Effect of Ultraviolet Irradiation on Polystyrene Containing Cephalexin Schiff Bases
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
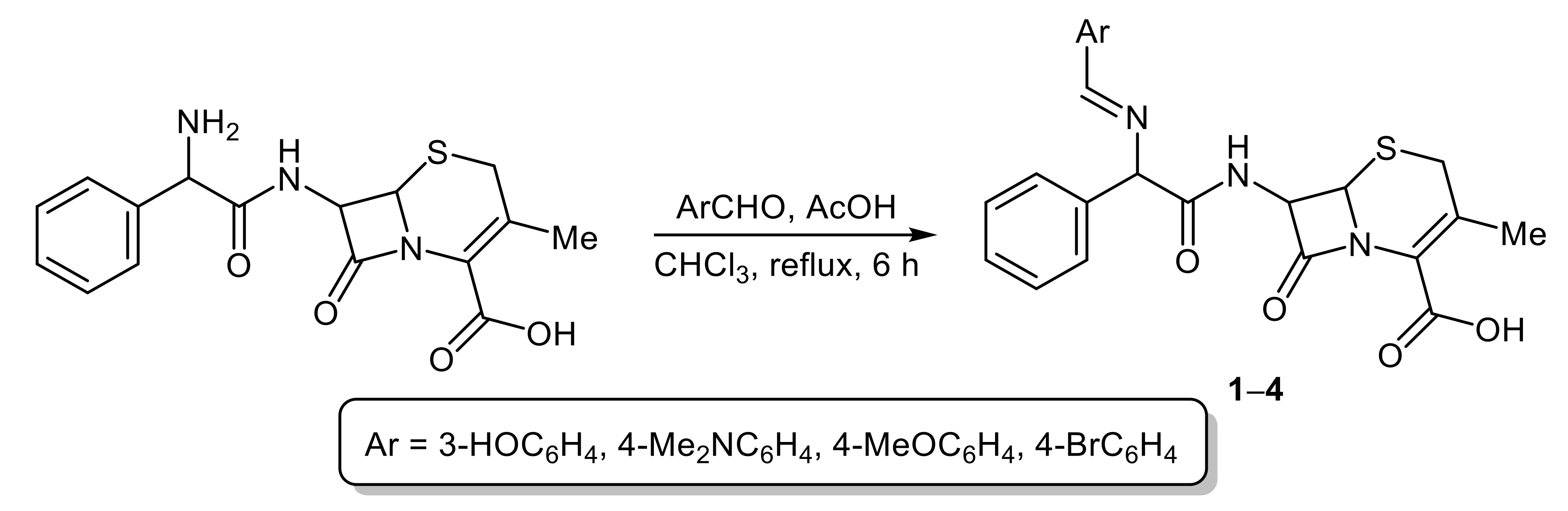
2.2. Synthesis of Cephalexin Schiff Bases 1–4
2.3. Preparation of PS Films
3. Results and Discussion
3.1. Synthesis of Cephalexin Schiff Bases 1–4
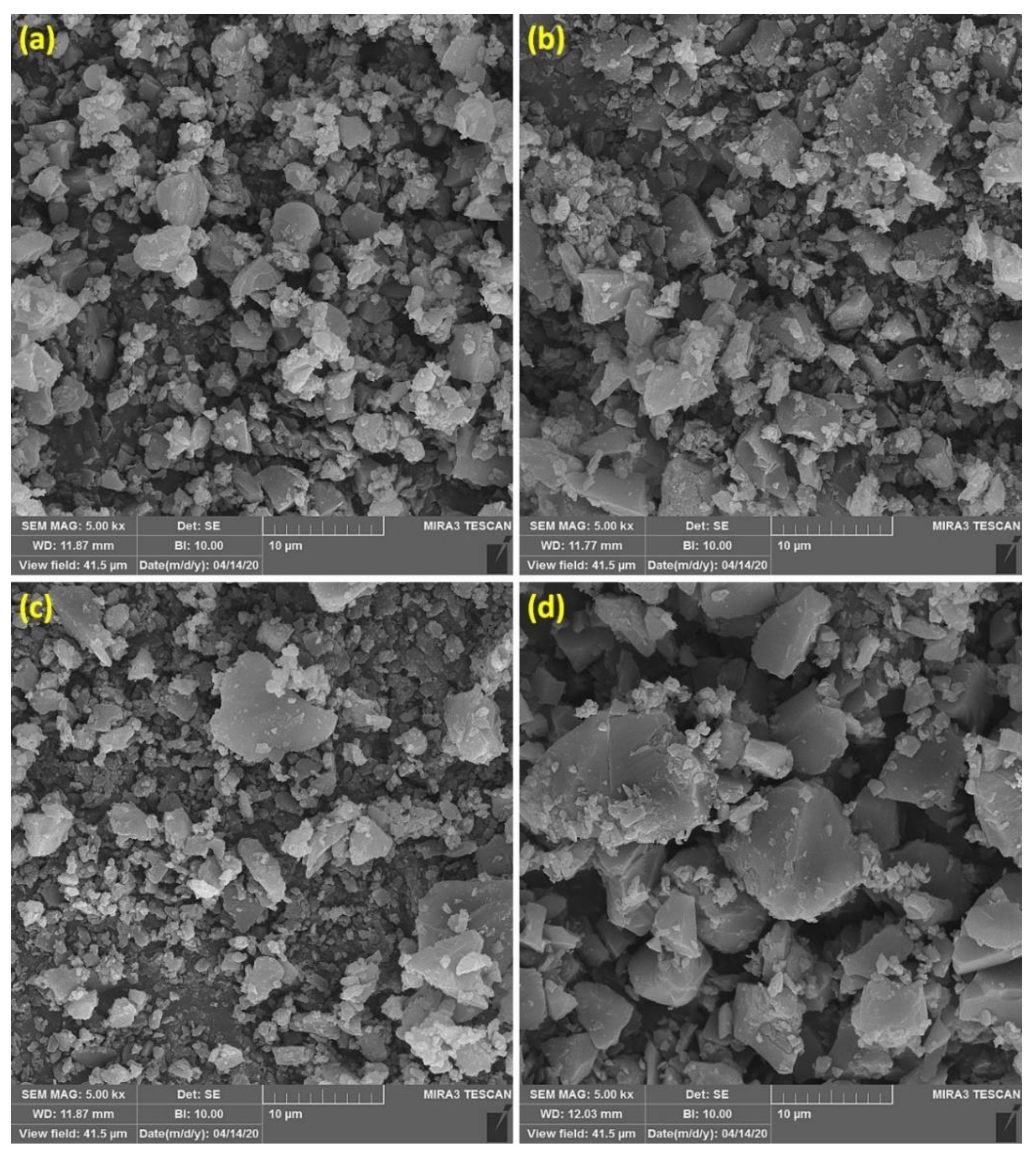
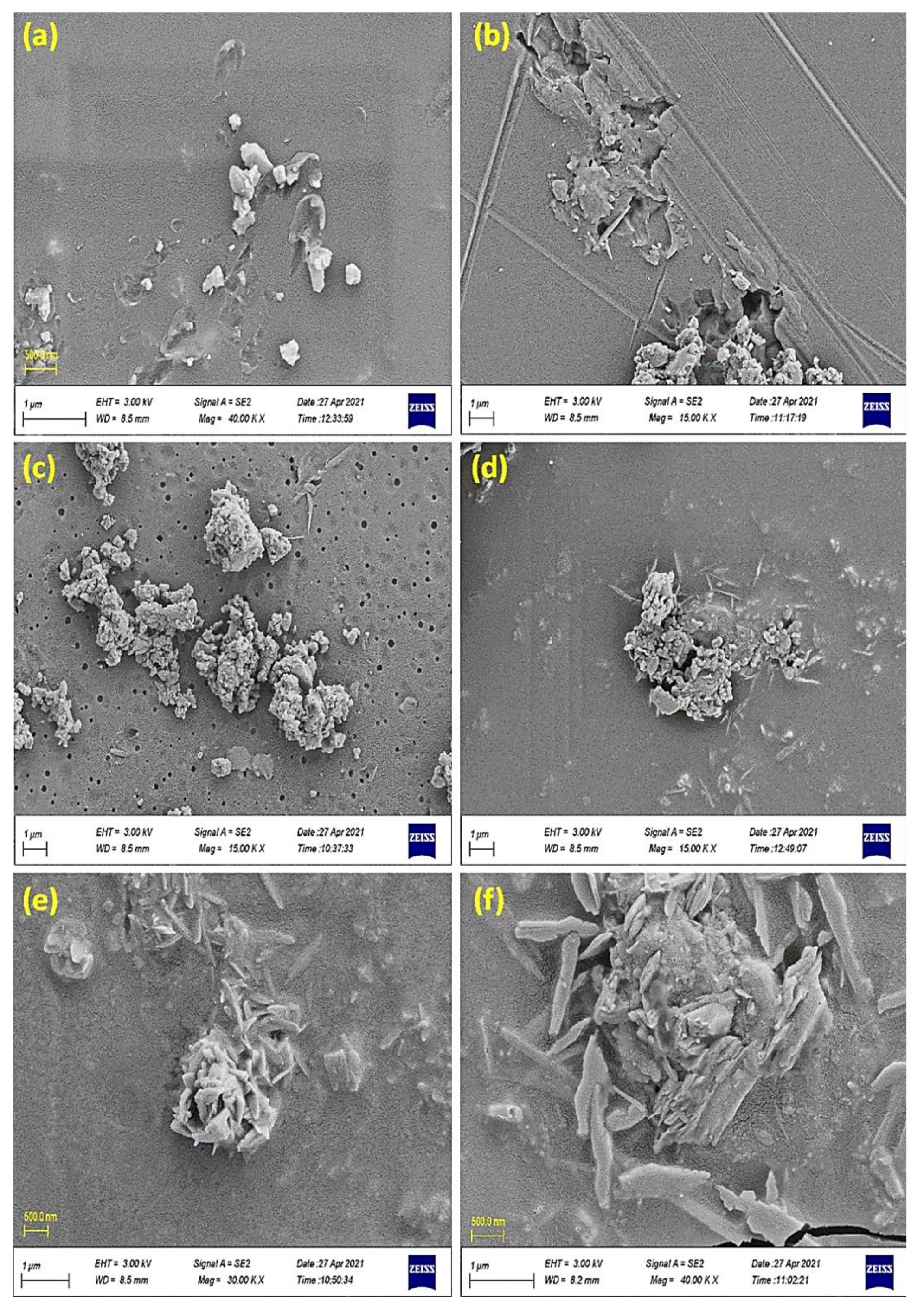
3.2. SEM of Cephalexin Schiff Bases 1–4
3.3. Effects of UV Irradiation on FTIR Spectra
3.4. Effect of UV Irradiation on Weight
3.5. Effect of UV Irradiation on Molecular Weight ()
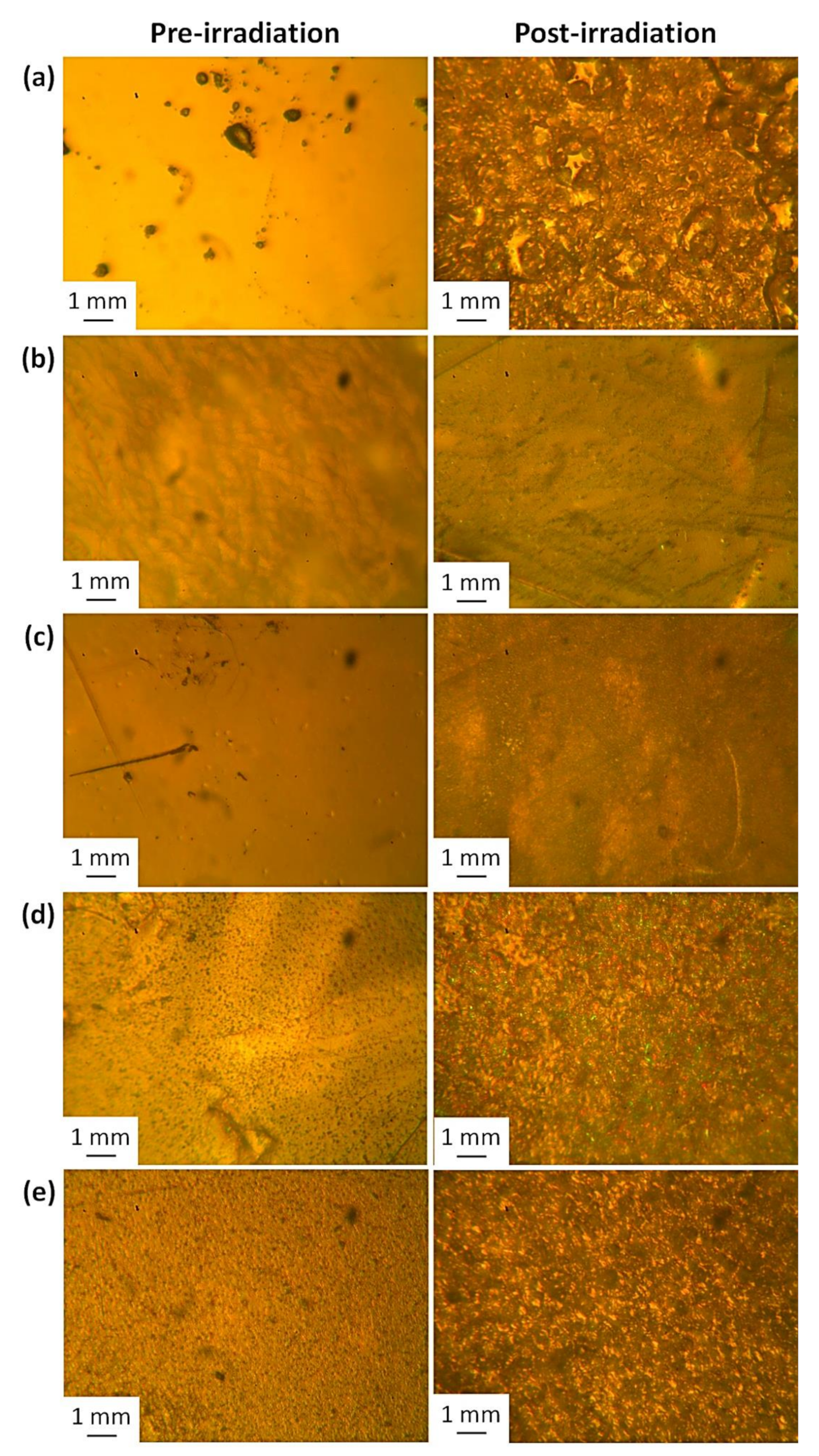
3.6. Optical Microscopy of PS
3.7. FESEM of PS
3.8. AFM of PS
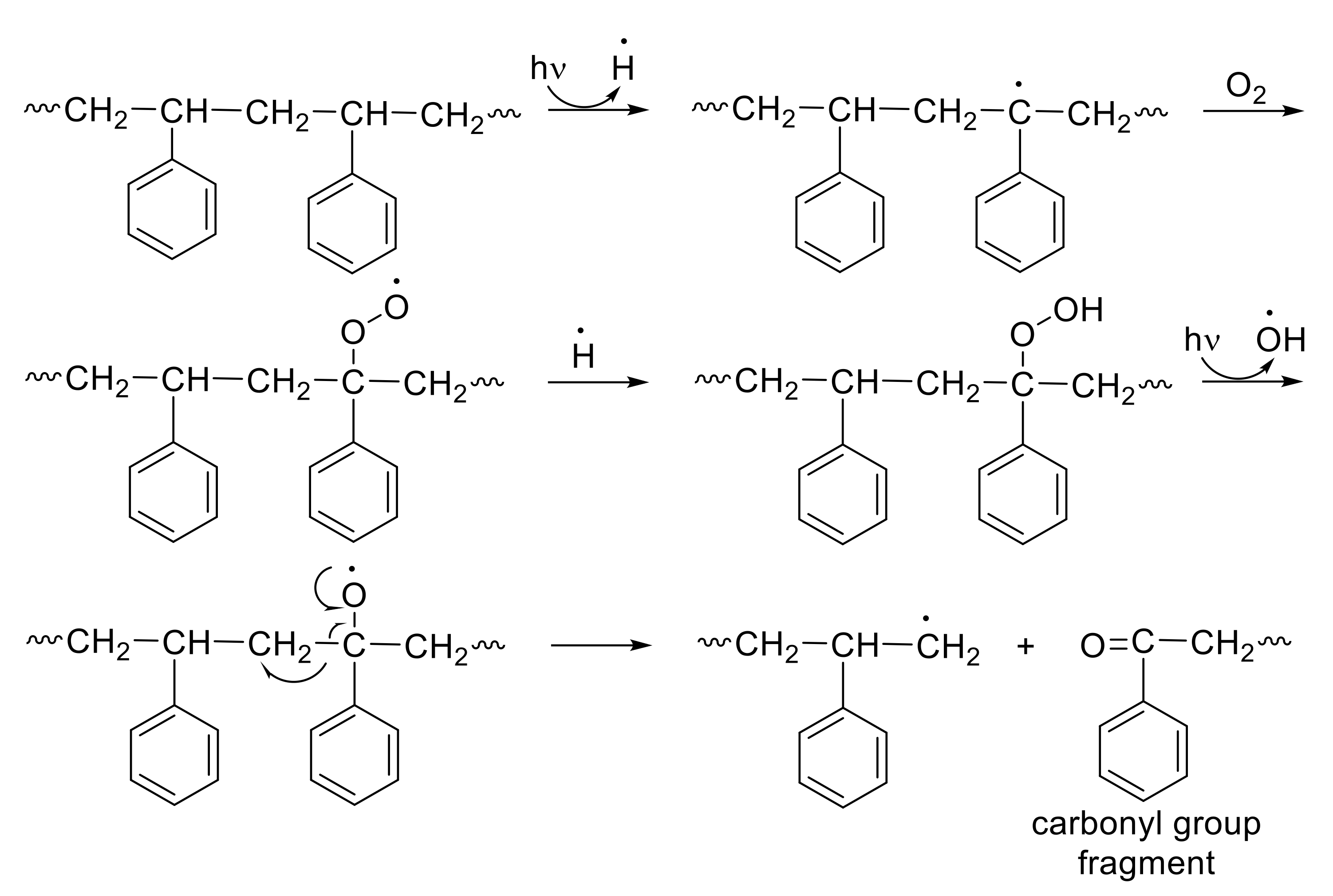
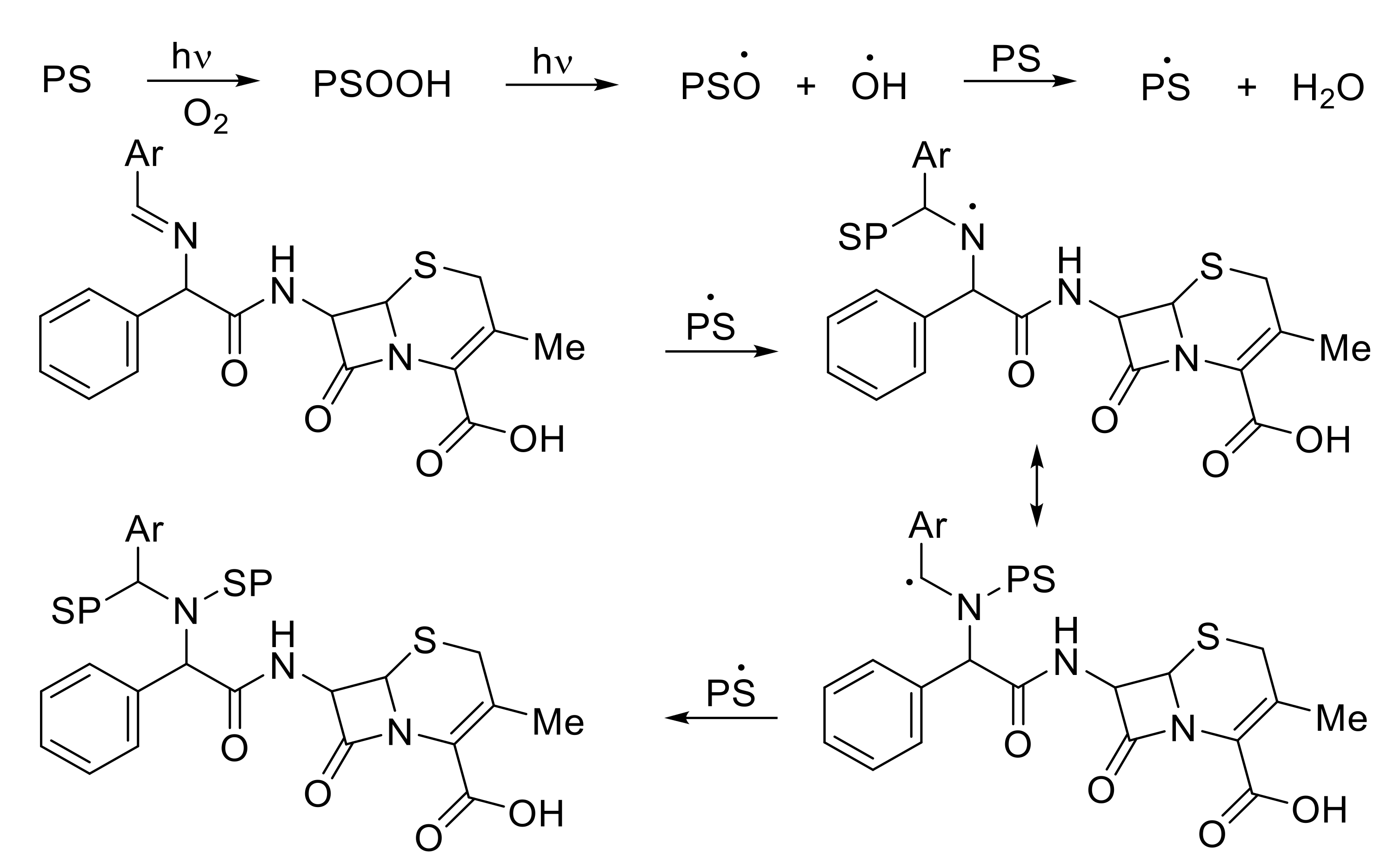
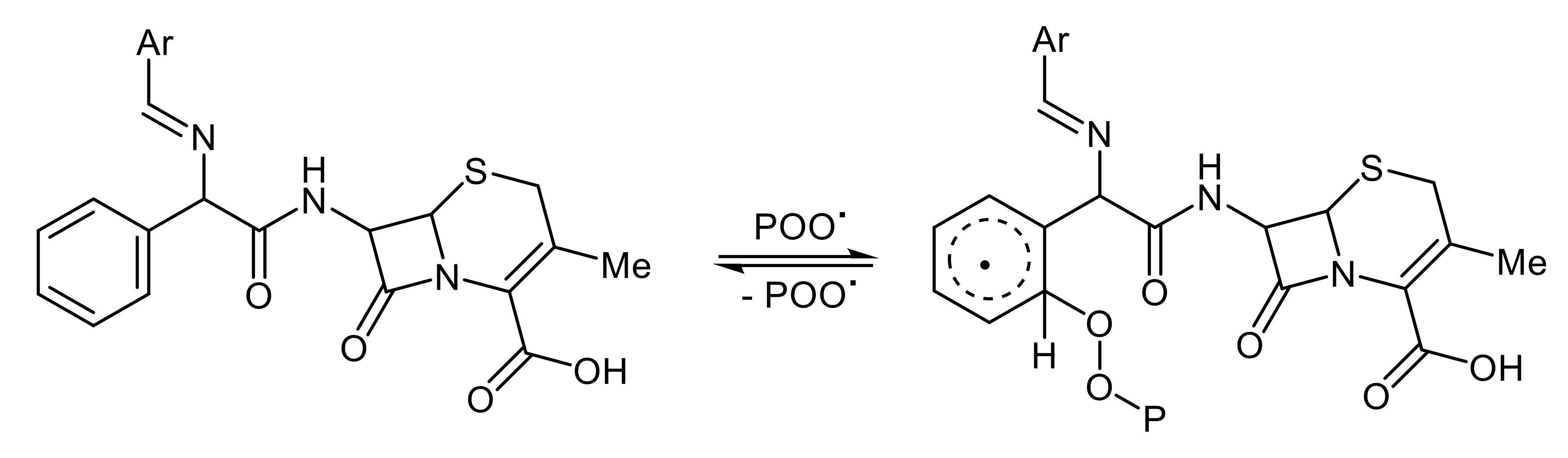
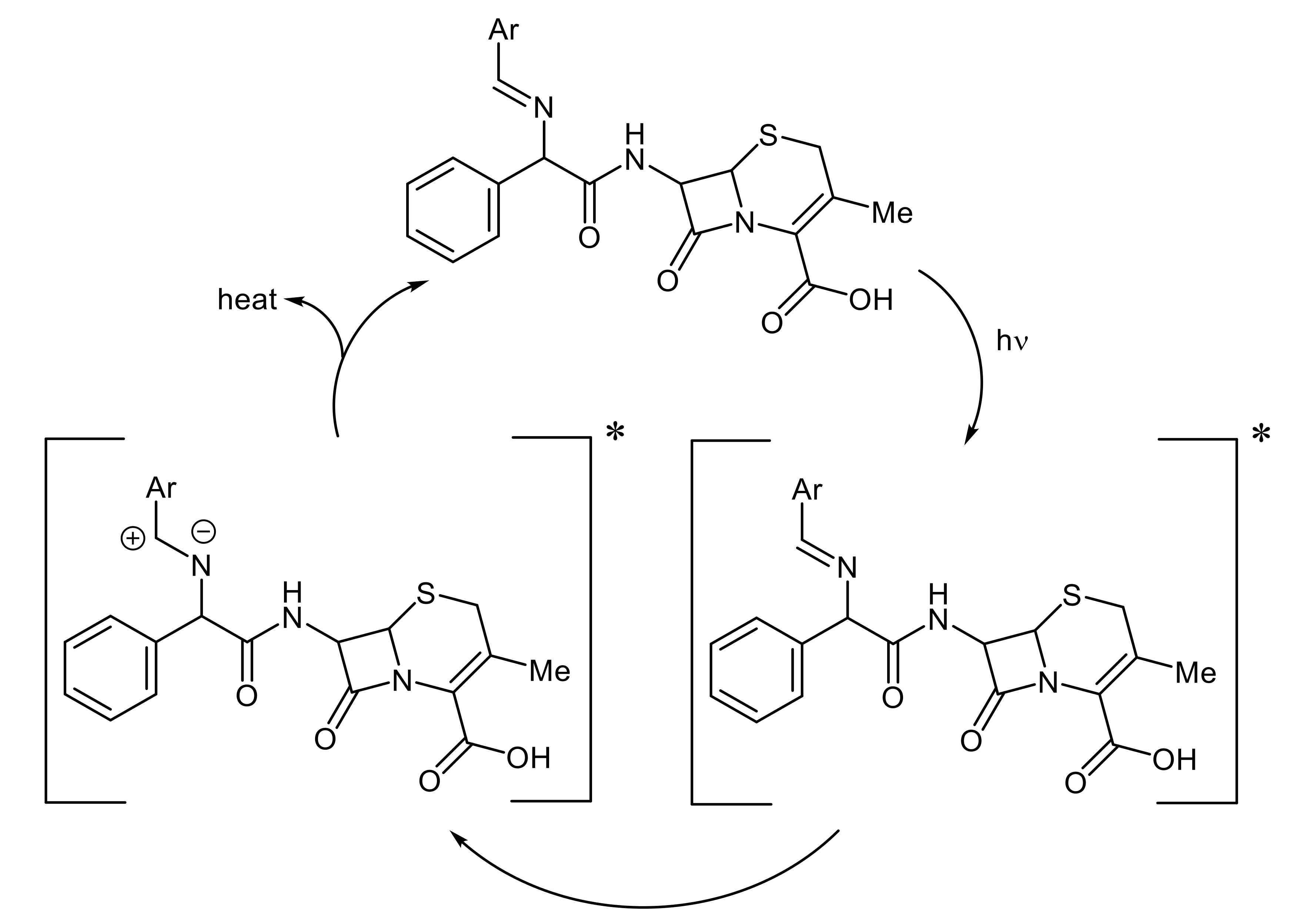
3.9. Photostabilization Mechanisms of PS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plastics—The Facts 2019. An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Plastics_the_facts2019_14102019.pdf (accessed on 1 August 2021).
- Marfa, I.M. Pyrolysis of polystyrene waste: A review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef]
- Maul, J.; Frushour, B.G.; Kontoff, J.R.; Eichenauer, H.; Ott, K.-H.; Schade, C. Polystyrene and Styrene Copolymers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2007; pp. 477–484. [Google Scholar]
- Gary, J.E. Polystyrene: Properties, Performance and Applications; Nova Science: New York, NY, USA, 2011; pp. 1–198. [Google Scholar]
- Vilaplana, F.; Ribes-Greus, A.; Karlsson, S. Degradation of recycled high-impact polystyrene. Simulation by reprocessing and thermo-oxidation. Polym. Degrad. Stab. 2006, 91, 2163–2170. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F. Structure and physical properties of syndiotactic polypropylene: A highly crystalline thermoplastic elastomer. Prog. Polym. Sci. 2006, 31, 145–237. [Google Scholar] [CrossRef]
- Rabek, J.F. Photodegradation of Polymers: Physical Characteristics and Applications; Springer Science & Business Media: Berlin, Germany, 2012; pp. 1–212. [Google Scholar]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [Green Version]
- White, J.R.; Turnbull, A. Weathering of polymers: Mechanisms of degradation and stabilization: Testing strategies and modelling. J. Mater. Sci. 1994, 29, 584–613. [Google Scholar] [CrossRef]
- Hernández, C.G.; González, R.; Soto, J.J.; Rosales, I. Photo-Oxidation of Polystyrene Film Irradiated with UV-B; Springer: Gewerbestasse, Switzerland, 2016; pp. 1–372. [Google Scholar]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.F.A.; Goi, B.E.; Schmitt, C.C.; Neumann, M.G. Photodegradation of polystyrene films containing UV-visible sensitizers. J. Res. Updates Polym. Sci. 2013, 2, 39–47. [Google Scholar] [CrossRef]
- Cadogan, D.F.; Howick, C.J. Plasticizers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000; pp. 599–603. [Google Scholar]
- Torikai, A.; Kobatake, T.; Okisaki, F.; Shuyama, H. Photodegradation of polystyrene containing flame-retardants: Wavelength sensitivity and efficiency of degradation. Polym. Degrad. Stab. 1995, 50, 261–267. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Ahmed, D.S.; El-Hiti, G.A.; Alotaibi, M.H.; Hashim, H.; Yousif, E. SEM morphological analysis of irradiated polystyrene film doped by a Schiff base containing a 1,2,4-triazole ring system. Appl. Petrochem. Res. 2019, 9, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Ali, G.Q.; El-Hiti, G.A.; Tomi, I.H.R.; Haddad, R.; Al-Qaisi, A.J.; Yousif, E. Photostability and performance of polystyrene films containing 1,2,4-triazole-3-thiol ring system Schiff bases. Molecules 2016, 21, 1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldshtein, J.; Margel, S. Synthesis and characterization of polystyrene/2-(5-chloro-2H-benzotriazole-2-yl)-6-(1,1-dimethylethyl)-4-methylphenol composite microspheres of narrow size distribution for UV irradiation protection. Colloid Polym. Sci. 2011, 289, 1863–1874. [Google Scholar] [CrossRef]
- Yousif, E.; Salimon, J.; Salih, N. New stabilizer for polystyrene based on 2-N-salicylidene-5-(substituted)-1,3,4-thiadiazole compounds. J. Saudi Chem. Soc. 2011, 16, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Rabie, S.T.; Ahmed, A.E.; Sabaa, M.W.; Abd El-Ghaffar, M.A. Maleic diamides as photostabilizers for polystyrene. J. Ind. Eng. Chem. 2013, 19, 1869–1878. [Google Scholar] [CrossRef]
- Torikai, A.; Takeuchi, T.; Fueki, K. Photodegradation of polystyrene and polystyrene containing benzophenone. Polym. Photochem. 1983, 3, 307–320. [Google Scholar] [CrossRef]
- Yousif, E.; Ahmed, D.S.; El-Hiti, G.A.; Alotaibi, M.H.; Hashim, H.; Hameed, A.S.; Ahmed, A. Fabrication of novel ball-like polystyrene films containing Schiff bases microspheres as photostabilizers. Polymers 2018, 10, 1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alotaibi, M.H.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Hashim, H.; Hameed, A.S.; Ahmed, A. Evaluation of the use of polyphosphates as photostabilizers and in the formation of ball-like polystyrene materials. J. Polym. Res. 2019, 26, 161. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R.; El-Hiti, G.A.; Yusop, R.M. Spectroscopic and photochemical stability of polystyrene films in the presence of metal complexes. J. Taibah Univ. Sci. 2017, 11, 997–1007. [Google Scholar] [CrossRef] [Green Version]
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers 2016, 8, 204. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, D.S.; El-Hiti, G.A.; Hameed, A.S.; Yousif, E.; Ahmed, A. New tetra-Schiff bases as efficient photostabilizers for poly(vinyl chloride). Molecules 2017, 22, 1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaalan, N.; Laftah, N.; El-Hiti, G.A.; Alotaibi, M.H.; Muslih, R.; Ahmed, D.S.; Yousif, E. Poly(vinyl chloride) photostabilization in the presence of Schiff bases containing a thiadiazole moiety. Molecules 2018, 23, 913. [Google Scholar] [CrossRef] [Green Version]
- Hashim, H.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S.; Yousif, E. Fabrication of ordered honeycomb porous poly(vinyl chloride) thin film doped with a Schiff base and nickel(II) chloride. Heliyon 2018, 4, e00743. [Google Scholar] [CrossRef] [Green Version]
- El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Hamad, B.A.; Ahmed, D.S.; Ahmed, A.; Hashim, H.; Yousif, E. The morphology and performance of poly(vinyl chloride) containing melamine Schiff bases against ultraviolet light. Molecules 2019, 24, 803. [Google Scholar] [CrossRef] [Green Version]
- Balakit, A.A.; Ahmed, A.; El-Hiti, G.A.; Smith, K.; Yousif, E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride). Int. J. Polym. Sci. 2015, 2015, 510390. [Google Scholar] [CrossRef]
- Cano, M.; Oriol, L.; Piñol, M.; Serrano, J.L. Photopolymerization of reactive mesogenic Schiff bases and related metallomesogens. Chem. Mater. 1999, 11, 94–100. [Google Scholar] [CrossRef]
- Li, Z.; Hu, P.; Zhu, J.; Gao, Y.; Xiong, X.; Liu, R. Conjugated carbazole-based Schiff bases as photoinitiators: From facile synthesis to efficient two-photon polymerization. J. Polym. Sci. A Polym. Chem. 2018, 56, 2692–2700. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Graber, C.J. A critical review of cephalexin and cefadroxil for the treatment of acute uncomplicated lower urinary tract infection in the era of “bad bugs, few drugs”. Int. J. Antimicrob. Agents 2020, 56, 106085. [Google Scholar] [CrossRef]
- Mehmood, N.; Andreasson, E.; Kao-Walter, S. SEM observations of a metal foil laminated with a polymer film. Procedia Mater. Sci. 2014, 3, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Biazar, E.; Zeinali, R.; Montazeri, N.; Pourshamsian, K.; Behrouz, M.; Asefnejad, A.; Khoshzaban, A.; Shahhosseini, G.; Najafabadi, M.S.; Abyani, R.; et al. Cell engineering: Nanometric grafting of poly-N-isopropylacrylamide onto polystyrene film by different doses of gamma radiation. Int. J. Nanomed. 2010, 5, 549–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, T.; Aggarwal, S.; Kumar, S.; Mittal, V.K.; Kalsi, P.C.; Manchanda, V.K. Effect of gamma irradiation on the optical properties of CR-39 polymer. J. Mater. Sci. 2007, 42, 1127–1130. [Google Scholar] [CrossRef]
- Lucki, J.; Rånby, B. Photo-oxidation of polystyrene–Part 2: Formation of carbonyl groups in photo-oxidised polystyrene. Polym. Degrad. Stab. 1979, 1, 165–179. [Google Scholar] [CrossRef]
- Kiatkamjornwong, S.; Sonsuk, M.; Wittayapichet, S.; Prasassarakich, P.; Vejjanukroh, P.-C. Degradation of styrene-g-cassava starch filled polystyrene plastics. Polym. Degrad. Stab. 1999, 66, 323–334. [Google Scholar] [CrossRef]
- Rabek, J.F. Polymer Photodegradation: Mechanisms and Experimental Methods; Springer Science & Business Media: Berlin, Germany, 1994; pp. 1–664. [Google Scholar]
- Rabek, J.F.; Ranby, B.G. Photodegradation, Photooxidation and Photostabilization of Polymer; John Wiley: New York, NY, USA, 1975; pp. 501–554. [Google Scholar]
- Gaumet, S.; Gardette, J.-L. Photo-oxidation of poly(vinyl chloride): Part 2—A comparative study of the carbonylated products in photo-chemical and thermal oxidations. Polym. Degrad. Stab. 1991, 33, 17–34. [Google Scholar] [CrossRef]
- Jellinek, H.H.G. Aspects of Degradation and Stabilization of Polymers; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Erlandsson, B.; Albertsson, A.-C.; Karlsson, S. Molecular weight determination in degraded oxidizable and hydrolyzable polymers giving deviation from accurate using calibration and the Mark-Houwink-Sakaruda (MHS) equation. Polym. Degrad. Stab. 1997, 57, 15–23. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R.; Noaman, R. Photostabilization of Polystyrene Films: Photostabilization Activity of Polystyrene; Lambert Academic Publishing: Beau Bassin, Mauritius, 2014; p. 56. [Google Scholar]
- Schmitt, T.; Guttmann, P.; Schmidt, O.; Müller-Buschbaum, P.; Stamm, M.; Schönhense, G.; Schmahl, G. Microscopy of thin polymer blend films of polystyrene and poly-n-butyl-methacrylate. AIP Conf. Proc. 2000, 507, 245. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Ahmadi, M.; Mirmohseni, A.; Taseidifar, M.; Naebe, M. The effects of UV light on the chemical and mechanical properties of a transparent epoxy-diamine system in the presence of an organic UV absorber. Materials 2017, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Shyichuk, A.V.; White, J.R. Analysis of chain-scission and crosslinking rates in the photo-oxidation of polystyrene. J. Appl. Polym. Sci. 2000, 77, 3015–3023. [Google Scholar] [CrossRef]
- Shinato, K.W.; Huang, F.; Jin, Y. Principle and application of atomic force microscopy (AFM) for nanoscale investigation of metal corrosion. Corros. Rev. 2020, 38, 423–432. [Google Scholar] [CrossRef]
- See, C.H.; O’Haver, J. Atomic force microscopy characterization of ultrathin polystyrene films formed by admicellar polymerization on silica disks. J. Appl. Polym. Sci. 2003, 89, 36–46. [Google Scholar] [CrossRef]
- Hadi, A.G.; Yousif, E.; El-Hiti, G.A.; Ahmed, D.S.; Jawad, K.; Alotaibi, M.H.; Hashim, H. Long-term effect of ultraviolet irradiation on poly(vinyl chloride) films containing naproxen diorganotin(IV) complexes. Molecules 2019, 24, 2396. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, R.; El-Hiti, G.A.; Ahmed, A.; Yousif, E. Poly(vinyl chloride) doped by 2-(4-isobutylphenyl)propanoate metal complexes: Enhanced resistance to UV irradiation. Arab. J. Sci. Eng. 2017, 42, 4307–4315. [Google Scholar] [CrossRef]
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Photostabilizing efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules 2016, 21, 1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, A.G.; Jawad, K.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Photostabilization of poly(vinyl chloride) by organotin(IV) compounds against photodegradation. Molecules 2019, 24, 3557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghazi, D.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Alotaibi, M.H. The effect of ultraviolet irradiation on the physicochemical properties of poly(vinyl chloride) films containing organotin(IV) complexes as photostabilizers. Molecules 2018, 23, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S. Polyphosphates as inhibitors for poly(vinyl chloride) photodegradation. Molecules 2017, 22, 1849. [Google Scholar] [CrossRef] [Green Version]
- Pospíšil, J.; Klemchuk, P.P. Oxidation Inhibition in Organic Materials; CRC Press: Boca Raton, FL, USA, 1989; pp. 1–384. [Google Scholar]
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]





| Schiff Base | Ar | Color | M.P. (°C) | Yield (%) | Calculated (Found; %) | |||
|---|---|---|---|---|---|---|---|---|
| C | H | N | S | |||||
| 1 | 3-HOC6H4 | Yellow | 234–235 | 82 | 61.19 (61.28) | 4.69 (4.72) | 9.31 (9.24) | 7.10 (7.02) |
| 2 | 4-Me2NC6H4 | Brown | 201–203 | 73 | 62.74 (62.56) | 4.48 (4.52) | 11.71 (11.62) | 6.70 (6.62) |
| 3 | 4-MeOC6H4 | White | 222–225 | 70 | 61.92 (61.80) | 4.98 (5.02) | 9.03 (8.96) | 6.89 (6.81) |
| 4 | 4-BrC6H4 | Orange | 131–132 | 78 | 53.70 (53.58) | 3.92 (3.95) | 8.17 (8.10) | 6.23 (6.15) |
| Schiff Base | OH | CH (Ar) | NH | C=O (Amide) | C=O (Carboxyl) | CH=N | C=C (Aromatic) | C–S |
|---|---|---|---|---|---|---|---|---|
| 1 | 3489 | 3041 | 3207 | 1759 | 1693 | 1568 | 1546 | 817 |
| 2 | 3464 | 3059 | 3271 | 1768 | 1683 | 1620 | 1564 | 858 |
| 3 | 3477 | 3039 | 3272 | 1758 | 1693 | 1587 | 1569 | 817 |
| 4 | 3320 | 3041 | 3288 | 1768 | 1728 | 1643 | 1541 | 727 |
| Schiff Base | 1H NMR (500 MHz: δ, ppm, J in Hz) |
|---|---|
| 1 | 13.13 (s, exch., 1H), 9.63 (s, exch., 1H), 8.64 (s, 1H), 7.66 (s, exch., 1H), 7.52–6.98 (m, 9H), 5.24 (d, J = 4.5 Hz, 1H), 5.03 (s, 1H), 4.98 (d, J = 4.5 Hz, 1H), 3.41 (d, J = 18.0 Hz, 1H), 3.30 (d, J = 18.0 Hz, 1H), 2.00 (s, 3H) |
| 2 | 12.60 (s, exch., 1H), 8.89 (s, 1H), 8.60 (s, exch., 1H), 7.75–7.69 (m, 9H), 4.75 (d, J = 4.6 Hz, 1H), 4.68 (s, 1H), 4.52 (d, J = 4.6 Hz, 1H), 3.45 (d, J = 18.2 Hz, 1H), 3.34 (d, J = 18.2 Hz, 1H), 3.12 (s, 6H), 1.96 (s, 3H) |
| 3 | 13.19 (s, exch., 1H), 9.31 (s, 1H), 8.61 (s, exch., 1H), 7.46–7.27 (m, 9H), 5.71 (d, J = 4.4 Hz, 1H), 5.63 (d, J = 4.6 Hz, 1H), 4.97 (s, 1H), 3.49 (d, J = 18.4 Hz, 1H), 3.46 (s, 3H), 3.41 (d, J = 18.2 Hz, 1H), 2.00 (s, 3H) |
| 4 | 12.82 (s, exch. 1H), 9.00 (s, 1H), 7.92 (s, exch., 1H), 7.75–7.22 (s, 9H), 5.14 (d, J = 4.3 Hz, 1H), 4.92 (s, 1H), 4.76 (d, J = 4.3 Hz, 1H), 3.27 (d, J = 18.3 Hz, 1H), 3.16 (d, J = 18.3 Hz, 1H), 1.97 (s, 3H) |
| Schiff Base | 13C NMR (125 MHz: δ, ppm, J in Hz) |
|---|---|
| 1 | 173.7, 171.7, 169.1, 164.2, 143.7, 139.5, 129.0, 128.7, 127.9, 127.4, 126.6, 126.4, 126.2, 118.3, 122.0, 115.6, 79.3, 58.0, 56.9, 30.5, 19.4 |
| 2 | 169.7, 164.7, 161.2, 155.0, 151.2, 146.6, 136.2, 129.6, 128.2, 127.5, 126.1, 124.6, 121.3, 105.0, 74.9, 68.3, 56.3, 44.2, 28.0, 20.2 |
| 3 | 168.3, 166.7, 163.9, 163.2, 158.0, 138.9, 130.3, 129.1, 128.6, 128.4, 127.8, 126.3, 122.0, 116.6, 73.0, 64.5, 63.5, 55.9, 28.7, 19.3 |
| 4 | 173.6, 162.7, 161.1, 158.8, 141.3, 135.9, 132.7, 130.0, 129.2, 128.8, 128.5, 127.6, 126.2, 121.7, 72.4, 62.6, 60.1, 30.5, 20.1 |
| Polymeric Materials | Additives | Fold-Reduction in Rq | Reference |
|---|---|---|---|
| PS | Cephalexin Schiff bases | 27.1 | Current research |
| PS | 1,2,3,4-Triazole-3-thiol Schiff bases | 3.3 | [17] |
| PS | Biphenyl-3,3′,4,4′-tetraamine Schiff bases | 8.3 | [22] |
| PVC | Biphenyl-3,3′,4,4′-tetraamine Schiff bases | 3.6 | [26] |
| PVC | Melamine Schiff bases | 6.0 | [29] |
| PVC | Tin complexes | 5.2–16.6 | [50,51,52,53,54] |
| PVC | Polyphosphates | 16.7 | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaseen, A.A.; Al-Tikrity, E.T.B.; Yousif, E.; Ahmed, D.S.; Kariuki, B.M.; El-Hiti, G.A. Effect of Ultraviolet Irradiation on Polystyrene Containing Cephalexin Schiff Bases. Polymers 2021, 13, 2982. https://doi.org/10.3390/polym13172982
Yaseen AA, Al-Tikrity ETB, Yousif E, Ahmed DS, Kariuki BM, El-Hiti GA. Effect of Ultraviolet Irradiation on Polystyrene Containing Cephalexin Schiff Bases. Polymers. 2021; 13(17):2982. https://doi.org/10.3390/polym13172982
Chicago/Turabian StyleYaseen, Anaheed A., Emaad T. B. Al-Tikrity, Emad Yousif, Dina S. Ahmed, Benson M. Kariuki, and Gamal A. El-Hiti. 2021. "Effect of Ultraviolet Irradiation on Polystyrene Containing Cephalexin Schiff Bases" Polymers 13, no. 17: 2982. https://doi.org/10.3390/polym13172982
APA StyleYaseen, A. A., Al-Tikrity, E. T. B., Yousif, E., Ahmed, D. S., Kariuki, B. M., & El-Hiti, G. A. (2021). Effect of Ultraviolet Irradiation on Polystyrene Containing Cephalexin Schiff Bases. Polymers, 13(17), 2982. https://doi.org/10.3390/polym13172982









