Foamed Phase Change Materials Based on Recycled Polyethylene/Paraffin Wax Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Differential Scanning Calorimetry (DSC)
2.2. Thermal Conductivity
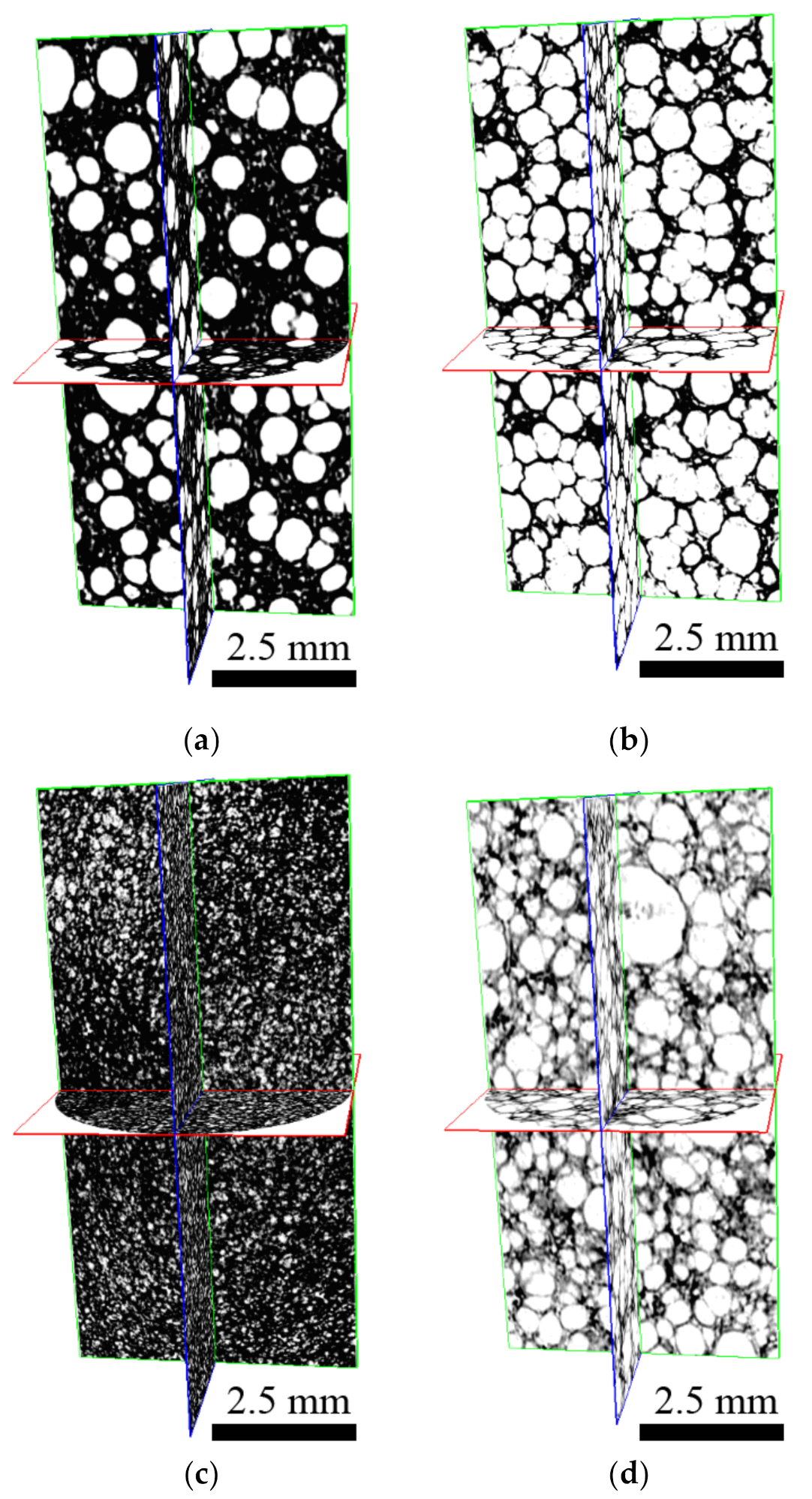
2.3. Internal Foam Structure Investigation
2.4. Mechanical Investigation during Phase Change Cycling
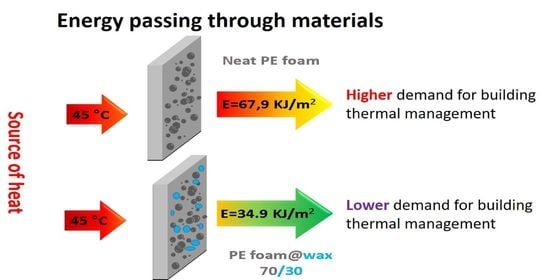
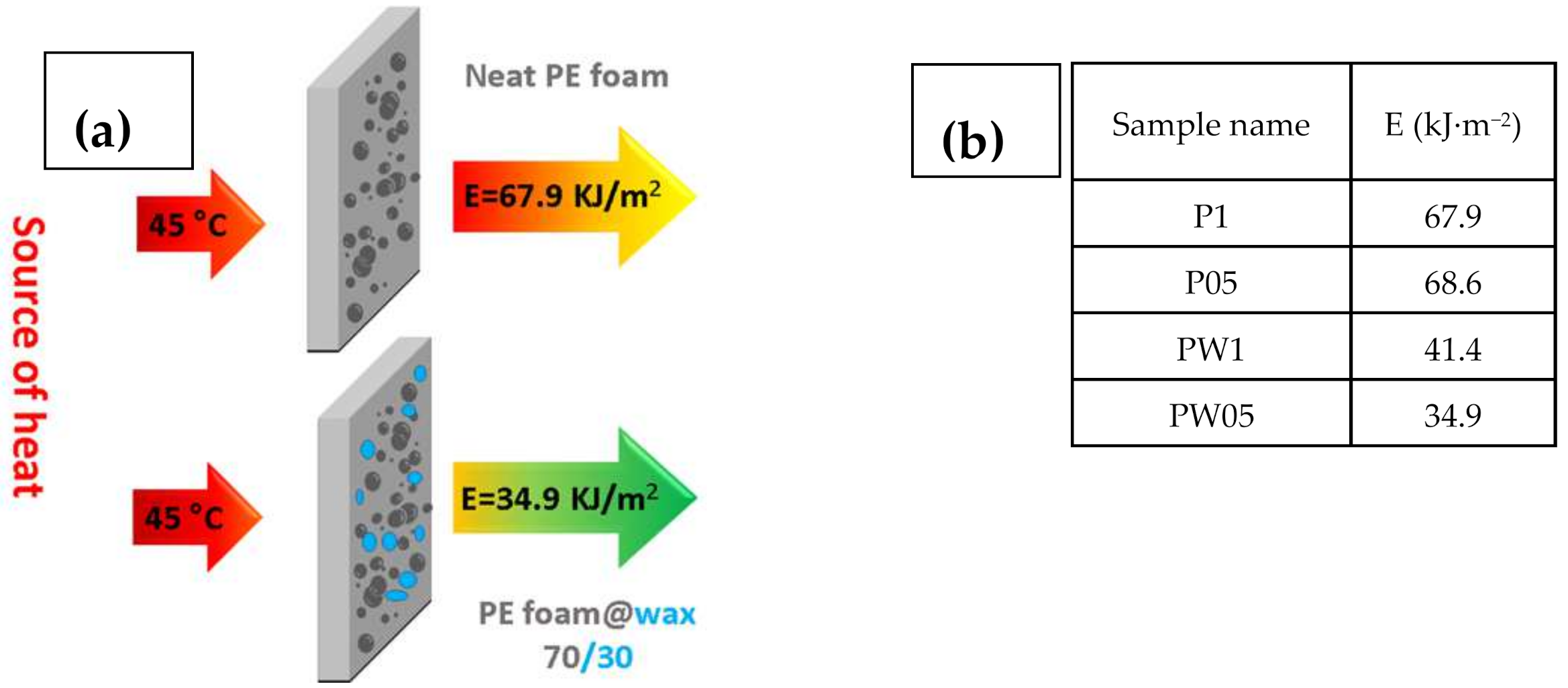
2.5. Capability of the Energy Transfer
3. Results
3.1. Internal Foam Structures Investigation
3.2. Thermophysical Properties
3.3. DMA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Climate Projects with Impact. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/7783/-BuildingsandClimateChange-Status,ChallengesandOpportunities-20073934.pdf?sequence=3&isAllowed=y (accessed on 10 March 2021).
- Cunningham, A. Physical behaviour of polymeric foams—An overview. In Low Density Cellular Plastics; Springer: Amsterdam, The Netherlands, 1994; pp. 1–24. [Google Scholar]
- Lee, Y.; Choi, S.; Choe, K.; Kim, S. Physical and Mechanical Characteristics of Polyurethane Foam Insulators Blown by HFC. In Proceedings of the Fifteenth International Offshore and Polar Engineering Conference, Soeul, Korea, 19–24 June 2005. [Google Scholar]
- Khudhair, A.M.; Farid, M.M. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers. Manag. 2004, 45, 263–275. [Google Scholar] [CrossRef]
- Hasnain, S.M. Review on sustainable thermal energy storage technologies, Part I: Heat storage materials and techniques. Energy Convers. Manag. 1998, 39, 1127–1138. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Solar energy storage using phase change materials. Renew. Sustain. Energy Rev. 2007, 11, 1913–1965. [Google Scholar] [CrossRef]
- Hong, Y.; Xin-shi, G. Preparation of polyethylene-paraffin compound as a form-stable solid-liquid phase change material. Sol. Energy Mater. Sol. Cells 2000, 64, 37–44. [Google Scholar] [CrossRef]
- Fan, Y.F.; Zhang, X.X.; Wu, S.Z.; Wang, X.C. Thermal stability and permeability of microencapsulated n-octadecane and cyclohexane. Thermochim. Acta 2005, 429, 25–29. [Google Scholar] [CrossRef]
- Zhang, X.X.; Fan, Y.F.; Tao, X.M.; Yick, K.L. Fabrication and properties of microcapsules and nanocapsules containing n-octadecane. Mater. Chem. Phys. 2004, 88, 300–307. [Google Scholar] [CrossRef]
- Sari, A. Form-stable paraffin/high density polyethylene composites as solid-liquid phase change material for thermal energy storage: Preparation and thermal properties. Energy Convers. Manag. 2004, 45, 2033–2042. [Google Scholar] [CrossRef]
- Inaba, H.; Tu, P. Evaluation of thermophysical characteristics on shape-stabilized paraffin as a solid-liquid phase change material. Heat Mass Transf. Stoffuebertragung 1997, 32, 307–312. [Google Scholar] [CrossRef]
- Peng, S.; Fuchs, A.; Wirtz, R.A. Polymeric phase change composites for thermal energy storage. J. Appl. Polym. Sci. 2004, 93, 1240–1251. [Google Scholar] [CrossRef]
- Krupa, I.; Luyt, A.S. Physical properties of blends of LLDPE and an oxidized paraffin wax. Polymer 2001, 42, 7285–7289. [Google Scholar] [CrossRef]
- Krupa, I.; Miková, G.; Luyt, A.S. Polypropylene as a potential matrix for the creation of shape stabilized phase change materials. Eur. Polym. J. 2007, 43, 895–907. [Google Scholar] [CrossRef]
- Krupa, I.; Miková, G.; Luyt, A.S. Phase change materials based on low-density polyethylene/paraffin wax blends. Eur. Polym. J. 2007, 43, 4695–4705. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Thermal characteristics of polyurethane foams incorporated with phase change materials. Thermochim. Acta 2007, 454, 90–98. [Google Scholar] [CrossRef]
- You, M.; Zhang, X.; Wang, J.; Wang, X. Polyurethane foam containing microencapsulated phase-change materials with styrene-divinybenzene co-polymer shells. J. Mater. Sci. 2009, 44, 3141–3147. [Google Scholar] [CrossRef]
- You, M.; Zhang, X.X.; Li, W.; Wang, X.C. Effects of MicroPCMs on the fabrication of MicroPCMs/polyurethane composite foams. Thermochim. Acta 2008, 472, 20–24. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Rodríguez, J.F.; Valverde, J.L.; Arevalo, R.; Peijs, T.; Carmona, M. Characterization of rigid polyurethane foams containing microencapsulated Rubitherm® RT27: Catalyst effect. Part II. J. Mater. Sci. 2011, 46, 347–356. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Valverde, J.L.; Peijs, T.; Rodríguez, J.F.; Carmona, M. Characterization of rigid polyurethane foams containing microencapsulated Rubitherm® RT27. Part i. J. Mater. Sci. 2010, 45, 4462–4469. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Rodríguez, J.F.; Valverde, J.L.; Peijs, T.; Carmona, M. Characterization of rigid polyurethane foams containing microencapsulted phase change materials: Microcapsules type effect. J. Appl. Polym. Sci. 2013, 128, 582–590. [Google Scholar] [CrossRef]
- Aydin, A.A.; Okutan, H. Polyurethane rigid foam composites incorporated with fatty acid ester-based phase change material. Energy Convers. Manag. 2013, 68, 74–81. [Google Scholar] [CrossRef]
- Serrano, A.; Borreguero, A.M.; Garrido, I.; Rodríguez, J.F.; Carmona, M. The role of microstructure on the mechanical properties of polyurethane foams containing thermoregulating microcapsules. Polym. Test. 2017, 60, 274–282. [Google Scholar] [CrossRef]
- Beretta, E.M.; Rossi, W.S.; Kindlein Jr, W.; Roldo, L.; Avila De Campos, T.L. Engineering design: Eicosane microcapsules synthesis and application in polyurethane forms aiming to diminish weelchair cushion effect on skin temperature. J. Eng. Sci. Technol. 2016, 11, 1818–1834. [Google Scholar]
- Ikutegbe, C.A.; Farid, M.M. Application of phase change material foam composites in the built environment: A critical review. Renew. Sustain. Energy Rev. 2020, 131, 110008. [Google Scholar] [CrossRef]
- Zhong, X.; Jing, H.; Shuquan, W.; Jun, L.; Enhui, W.; Qianshu, L.; Xin, G. Preparation and thermal properties of metal foam/ paraffin composite phase change materials. Energy Storage Sci. Technol. 2020, 9, 109. [Google Scholar]
- Kausar, A. Polyurethane Composite Foams in High-Performance Applications: A Review. Polym. Plast. Technol. Eng. 2018, 57, 346–369. [Google Scholar] [CrossRef]
- Yang, C.; Fischer, L.; Maranda, S.; Worlitschek, J. Rigid polyurethane foams incorporated with phase change materials: A state-of-the-art review and future research pathways. Energy Build. 2015, 87, 25–36. [Google Scholar] [CrossRef]
- Qu, L.; Li, A.; Gu, J.; Zhang, C. Thermal Energy Storage Capability of Polyurethane Foams Incorporated with Microencapsulated Phase Change Material. ChemistrySelect 2018, 3, 3180–3186. [Google Scholar] [CrossRef]
- Chen, P.; Gao, X.; Wang, Y.; Xu, T.; Fang, Y.; Zhang, Z. Metal foam embedded in SEBS/paraffin/HDPE form-stable PCMs for thermal energy storage. Sol. Energy Mater. Sol. Cells 2016, 149, 60–65. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L. Microencapsulated paraffin as a phase change material with polyurea/polyurethane/poly(lauryl methacrylate) hybrid shells for thermal energy storage applications. J. Renew. Sustain. Energy 2021, 13, 014104. [Google Scholar] [CrossRef]
- Krupa, I.; Nógellová, Z.; Špitalský, Z.; Janigová, I.; Boh, B.; Sumiga, B.; Kleinová, A.; Karkri, M.; Almaadeed, M.A. Phase change materials based on high-density polyethylene filled with microencapsulated paraffin wax. Energy Convers. Manag. 2014, 87, 400–409. [Google Scholar] [CrossRef]
- Tao, Z.; Wang, H.; Liu, J.; Zhao, W.; Liu, Z.; Guo, Q. Dual-level packaged phase change materials–thermal conductivity and mechanical properties. Sol. Energy Mater. Sol. Cells 2017, 169, 222–225. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.; Wu, M.; Xu, J.; Hu, Y.; Chao, J.; Yan, T.; Wang, R. Highly thermally conductive and flexible phase change composites enabled by polymer/graphite nanoplatelet-based dual networks for efficient thermal management. J. Mater. Chem. A 2020, 8, 20011–20020. [Google Scholar] [CrossRef]
- Jiang, Q.; Bismarck, A. A perspective: Is viscosity the key to open the next door for foam templating? React. Funct. Polym. 2021, 162, 104877. [Google Scholar] [CrossRef]
- Eiermann, K. Modellmäßige Deutung der Wärmeleitfähigkeit von Hochpolymeren—Teil 3: Teilkristalline Hochpolymere. Kolloid Z. Z. Polym. 1965, 201, 3–15. [Google Scholar] [CrossRef]
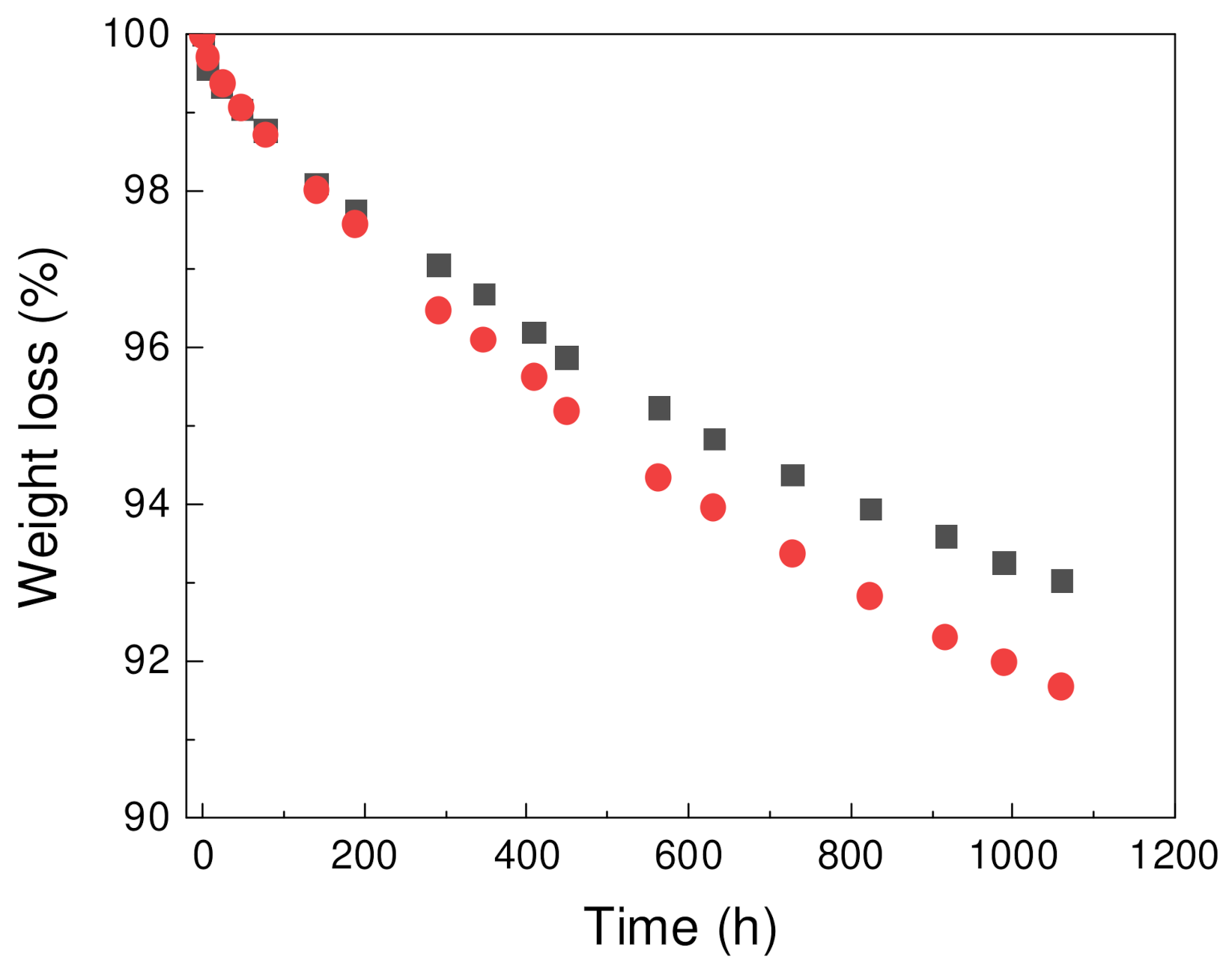
- Sobolčiak, P.; Abdelrazeq, H.; Ouederni, M.; Karkri, M.; Al-Maadeed, M.A.; Krupa, I. The stabilizing effect of expanded graphite on the artificial aging of shape stabilized phase change materials. Polym. Test. 2015, 46. [Google Scholar] [CrossRef]
- Sobolciak, P.; Mrlík, M.; Almaadeed, M.A.; Krupa, I. Calorimetric and dynamic mechanical behavior of phase change materials based on paraffin wax supported by expanded graphite. Thermochim. Acta 2015, 617. [Google Scholar] [CrossRef]
- Velasco, J.I.; Antunes, M.; Ayyad, O.; Saiz-Arroyo, C.; Rodríguez-Pérez, M.A.; Hidalgo, F.; De Saja, J.A. Foams based on low density polyethylene/ hectorite nanocomposites: Thermal stability and thermomechanical properties. J. Appl. Polym. Sci. 2007, 105, 1658–1667. [Google Scholar] [CrossRef]
- Popelka, A.; Sobolčiak, P.; Mrlík, M.; Nogellova, Z.; Chodák, I.; Ouederni, M.; Al-Maadeed, M.A.; Krupa, I. Foamy phase change materials based on linear low-density polyethylene and paraffin wax blends. Emergent Mater. 2018, 1, 47–54. [Google Scholar] [CrossRef]
- Sobolčiak, P.; Abdelrazeq, H.; Özerkan, N.G.; Ouederni, M.; Nógellová, Z.; AlMaadeed, M.A.; Karkri, M.; Krupa, I. Heat transfer performance of paraffin wax based phase change materials applicable in building industry. Appl. Therm. Eng. 2016, 107. [Google Scholar] [CrossRef]
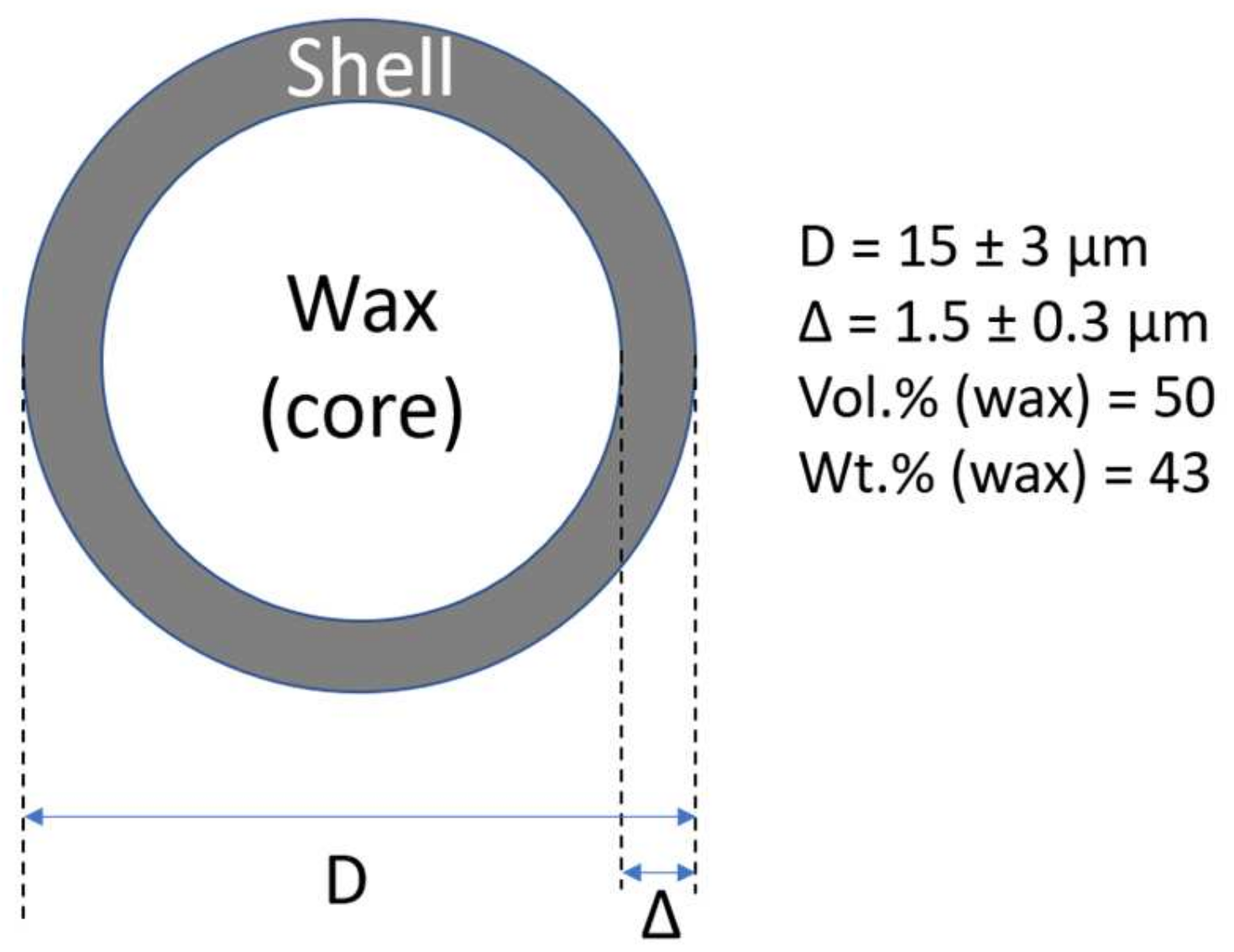




| Sample Name | LLDPE | PW | Blowing Agent | Luperox130 |
|---|---|---|---|---|
| wt.% | wt.% | wt.% | wt.% | |
| PW1 | 70 | 30 | 10 | 1 |
| PW05 | 70 | 30 | 10 | 0.5 |
| P1 | 100 | 0 | 10 | 1 |
| P05 | 100 | 0 | 10 | 0.5 |
| Description | Abbreviation | Unit | Samples’ Codes | |||
|---|---|---|---|---|---|---|
| PW1 | PW05 | P1 | P05 | |||
| Total analyzed volume | TV | mm3 | 193 | 193 | 193 | 193 |
| Foam volume in analyzed volume | vol. TV | mm3 | 120 | 69 | 162 | 83 |
| Number of closed pores | No. | / | 22,422 | 11,199 | 325,625 | 12,985 |
| Sample | Density (g/cm3) | φpores (vol.%) | Gel Portion (wt.%) |
|---|---|---|---|
| LLDPE | 0.941 | / | 0 |
| PW1 | 0.084 | 86.8 | 59.7 (0.1) |
| PW05 | 0.080 | 85.0 | 43.9 (0.8) |
| P1 | 0.143 | 83.6 | 84.1 (0.4) |
| P05 | 0.128 | 84.1 | 76.0 (1.0) |
| Sample | λ, W·m−1·K−1 | k, mm2/s | cp, J·g−1·K−1 |
|---|---|---|---|
| PW1 | 0.066 | 0.269 | 2.92 |
| PW05 | 0.054 | 0.393 | 1.72 |
| P1 | 0.086 | 0.459 | 1.31 |
| P05 | 0.073 | 0.460 | 1.24 |
| Heating | Cooling | |||||||
|---|---|---|---|---|---|---|---|---|
| Paraffin | LLDPE | Paraffin | LLDPE | |||||
| Tm (°C) | ΔHm (J/g) | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | Tc (°C) | ΔHc (J/g) | |
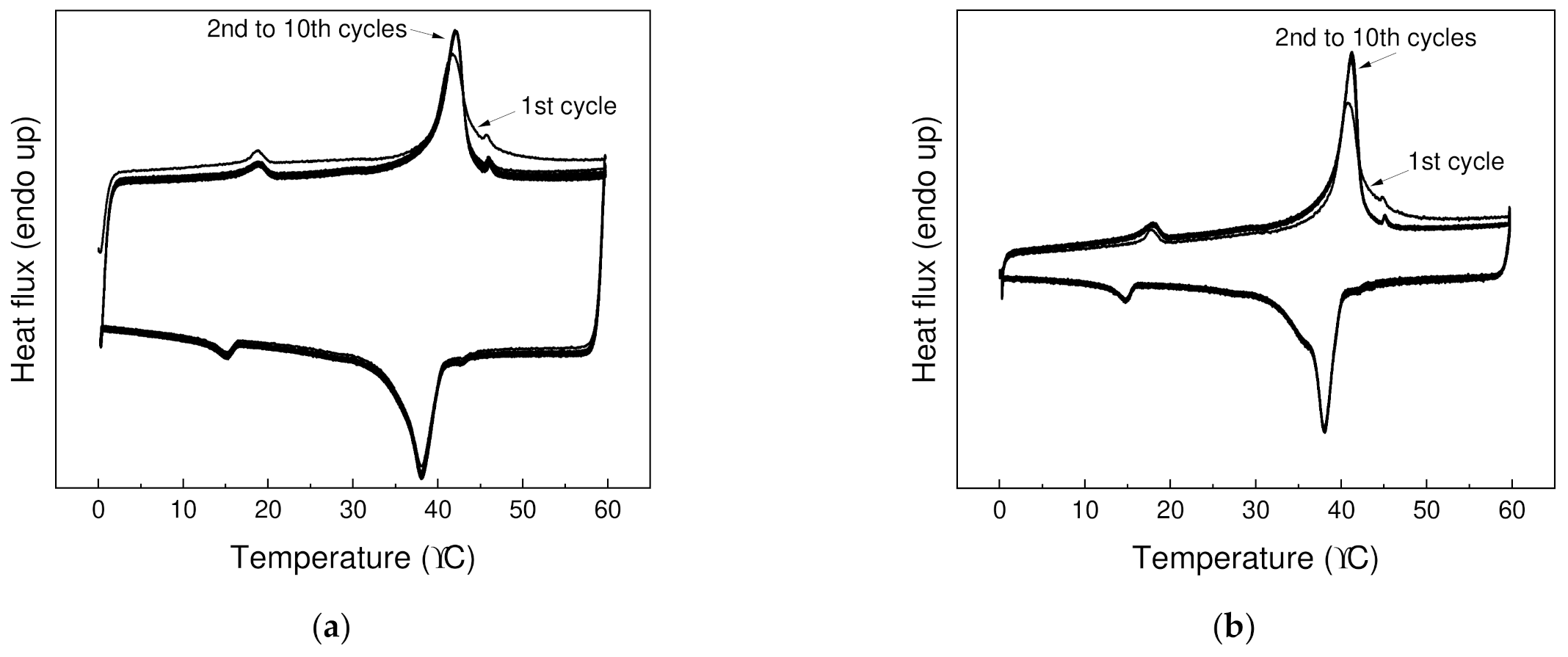
| PW1 | 42.7 (0.4) | 22.4 (1.1) | 117.2 (0.5) | 81.1 (4.0) | 38.4 (0.1) | 23.3 (0.1) | 110.2 (0.6) | 82.7 (3.8) |
| PW05 | 42.9 (0.4) | 25.1 (1.1) | 121.6 (0.6) | 80.8 (3.4) | 37.1 (0.4) | 24.7 (0.2) | 112.4 (0.6) | 80.9 (0.1) |
| P1 | N/A | N/A | 126.8 (0.4) | 114.4 (2.7) | N/A | N/A | 114.4 (1.1) | 115.0 (1.6) |
| P05 | N/A | N/A | 128.2 (0.7) | 113.1 (3.8) | N/A | N/A | 116.5 (0.6) | 116.8 (4.2) |
| Sample | Heating | Cooling | ||||||
|---|---|---|---|---|---|---|---|---|
| Ts-s (°C) | ∆Hs-s (J/g) | Ts-l (°C) | ∆Hs-l (J/g) | Ts-s (°C) | ∆Hs-s (J/g) | Ts-l (°C) | ∆Hs-l (J/g) | |
| PW1 | N/A | N/A | 39.9 | 19.5 | 12.0 | 0.9 | 36.6 | 19.9 |
| PW1afterL | 16.1 | 0.9 | 39.7 | 14.8 | 12.4 | 0.8 | 36.3 | 15.0 |
| PW05 | N/A | N/A | 41.4 | 20.6 | N/A | N/A | 36.7 | 19.7 |
| PW05afterL | 16.6 | 0.8 | 40.1 | 14.0 | 13.0 | 0.6 | 36.9 | 13.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolčiak, P.; Mrlik, M.; Popelka, A.; Minařík, A.; Ilcikova, M.; Srnec, P.; Nogellova, Z.; Ouederni, M.; Krupa, I. Foamed Phase Change Materials Based on Recycled Polyethylene/Paraffin Wax Blends. Polymers 2021, 13, 1987. https://doi.org/10.3390/polym13121987
Sobolčiak P, Mrlik M, Popelka A, Minařík A, Ilcikova M, Srnec P, Nogellova Z, Ouederni M, Krupa I. Foamed Phase Change Materials Based on Recycled Polyethylene/Paraffin Wax Blends. Polymers. 2021; 13(12):1987. https://doi.org/10.3390/polym13121987
Chicago/Turabian StyleSobolčiak, Patrik, Miroslav Mrlik, Anton Popelka, Antonín Minařík, Marketa Ilcikova, Peter Srnec, Zuzana Nogellova, Mabrouk Ouederni, and Igor Krupa. 2021. "Foamed Phase Change Materials Based on Recycled Polyethylene/Paraffin Wax Blends" Polymers 13, no. 12: 1987. https://doi.org/10.3390/polym13121987
APA StyleSobolčiak, P., Mrlik, M., Popelka, A., Minařík, A., Ilcikova, M., Srnec, P., Nogellova, Z., Ouederni, M., & Krupa, I. (2021). Foamed Phase Change Materials Based on Recycled Polyethylene/Paraffin Wax Blends. Polymers, 13(12), 1987. https://doi.org/10.3390/polym13121987