extract as an antioxidant additive on the properties of active gelatin films. Food Chem. 2021, 355, 129631. [Google Scholar] [CrossRef]
- Mardijanti, D.S.; Megantara, E.N.; Bahtiar, A.; Sunardi, S. Turning the Cocopith Waste into Myceliated Biocomposite to Make an Insulator. Int. J. Biomater. 2021, 2021, 6630657. [Google Scholar] [CrossRef]
- Nagarjun, J.; Kanchana, J.; RajeshKumar, G.; Manimaran, S.; Krishnaprakash, M. Enhancement of Mechanical Behavior of PLA Matrix Using Tamarind and Date Seed Micro Fillers. J. Nat. Fibers 2021, 1–13. [Google Scholar] [CrossRef]
- Reinaldo, J.S.; Milfont, C.H.; Gomes, F.P.; Mattos, A.L.; Medeiros, F.G.; Lopes, P.F.; Matsui, K.N.; Ito, E.N. Influence of grape and acerola residues on the antioxidant, physicochemical and mechanical properties of cassava starch biocomposites. Polym. Test. 2021, 93, 107015. [Google Scholar] [CrossRef]
- Marzuki, M.N.A.; Tawakkal, I.S.M.A.; Basri, M.S.M.; Othman, S.H.; Kamarudin, S.H.; Lee, C.H.; Khalina, A. The effect of jackfruit skin powder and fiber bleaching treatment in pla composites with incorporation of thymol. Polymers 2020, 12, 2622. [Google Scholar] [CrossRef]
- Farhadi, S.; Javanmard, M. Mechanical and physical properties of polyethylene/sour cherry shell powder bio-composite as potential food packaging. Food Sci. Nutr. 2021, 9, 3071–3077. [Google Scholar] [CrossRef]
- Torres, F.G.; Mayorga, J.P.; Vilca, C.; Arroyo, J.; Castro, P.; Rodriguez, L. Preparation and characterization of a novel starch–chestnut husk biocomposite. SN Appl. Sci. 2019, 1, 1–7. [Google Scholar] [CrossRef]
- Peças, P.; Carvalho, H.; Salman, H.; Leite, M. Natural fibre composites and their applications: A review. J. Compos. Sci. 2018, 2, 66. [Google Scholar] [CrossRef]
- Soofi, M.; Alizadeh, A.; Hamishehkar, H.; Almasi, H.; Roufegarinejad, L. Preparation of nanobiocomposite film based on lemon waste containing cellulose nanofiber and savory essential oil: A new biodegradable active packaging system. Int. J. Biol. Macromol. 2021, 169, 352–361. [Google Scholar] [CrossRef]
- Rasid, N.; Nazmi, N.; Isa, M.; Sarbon, N. Rheological, functional and antioxidant properties of films forming solution and active gelatin films incorporated with Centella asiatica (L.) urban extract. Food Packag. Shelf Life 2018, 18, 115–124. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Kaanin-Boudraa, G.; Brahmi, F.; Wrona, M.; Nerín, C.; Moudache, M.; Mouhoubi, K.; Madani, K.; Boulekbache-Makhlouf, L. Response surface methodology and UPLC-QTOF-MSE analysis of phenolic compounds from grapefruit (Citrus × paradisi) by-products as novel ingredients for new antioxidant packaging. LWT 2021, 151, 112158. [Google Scholar] [CrossRef]
- Lalnunthari, C.; Devi, L.M.; Amami, E.; Badwaik, L.S. Valorisation of pumpkin seeds and peels into biodegradable packaging films. Food Bioprod. Process. 2019, 118, 58–66. [Google Scholar] [CrossRef]
- Hanani, Z.N.; Husna, A.A.; Syahida, S.N.; Khaizura, M.N.; Jamilah, B. Effect of different fruit peels on the functional properties of gelatin/polyethylene bilayer films for active packaging. Food Packag. Shelf Life 2018, 18, 201–211. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Herniou-Julien, C.; Álvarez, K.; Alvarez, V.A. Structural properties and in vitro digestibility of edible and pH-sensitive films made from guinea arrowroot starch and wastes from wine manufacture. Carbohydr. Polym. 2018, 184, 135–143. [Google Scholar] [CrossRef]
- Deng, Q.; Zhao, Y. Physicochemical, nutritional, and antimicrobial properties of wine grape (cv. Merlot) pomace extract-based films. J. Food Sci. 2011, 76, E309–E317. [Google Scholar] [CrossRef]
- Suradi, S.; Yunus, R.; Beg, M.; Rivai, M.; Yusof, Z. Oil palm bio-fiber reinforced thermoplastic composites-effects of matrix modification on mechanical and thermal properties. J. Appl. Sci. 2010, 10, 3271–3276. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Sangnitidej, P.; Boonpasith, P. Properties of thermoplastic rice starch composites reinforced by cotton fiber or low-density polyethylene. Carbohydr. Polym. 2010, 81, 425–433. [Google Scholar] [CrossRef]
- Cheng, W. Preparation and properties of lignocellulosic fiber/CaCO3/thermoplastic starch composites. Carbohydr. Polym. 2019, 211, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Ochi, S. Fabrication of Manila Hemp Fiber Reinforced Cross Ply Biodegradable Composites and Their Tensile Properties. Open J. Compos. Mater. 2018, 8, 75. [Google Scholar] [CrossRef][Green Version]
- Leites, L.C.; Frick, P.J.M.; Cristina, T.I. Influence of the incorporation form of waste from the production of orange juice in the properties of cassava starch-based films. Food Hydrocoll. 2021, 117, 106730. [Google Scholar] [CrossRef]
- Tavker, N.; Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.; Alam, J.; Shukla, A.K.; Ali, F.A.A.; Alhoshan, M. Removal of cadmium and chromium by mixture of silver nanoparticles and nano-fibrillated cellulose isolated from waste peels of citrus sinensis. Polymers 2021, 13, 234. [Google Scholar] [CrossRef]
- Phirom-on, K.; Apiraksakorn, J. Development of cellulose-based prebiotic fiber from banana peel by enzymatic hydrolysis. Food Biosci. 2021, 41, 101083. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.; Siddiqui, M.W.; Dávila-Aviña, J.; González-Aguilar, G. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Khoozani, A.A.; Birch, J.; Bekhit, A.E.-D.A. Production, application and health effects of banana pulp and peel flour in the food industry. J. Food Sci. Technol. 2019, 56, 548–559. [Google Scholar] [CrossRef]
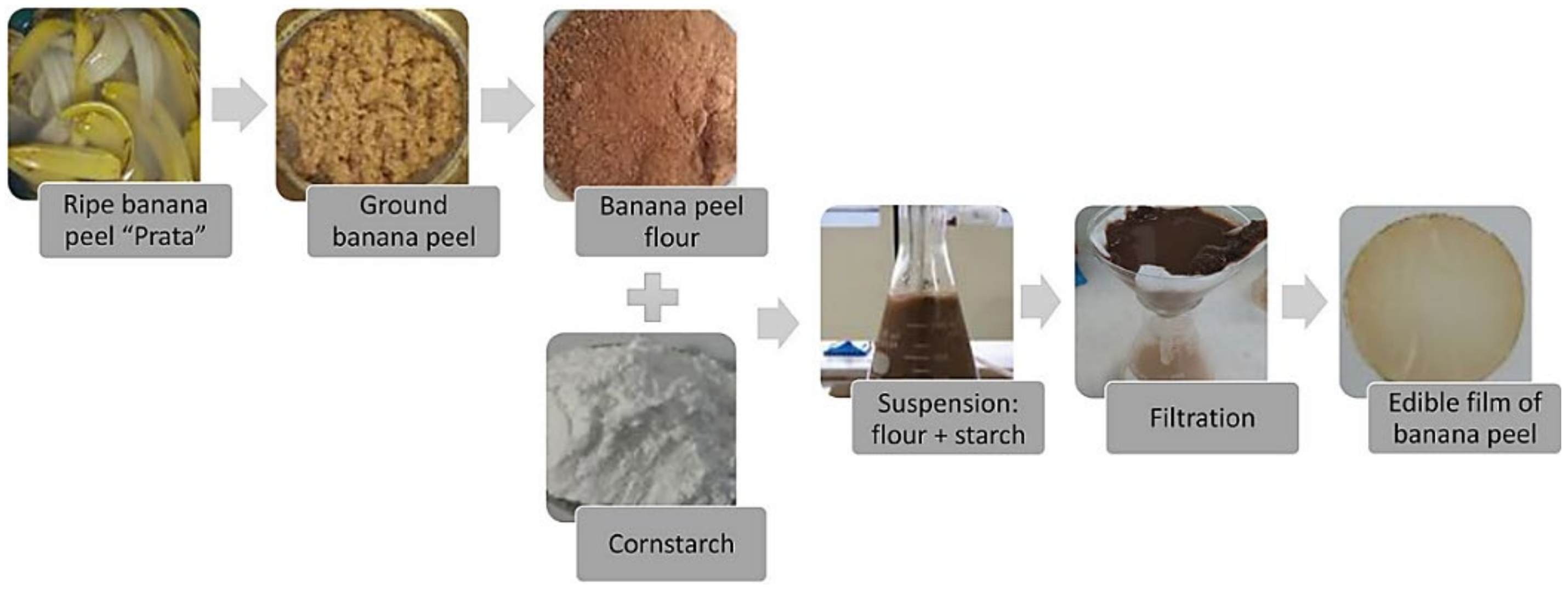
- De Faria Arquelau, P.B.; Silva, V.D.M.; Garcia, M.A.V.T.; de Araújo, R.L.B.; Fante, C.A. Characterization of edible coatings based on ripe “Prata” banana peel flour. Food Hydrocoll. 2019, 89, 570–578. [Google Scholar] [CrossRef]
- Reißner, A.M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and physicochemical properties of dried berry pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, R.M.S.; Silva, S.; Costa, C.M.d.S.F.; Veiga, M.; Costa, E.; Ferreira, M.S.L.; de Andrade Gonçalves, E.C.B.; Pintado, M.E. Potential prebiotic effect of fruit and vegetable byproducts flour using in vitro gastrointestinal digestion. Food Res. Int. 2020, 137, 109354. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Gupta, A.; Issar, K. Effect of packaging and storage on dried apple pomace and fiber extracted from pomace. J. Food Process. Preserv. 2017, 41, e12913. [Google Scholar] [CrossRef]
- Mohankumararadhya, H.; Wadappi, P.; Chandrashekar, A.; Naik, Y. Studies on bio waste product particle reinforced polymer composites. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2020; p. 030047. [Google Scholar]
- Begum, Y.A.; Deka, S.C. Effect of processing on structural, thermal, and physicochemical properties of dietary fiber of culinary banana bracts. J. Food Process. Preserv. 2019, 43, e14256. [Google Scholar] [CrossRef]
- Mir, S.A.; Bosco, S.J.D.; Shah, M.A.; Santhalakshmy, S.; Mir, M.M. Effect of apple pomace on quality characteristics of brown rice based cracker. J. Saudi Soc. Agric. Sci. 2017, 16, 25–32. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of pectin extracted from banana peels of different varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B.; Al-Dhabi, N.A.; Ponmurugan, K.; Moorthy, I.G.; Sivarajasekar, N. Ultrasound assisted citric acid mediated pectin extraction from industrial waste of Musa balbisiana. Ultrason. Sonochem. 2017, 35, 204–209. [Google Scholar] [CrossRef]
- Moorthy, I.G.; Maran, J.P.; Ilakya, S.; Anitha, S.; Sabarima, S.P.; Priya, B. Ultrasound assisted extraction of pectin from waste Artocarpus heterophyllus fruit peel. Ultrason. Sonochem. 2017, 34, 525–530. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Najari, Z. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int. J. Biol. Macromol. 2019, 125, 621–629. [Google Scholar] [CrossRef]
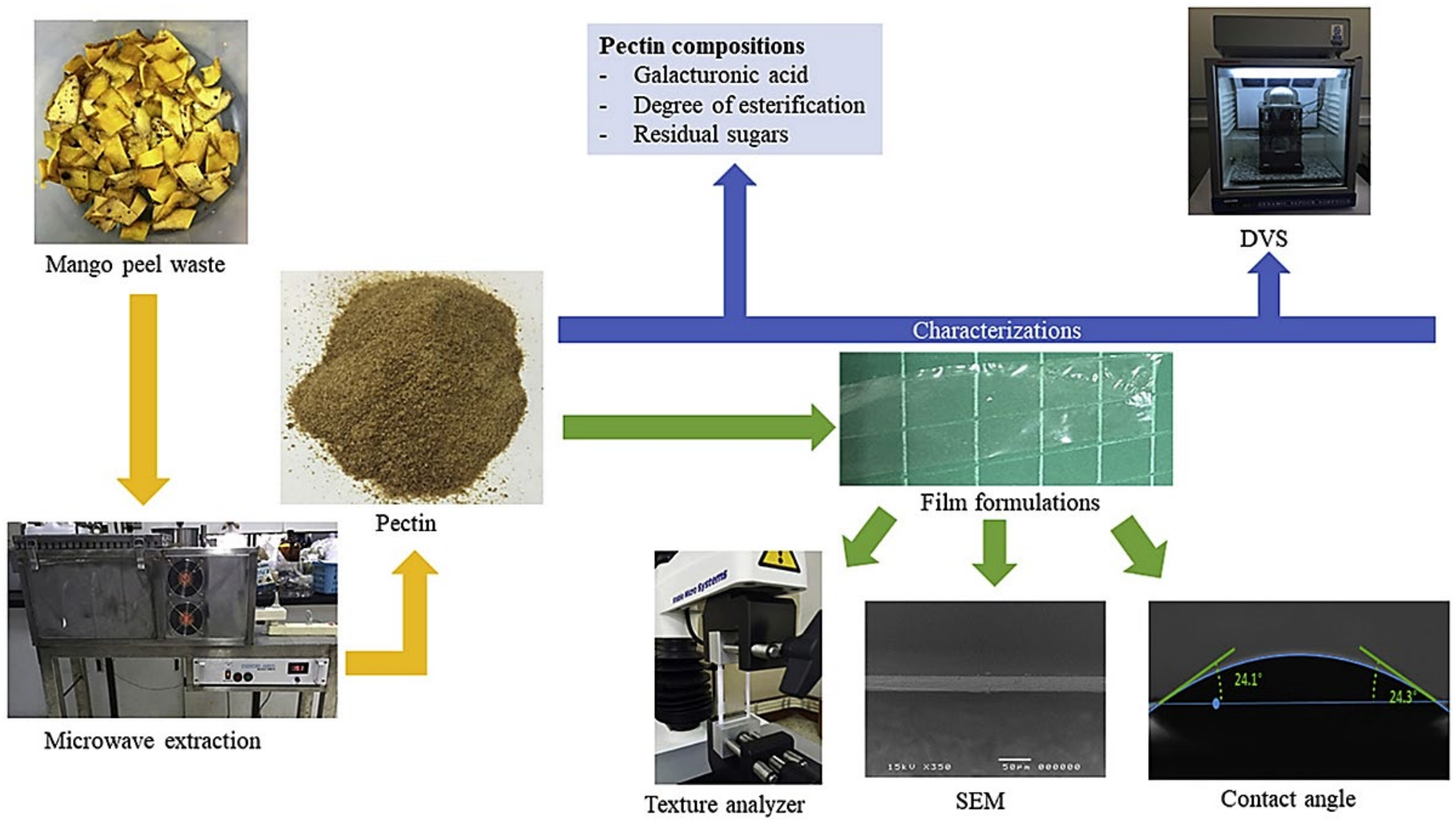
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res. Int. 2019, 119, 455–461. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Masavang, S.; Mahe, J.; Sommano, S.; Ruksiriwanich, W.; Brachais, C.-H.; Chambin, O.; Jantrawut, P. Mango (cv. Nam Dokmai) peel as a source of pectin and its potential use as a film-forming polymer. Food Hydrocoll. 2020, 102, 105611. [Google Scholar] [CrossRef]
- Laysandra, L.; Santosa, F.H.; Austen, V.; Soetaredjo, F.E.; Foe, K.; Putro, J.N.; Ju, Y.-H.; Ismadji, S. Rarasaponin-bentonite-activated biochar from durian shells composite for removal of crystal violet and Cr (VI) from aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 30680–30695. [Google Scholar] [CrossRef]
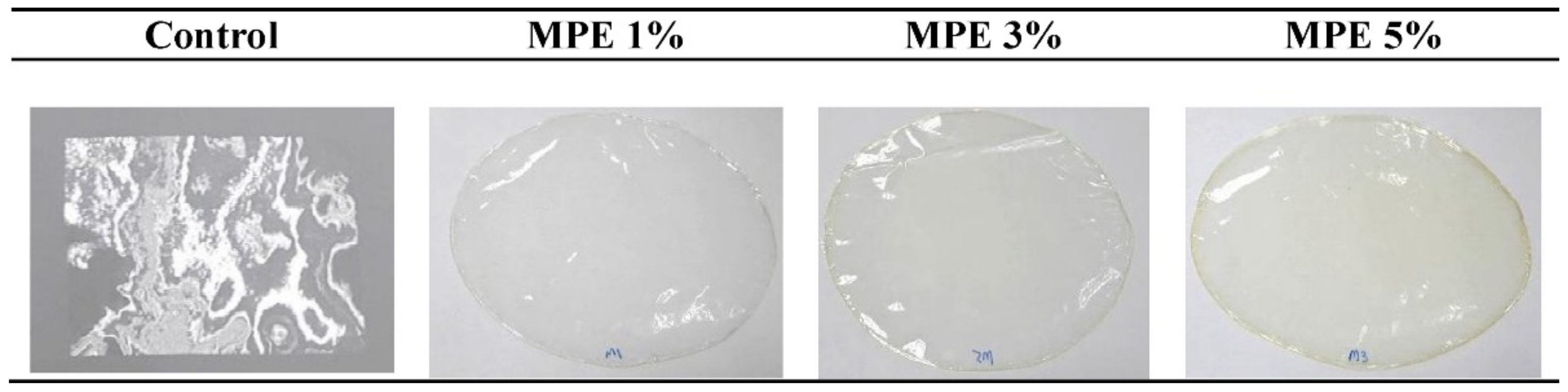
- Adilah, A.N.; Jamilah, B.; Noranizan, M.; Hanani, Z.N. Utilization of mango peel extracts on the biodegradable films for active packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Isopencu, G.O.; Stoica-Guzun, A.; Busuioc, C.; Stroescu, M.; Deleanu, I.M. Development of antioxidant and antimicrobial edible coatings incorporating bacterial cellulose, pectin, and blackberry pomace. Carbohydr. Polym. Technol. Appl. 2021, 2, 100057. [Google Scholar]
- Priyadarshi, R.; Riahi, Z.; Rhim, J.-W. Antioxidant pectin/pullulan edible coating incorporated with Vitis vinifera grape seed extract for extending the shelf life of peanuts. Postharvest Biol. Technol. 2022, 183, 111740. [Google Scholar] [CrossRef]
- Amofa-Diatuo, T.; Anang, D.M.; Barba, F.J.; Tiwari, B.K. Development of new apple beverages rich in isothiocyanates by using extracts obtained from ultrasound-treated cauliflower by-products: Evaluation of physical properties and consumer acceptance. J. Food Compos. Anal. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Majumdar, S.; Naha, A.; Bhattacharyya, D.; Bhowal, J. Effective delignification and decrystallization of cauliflower wastes by using dilute phosphoric acid for efficient enzymatic digestibility to produce fermentable sugars. Biomass Bioenergy 2019, 125, 169–179. [Google Scholar] [CrossRef]
- Iwassa, I.J.; Piai, J.F.; Bolanho, B.C. Fiber concentrates from asparagus by-products: Microstructure, composition, functional and antioxidant properties. Ciência Agrotecnol. 2019, 43, e007319. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Eggplant peel as a high potential source of high methylated pectin: Ultrasonic extraction optimization and characterization. LWT 2019, 105, 182–189. [Google Scholar] [CrossRef]
- Chiboub, W.; Sassi, A.B.; Amina, C.M.h.; Souilem, F.; El Ayeb, A.; Djlassi, B.; Ascrizzi, R.; Flamini, G.; Harzallah-Skhiri, F. Valorization of the green waste from two varieties of fennel and carrot cultivated in tunisia by identification of the phytochemical profile and evaluation of the antimicrobial activities of their essentials oils. Chem. Biodivers. 2019, 16, e1800546. [Google Scholar] [CrossRef]
- Caliceti, C.; Capriotti, A.; Calabria, D.; Bonvicini, F.; Zenezini Chiozzi, R.; Montone, C.; Piovesana, S.; Zangheri, M.; Mirasoli, M.; Simoni, P. Peptides from cauliflower by-products, obtained by an efficient, ecosustainable, and semi-industrial method, exert protective effects on endothelial function. Oxidative Med. Cell. Longev. 2019, 2019, 1046504. [Google Scholar] [CrossRef]
- Otoni, C.G.; Lodi, B.D.; Lorevice, M.V.; Leitão, R.C.; Ferreira, M.D.; de Moura, M.R.; Mattoso, L.H. Optimized and scaled-up production of cellulose-reinforced biodegradable composite films made up of carrot processing waste. Ind. Crop. Prod. 2018, 121, 66–72. [Google Scholar] [CrossRef]
- Uçak, I. Physicochemical and antimicrobial effects of gelatin-based edible films incorporated with garlic peel extract on the rainbow trout fillets. Prog. Nutr. 2019, 21, 232–240. [Google Scholar]
- Liu, C.; Jin, T.; Liu, W.; Hao, W.; Yan, L.; Zheng, L. Effects of hydroxyethyl cellulose and sodium alginate edible coating containing asparagus waste extract on postharvest quality of strawberry fruit. LWT 2021, 148, 111770. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Rakariyatham, K.; Zhou, D.; Rakariyatham, N.; Shahidi, F. Sapindaceae (Dimocarpus longan and Nephelium lappaceum) seed and peel by-products: Potential sources for phenolic compounds and use as functional ingredients in food and health applications. J. Funct. Foods 2020, 67, 103846. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aslam Maan, A.; Khan, M.K.I.; Nadeem, M.; Khalil, A.A.; Din, A.; Aadil, R.M. An inclusive overview of advanced thermal and nonthermal extraction techniques for bioactive compounds in food and food-related matrices. Food Rev. Int. 2020, 1–31. [Google Scholar] [CrossRef]
- RedCorn, R.; Fatemi, S.; Engelberth, A.S. Comparing end-use potential for industrial food-waste sources. Engineering 2018, 4, 371–380. [Google Scholar] [CrossRef]
- Dao, T.A.T.; Webb, H.K.; Malherbe, F. Optimization of pectin extraction from fruit peels by response surface method: Conventional versus microwave-assisted heating. Food Hydrocoll. 2021, 113, 106475. [Google Scholar]
- Kwiatkowski, M.; Kravchuk, O.; Skouroumounis, G.K.; Taylor, D.K. Response surface parallel optimization of extraction of total phenolics from separate white and red grape skin mixtures with microwave-assisted and conventional thermal methods. J. Clean. Prod. 2020, 251, 119563. [Google Scholar] [CrossRef]
- Misra, N.; Yadav, S.K. Extraction of pectin from black carrot pomace using intermittent microwave, ultrasound and conventional heating: Kinetics, characterization and process economics. Food Hydrocoll. 2020, 102, 105592. [Google Scholar]
- Cherif, M.M.; Grigorakis, S.; Halahlah, A.; Loupassaki, S.; Makris, D.P. High-efficiency extraction of phenolics from wheat waste biomass (bran) by combining deep eutectic solvent, ultrasound-assisted pretreatment and thermal treatment. Environ. Process. 2020, 7, 845–859. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Brnčić, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019, 278, 364–372. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.F.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F.; Karwe, M. Combined effect of high-pressure and conventional heating on pectin extraction from passion fruit peel. Food Bioprocess Technol. 2016, 9, 1021–1030. [Google Scholar] [CrossRef]
- Hossain, S.; Taher, S.; Khan, A.; Sultana, N.; Irfan, M.; Haq, B.; Razzak, S. Experimental study and modeling approach of response surface methodology coupled with crow search algorithm for optimizing the extraction conditions of papaya seed waste oil. Arab. J. Sci. Eng. 2020, 45, 7371–7383. [Google Scholar] [CrossRef]
- De Menezes, M.L.; Johann, G.; Diório, A.; Pereira, N.C.; da Silva, E.A. Phenomenological determination of mass transfer parameters of oil extraction from grape biomass waste. J. Clean. Prod. 2018, 176, 130–139. [Google Scholar] [CrossRef]
- Hu, B.; Xi, X.; Li, H.; Qin, Y.; Li, C.; Zhang, Z.; Liu, Y.; Zhang, Q.; Liu, A.; Liu, S. A comparison of extraction yield, quality and thermal properties from Sapindus mukorossi seed oil between microwave assisted extraction and Soxhlet extraction. Ind. Crop. Prod. 2021, 161, 113185. [Google Scholar] [CrossRef]
- Flórez Montes, C.; Felipe Rojas-González, A.; Rodríguez-Barona, S. Evaluation of Extracts Obtained from Fruit Wastes Using Different Methods. Ingeniería 2021, 26, 1–11. [Google Scholar]
- Fernandez-Pastor, I.; Fernandez-Hernandez, A.; Perez-Criado, S.; Rivas, F.; Martinez, A.; Garcia-Granados, A.; Parra, A. Microwave-assisted extraction versus Soxhlet extraction to determine triterpene acids in olive skins. J. Sep. Sci. 2017, 40, 1209–1217. [Google Scholar] [CrossRef]
- Alias, N.H.; Abbas, Z. Microwave-assisted extraction of phenolic compound from pineapple skins: The optimum operating condition and comparision with soxhlet extraction. Malays. J. Anal. Sci. 2017, 21, 690–699. [Google Scholar]
- Khandare, R.D.; Tomke, P.D.; Rathod, V.K. Kinetic modeling and process intensification of ultrasound-assisted extraction of d-limonene using citrus industry waste. Chem. Eng. Process. Process. Intensif. 2021, 159, 108181. [Google Scholar] [CrossRef]
- Garude, H.; Ade, V.; Gadhave, R. Comparative Study of Various Methods for Extraction of Antioxidants Compound from Bitter Gourd Peel. Adv. Life Sci. 2016, 24, 11189–11192. [Google Scholar]
- Sukatta, U.; Rugthaworn, P.; Khanoonkon, N.; Anongjanya, P.; Kongsin, K.; Sukyai, P.; Harnkarnsujarit, N.; Sothornvit, R.; Chollakup, R. Rambutan (Nephelium lappaceum) peel extract: Antimicrobial and antioxidant activities and its application as a bioactive compound in whey protein isolate film. Songklanakarin J. Sci. Technol. 2021, 43, 37–44. [Google Scholar]
- Jridi, M.; Boughriba, S.; Abdelhedi, O.; Nciri, H.; Nasri, R.; Kchaou, H.; Kaya, M.; Sebai, H.; Zouari, N.; Nasri, M. Investigation of physicochemical and antioxidant properties of gelatin edible film mixed with blood orange (Citrus sinensis) peel extract. Food Packag. Shelf Life 2019, 21, 100342. [Google Scholar] [CrossRef]
- Košťálová, Z.; Aguedo, M.; Hromádková, Z. Microwave-assisted extraction of pectin from unutilized pumpkin biomass. Chem. Eng. Process. Process. Intensif. 2016, 102, 9–15. [Google Scholar] [CrossRef]
- Narkprasom, K.; Tanongkankit, Y.; Saenscharoenrat, P.; Narkprasom, N. Optimization of Total Phenolic from Euphoria longana Lam. Seed by Microwave Assisted Extraction. Burapha Sci. J. 2019, 24, 48–63. [Google Scholar]
- Bakić, M.T.; Pedisić, S.; Zorić, Z.; Dragović-Uzelac, V.; Grassino, A.N. Effect of microwave-assisted extraction on polyphenols recovery from tomato peel waste. Acta Chim. Slov. 2019, 66, 367–377. [Google Scholar] [CrossRef]
- Zheng, J.; Li, H.; Wang, D.; Li, R.; Wang, S.; Ling, B. Radio frequency assisted extraction of pectin from apple pomace: Process optimization and comparison with microwave and conventional methods. Food Hydrocoll. 2021, 121, 107031. [Google Scholar] [CrossRef]
- Rivadeneira, J.P.; Wu, T.; Ybanez, Q.; Dorado, A.A.; Migo, V.P.; Nayve, F.R.P.; Castillo-Israel, K.A.T. Microwave-assisted extraction of pectin from “Saba” banana peel waste: Optimization, characterization, and rheology study. Int. J. Food Sci. 2020, 2020, 8879425. [Google Scholar] [CrossRef]
- Zain, N.; Nazeri, M. Antioxidant and mineral content of pitaya peel extract obtained using microwave assisted extraction (MAE). Aust. J. Basic Appl. Sci. 2016, 10, 63–68. [Google Scholar]
- Karbuz, P.; Tugrul, N. Microwave and ultrasound assisted extraction of pectin from various fruits peel. J. Food Sci. Technol. 2021, 58, 641–650. [Google Scholar] [CrossRef]
- Wongkaew, M.; Sommano, S.R.; Tangpao, T.; Rachtanapun, P.; Jantanasakulwong, K. Mango peel pectin by microwave-assisted extraction and its use as fat replacement in dried Chinese sausage. Foods 2020, 9, 450. [Google Scholar] [CrossRef]
- Hayat, K.; Hussain, S.; Abbas, S.; Farooq, U.; Ding, B.; Xia, S.; Jia, C.; Zhang, X.; Xia, W. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep. Purif. Technol. 2009, 70, 63–70. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: Optimization, characterization and bioactivity. Carbohydr. Polym. 2019, 222, 114992. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Robinson, J.; Binner, E. Understanding heat and mass transfer processes during microwave-assisted and conventional solvent extraction. Chem. Eng. Sci. 2021, 233, 116418. [Google Scholar] [CrossRef]
- Swamy, G.J.; Muthukumarappan, K. Optimization of continuous and intermittent microwave extraction of pectin from banana peels. Food Chem. 2017, 220, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Zin, M.M.; Anucha, C.B.; Bánvölgyi, S. Recovery of phytochemicals via electromagnetic irradiation (microwave-assisted-extraction): Betalain and phenolic compounds in perspective. Foods 2020, 9, 918. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C.; Varghese, E.; Saha, S.; Sairam, K. Valorisation of black carrot pomace: Microwave assisted extraction of bioactive phytoceuticals and antioxidant activity using Box–Behnken design. J. Food Sci. Technol. 2019, 56, 995–1007. [Google Scholar] [CrossRef]
- Torres-León, C.; Rojas, R.; Serna-Cock, L.; Belmares-Cerda, R.; Aguilar, C.N. Extraction of antioxidants from mango seed kernel: Optimization assisted by microwave. Food Bioprod. Process. 2017, 105, 188–196. [Google Scholar] [CrossRef]
- Reis, L.C.; Carneiro, L.M.; Branco, C.R.; Branco, A. Comparison of conventional microwave and focused microwave-assisted extraction to enhance the efficiency of the extraction of antioxidant flavonols from jocote pomace (Spondias purpurea L.). Plant Foods Hum. Nutr. 2015, 70, 160–169. [Google Scholar] [CrossRef]
- Varadharajan, V.; Shanmugam, S.; Ramaswamy, A. Model generation and process optimization of microwave-assisted aqueous extraction of anthocyanins from grape juice waste. J. Food Process. Eng. 2017, 40, e12486. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel extraction of polyphenols from sour cherry pomace using natural deep eutectic solvents–Ultrafast microwave-assisted NADES preparation and extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Ramos, M.; Hamzaoui, M.; Kohnen, S.; Jiménez, A.; Garrigós, M.C. Optimisation of sequential microwave-assisted extraction of essential oil and pigment from lemon peels waste. Foods 2020, 9, 1493. [Google Scholar] [CrossRef] [PubMed]
- Casas, E.V.; Comedia, V.J.G.; Gilbuena, A.G.; Yaptenco, K.F. Optimizing Microwave-assisted Crude Butter Extraction from Carabao Mango (Mangifera indica) Kernels. Sci. Diliman 2015, 27, 41–75. [Google Scholar]
- Utama-Ang, N.; Sida, S.; Wanachantararak, P.; Kawee-Ai, A. Development of edible Thai rice film fortified with ginger extract using microwave-assisted extraction for oral antimicrobial properties. Sci. Rep. 2021, 11, 14870. [Google Scholar] [CrossRef] [PubMed]
- Sepelevs, I.; Zagorska, J.; Galoburda, R. A food-grade antioxidant production using industrial potato peel by–products. Agron. Res. 2020, 18, 2. [Google Scholar]
- Sari, A.; Ishartani, D.; Dewanty, P. Effects of microwave power and irradiation time on pectin extraction from watermelon rinds (Citrullus lanatus) with acetic acid using microwave assisted extraction method. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; p. 012085. [Google Scholar]
- Herrero, M.; del Pilar Sánchez-Camargo, A.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Allcca-Alca, E.E.; León-Calvo, N.C.; Luque-Vilca, O.M.; Martínez-Cifuentes, M.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Huamán-Castilla, N.L. Hot Pressurized Liquid Extraction of Polyphenols from the Skin and Seeds of Vitis vinifera L. cv. Negra Criolla Pomace a Peruvian Native Pisco Industry Waste. Agronomy 2021, 11, 866. [Google Scholar] [CrossRef]
- Viganó, J.; Brumer, I.Z.; de Campos Braga, P.A.; da Silva, J.K.; Júnior, M.R.M.; Reyes, F.G.R.; Martínez, J. Pressurized liquids extraction as an alternative process to readily obtain bioactive compounds from passion fruit rinds. Food Bioprod. Process. 2016, 100, 382–390. [Google Scholar] [CrossRef]
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of Bioactive Compounds from Pomegranate (Punica granatum L.) Peel Using Pressurized Liquid Extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Katsinas, N.; Bento da Silva, A.; Enríquez-de-Salamanca, A.; Fernández, N.; Bronze, M.R.; Rodríguez-Rojo, S. Pressurized Liquid Extraction Optimization from Supercritical Defatted Olive Pomace: A Green and Selective Phenolic Extraction Process. ACS Sustain. Chem. Eng. 2021, 9, 5590–5602. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Carrera, C.; Palma, M.; Barroso, C.G. Alternative extraction method of bioactive compounds from mulberry (Morus nigra L.) pulp using pressurized-liquid extraction. Food Anal. Methods 2018, 11, 2384–2395. [Google Scholar] [CrossRef]
- Lasta, H.F.B.; Lentz, L.; Rodrigues, L.G.G.; Mezzomo, N.; Vitali, L.; Ferreira, S.R.S. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatal. Agric. Biotechnol. 2019, 21, 101353. [Google Scholar] [CrossRef]
- Cejudo-Bastante, C.; Arjona-Mudarra, P.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J.; Pereyra, C. Application of a Natural Antioxidant from Grape Pomace Extract in the Development of Bioactive Jute Fibers for Food Packaging. Antioxidants 2021, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoez, W.; Abdelfatah, R.; Tayeb, A.; Yoshida, H. Extraction of cottonseed oil using subcritical water technology. AIChE J. 2011, 57, 2353–2359. [Google Scholar] [CrossRef]
- Tunchaiyaphum, S.; Eshtiaghi, M.; Yoswathana, N. Extraction of bioactive compounds from mango peels using green technology. Int. J. Chem. Eng. Appl. 2013, 4, 194. [Google Scholar] [CrossRef]
- Ravber, M.; Knez, Ž.; Škerget, M. Simultaneous extraction of oil-and water-soluble phase from sunflower seeds with subcritical water. Food Chem. 2015, 166, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical water extraction of bioactive compounds from plants and algae: Applications in pharmaceutical and food ingredients. Food Eng. Rev. 2016, 8, 23–34. [Google Scholar] [CrossRef]
- King, J.W.; Grabiel, R.D. Isolation of Polyphenolic Compounds from Fruits or Vegetables Utilizing Sub-Critical Water Extraction. U.S. Patent 7,208,181, 24 April 2007. [Google Scholar]
- Lu, J.; Feng, X.; Han, Y.; Xue, C. Optimization of subcritical fluid extraction of carotenoids and chlorophyll a from Laminaria japonica Aresch by response surface methodology. J. Sci. Food Agric. 2014, 94, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cheigh, C.-I.; Yoo, S.-Y.; Ko, M.-J.; Chang, P.-S.; Chung, M.-S. Extraction characteristics of subcritical water depending on the number of hydroxyl group in flavonols. Food Chem. 2015, 168, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Amashukeli, X.; Pelletier, C.C.; Kirby, J.P.; Grunthaner, F.J. Subcritical water extraction of amino acids from Atacama Desert soils. J. Geophys. Res. Biogeosci. 2007, 112, G04S16. [Google Scholar] [CrossRef]
- Singh, P.P.; Saldaña, M.D. Subcritical water extraction of phenolic compounds from potato peel. Food Res. Int. 2011, 44, 2452–2458. [Google Scholar] [CrossRef]
- Pourali, O.; Asghari, F.S.; Yoshida, H. Simultaneous rice bran oil stabilization and extraction using sub-critical water medium. J. Food Eng. 2009, 95, 510–516. [Google Scholar] [CrossRef]
- Marcet, I.; Álvarez, C.; Paredes, B.; Díaz, M. The use of sub-critical water hydrolysis for the recovery of peptides and free amino acids from food processing wastes. Review of sources and main parameters. Waste Manag. 2016, 49, 364–371. [Google Scholar] [CrossRef]
- Pedras, B.M.; Regalin, G.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Fractionation of red wine grape pomace by subcritical water extraction/hydrolysis. J. Supercrit. Fluids 2020, 160, 104793. [Google Scholar] [CrossRef]
- Guthrie, F.; Wang, Y.; Neeve, N.; Quek, S.Y.; Mohammadi, K.; Baroutian, S. Recovery of phenolic antioxidants from green kiwifruit peel using subcritical water extraction. Food Bioprod. Process. 2020, 122, 136–144. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lim, S.-B. Semi-continuous subcritical water extraction of flavonoids from citrus unshiu peel: Their antioxidant and enzyme inhibitory activities. Antioxidants 2020, 9, 360. [Google Scholar] [CrossRef]
- Limsangouan, N.; Milasing, N.; Thongngam, M.; Khuwijitjaru, P.; Jittanit, W. Physical and chemical properties, antioxidant capacity, and total phenolic content of xyloglucan component in tamarind (Tamarindus indica) seed extracted using subcritical water. J. Food Process. Preserv. 2019, 43, e14146. [Google Scholar] [CrossRef]
- Li, B.; Akram, M.; Al-Zuhair, S.; Elnajjar, E.; Munir, M.T. Subcritical water extraction of phenolics, antioxidants and dietary fibres from waste date pits. J. Environ. Chem. Eng. 2020, 8, 104490. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef]
- Kronholm, J.; Hartonen, K.; Riekkola, M.-L. Analytical extractions with water at elevated temperatures and pressures. TrAC Trends Anal. Chem. 2007, 26, 396–412. [Google Scholar] [CrossRef]
- Ndlela, S.; De Moura, J.; Olson, N.; Johnson, L. Aqueous extraction of oil and protein from soybeans with subcritical water. J. Am. Oil Chem. Soc. 2012, 89, 1145–1153. [Google Scholar] [CrossRef]
- Thani, N.M.; Kamal, S.M.M.; Taip, F.S.; Sulaiman, A.; Omar, R. Effect of sub-critical water hydrolysis on sugar recovery from bakery leftovers. Food Bioprod. Process. 2019, 117, 105–112. [Google Scholar] [CrossRef]
- Ho, T.C.; Kim, M.H.; Cho, Y.-J.; Park, J.-S.; Nam, S.Y.; Chun, B.-S. Gelatin-sodium alginate based films with Pseuderanthemum palatiferum (Nees) Radlk. freeze-dried powder obtained by subcritical water extraction. Food Packag. Shelf Life 2020, 24, 100469. [Google Scholar] [CrossRef]
- Adilah, Z.M.; Jamilah, B.; Hanani, Z.N. Functional and antioxidant properties of protein-based films incorporated with mango kernel extract for active packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaïeb, N.; Jridi, M.; Abdelhedi, O.; Karbowiak, T.; Brachais, C.-H.; Léonard, M.-L.; Debeaufort, F.; Nasri, M. Enhancement of structural, functional and antioxidant properties of fish gelatin films using Maillard reactions. Food Hydrocoll. 2018, 83, 326–339. [Google Scholar] [CrossRef]
- Ho, T.C.; Chun, B.S. Extraction of bioactive compounds from Pseuderanthemum palatiferum (Nees) Radlk. using subcritical water and conventional solvents: A Comparison Study. J. Food Sci. 2019, 84, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Mohd Thani, N.; Mustapa Kamal, S.M.; Sulaiman, A.; Taip, F.S.; Omar, R.; Izhar, S. Sugar Recovery from Food Waste via Sub-critical Water Treatment. Food Rev. Int. 2020, 36, 241–257. [Google Scholar] [CrossRef]
- Khuwijitjaru, P.; Watsanit, K.; Adachi, S. Carbohydrate content and composition of product from subcritical water treatment of coconut meal. J. Ind. Eng. Chem. 2012, 18, 225–229. [Google Scholar] [CrossRef]
- Cantero, D.A.; Bermejo, M.D.; Cocero, M.J. Reaction engineering for process intensification of supercritical water biomass refining. J. Supercrit. Fluids 2015, 96, 21–35. [Google Scholar] [CrossRef]
- Vladić, J.; Jakovljević, M.; Molnar, M.; Vidović, S.; Tomić, M.; Drinić, Z.; Jokić, S. Valorization of Yarrow (Achillea millefolium L.) By-Product through Application of Subcritical Water Extraction. Molecules 2020, 25, 1878. [Google Scholar] [CrossRef] [PubMed]
- Švarc-Gajić, J.; Morais, S.; Delerue-Matos, C.; Vieira, E.F.; Spigno, G. Valorization Potential of Oilseed Cakes by Subcritical Water Extraction. Appl. Sci. 2020, 10, 8815. [Google Scholar] [CrossRef]
- He, L.; Zhang, X.; Xu, H.; Xu, C.; Yuan, F.; Knez, Ž.; Novak, Z.; Gao, Y. Subcritical water extraction of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant activities with HPLC–ABTS+ assay. Food Bioprod. Process. 2012, 90, 215–223. [Google Scholar] [CrossRef]
- Pedras, B.; Salema-Oom, M.; Sa-Nogueira, I.; Simoes, P.; Paiva, A.; Barreiros, S. Valorization of white wine grape pomace through application of subcritical water: Analysis of extraction, hydrolysis, and biological activity of the extracts obtained. J. Supercrit. Fluids 2017, 128, 138–144. [Google Scholar] [CrossRef]
- Giombelli, C.; Iwassa, I.J.; da Silva, C.; Barros, B.C.B. Valorization of peach palm by-product through subcritical water extraction of soluble sugars and phenolic compounds. J. Supercrit. Fluids 2020, 165, 104985. [Google Scholar] [CrossRef]
- Yan, L.-G.; Xi, J. Micro-mechanism analysis of ultrahigh pressure extraction from green tea leaves by numerical simulation. Sep. Purif. Technol. 2017, 180, 51–57. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Giovagnoli-Vicu, C.; Figueroa-Alvarez, P.; Quispe-Fuentes, I.; Pérez-Won, M. Extraction of β-carotene, vitamin C and antioxidant compounds from Physalis peruviana (Cape Gooseberry) assisted by high hydrostatic pressure. Food Nutr. Sci. 2013, 4, 35284. [Google Scholar]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive phenolic compounds from agri-food wastes: An update on green and sustainable extraction methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.; Jaime, L.; Santoyo, S.; Reglero, G.; Cifuentes, A.; Ibañez, E.; Señoráns, F. Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chem. 2007, 102, 1357–1367. [Google Scholar] [CrossRef]
- Puértolas, E.; Barba, F.J. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Puértolas, E.; Luengo, E.; Álvarez, I.; Raso, J. Improving mass transfer to soften tissues by pulsed electric fields: Fundamentals and applications. Annu. Rev. Food Sci. Technol. 2012, 3, 263–282. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Houska, M.; Pravda, P. Examples of Commercial Fruit and Vegetable Juices and Smoothies Cold Pasteurized by High Pressure. In High Pressure Processing of Fruit and Vegetable Products; CRC Press: Boca Raton, FL, USA, 2017; pp. 147–154. [Google Scholar]
- Silva, F.V. Heat assisted HPP for the inactivation of bacteria, moulds and yeasts spores in foods: Log reductions and mathematical models. Trends Food Sci. Technol. 2019, 88, 143–156. [Google Scholar]
- Silva, F.V.M.; Sulaiman, A. Polyphenoloxidase in Fruit and Vegetables: Inactivation by Thermal and Non-Thermal Processes; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Da Silva, F.V.M. High-Pressure Processing Effect on Microorganisms in Fruit and Vegetable Products. In High Pressure Processing of Fruit and Vegetable Products; CRC Press: Boca Raton, FL, USA, 2017; pp. 3–38. [Google Scholar]
- Zhu, Z.; Gavahian, M.; Barba, F.J.; Roselló-Soto, E.; Kovačević, D.B.; Putnik, P.; Denoya, G.I. Valorization of waste and by-products from food industries through the use of innovative technologies. In Agri-Food Industry Strategies for Healthy Diets and Sustainability; Elsevier: Amsterdam, The Netherlands, 2020; pp. 249–266. [Google Scholar]
- El-Shamy, S.; Farag, M.A. Novel trends in extraction and optimization methods of bioactives recovery from pomegranate fruit biowastes: Valorization purposes for industrial applications. Food Chem. 2021, 365, 130465. [Google Scholar] [CrossRef]
- Jun, X. High-pressure processing as emergent technology for the extraction of bioactive ingredients from plant materials. Crit. Rev. Food Sci. Nutr. 2013, 53, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Scepankova, H.; Martins, M.; Estevinho, L.; Delgadillo, I.; Saraiva, J.A. Enhancement of bioactivity of natural extracts by non-thermal high hydrostatic pressure extraction. Plant Foods Hum. Nutr. 2018, 73, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Richard, J. High Pressure Phase Behaviour of Multicomponent Fluid Mixtures; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Samaranayake, C.P.; Sastry, S.K. In-situ pH measurement of selected liquid foods under high pressure. Innov. Food Sci. Emerg. Technol. 2013, 17, 22–26. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Carciochi, R.A.; D’Alessandro, L.G.; Vauchel, P.; Rodriguez, M.M.; Nolasco, S.M.; Dimitrov, K. Valorization of agrifood by-products by extracting valuable bioactive compounds using green processes. In Ingredients Extraction by Physicochemical Methods in Food; Elsevier: Amsterdam, The Netherlands, 2017; pp. 191–228. [Google Scholar]
- Armenta, S.; Garrigues, S.; Esteve-Turrillas, F.A.; de la Guardia, M. Green extraction techniques in green analytical chemistry. TrAC Trends Anal. Chem. 2019, 116, 248–253. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Evrendilek, G.A. Change regime of aroma active compounds in response to pulsed electric field treatment time, sour cherry juice apricot and peach nectars, and physical and sensory properties. Innov. Food Sci. Emerg. Technol. 2016, 33, 195–205. [Google Scholar] [CrossRef]
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Kowalski, R. The application of PEF technology in food processing and human nutrition. J. Food Sci. Technol. 2021, 58, 397–411. [Google Scholar] [CrossRef]
- Vaessen, E.; Timmermans, R.; Tempelaars, M.; Schutyser, M.; den Besten, H. Reversibility of membrane permeabilization upon pulsed electric field treatment in lactobacillus plantarum WCFS1. Sci. Rep. 2019, 9, 19990. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, J.R.; Boussetta, N.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Application of pulsed electric fields and high voltage electrical discharges for oil extraction from sesame seeds. J. Food Eng. 2015, 153, 20–27. [Google Scholar] [CrossRef]
- Fincan, M.; Dejmek, P. In Situ visualization of the effect of a pulsed electric field on plant tissue. J. Food Eng. 2002, 55, 223–230. [Google Scholar] [CrossRef]
- Ade-Omowaye, B.; Taiwo, K.; Eshtiaghi, N.; Angersbach, A.; Knorr, D. Comparative evaluation of the effects of pulsed electric field and freezing on cell membrane permeabilisation and mass transfer during dehydration of red bell peppers. Innov. Food Sci. Emerg. Technol. 2003, 4, 177–188. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.-A.; Sun, D.-W.; Wang, M.-S.; Liu, Z.-W.; Zhang, Z.-H. Combined effects of sonication and pulsed electric field on selected quality parameters of grapefruit juice. LWT-Food Sci. Technol. 2015, 62, 890–893. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Huang, H. Effects of pulsed electric fields on anthocyanin extraction yield of blueberry processing by-products. J. food Process. Preserv. 2015, 39, 1898–1904. [Google Scholar] [CrossRef]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crop. Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov. Food Sci. Emerg. Technol. 2013, 17, 120–129. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crop. Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Milella, R.A.; Basile, T.; Alba, V.; Gasparro, M.; Giannandrea, M.A.; Debiase, G.; Genghi, R.; Antonacci, D. Optimized ultrasonic-assisted extraction of phenolic antioxidants from grape (Vitis vinifera L.) skin using response surface methodology. J. Food Sci. Technol. 2019, 56, 4417–4428. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A.S.; Rawson, A.; Muthiah, M.; Kalakandan, S.K. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason. Sonochem. 2020, 61, 104812. [Google Scholar] [CrossRef] [PubMed]
- Medina-Meza, I.G.; Barbosa-Cánovas, G.V. Assisted extraction of bioactive compounds from plum and grape peels by ultrasonics and pulsed electric fields. J. Food Eng. 2015, 166, 268–275. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High voltage electrical discharges, pulsed electric field, and ultrasound assisted extraction of protein and phenolic compounds from olive kernel. Food Bioprocess Technol. 2015, 8, 885–894. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Manrique, G.D.; Dimitrov, K. Optimization of antioxidant phenolic compounds extraction from quinoa (Chenopodium quinoa) seeds. J. Food Sci. Technol. 2015, 52, 4396–4404. [Google Scholar] [CrossRef]
- Rodríguez De Luna, S.L.; Ramírez-Garza, R.; Serna Saldívar, S.O. Environmentally friendly methods for flavonoid extraction from plant material: Impact of their operating conditions on yield and antioxidant properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Raes, K.; Van Camp, J. Combined alkaline hydrolysis and ultrasound-assisted extraction for the release of nonextractable phenolics from cauliflower (Brassica oleracea var. botrytis) waste. J. Agric. Food Chem. 2014, 62, 3371–3376. [Google Scholar] [CrossRef]
- Leong, T.; Ashokkumar, M.; Kentish, S. The fundamentals of power ultrasound—A review. Acoust. Aust. 2011, 39, 2. [Google Scholar]
- Achat, S.; Tomao, V.; Madani, K.; Chibane, M.; Elmaataoui, M.; Dangles, O.; Chemat, F. Direct enrichment of olive oil in oleuropein by ultrasound-assisted maceration at laboratory and pilot plant scale. Ultrason. Sonochem. 2012, 19, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Patiño, J.C.; Gullón, B.; Romero, I.; Ruiz, E.; Brnčić, M.; Žlabur, J.Š.; Castro, E. Optimization of ultrasound-assisted extraction of biomass from olive trees using response surface methodology. Ultrason. Sonochem. 2019, 51, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Bailina, Y.; Ge, Z.; Ding, T.; Ye, X.; Liu, D. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J. Food Eng. 2014, 126, 72–81. [Google Scholar] [CrossRef]


| Film Type | Polymers | Fruit/Vegetable Waste | Applications | Findings | Ref. |
|---|---|---|---|---|---|
| Active film | Hydroxypropyl high-amylose starch | Pomegranate peel (PGP) | - | PGP inhibited the growth of S. aureus and Salmonella bacteria. | [34] |
| Active film | Cassava starch | Blueberry pomace (BP) | - | BP improved the barrier properties and released active compounds into acidic medium. | [39] |
| Active film | Gelatin capsule waste | Fiber and ethanolic extract from blueberry juice processing waste | Sunflower oil | Improved light barrier properties; reduced the lipid oxidation of sunflower oil and stability in antioxidant activity. | [40] |
| Active film | Tapioca starch thymol | Jackfruit skin and straw as filler | Cherry tomato | Jackfruit skin and straw resulted in higher tensile strength with lower water solubility and water vapor permeability. | [41] |
| Active packaging | Poly(butylene adipate-co-terephthalate) (PBAT) Cinnamon essential oil | Cellulose nanofibers (CNF) | Strawberry | High thermal stability, decreased water vapor permeability. | [37] |
| Active packaging | Chitosan | Apricot kernel essential oil (AKEO) | Bread slices | AKEO improved water resistance, water vapor barrier, and mechanical properties. Inhibited E. coli, B. subtilis and fungal growth. | [42] |
| Biodegradable film | Tapioca starch | Banana pseudostems (BP) | - | BP reduced the mechanical and optical properties, improved the barrier properties. | [43] |
| Active film | Mango kernel fat (MKF), phenolic extract from mango kernel (MKPE), mango kernel starch (MKS), | Mango kernel | - | Films exhibited antioxidant activity, UV-absorbing capacity, and good barrier properties. | [44] |
| Biodegradable film | Pectin, sodium carboxymethyl cellulose (CMC− Na) Thyme essential oil | Okara soluble dietary fiber | - | Higher mechanical, barrier, optical, and antioxidant properties. Antimicrobial activity against E. coli and S. aureus was not significant. | [45] |
| Active edible film | Basil seed gum Zataria multiflora essential oil | Basil seed gum (BSG) | - | Efficient antimicrobial activity against E. coli and B. cereus. | [46] |
| Polymers | Fruit/Vegetable Waste | Applications | Findings | Ref. |
|---|---|---|---|---|
| Cassava starch | Blueberry residue (BR) powder, two particle sizes | Distilled water, sucrose, sodium chloride, soybean protein, milk protein, whole milk powder, orange juice, corn oil, chicken meat | BR films displayed changes in color responding to the pH of tested samples. | [50] |
| Corn starch | Blueberry juice processing waste | - | The blueberry residue film showed visual color differences in different pH ranges. | [51] |
| Sweet potato starch | Sweet potato peel | Chicken flesh | SPP reduced the mechanical and barrier properties, did not influence the color response of the films. | [52] |
| Residue | Fruit/Vegetable | Matrix | Properties | Ref. |
|---|---|---|---|---|
| Cellulose nanofibrils (CCNF) | Carrot pomace | PLA | Mechanical, hydrophilic, thermal, and antibacterial | [36] |
| Nanofiber | Durian skin | PLA | Tensile strength | [68] |
| Fiber | Banana peel | PP | Sound insulation | [69] |
| Fiber | Durian skin | PLA | Production energy | [70] |
| Fiber | Durian skin | PLA | Tensile strength, modulus of elasticity, and enzymatic degradation | [71] |
| Fiber | Sea mango peel | PP | Flexural strength, flexural modulus, and thermal | [72] |
| Pomace extract | Chokeberry, blackcurrant, apple, and raspberry | Rapeseed meal, microcrystalline cellulose | Flexural strength and water contact angle | [73] |
| Extract | Coconut shell | Polyvinyl alcohol (PVA) and corn starch | Antioxidant activity and thermal | [74] |
| Extract | Date fruit | Gelatin | Moisture content and water solubility | [75] |
| Powder | Cocopith | Wood powder, tapioca | Thermal conductivity | [76] |
| Powder | Date and tamarind seed | PLA | Tensile, flexural, and impact strength | [77] |
| Powder | Grape and acerola | Cassava starch | Antioxidant, physicochemical, and mechanical | [78] |
| Powder and fiber | Jackfruit skin | PLA | Tensile strength and tensile modulus | [79] |
| Powder | Sour cherry shell | PE | Elastic modulus, tensile strength, moisture absorption, and water vapor transmission rate | [80] |
| Husks | Chestnut | Starch | Elastic modulus and tensile strength | [81] |
| Fruit and Vegetables Waste | Bioactive Extraction | Process Condition | Agent | Optimum Yield (%) | Ref. |
|---|---|---|---|---|---|
| Red and white dragon fruit peel, passion fruit peel | Pectin | 43—107 min, 60–80 °C (Dragon Fruit), 60–120 °C (Passion Fruit) | Citric acid | 15.12% (red dragon fruit), 14.11% (white dragon fruit), 13.18% (passion fruit) | [130] |
| Grape (white and red) skin | TPC | 60–90 min, 40–70 °C | Ethanol | 1.74–2.12 gGAE/L | [131] |
| Black carrot pomace | Pectin | 90 min, 110 °C | Acidic solution | 0.22 kg pectin/kg pomace | [132] |
| Wheat bran | Phenolics | 3, 6, and 24 h, 50–90 °C | 99% glycerol, citric acid, Folin–Ciacalteu | 4.57–16.11 mgFAE/gdm | [133] |
| Grapefruit peel | Pectin | 90 min, 80 °C | HCl | MW 385.55 kDa | [135] |
| Lime peel | Pectin | 60 min, 95 °C | HCl or citric acid | 16.12–23.52% | [136] |
| Fruit and Vegetables Waste | Bioactive Extraction | Process Condition | Agent | Optimum Yield (%) | Ref. |
|---|---|---|---|---|---|
| Olive Oil (Alperujo) solid waste | TPC | 4 h, 70 °C | n-Hexane | 0.75–3.76 g/kg raw alperujo | [139] |
| Indian Soapberry seed | Oil | 6 h, 80 °C | n-Hexane | 40.63% | [140] |
| Mango peel, Soursop peel, Grape peel, Grape seed | AOA, TPC, TFC | 8 h, 40 °C | 60% Ethanol; 1:25 solid/liquid ratio | 52.28% (Mango peel), 50.63% (Soursop peel), 64.65% (Grape peel), 18.45% (Grape seed) | [141] |
| Olive skin/peel | Triterpene acids | unmilled/milled, 30–90 min, 65–70 °C | Ethyl Acetate/Methanol (1:40, 1:20, 1:10 sample/solvent ratio) | unmilled, ratio 1:40 g/mL: 22.24% | [142] |
| Bitter Gourd peel | AOA, TPC, TFC | 6 h, 40–50 °C | Methanol | 26.48% | [145] |
| Fruit and Vegetables Waste | Bioactive Extraction | Process Condition | Agent | Optimum Yield (%) | Ref. |
|---|---|---|---|---|---|
| Red and White Dragon fruit peels, Passion Fruit peel | Pectin | 10–12 min, 75 °C, 153–218 W | Methanol (pH: 2.9–3.0) | 17.01 ± 0.32% (Red Dragon Fruit), 13.22 ± 1.42% (White Dragon Fruit), 18.73 ± 0.06 (Passion Fruit) | [130] |
| Black Carrot pomace | Phenolic, Antioxidants, Anthocyanins | 5 min, 110 °C, 20% output power of 900 W | Hot Acidic Water (pH: 2.5) | phenolic content: 1692 ± 79.4 mg GAE/l (0.17 kg pectin/kg pomace); antioxidant: 60 ± 9.6 MTE/mL; anthocyanins: 456.8 ± 38.2 mg/L | [132] |
| Longan seeds | Pectin | 3.5 min, 700 W | 50% Ethanol | 64.95 + 20.56 mgGAE/gdw | [149] |
| Lemon, Mandarin and Kiwi peels | Pectin | 1–3 min, 60–75 °C, 360–600 W | HCl, Nitric Acid (HCl) | 17.97% (kiwi peels), 7.47% (mandarin peels), 7.31% (lemon peels) | [154] |
| Apple pomace; Orange peel; Mango peel; Carrot pulp | Pectin | 10–180 min, 90 °C, 50–200 W | Water | Orange peel, 60 min, 200 W: 12.9 ± 1.0%; Mango peel, 120 min, 200 W: 14.7 ± 0.6%; Apple pomace, 120 min, 200 W: 14.7 ± 0.1%; Carrot pulp, 60 min, 200 W: 6.3 ± 0.7% | [158] |
| Black Carrot pomace | Phenolic, Flavonoid, Anthocyanins, AOA | 9.8 min, 348.07 W | 20% Ethanol | polyphenolic content: 264.9 ± 10.025 mg GAE/100 mL; flavonoid: 1662.22 ± 47.3 mgQE/L; AOA: 13.14 ± 1.05 MTE/mL; anthocyanins: 753.40 ± 31.6 mg/L; color density; 68.63 ± 5.40 | [161] |
| Jocote (Spondias purpurea L.) pomace | Pectin, Flavonoid | 20 min, 68 °C, 100 W | 80% Ethanol | phenol: 0.897 g GAE/g (3.42%), flavonoid: 1.271 g QE/g, | [163] |
| Mango kernel | Crude butter | 3.5 min, 160 W | Water | 48.85% | [167] |
| Lemon peel | Essential oil, Pigment | 50 min, 20 °C/min, 500 W | 80% Methanol | Essential oil: 2 wt.%, Pigment: 6 wt.% | [166] |
| Watermelon rind | Pectin | 12 min, 279.3 W | Acetic Acid | 3.93–5.77% (DE: 56.86–85.76%) | [170] |
| Fruit and Vegetables Waste | Bioactive Extraction | Process Condition | Agent | Optimum Yield (%) | Ref. |
|---|---|---|---|---|---|
| Grape pomace: skin, seed | Polyphenols, Antioxidants | 5 min with 250 s nitrogen purge, 100–160 °C, ~10 atm | 20–60% Ethanol | Polyphenols content, 160 °C, 60% ethanol, Skin:1.98 ± 0.06 mgGAE/gdw; Seeds: 12.54 8 ± 0.02 mgGAE/gdw; Antioxidant by DPPH, 100 °C, 20% Ethanol, skin: 121.91 8 ± 0.08 mg/mL, Seeds: 39.63 8 ± 0.01 mg/mL; Antioxidant by ORAC, 160 °C, 60% Ethanol, Skin: 36.33 8 ± 0.06 MTE/gdw, Seeds: 137.65 ± 0.11 MTE/gdw | [172] |
| Pomegranate peel | TPC, Punicalagin content, antimicrobial activity | 200 °C | 77% Ethanol | Polyphenols content: 164.3 ± 10.7 mgGAE/gdw; Punicalagin content: 17 ± 3.6 mg/gdw | [174] |
| Olive pomace | TPC, AOA, TFC | 65–18 °C, supercritical carbon dioxide (scCO2) | 8–92% Ethanol | TPC, 160.7 °C, 75% Ethanol: 280.37 mgGAE/gDE; AOA, 125 °C, 50% Ethanol: 6.88 MTE/gDE; TFC, 89.3 °C, 25% Ethanol: 15.82 mgRE/gDE | [175] |
| Mulberry pulp | TPC, Anthocyanins | 10 min, 75.5 °C, 200 atm, purge time 90 s | 47.2% Methanol (pH3.01) | TPC: 2186.09 ug/g, AOA: 164.53 ug/g | [176] |
| Beetroot waste: residues, leaves and stems | TPC, AOA | 40 °C, 7.5–12.5 MPa, 3 mL/min | 70–100% Ethanol | Leaves- TPC, 40 °C, 10 MPa, 100% Ethanol: 252 ± 2 mgGAE/g; AOA by ABTS, 40 °C, 12.5 MPa, 100% Ethanol: 823 ± 48 MTE/g; Stems- TPC, 40 °C, 10 MPa, 100% Ethanol: 14 ± 2 mgGAE/g; AOA by DPPH, 40 °C, 10 MPa, 100% Ethanol: 515 ± 89 G/mL (Leaves > Stems) | [177] |
| Fruit and Vegetables Waste | Bioactive Extraction | Process Condition | Agent | Optimum Yield (%) | Ref. |
|---|---|---|---|---|---|
| Grape pomace | Phenolic compounds | 50–190 °C | Water | 29 g/100 g extracts | [190] |
| Kiwifruit peel | TPC, TFC, AOA | 5–30 min (20 min) 120–160 °C (160 °C) | Aqueous mixture | TPC: 51.24 mg GAE/gdw, TFC: 22.49 mgCE/gde AOA by ABTS: 269.4 mM TE/gdw | [191] |
| Citrus (C. unshiu) peel | TFC | 145–175 °C 15 min | Water | TFC: 59,490 g/gdb | [192] |
| Tamarind seed | Xyloglucan component, TPC, AOA | 100–200 °C (175 °C) 5.03–13.55 min | Distilled Water | Xyloglucan component: 62.28%, TPC: 14.65–42.00 gGAE/g, AOA: 1.93–3.20 MTE/g | [193] |
| Dates seed | TPC, AOA, TFC, Dietary fiber | 120–180 °C (144 °C) 10–30 min (18.4 min) | Aqueous mixture | TPC: 9.97 mgGAE/g, TFC: 3.52 mgQE/g, AOA 1.67 mgTE/g, Dietary fibers: 29 g/mg | [194] |
| Extraction Method | Working Principles | Parameters | Advantages | Disadvantages |
|---|---|---|---|---|
| High Pressure Processing (HPP) |
|
|
|
|
| Ultrasound-assisted extraction (UAE) |
|
|
|
|
| Supercritical fluid extraction (SFE) |
|
|
|
|
| Pulsed electric field (PEF) |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Basri, M.S.; Abdul Karim Shah, N.N.; Sulaiman, A.; Mohamed Amin Tawakkal, I.S.; Mohd Nor, M.Z.; Ariffin, S.H.; Abdul Ghani, N.H.; Mohd Salleh, F.S. Progress in the Valorization of Fruit and Vegetable Wastes: Active Packaging, Biocomposites, By-Products, and Innovative Technologies Used for Bioactive Compound Extraction. Polymers 2021, 13, 3503. https://doi.org/10.3390/polym13203503
Mohd Basri MS, Abdul Karim Shah NN, Sulaiman A, Mohamed Amin Tawakkal IS, Mohd Nor MZ, Ariffin SH, Abdul Ghani NH, Mohd Salleh FS. Progress in the Valorization of Fruit and Vegetable Wastes: Active Packaging, Biocomposites, By-Products, and Innovative Technologies Used for Bioactive Compound Extraction. Polymers. 2021; 13(20):3503. https://doi.org/10.3390/polym13203503
Chicago/Turabian StyleMohd Basri, Mohd Salahuddin, Nor Nadiah Abdul Karim Shah, Alifdalino Sulaiman, Intan Syafinaz Mohamed Amin Tawakkal, Mohd Zuhair Mohd Nor, Siti Hajar Ariffin, Nur Hamizah Abdul Ghani, and Faiqa Shazeaa Mohd Salleh. 2021. "Progress in the Valorization of Fruit and Vegetable Wastes: Active Packaging, Biocomposites, By-Products, and Innovative Technologies Used for Bioactive Compound Extraction" Polymers 13, no. 20: 3503. https://doi.org/10.3390/polym13203503
APA StyleMohd Basri, M. S., Abdul Karim Shah, N. N., Sulaiman, A., Mohamed Amin Tawakkal, I. S., Mohd Nor, M. Z., Ariffin, S. H., Abdul Ghani, N. H., & Mohd Salleh, F. S. (2021). Progress in the Valorization of Fruit and Vegetable Wastes: Active Packaging, Biocomposites, By-Products, and Innovative Technologies Used for Bioactive Compound Extraction. Polymers, 13(20), 3503. https://doi.org/10.3390/polym13203503







