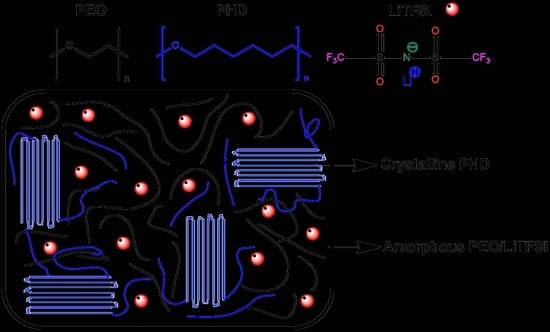
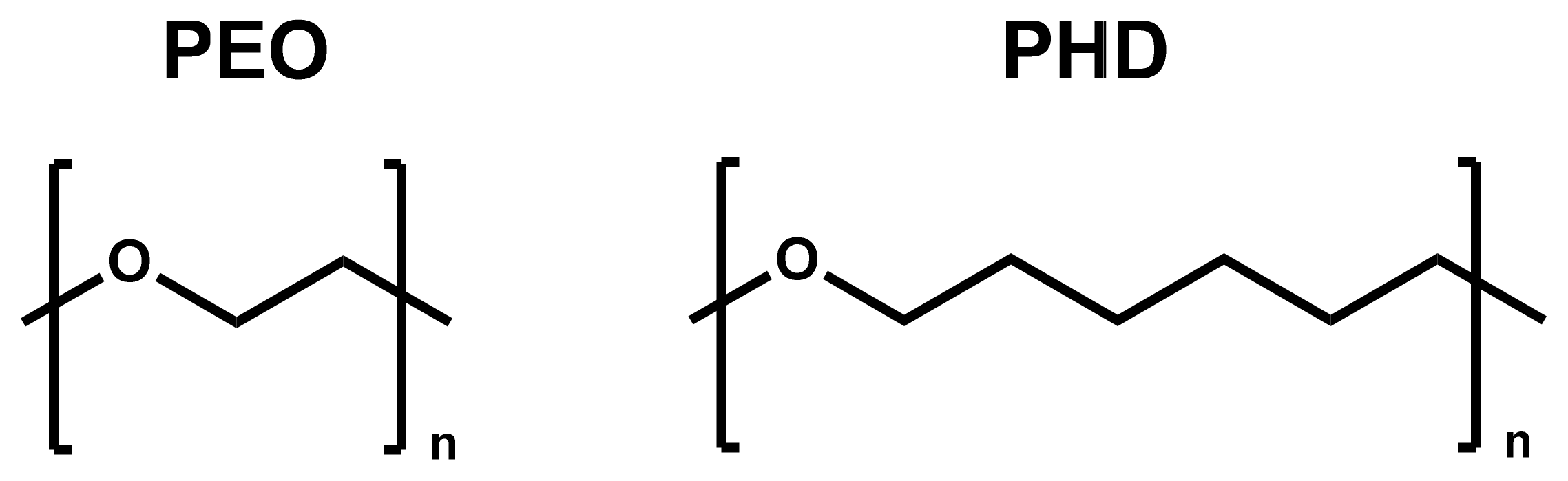
Polyether Single and Double Crystalline Blends and the Effect of Lithium Salt on Their Crystallinity and Ionic Conductivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PHD: Bulk Self-Condensation of 1,6-Hexanediol
2.2. Blends Preparation
2.3. Characterization
3. Results and Discussion
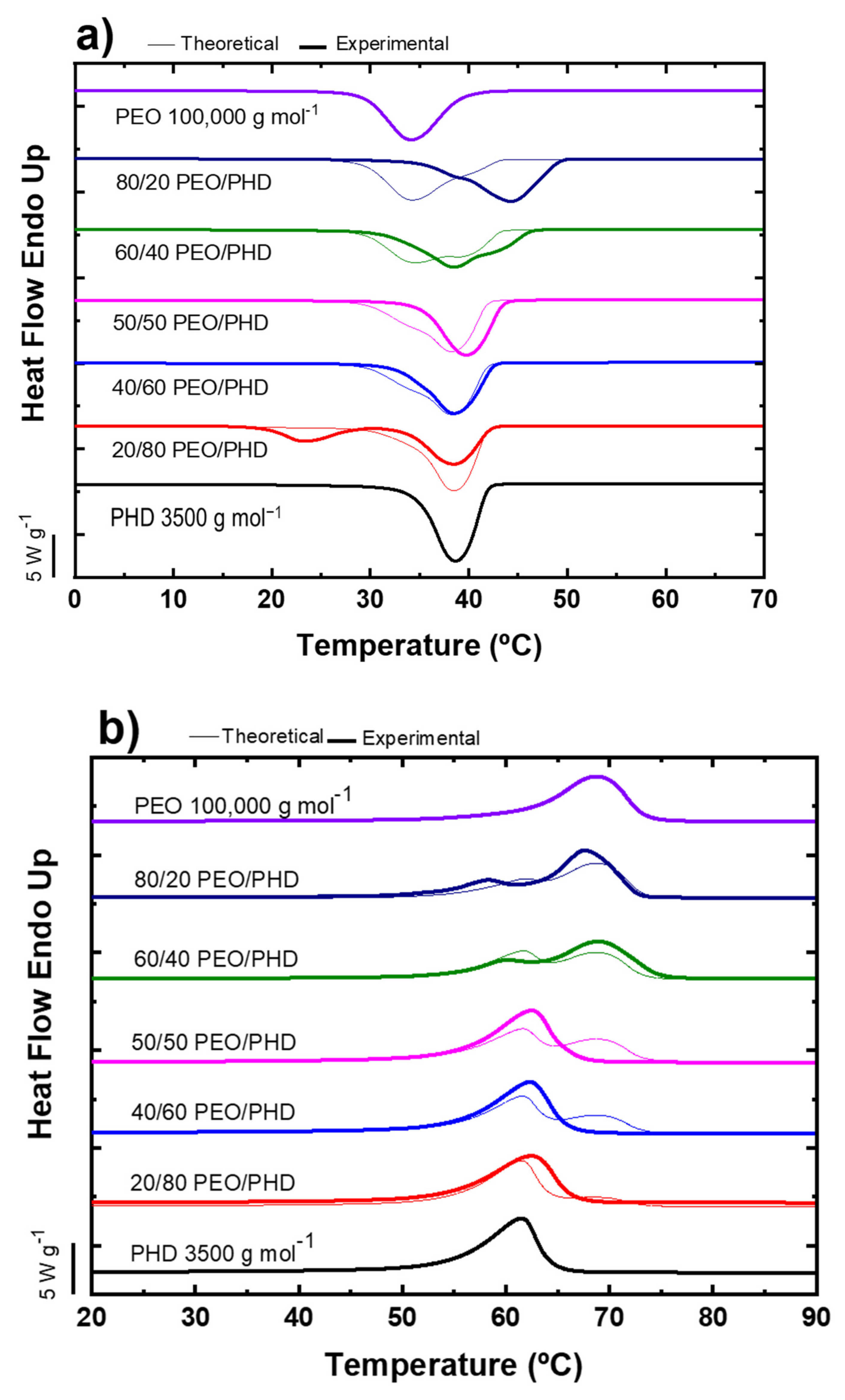
3.1. Non-Isothermal DSC of PEO/PHD Blends
3.2. Wide Angle X-ray Scattering of PEO/PHD Blends
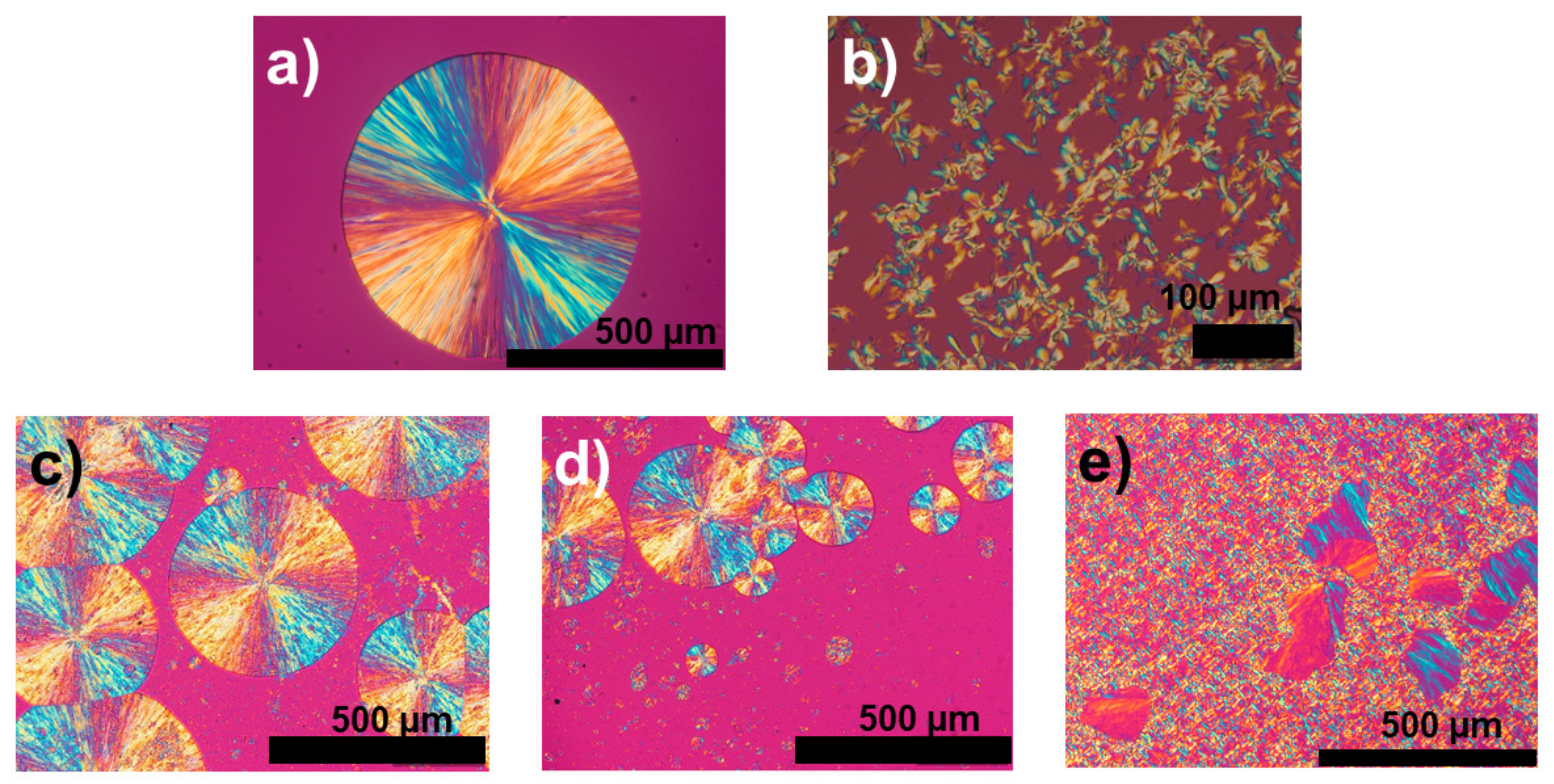
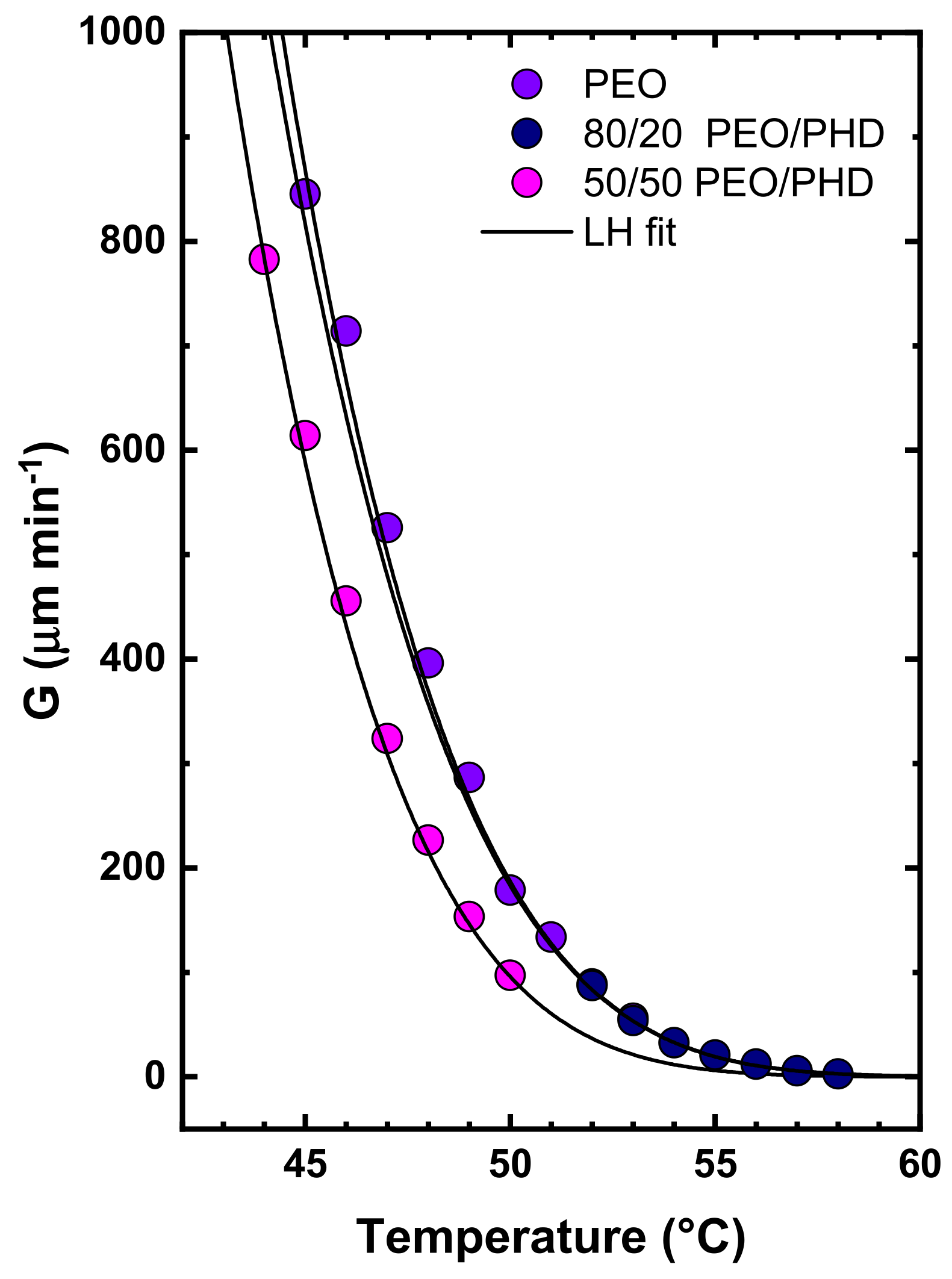
3.3. Morphology and Crystal Growth Rate
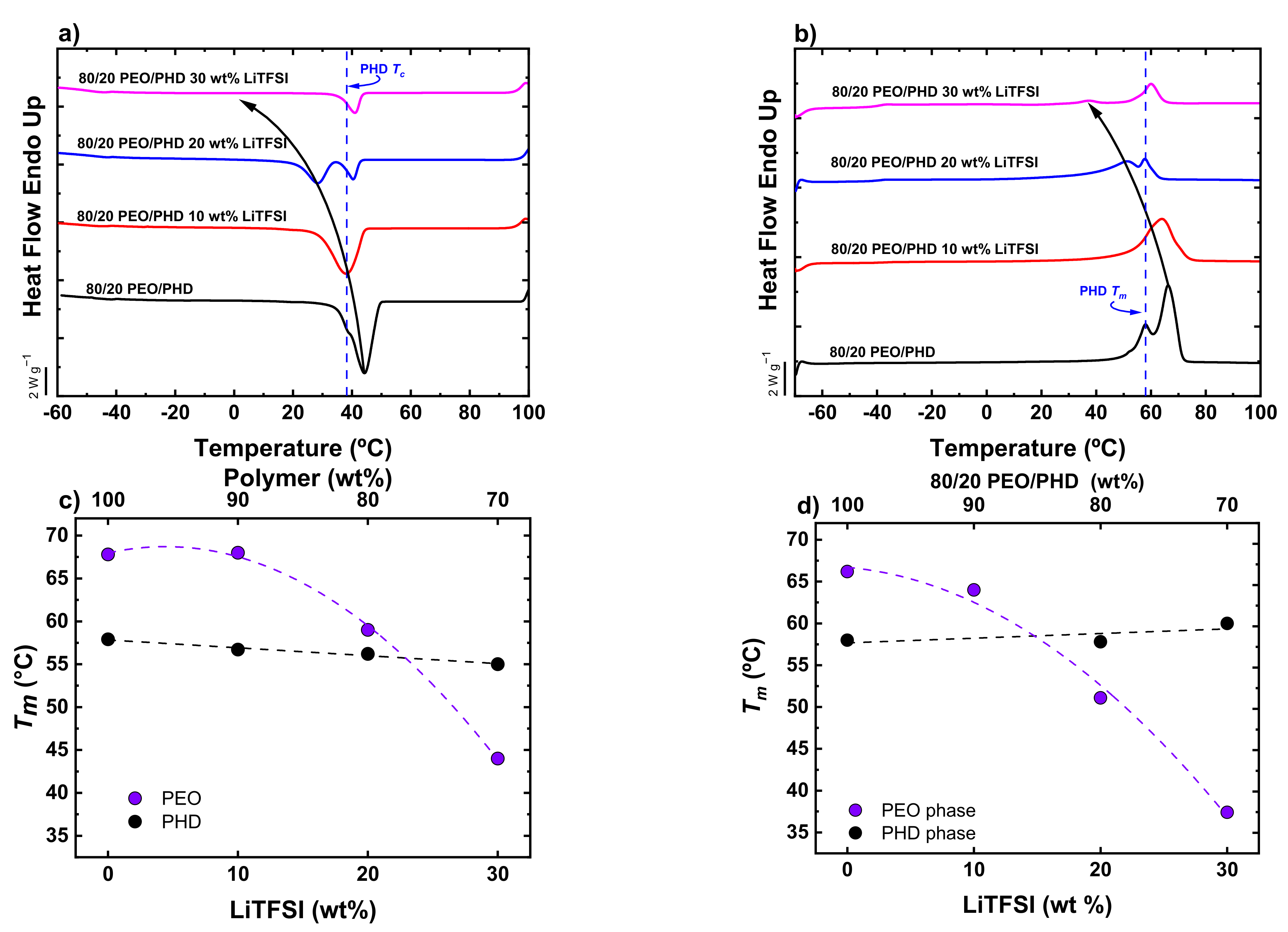
3.4. Non-Isothermal DSC of PEO/PHD Blends with LiTFSI
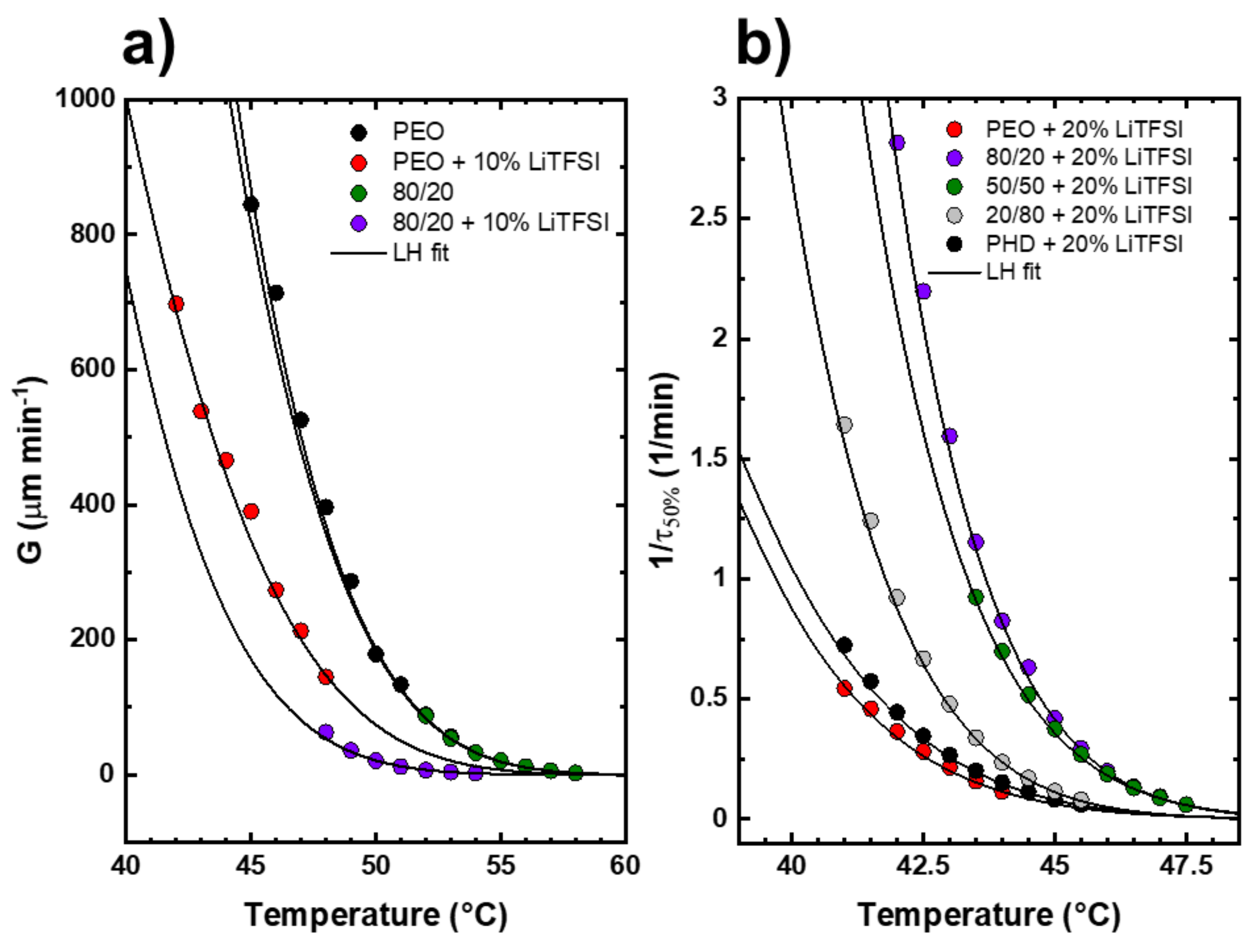
3.5. Isothermal Crystallization Studies of the PEO/PHD Blends with LiTFSI
3.6. Ionic Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Klein, R.; Wurm, F.R. Aliphatic polyethers: Classical polymers for the 21st century. Macromol. Rapid Commun. 2015. [Google Scholar] [CrossRef]
- Evans, T.H.; Hawkins, W.L.; Hibbert, H. Studies on reactions relating to Carbohydrates and Polysaccharides. J. Exp. Med. 1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenberg, E.J. Organometallic catalysts for polymerizing monosubstituted epoxides. J. Polym. Sci. 1960, 47, 486–489. [Google Scholar] [CrossRef]
- Dreyfuss, M.P.; Dreyfuss, P. A “living” polymer after cationic initiation. Polymer 1965, 6, 93–95. [Google Scholar] [CrossRef]
- Basterretxea, A.; Gabirondo, E.; Jehanno, C.; Zhu, H.; Flores, I.; Müller, A.J.; Etxeberria, A.; Mecerreyes, D.; Coulembier, O.; Sardon, H. Polyether Synthesis by Bulk Self-Condensation of Diols Catalyzed by Non-Eutectic Acid-Base Organocatalysts. ACS Sustain. Chem. Eng. 2019, 7, 4103–4111. [Google Scholar] [CrossRef]
- Jérôme, C.; Lecomte, P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.J.A.; Shaver, M.P. Aliphatic polyester polymer stars: Synthesis, properties and applications in biomedicine and nanotechnology. Chem. Soc. Rev. 2011, 40, 1761–1776. [Google Scholar] [CrossRef] [Green Version]
- Lécuyer, M.; Deschamps, M.; Guyomard, D.; Gaubicher, J.; Poizot, P. Electrochemical Assessment of Indigo Carmine Dye in Lithium Metal Polymer Technology. Molecules 2021, 26, 3079. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO based solid polymer electrolytes for all-solid state lithium ion batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Zhang, H.; Zhou, C.; Zheng, L.; Cheng, P.; Nie, J.; Feng, W.; Hu, Y.; Li, H.; Huang, X.; et al. Ion Conductors Single Lithium-Ion Conducting Polymer Electrolytes Based on a Super- Delocalized Polyanion. Angew. Chem. Int. Ed. 2016, 55, 2521–2525. [Google Scholar] [CrossRef]
- Geiculescu, O.E.; Yang, J.; Zhou, S.; Shafer, G.; Xie, Y.; Albright, J.; Creager, S.E.; Pennington, W.T.; DesMarteau, D.D. Solid Polymer Electrolytes from Polyanionic Lithium Salts Based on the LiTFSI Anion Structure. J. Electrochem. Soc. 2004, 151, A1363. [Google Scholar] [CrossRef]
- Tao, C.; Gao, M.H.; Yin, B.H.; Li, B.; Huang, Y.P.; Xu, G.; Bao, J.J. A promising TPU/PEO blend polymer electrolyte for all-solid-state lithium ion batteries. Electrochim. Acta 2017, 257, 31–39. [Google Scholar] [CrossRef]
- Marzantowicz, M.; Dygas, J.R.; Krok, F.; Łasińska, A.; Florjańczyk, Z.; Zygadło-Monikowska, E.; Affek, A. Crystallization and melting of PEO:LiTFSI polymer electrolytes investigated simultaneously by impedance spectroscopy and polarizing microscopy. Electrochim. Acta 2005, 50, 3969–3977. [Google Scholar] [CrossRef]
- Vignarooban, K.; Mellander, B.-E.; Albinson, I.; Dissanayake, M. Effect of Different Types of Ceramic Nano-Fillers on Thermal and Transport Properties of PEO 9 LiTf Solid Polymer Electrolyte. In Solid State Ionics: Advanced Materials for Emerging Technologies; World Scientific: Singapore, 2006; pp. 623–630. [Google Scholar]
- Borgohain, M.M.; Joykumar, T.; Bhat, S.V. Studies on a nanocomposite solid polymer electrolyte with hydrotalcite as a filler. Solid State Ionics 2010, 181, 964–970. [Google Scholar] [CrossRef]
- Niitani, T.; Shimada, M.; Kawamura, K.; Kanamura, K. Characteristics of new-type solid polymer electrolyte controlling nano-structure. J. Power Sources 2005, 146, 386–390. [Google Scholar] [CrossRef]
- Sadoway, D.R. Block and graft copolymer electrolytes for high-performance, solid-state, lithium batteries. J. Power Sources 2004, 129, 1–3. [Google Scholar] [CrossRef]
- Snyder, J.F.; Carter, R.H.; Wetzel, E.D. Electrochemical and mechanical behavior in mechanically robust solid polymer electrolytes for use in multifunctional structural batteries. Chem. Mater. 2007, 19, 3793–3801. [Google Scholar] [CrossRef]
- Meabe, L.; Huynh, T.V.; Lago, N.; Sardon, H.; Li, C.; O’Dell, L.A.; Armand, M.; Forsyth, M.; Mecerreyes, D. Poly(ethylene oxide carbonates) solid polymer electrolytes for lithium batteries. Electrochim. Acta 2018, 264, 367–375. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Progress in Polymer Science Beyond PEO—Alternative host materials for Li + -conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Xu, H.; Xie, J.; Liu, Z.; Wang, J.; Deng, Y. Carbonyl-coordinating polymers for high-voltage solid-state lithium batteries: Solid polymer electrolytes. MRS Energy Sustain. 2020, 7. [Google Scholar] [CrossRef]
- Gao, K.W.; Loo, W.S.; Snyder, R.L.; Abel, B.A.; Choo, Y.; Lee, A.; Teixeira, S.C.M.; Garetz, B.A.; Coates, G.W.; Balsara, N.P. Miscible Polyether/Poly (ether–acetal) Electrolyte Blends. Macromolecules 2020, 53, 5728–5739. [Google Scholar] [CrossRef]
- Olmedo-Martínez, J.L.; Meabe, L.; Basterretxea, A.; Mecerreyes, D.; Müller, A.J. Effect of chemical structure and salt concentration on the crystallization and ionic conductivity of aliphatic polyethers. Polymers 2019, 11, 452. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, A.T.; Arnal, M.L.; Albuerne, J.; Müller, A.J. DSC isothermal polymer crystallization kinetics measurements and the use of the Avrami equation to fit the data: Guidelines to avoid common problems. Polym. Test. 2007, 26, 222–231. [Google Scholar] [CrossRef]
- Faucher, J.A.; Koleske, J.V.; Santee, E.R., Jr.; Stratta, J.J.; Wilson, C.W., III. Glass transitions of ethylene oxide polymers. J. Appl. Phys. 1966, 37, 3962–3964. [Google Scholar] [CrossRef]
- Mandelkern, L. Crystallization of Polymers, 2nd ed.; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Sangroniz, L.; Wang, B.; Su, Y.; Liu, G.; Cavallo, D.; Wang, D.; Müller, A.J. Fractionated crystallization in semicrystalline polymers. Prog. Polym. Sci. 2021, 101376. [Google Scholar] [CrossRef]
- Bartczak, Z.; Galeski, A.; Krasnikova, N.P. Primary nucleation and spherulite growth rate in isotactic polypropylene-polystyrene blends. Polymer 1987, 28, 1627–1634. [Google Scholar] [CrossRef]
- Su, Z.; Dong, M.; Guo, Z.; Yu, J. Study of polystyrene and acrylonitrile−styrene copolymer as special β-nucleating agents to induce the crystallization of isotactic polypropylene. Macromolecules 2007, 40, 4217–4224. [Google Scholar] [CrossRef]
- Yang, B.; Ni, H.; Huang, J.; Luo, Y. Effects of Poly (vinyl butyral) as a Macromolecular Nucleating Agent on the Nonisothermal Crystallization and Mechanical Properties of Biodegradable Poly (butylene succinate). Macromolecules 2014, 47, 284–296. [Google Scholar] [CrossRef]
- Azli, A.A.; Manan, N.S.A.; Kadir, M.F.Z. Conductivity and Dielectric Studies of Lithium Trifluoromethanesulfonate Doped Polyethylene Oxide-Graphene Oxide Blend Based Electrolytes. Adv. Mater. Sci. Eng. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Reddeppa, N.; Reddy, T.J.R.; Achari, V.B.S.; Rao, V.V.R.N.; Sharma, A.K. Electrical and optical characterization of (PEO+PVAc) polyblend films. Ionics 2009, 15, 255–259. [Google Scholar] [CrossRef]
- Kumar, K.K.; Ravi, M.; Pavani, Y.; Bhavani, S.; Sharma, A.K.; Rao, V.V.R.N. Investigations on PEO/PVP/NaBr complexed polymer blend electrolytes for electrochemical cell applications. J. Memb. Sci. 2014, 454, 200–211. [Google Scholar] [CrossRef]
- Basterretxea, A.; Gabirondo, E.; Flores, I.; Etxeberria, A.; Gonzalez, A.; Müller, A.J.; Mecerreyes, D.; Coulembier, O.; Sardon, H. Isomorphic Polyoxyalkylene Copolyethers Obtained by Copolymerization of Aliphatic Diols. Macromolecules 2019, 52, 3506–3515. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tadokoro, H.; Chatani, Y. Structural studies on polyethers, [-(CH2)m-O-] n. VI. The higher members with m = 6–10, 12. Die Makromol. Chem. Macromol. Chem. Phys. 1968, 112, 225–241. [Google Scholar] [CrossRef]
- Müller, A.J.; Michell, R.M.; Lorenzo, A.T. Isothermal Crystallization Kinetics of Polymers. In Polymer Morphology: Principles, Characterization, and Processing; Guo, Q., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 181–203. [Google Scholar]
- Mary, R.; Müller, A.J. Progress in Polymer Science Confined crystallization of polymeric materials. Prog. Polym. Sci. 2016, 54–55, 183–213. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Davis, G.T.; Lauritzen, J.I. The Rate of Crystallization of Linear Polymers with Chain Folding. In Treatise on Solid State Chemistry; Springer: Berlin/Heidelberg, Germany, 1976; pp. 497–614. [Google Scholar]
- Safari, M.; Mugica, A.; Zubitur, M.; de Ilarduya, A.M.; Muñoz-Guerra, S.; Müller, A.J. Controlling the isothermal crystallization of isodimorphic PBS-ran-PCL random copolymers by varying composition and supercooling. Polymers 2020, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Lascaud, S.; Perrier, M.; Vallee, A.; Besner, S.; Prud’Homme, J.; Armand, M. Phase diagrams and conductivity behavior of poly (ethylene oxide)-molten salt rubbery electrolytes. Macromolecules 1994, 27, 7469–7477. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedo-Martínez, J.L.; Pastorio, M.; Gabirondo, E.; Lorenzetti, A.; Sardon, H.; Mecerreyes, D.; Müller, A.J. Polyether Single and Double Crystalline Blends and the Effect of Lithium Salt on Their Crystallinity and Ionic Conductivity. Polymers 2021, 13, 2097. https://doi.org/10.3390/polym13132097
Olmedo-Martínez JL, Pastorio M, Gabirondo E, Lorenzetti A, Sardon H, Mecerreyes D, Müller AJ. Polyether Single and Double Crystalline Blends and the Effect of Lithium Salt on Their Crystallinity and Ionic Conductivity. Polymers. 2021; 13(13):2097. https://doi.org/10.3390/polym13132097
Chicago/Turabian StyleOlmedo-Martínez, Jorge L., Michele Pastorio, Elena Gabirondo, Alessandra Lorenzetti, Haritz Sardon, David Mecerreyes, and Alejandro J. Müller. 2021. "Polyether Single and Double Crystalline Blends and the Effect of Lithium Salt on Their Crystallinity and Ionic Conductivity" Polymers 13, no. 13: 2097. https://doi.org/10.3390/polym13132097
APA StyleOlmedo-Martínez, J. L., Pastorio, M., Gabirondo, E., Lorenzetti, A., Sardon, H., Mecerreyes, D., & Müller, A. J. (2021). Polyether Single and Double Crystalline Blends and the Effect of Lithium Salt on Their Crystallinity and Ionic Conductivity. Polymers, 13(13), 2097. https://doi.org/10.3390/polym13132097