Structural Geometry Variation of 1,4-Naphthalene-Based Co-Polymers to Tune the Device Performance of PVK-Host-Based OLEDs
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
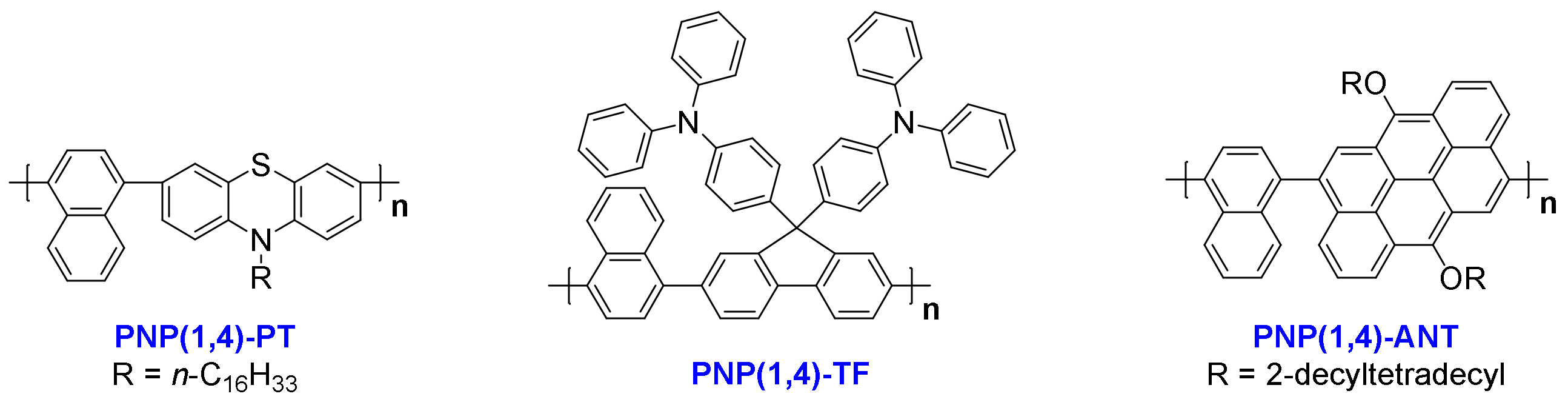
3.1. Design and Synthesis
3.2. Thermal Properties
3.3. Optical Properties
3.4. Energy Levels Estimation
3.5. Density Functional Theory (DFT) Calculations
3.6. OLED Device Performance and Discussion
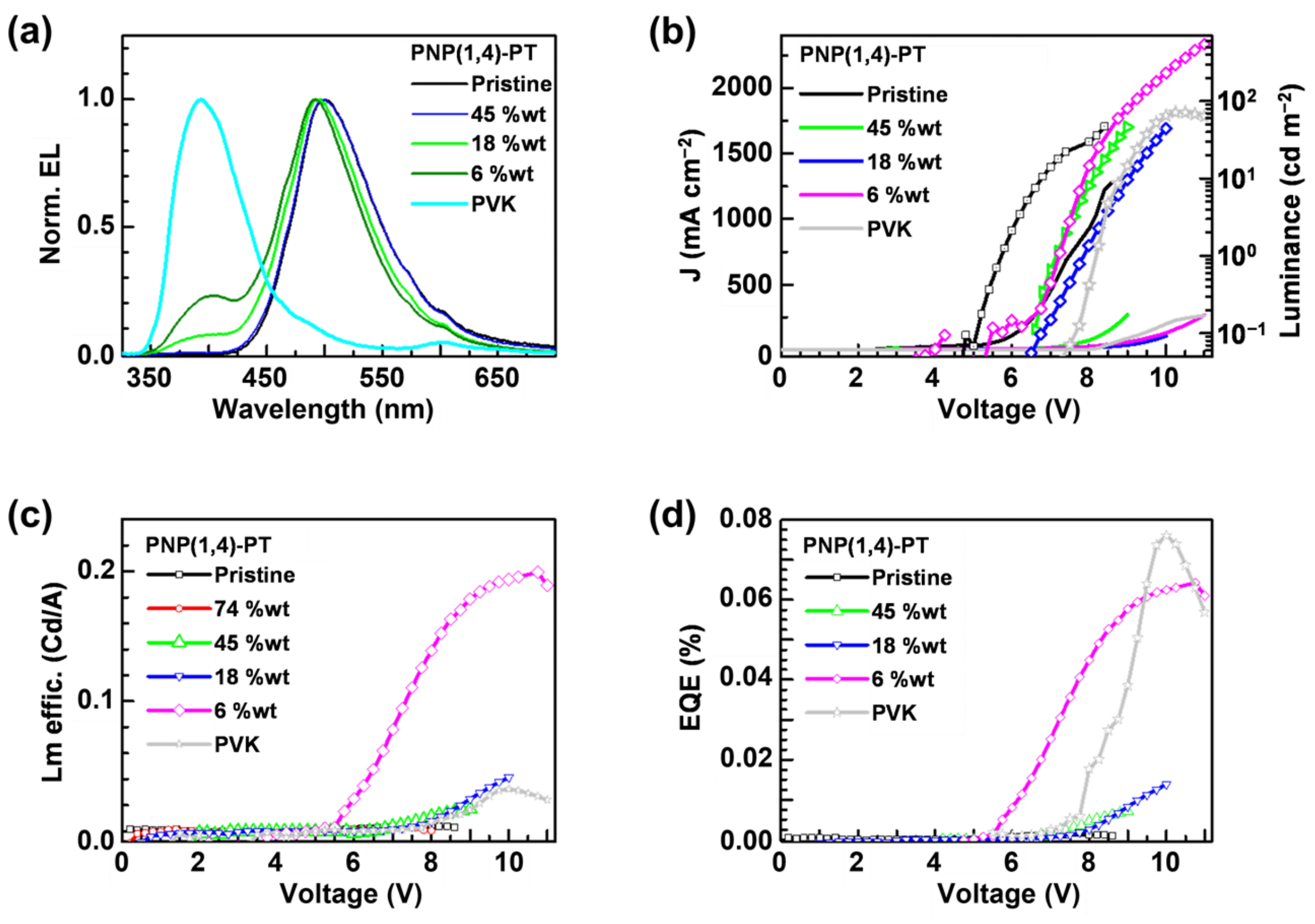
3.6.1. PNP(1,4)-PT Polymer Results
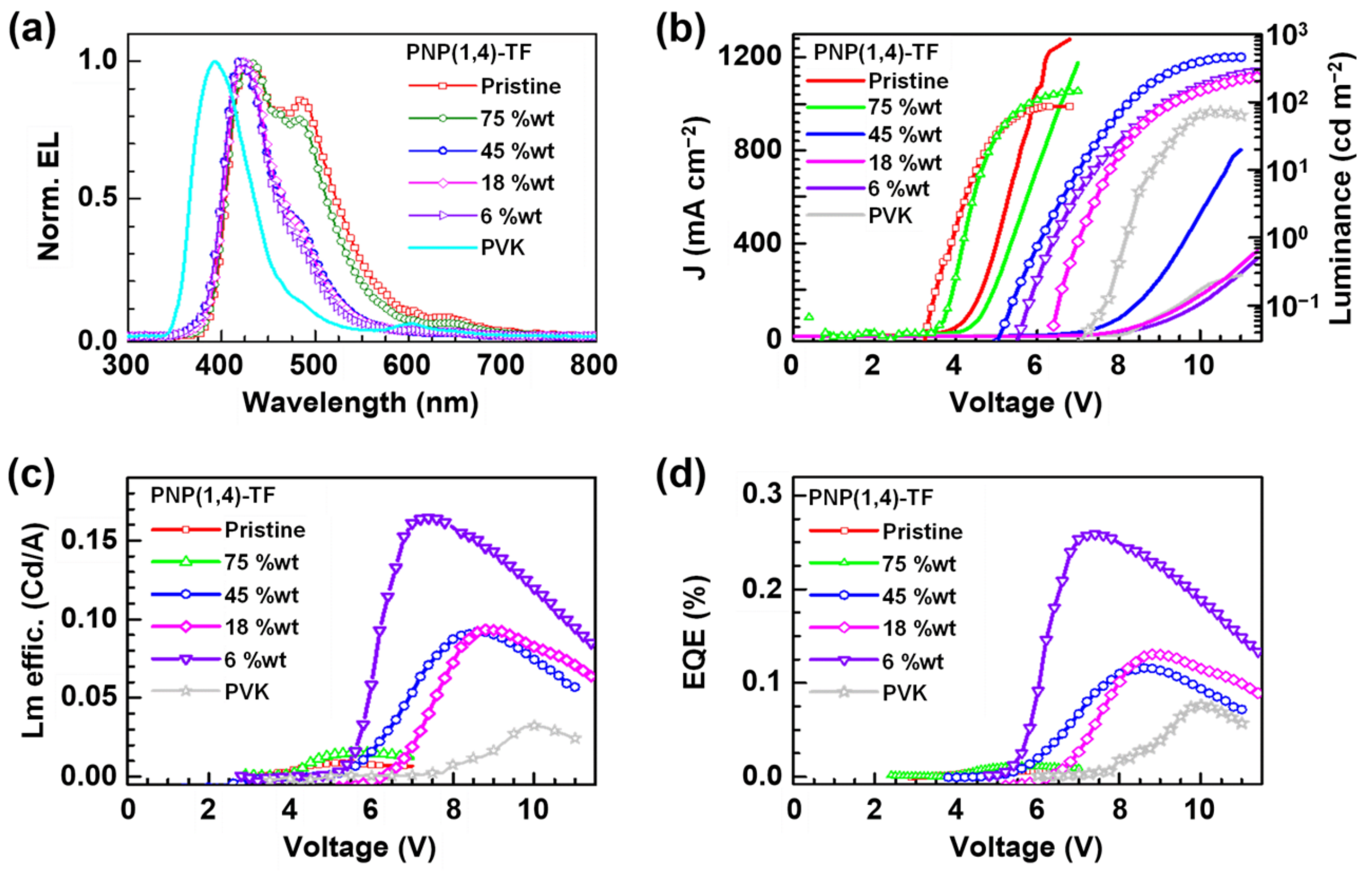
3.6.2. PNP(1,4)-TF Polymer Results
3.6.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Liu, Q.; Zhang, Y.; Li, C.; He, Z.; Choy, W.C.H.; Low, P.J.; Sonar, P.; Kyaw, A.K.K. Biodegradable Materials and Green Processing for Green Electronics. Adv. Mater. 2020, 32, 2001591. [Google Scholar] [CrossRef]
- Yan, C.; Barlow, S.; Wang, Z.; Yan, H.; Jen, A.K.Y.; Marder, S.R.; Zhan, X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 18003. [Google Scholar] [CrossRef]
- Yuvaraja, S.; Nawaz, A.; Liu, Q.; Dubal, D.; Surya, S.G.; Salama, K.N.; Sonar, P. Organic field-effect transistor-based flexible sensors. Chem. Soc. Rev. 2020, 49, 3423–3460. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Bottle, S.E.; Sonar, P. Developments of Diketopyrrolopyrrole-Dye-Based Organic Semiconductors for a Wide Range of Applications in Electronics. Adv. Mater. 2020, 32, 1903882. [Google Scholar] [CrossRef] [PubMed]
- Slodek, A.; Zych, D.; Maroń, A.; Golba, S.; Schab-Balcerzak, E.; Janeczek, H.; Siwy, M.; Maćkowski, S. Fluorene vs carbazole substituent at quinoline core toward organic electronics. Dyes Pigm. 2019, 166, 98–106. [Google Scholar] [CrossRef]
- Moghe, D.; Kabra, D. Polymer Light-Emitting Diodes. In Advanced Nanomaterials for Solar Cells and Light-Emitting Diodes, 1st ed.; Gao, F., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 9; pp. 343–369. [Google Scholar] [CrossRef]
- Trung, T.Q.; Kim, C.; Lee, H.B.; Cho, S.M.; Lee, N.E. Toward a Stretchable Organic Light-Emitting Diode on 3D Microstructured Elastomeric Substrate and Transparent Hybrid Anode. Adv. Mater. Technol. 2020, 5, 1900995. [Google Scholar] [CrossRef]
- Pode, R. Organic light emitting diode devices: An energy efficient solid state lighting for applications. Renew. Sustain. Energy Rev. 2020, 133, 110043. [Google Scholar] [CrossRef]
- Kang, C.-M.; Lee, H. Recent progress of organic light-emitting diode microdisplays for augmented reality/virtual reality applications. J. Inf. Disp. 2021, 1–14. [Google Scholar] [CrossRef]
- Jeon, Y.; Choi, H.R.; Park, K.C.; Choi, K.C. Flexible organic light-emitting-diode-based photonic skin for attachable phototherapeutics. J. Soc. Inf. Disp. 2020, 28, 324–332. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, J.-W. Intrinsically stretchable organic light-emitting diodes. Sci. Adv. 2021, 7, eabd9715. [Google Scholar] [CrossRef] [PubMed]
- Divayana, Y.; Liu, S.; Kyaw, A.K.K.; Sun, X.W. Efficient extraction of singlet–triplet excitons for high-efficient white organic light-emitting diode with a multilayer emission region. Org. Electron. 2011, 12, 1–7. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, J.Y.; Kim, J.; Kwak, J.; Park, J.-H. Progress of display performances: AR, VR, QLED, and OLED. J. Inf. Disp. 2020, 21, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Moghe, D.A.; Dey, A.; Johnson, K.; Lu, L.P.; Friend, R.H.; Kabra, D. Ultrafast endothermic transfer of non-radiative exciplex state to radiative excitons in polyfluorene random copolymer for blue electroluminescence. Appl. Phys. Lett. 2018, 112, 163301. [Google Scholar] [CrossRef]
- Wei, B.; Xu, H.; Zhao, N.; Gao, X.; Ye, Y.; Wang, Y.; Yao, D.; Zhang, X.; Liu, X. Deep-blue organic light-emitting diodes based on multi-tert-butyl modified naphthylene. J. Ind. Eng. Chem. 2021, 102, 44–50. [Google Scholar] [CrossRef]
- Zou, S.-J.; Shen, Y.; Xie, F.-M.; Chen, J.-D.; Li, Y.-Q.; Tang, J.-X. Recent advances in organic light-emitting diodes: Toward smart lighting and displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Samanta, S.K.; Kumar, G.S.; Ghorai, U.K.; Scherf, U.; Acharya, S.; Bhattacharya, S. Synthesis of High Molecular Weight 1,4-Polynaphthalene for Solution-Processed True Color Blue Light Emitting Diode. Macromolecules 2018, 51, 8324–8329. [Google Scholar] [CrossRef]
- Elkassih, S.A.; Sista, P.; Magurudeniya, H.D.; Papadimitratos, A.; Zakhidov, A.A.; Biewer, M.C.; Stefan, M.C. Phenothiazine Semiconducting Polymer for Light-Emitting Diodes. Macromol. Chem. Phys. 2013, 214, 572–577. [Google Scholar] [CrossRef]
- Slodek, A.; Zych, D.; Kotowicz, S.; Szafraniec-Gorol, G.; Zimosz, S.; Schab-Balcerzak, E.; Siwy, M.; Grzelak, J.; Maćkowski, S. “Small in size but mighty in force”—The first principle study of the impact of A/D units in A/D-phenyl-π-phenothiazine-π-dicyanovinyl systems on photophysical and optoelectronic properties. Dyes Pigm. 2021, 189, 109248. [Google Scholar] [CrossRef]
- Slodek, A.; Zych, D.; Szafraniec-Gorol, G.; Gnida, P.; Vasylieva, M.; Schab-Balcerzak, E. Investigations of New Phenothiazine-Based Compounds for Dye-Sensitized Solar Cells with Theoretical Insight. Materials 2020, 13, 2292. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.; May, L.; Müller, T.J.J. The interplay of conformations and electronic properties in N-aryl phenothiazines. Org. Chem. Front. 2020, 7, 1206–1217. [Google Scholar] [CrossRef]
- Truong, N.T.T.; Nguyen, L.T.; Mai, H.L.T.; Doan, B.K.; Tran, D.H.; Truong, K.T.; Nguyen, V.Q.; Nguyen, L.-T.T.; Hoang, M.H.; Van Pham, T.; et al. Phenothiazine derivatives, diketopyrrolopyrrole-based conjugated polymers: Synthesis, optical and organic field effect transistor properties. J. Polym. Res. 2020, 27, 223. [Google Scholar] [CrossRef]
- Gangadhar, P.S.; Reddy, G.; Prasanthkumar, S.; Giribabu, L. Phenothiazine functional materials for organic optoelectronic applications. Phys. Chem. Chem. Phys. 2021, 23, 14969–14996. [Google Scholar] [CrossRef]
- Jadoun, S.; Riaz, U. Conjugated Polymer Light-Emitting Diodes. In Polymers for Light-Emitting Devices and Displays, 2nd ed.; Inamuddin, Boddula, R., Ahamed, M.I., Asiri, A.M., Eds.; Wiley: Hoboken, NJ, USA, 2020; Chapter 4; pp. 77–98. [Google Scholar] [CrossRef]
- Agarwala, P.; Kabra, D. A review on triphenylamine (TPA) based organic hole transport materials (HTMs) for dye sensitized solar cells (DSSCs) and perovskite solar cells (PSCs): Evolution and molecular engineering. J. Mater. Chem. A 2017, 5, 1348–1373. [Google Scholar] [CrossRef]
- Ego, C.; Grimsdale, A.C.; Uckert, F.; Yu, G.; Srdanov, G.; Müllen, K. Triphenylamine-Substituted Polyfluorene—A Stable Blue-Emitter with Improved Charge Injection for Light-Emitting Diodes. Adv. Mater. 2002, 14, 809–811. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Arunagiri, L.; Khatib, M.; Manzhos, S.; Feron, K.; Bottle, S.E.; Haick, H.; Yan, H.; Michinobu, T.; et al. Versatile nature of anthanthrone based polymers as active multifunctional semiconductors for various organic electronic devices. Mater. Adv. 2020, 1, 3428–3438. [Google Scholar] [CrossRef]
- Du, Y.; Lovell, H.B.; Lirette, F.; Morin, J.F.; Plunkett, K.N. Electron Acceptors Based on Cyclopentannulated Anthanthrenes. J. Org. Chem. 2021, 86, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.D.; Do, T.T.; Kim, J.; Charbonneau, C.; Manzhos, S.; Feron, K.; Tsoi, W.C.; Durrant, J.R.; Jain, S.M.; Sonar, P. Molecular Engineering Using an Anthanthrone Dye for Low-Cost Hole Transport Materials: A Strategy for Dopant-Free, High-Efficiency, and Stable Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1703007. [Google Scholar] [CrossRef]
- Shah, B.K.; Neckers, D.C. Anthanthrene Derivatives as Blue Emitting Materials for Organic Light-Emitting Diode Applications. Chem. Mater. 2006, 18, 603–608. [Google Scholar] [CrossRef]
- Giguère, J.-B.; Verolet, Q.; Morin, J.-F. 4,10-Dibromoanthanthrone as a New Building Block for p-Type, n-Type, and Ambipolar π-Conjugated Materials. Chem. Eur. J. 2013, 19, 372–381. [Google Scholar] [CrossRef]
- Tang, W.; Kietzke, T.; Vemulamada, P.; Chen, Z.-K. Synthesis, characterization, and photovoltaic properties of novel conjugated copolymers derived from phenothiazines. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 5266–5276. [Google Scholar] [CrossRef]
- Qian, Z.; Cao, Z.; Galuska, L.; Zhang, S.; Xu, J.; Gu, X. Glass Transition Phenomenon for Conjugated Polymers. Macromol. Chem. Phys. 2019, 220, 1900062. [Google Scholar] [CrossRef]
- Geng, Y.; Yi, C.; Bircher, M.P.; Decurtins, S.; Cascella, M.; Grätzel, M.; Liu, S.-X. Anthanthrene dye-sensitized solar cells: Influence of the number of anchoring groups and substitution motif. RSC Adv. 2015, 5, 98643–98652. [Google Scholar] [CrossRef]
- Pham, H.D.; Hu, H.; Wong, F.-L.; Lee, C.-S.; Chen, W.-C.; Feron, K.; Manzhos, S.; Wang, H.; Motta, N.; Lam, Y.M.; et al. Acene-based organic semiconductors for organic light-emitting diodes and perovskite solar cells. J. Mater. Chem. C 2018, 6, 9017–9029. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Kohara, A.; Matsumoto, H.; Manzhos, S.; Feron, K.; Bottle, S.E.; Bell, J.; Michinobu, T.; Sonar, P. Tuning the Charge Carrier Polarity of Organic Transistors by Varying the Electron Affinity of the Flanked Units in Diketopyrrolopyrrole-Based Copolymers. Adv. Funct. Mater. 2020, 30, 1907452. [Google Scholar] [CrossRef]
- Liu, Q.; Kumagai, S.; Manzhos, S.; Chen, Y.; Angunawela, I.; Nahid, M.M.; Feron, K.; Bottle, S.E.; Bell, J.; Ade, H.; et al. Synergistic Use of Pyridine and Selenophene in a Diketopyrrolopyrrole-Based Conjugated Polymer Enhances the Electron Mobility in Organic Transistors. Adv. Funct. Mater. 2020, 30, 2000489. [Google Scholar] [CrossRef]
- Moghe, D.; Dey, A.; Kaur, B.; Jacob, J.; Kabra, D. Enhancing the electroluminescence efficiency by controlling the migration of excited states to quenching sites in a truxene-based oligomer. J. Appl. Phys. 2019, 126, 125502. [Google Scholar] [CrossRef]




| Polymer | HOMO/LUMO | |||||
|---|---|---|---|---|---|---|
| PNP(1,4)-PT | 332 nm | 496 nm | 339 nm | 440 nm | 2.82 eV | −5.55/−2.73 eV |
| PNP(1,4)-TF | 310 nm 363 * nm | 426 nm | 310 nm | 413 nm | 3.00 eV | −5.81/−2.81 eV |
| PNP(1,4)-ANT | 327 nm 461 * nm | 474 nm 502 * nm | 327 nm | 483 nm | 2.57 eV | −5.53/−2.96 eV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Moghe, D.; Sardar, G.; Manzhos, S.; Bottle, S.E.; Kyaw, A.K.K.; Kabra, D.; Sonar, P. Structural Geometry Variation of 1,4-Naphthalene-Based Co-Polymers to Tune the Device Performance of PVK-Host-Based OLEDs. Polymers 2021, 13, 2914. https://doi.org/10.3390/polym13172914
Liu Q, Moghe D, Sardar G, Manzhos S, Bottle SE, Kyaw AKK, Kabra D, Sonar P. Structural Geometry Variation of 1,4-Naphthalene-Based Co-Polymers to Tune the Device Performance of PVK-Host-Based OLEDs. Polymers. 2021; 13(17):2914. https://doi.org/10.3390/polym13172914
Chicago/Turabian StyleLiu, Qian, Dhanashree Moghe, Gopa Sardar, Sergei Manzhos, Steven E. Bottle, Aung Ko Ko Kyaw, Dinesh Kabra, and Prashant Sonar. 2021. "Structural Geometry Variation of 1,4-Naphthalene-Based Co-Polymers to Tune the Device Performance of PVK-Host-Based OLEDs" Polymers 13, no. 17: 2914. https://doi.org/10.3390/polym13172914
APA StyleLiu, Q., Moghe, D., Sardar, G., Manzhos, S., Bottle, S. E., Kyaw, A. K. K., Kabra, D., & Sonar, P. (2021). Structural Geometry Variation of 1,4-Naphthalene-Based Co-Polymers to Tune the Device Performance of PVK-Host-Based OLEDs. Polymers, 13(17), 2914. https://doi.org/10.3390/polym13172914