Silicone Implants Immobilized with Interleukin-4 Promote the M2 Polarization of Macrophages and Inhibit the Formation of Fibrous Capsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
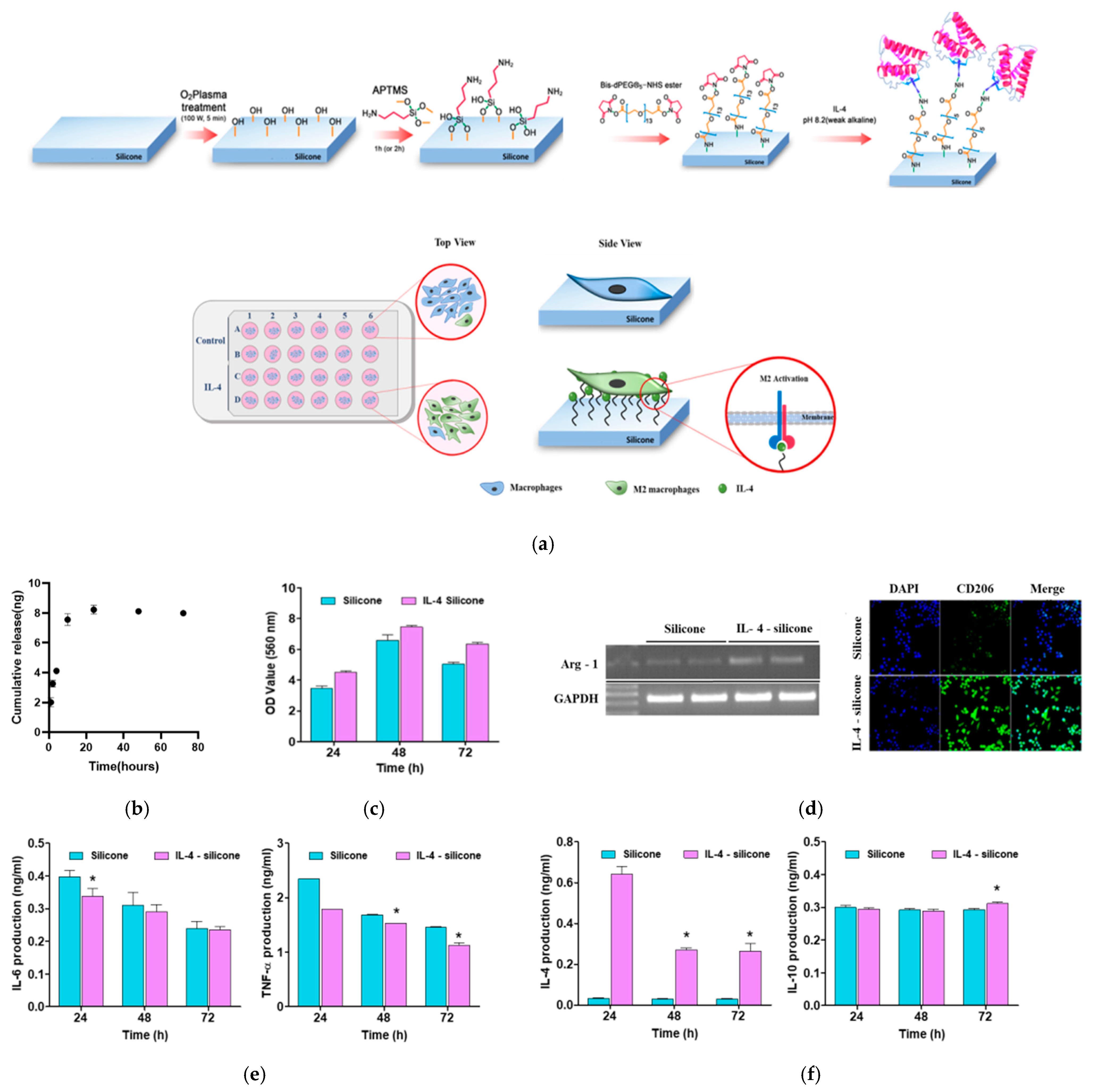
2.2. Immobilization of IL-4 on Silicone Implant
2.3. The Physicochemical Characterization of Modified Silicone Implants
2.4. IL-4 Release Profiles
2.5. Macrophage Cell Culture for In Vitro Analysis
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Immunofluorescence Staining and MTT Assay
2.8. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.9. In Vivo Experiment
2.10. In Vivo Evaluation of Capsule Thickness and Collagen Density
2.11. Statistical Analysis
3. Results
3.1. Characterization of the Modified Surface of Our Silicone Implants
3.2. In Vitro Release Profiles and the Effect of the IL-4-Coated Silicone Implant on Macrophage Polarization
3.3. Effect of the IL-4-Coated Silicone Implant on the Fibrous Capsule Formation In Vivo
3.4. The Effect of the IL-4-Coated Silicone Implant on Macrophage Polarization
3.5. Estimation of the Numbers of Fibroblasts and Myofibroblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, B.H.; Kim, B.H.; Kim, S.; Lee, K.; Bin Choy, Y.; Heo, C.Y. Silicone breast implant modification review: Overcoming capsular contracture. Biomater. Res. 2018, 22, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Siggelkow, W.; Faridi, A.; Spiritus, K.; Klinge, U.; Rath, W.; Klosterhalfen, B. Histological analysis of silicone breast implant capsules and correlation with capsular contracture. Biomaterials 2003, 24, 1101–1109. [Google Scholar] [CrossRef]
- O’Connell, S.G.; Kerkvliet, N.I.; Carozza, S.; Rohlman, D.; Pennington, J.; Anderson, K.A. In vivo contaminant partitioning to silicone implants: Implications for use in biomonitoring and body burden. Environ. Int. 2015, 85, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Headon, H.; Kasem, A.; Mokbel, K. Capsular Contracture after Breast Augmentation: An Update for Clinical Practice. Arch. Plast. Surg. 2015, 42, 532–543. [Google Scholar] [CrossRef] [Green Version]
- Sridharan, R.; Cameron, A.; Kelly, D.; Kearney, C.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Wolfram, D.; Rainer, C.; Niederegger, H.; Piza, H.; Wick, G. Corrigendum to “Cellular and molecular composition of fibrous capsules formed around silicone breast implants with special focus on local immune reactions” [J Autoimmun 23 (2004) 81–91]. J. Autoimmun. 2005, 24, 361. [Google Scholar] [CrossRef]
- Chazaud, B.; Brigitte, M.; Yacoub-Youssef, H.; Arnold, L.; Gherardi, R.; Sonnet, C.; Lafuste, P.; Chretien, F. Dual and Beneficial Roles of Macrophages During Skeletal Muscle Regeneration. Exerc. Sport Sci. Rev. 2009, 37, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-L.; Lassner, F.; Bader, A.; Becker, M.; Walter, G.F.; Berger, A. Cellular activity of resident macrophages during Wallerian degeneration. Microsurgery 2000, 20, 255–261. [Google Scholar] [CrossRef]
- Mueller, M.; Leonhard, C.; Wacker, K.; Ringelstein, E.B.; Okabe, M.; Hickey, W.F.; Kiefer, R. Macrophage Response to Peripheral Nerve Injury: The Quantitative Contribution of Resident and Hematogenous Macrophages. Lab. Investig. 2003, 83, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Edwards, J.P.; Zhang, X.; Frauwirth, K.A.; Mosser, D.M. Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 2006, 80, 1298–1307. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S.; Martinez, F.O. Alternative Activation of Macrophages: Mechanism and Functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [Green Version]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.N.; Londono, R.; Tottey, S.; Zhang, L.; Kukla, K.A.; Wolf, M.T.; Daly, K.A.; Reing, J.; Badylak, S. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012, 8, 978–987. [Google Scholar] [CrossRef] [Green Version]
- Zurawski, G.; de Vries, J.E. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol. Today 1994, 15, 19–26. [Google Scholar] [CrossRef]
- Chomarat, P.; Banchereau, J. Interleukin-4 and lnterleukin-13: Their Similarities and Discrepancies. Int. Rev. Immunol. 1998, 17, 1–52. [Google Scholar] [CrossRef]
- Mitchell, R.E.; Hassan, M.; Burton, B.R.; Britton, G.; Hill, E.V.; Verhagen, J.; Wraith, D.C. IL-4 enhances IL-10 production in Th1 cells: Implications for Th1 and Th2 regulation. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Luzina, I.G.; Keegan, A.D.; Heller, N.M.; Rook, G.; Shea-Donohue, T.; Atamas, S.P. Regulation of inflammation by interleukin-4: A review of “alternatives”. J. Leukoc. Biol. 2012, 92, 753–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.; Xu, G.; Gao, Y.; An, Y. Surface Wettability of (3-Aminopropyl)triethoxysilane Self-Assembled Monolayers. J. Phys. Chem. B 2011, 115, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S. Effects of biomaterial-induced inflammation on fibrosis and rejection. Semin. Immunol. 2008, 20, 130–136. [Google Scholar] [CrossRef]
- Ji, L.; Wang, T.; Tian, L.; Song, H.; Gao, M. Roxatidine inhibits fibrosis by inhibiting NF-κB and MAPK signaling in macrophages sensing breast implant surface materials. Mol. Med. Rep. 2019, 21, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Yoo, B.Y.; Kim, B.H.; Lee, J.S.; Shin, B.H.; Kwon, H.; Koh, W.-G.; Heo, C.Y. Dual surface modification of PDMS-based silicone implants to suppress capsular contracture. Acta Biomater. 2018, 76, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Scheller, J.; Garbers, C.; Rose-John, S. Interleukin-6: From basic biology to selective blockade of pro-inflammatory activities. Semin. Immunol. 2014, 26, 2–12. [Google Scholar] [CrossRef]
- Mauer, J.; Chaurasia, B.; Goldau, J.; Vogt, M.C.; Ruud, J.; Nguyen, K.; Theurich, S.; Hausen, A.C.; Schmitz, J.; Brönneke, H.S.; et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 2014, 15, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.; Garzetti, L.; Gatta, A.T.; Finardi, A.; Maiorino, C.; Ruffini, F.; Martino, G.; Muzio, L.; Furlan, R. IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. J. Neuroinflamm. 2016, 13, 139. [Google Scholar] [CrossRef] [Green Version]
- Dolores, W.; Christian, R.; Harald, N.; Hildegunde, P.; Georg, W. Cellular and molecular composition of fibrous capsules formed around silicone breast implants with special focus on local immune reactions. J. Autoimmun. 2004, 23, 81–91. [Google Scholar] [CrossRef]
- Di Vito, A.; Santise, G.; Mignogna, C.; Chiefari, E.; Cardillo, G.; Presta, I.; Arturi, F.; Malara, N.; Brunetti, F.; Donato, A.; et al. Innate immunity in cardiac myxomas and its pathological and clinical correlations. Innate Immun. 2017, 24, 47–53. [Google Scholar] [CrossRef]
- Grotendorst, G.R. Connective tissue growth factor: A mediator of TGF-β action on fibroblasts. Cytokine Growth Fact. Rev. 1997, 8, 171–179. [Google Scholar] [CrossRef]
- Champaneria, M.C.; Wong, W.W.; Hill, M.E.; Gupta, S.C. The Evolution of Breast Reconstruction: A Historical Perspective. World J. Surg. 2012, 36, 730–742. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign Body Reaction to Biomaterials. In Seminars in Immunology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 20, pp. 86–100. [Google Scholar]
- Hotaling, N.A.; Cummings, R.D.; Ratner, D.M.; Babensee, J.E. Molecular factors in dendritic cell responses to adsorbed glycoconjugates. Biomaterials 2014, 35, 5862–5874. [Google Scholar] [CrossRef] [Green Version]
- Kou, P.M.; Babensee, J.E. Macrophage and dendritic cell phenotypic diversity in the context of biomaterials. J. Biomed. Mater. Res. Part A 2010, 96, 239–260. [Google Scholar] [CrossRef]
- Park, J.; Babensee, J.E. Differential functional effects of biomaterials on dendritic cell maturation. Acta Biomater. 2012, 8, 3606–3617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.M. Biological Responses to Materials. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Bryers, J.D.; Giachelli, C.M.; Ratner, B.D. Engineering biomaterials to integrate and heal: The biocompatibility paradigm shifts. Biotechnol. Bioeng. 2012, 109, 1898–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/Tissue Interactions: Possible Solutions to Overcome Foreign Body Response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Luttikhuizen, D.T.; Harmsen, M.C.; Van Luyn, M.J. Cellular and Molecular Dynamics in the Foreign Body Reaction. Tissue Eng. 2006, 12, 1955–1970. [Google Scholar] [CrossRef]
- Jones, J.A.; Chang, D.T.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. Part A 2007, 83, 585–596. [Google Scholar] [CrossRef]
- Stout, R.D.; Jiang, C.; Matta, B.; Tietzel, I.; Watkins, S.K.; Suttles, J. Macrophages Sequentially Change Their Functional Phenotype in Response to Changes in Microenvironmental Influences. J. Immunol. 2005, 175, 342–349. [Google Scholar] [CrossRef]
- Porcheray, F.; Viaud, S.; Rimaniol, A.-C.; Leone, C.; Samah, B.; Dereuddre-Bosquet, N.; Dormont, D.; Gras, G. Macrophage activation switching: An asset for the resolution of inflammation. Clin. Exp. Immunol. 2005, 142, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.J.; D’acampora, A.J.; Marcos, A.B.W.; Di Giunta, G.; De Vasconcellos, Z.A.A.; Bins-Ely, J.; Neves, R.D.; Figueiredo, C.P. Vascular Endothelial Growth Factor Overexpression Positively Modulates the Characteristics of Periprosthetic Tissue of Polyurethane-Coated Silicone Breast Implant in Rats. Plast. Reconstr. Surg. 2010, 126, 1899–1910. [Google Scholar] [CrossRef]
- Weiss, M.; Blazek, K.; Byrne, A.; Perocheau, D.P.; Udalova, I.A. IRF5 Is a Specific Marker of Inflammatory Macrophages In Vivo. Mediat. Inflamm. 2013, 2013, 245804. [Google Scholar] [CrossRef] [Green Version]
- Kechagia, J.Z.; Ezra, D.G.; Burton, M.J.; Bailly, M. Fibroblasts profiling in scarring trachoma identifies IL-6 as a functional component of a fibroblast-macrophage pro-fibrotic and pro-inflammatory feedback loop. Sci. Rep. 2016, 6, 28261. [Google Scholar] [CrossRef] [Green Version]
- Piera-Velazquez, S.; Li, Z.; Jimenez, S.A. Role of Endothelial-Mesenchymal Transition (EndoMT) in the Pathogenesis of Fibrotic Disorders. Am. J. Pathol. 2011, 179, 1074–1080. [Google Scholar] [CrossRef]
- Darby, I.; Hewitson, T. Fibroblast Differentiation in Wound Healing and Fibrosis. Int. Rev. Cytol. 2007, 257, 143–179. [Google Scholar] [CrossRef]
- Prud’Homme, G.J. Pathobiology of transforming growth factor β in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab. Investig. 2007, 87, 1077–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, K.; Sim, H.B.; Huan, F.; Kim, D.J. Myofibroblasts and Capsular Tissue Tension in Breast Capsular Contracture. Aesthetic Plast. Surg. 2010, 34, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M. The fascia of the limbs and back—A review. J. Anat. 2009, 214, 1–18. [Google Scholar] [CrossRef]
- E Martin, K.; García, A.J. Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta Biomater. 2021. [Google Scholar] [CrossRef]





| Gene-Specific Primers | RT-PCR Primer Sequence (5′-3′) |
|---|---|
| Arg-1 forward | 5′-AAGAAAAGGCCGATTCACCT-3′ |
| Arg-1 reverse | 5′-CACCTCCTCT GCTGTCTTCC-3′ |
| GAPDH forward | 5′-GGC ATG GAC TGT GGT CAT GA-3′ |
| GAPDH reverse | 5′-TTC ACC ACC ATG GAG AAG GC-3′ |
| Sample | WCA (°) |
|---|---|
| (a) Bare silicone prosthetic material (Si) | 93.90 |
| (b) Si/O2 plasma | 0.16 |
| (c) Si/O2 plasma/APTMS | 97.80 |
| (d) Si/O2 plasma/APTMS/Bis diPEG@13NHS ester | 100.6 |
| (e) IL-4 (cytokine immobilization) | 78.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-S.; Kim, S.; Shin, B.-H.; Heo, C.-Y.; Faruq, O.; Van Anh, L.T.; Dönmez, N.; Chien, P.N.; Shin, D.-S.; Nam, S.-Y.; et al. Silicone Implants Immobilized with Interleukin-4 Promote the M2 Polarization of Macrophages and Inhibit the Formation of Fibrous Capsules. Polymers 2021, 13, 2630. https://doi.org/10.3390/polym13162630
Kim H-S, Kim S, Shin B-H, Heo C-Y, Faruq O, Van Anh LT, Dönmez N, Chien PN, Shin D-S, Nam S-Y, et al. Silicone Implants Immobilized with Interleukin-4 Promote the M2 Polarization of Macrophages and Inhibit the Formation of Fibrous Capsules. Polymers. 2021; 13(16):2630. https://doi.org/10.3390/polym13162630
Chicago/Turabian StyleKim, Hyun-Seok, Seongsoo Kim, Byung-Ho Shin, Chan-Yeong Heo, Omar Faruq, Le Thi Van Anh, Nilsu Dönmez, Pham Ngoc Chien, Dong-Sik Shin, Sun-Young Nam, and et al. 2021. "Silicone Implants Immobilized with Interleukin-4 Promote the M2 Polarization of Macrophages and Inhibit the Formation of Fibrous Capsules" Polymers 13, no. 16: 2630. https://doi.org/10.3390/polym13162630
APA StyleKim, H.-S., Kim, S., Shin, B.-H., Heo, C.-Y., Faruq, O., Van Anh, L. T., Dönmez, N., Chien, P. N., Shin, D.-S., Nam, S.-Y., & Baek, R.-M. (2021). Silicone Implants Immobilized with Interleukin-4 Promote the M2 Polarization of Macrophages and Inhibit the Formation of Fibrous Capsules. Polymers, 13(16), 2630. https://doi.org/10.3390/polym13162630







