A Review on Hydrogels with Photothermal Effect in Wound Healing and Bone Tissue Engineering
Abstract
:1. Introduction
2. Composition of Hydrogels with Photothermal Effects
2.1. Inorganic Materials Hybrid Hydrogels with Photothermal Effects
2.2. Organic Materials Hybrid Hydrogels with Photothermal Effects
3. Potential Mechanisms of Photothermal Hydrogels for Wound Healing and Tissue Engineering
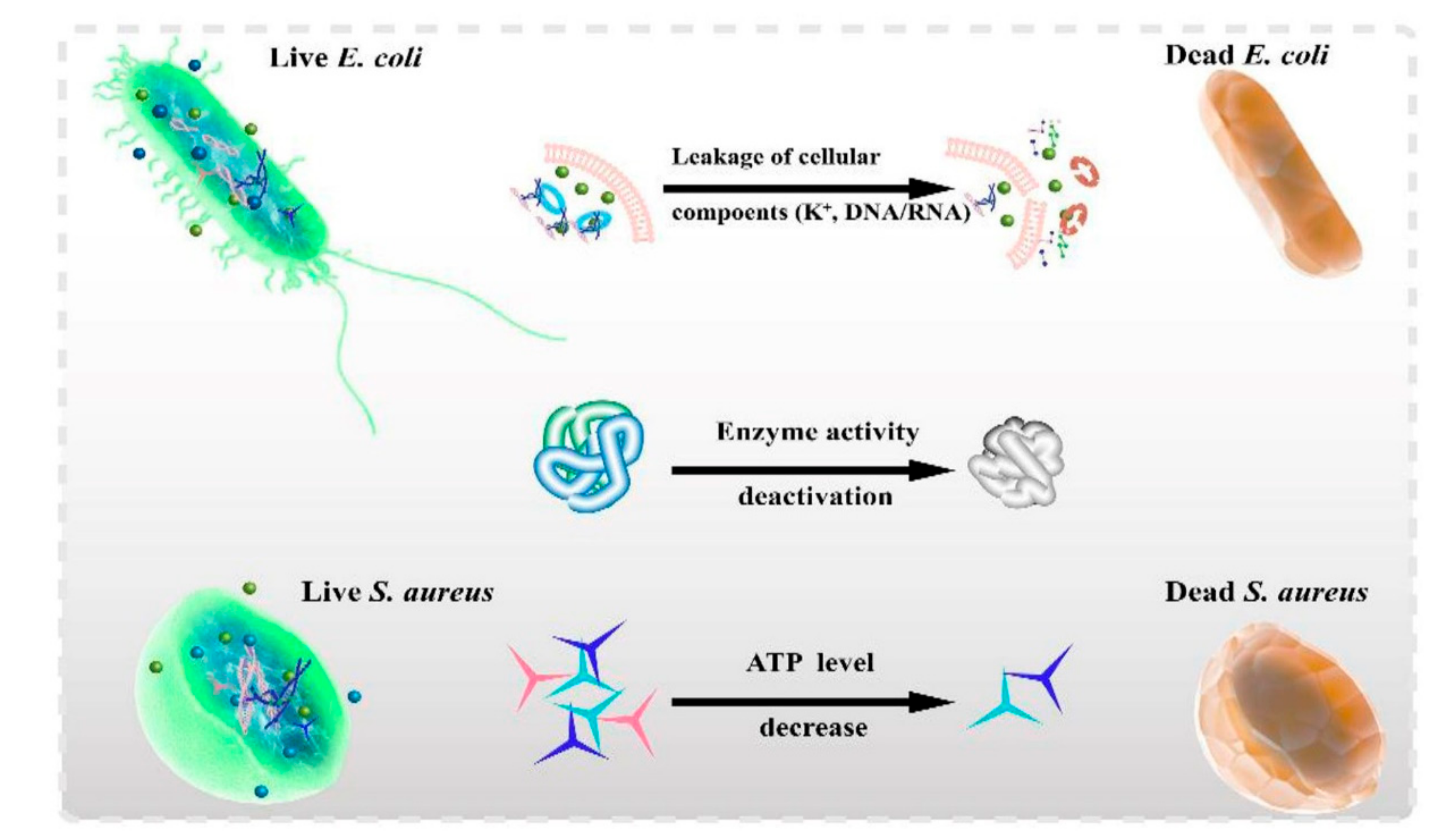
3.1. Antibacterial Effects
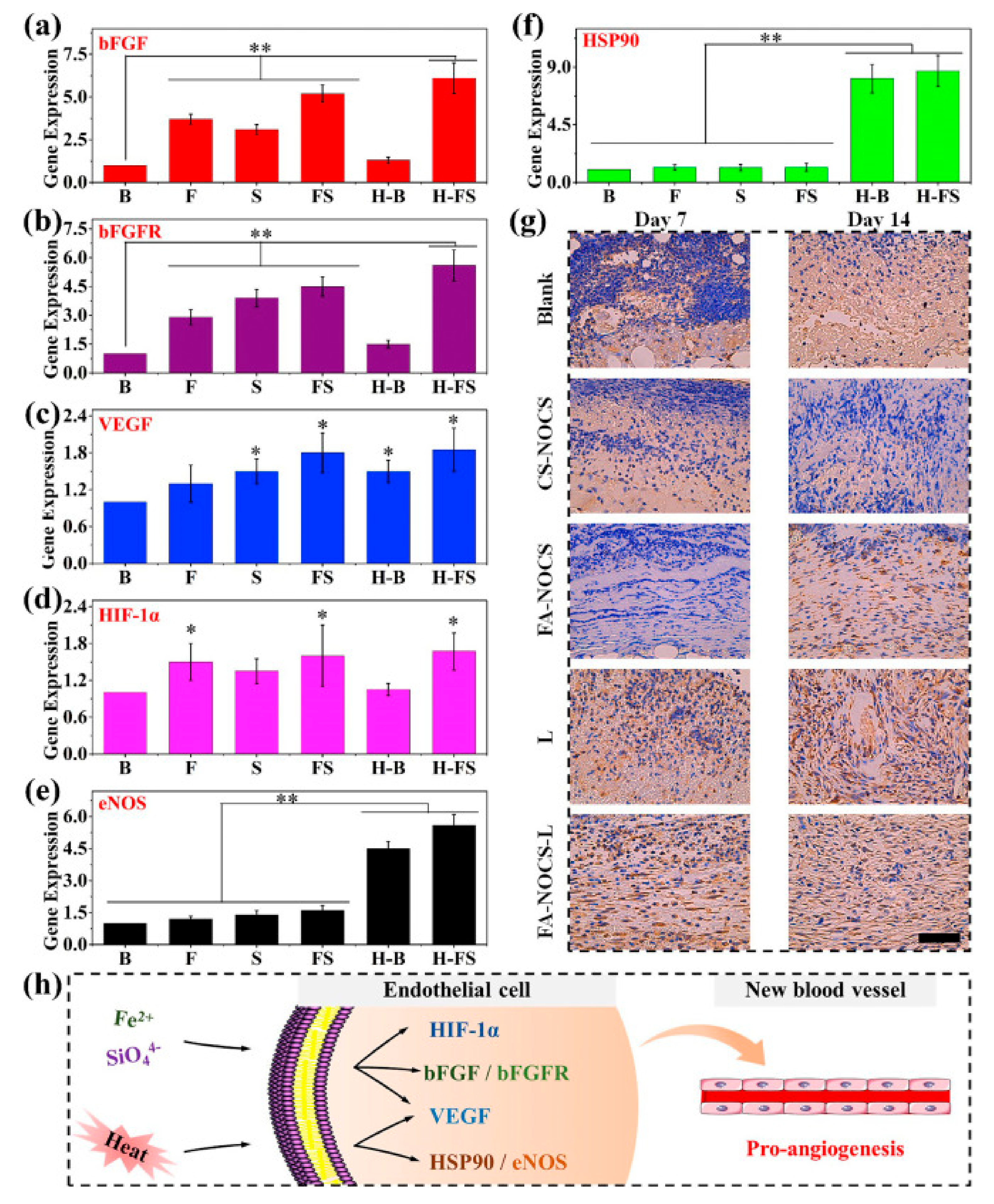
3.2. Angiogenesis
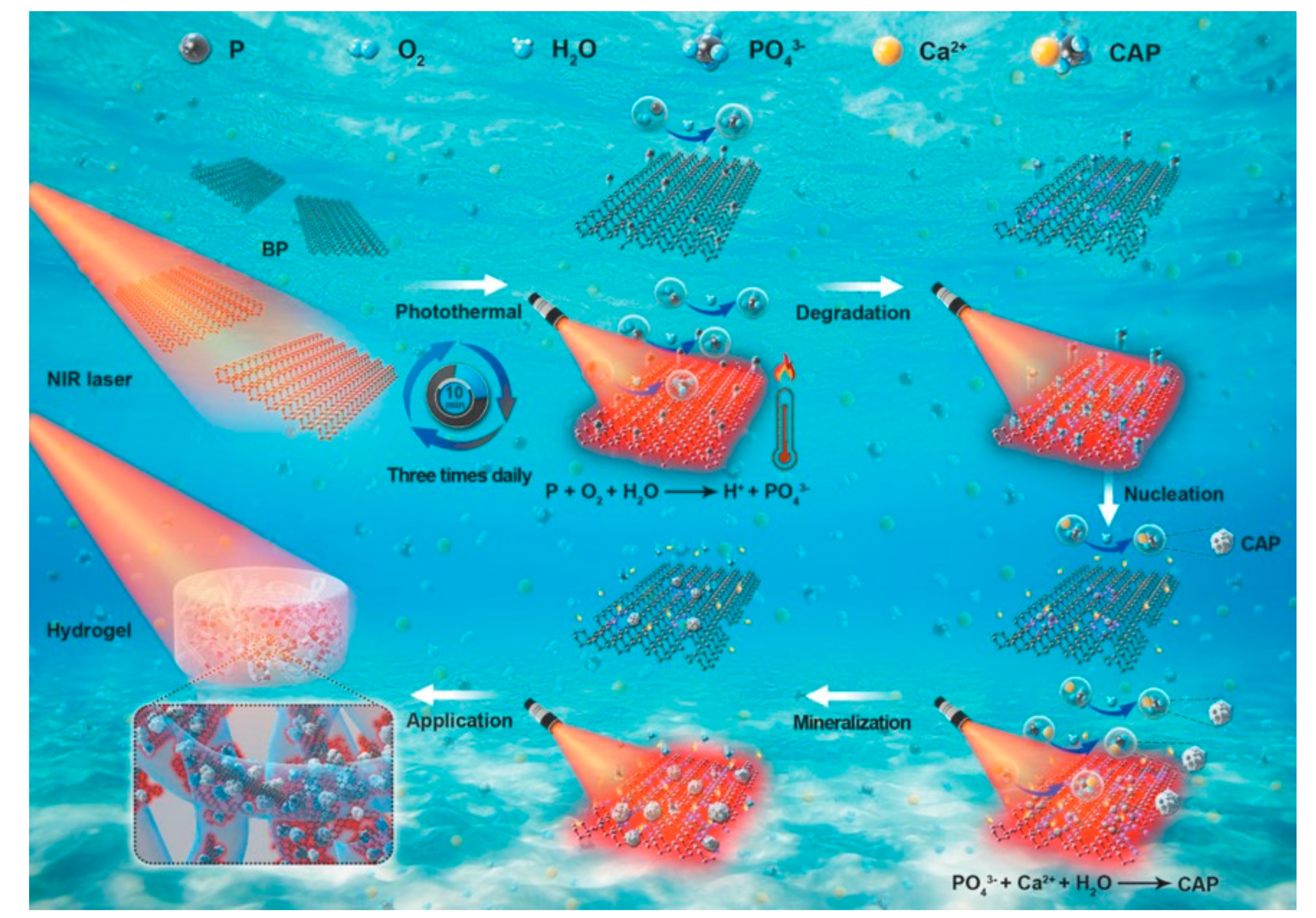
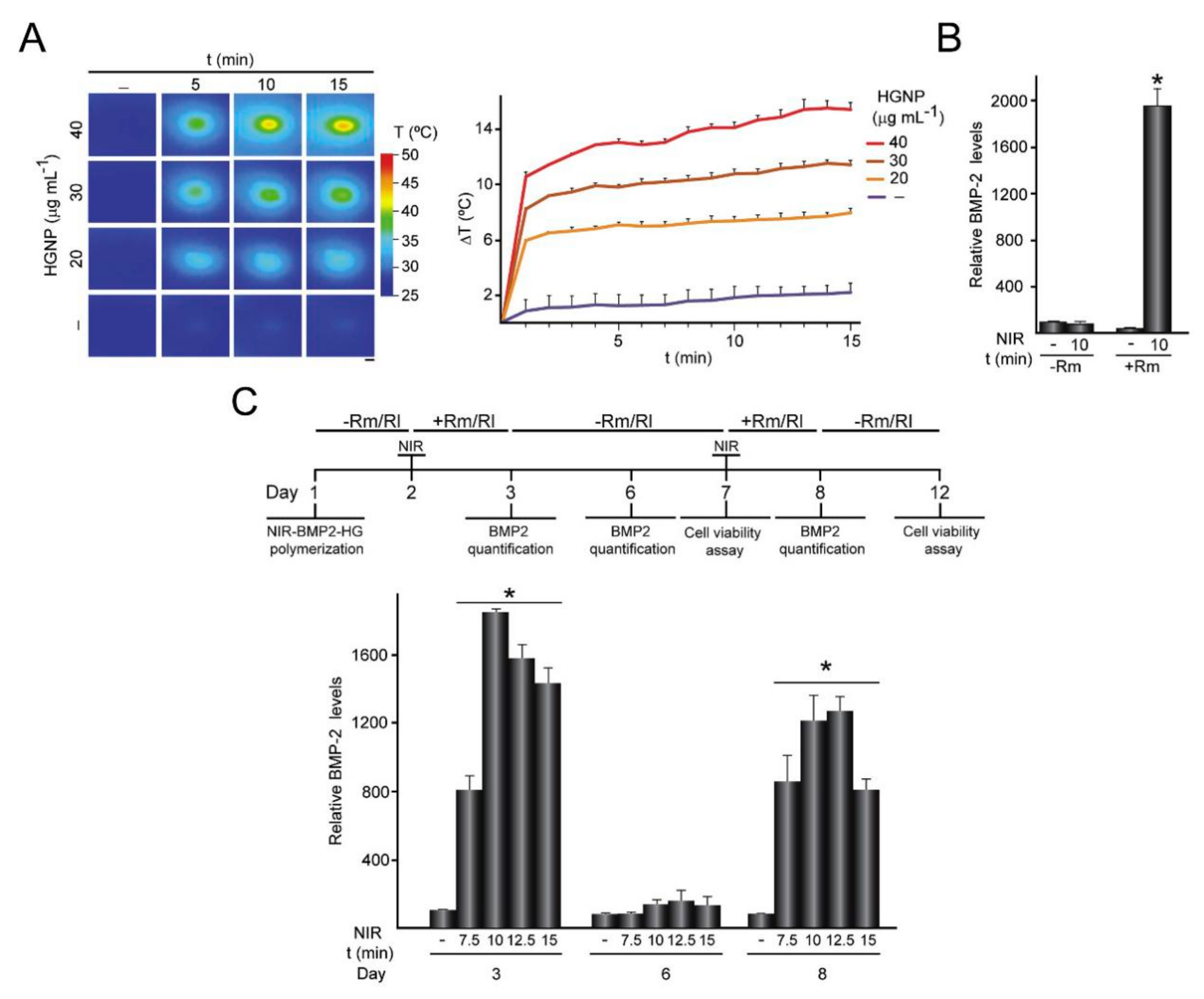
3.3. Osteogenesis
4. Photothermal Hydrogels in Wound Healing and Bone Repair
4.1. Wound Healing
4.2. Bone Regeneration
5. Prospective and Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tao, N.; Liu, Y.; Wu, Y.; Li, X.; Li, J.; Sun, X.; Chen, S.; Liu, Y.-N. Minimally Invasive Antitumor Therapy Using Biodegradable Nanocomposite Micellar Hydrogel with Functionalities of NIR-II Photothermal Ablation and Vascular Disruption. ACS Appl. Bio Mater. 2020, 3, 4531–4542. [Google Scholar] [CrossRef]
- Cahill, S.V.; Kwon, H.; Back, J.; Lee, I.; Lee, S.; Alder, K.D.; Hao, Z.; Yu, K.E.; Dussik, C.M.; Kyriakides, T.R.; et al. Locally delivered adjuvant biofilm-penetrating antibiotics rescue impaired endochondral fracture healing caused by MRSA infection. J. Orthop. Res. 2021, 39, 402–414. [Google Scholar] [CrossRef]
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Giacomazza, D.; Carzino, R.; Goldoni, L.; Scoponi, G.; et al. Electrospun polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. 2021, 409, 128144. [Google Scholar] [CrossRef]
- Ahmed, R.; Afreen, A.; Tariq, M.; Zahid, A.A.; Masoud, M.S.; Ahmed, M.; Ali, I.; Akram, Z.; Hasan, A. Bone marrow mesenchymal stem cells preconditioned with nitric-oxide-releasing chitosan/PVA hydrogel accelerate diabetic wound healing in rabbits. Biomed. Mater. 2021, 16, 035014. [Google Scholar] [CrossRef]
- Zhang, B.; He, J.; Shi, M.; Liang, Y.; Guo, B. Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chem. Eng. J. 2020, 400, 125994. [Google Scholar] [CrossRef]
- Gao, Y.; Du, H.; Xie, Z.; Li, M.; Zhu, J.; Xu, J.; Zhang, L.; Tao, J.; Zhu, J. Self-adhesive photothermal hydrogel films for solar-light assisted wound healing. J. Mater. Chem. B 2019, 7, 3644–3651. [Google Scholar] [CrossRef]
- Zhang, K.; Bai, X.; Yuan, Z.; Cao, X.; Jiao, X.; Li, Y.; Qin, Y.; Wen, Y.; Zhang, X. Layered nanofiber sponge with an improved capacity for promoting blood coagulation and wound healing. Biomaterials 2019, 204, 70–79. [Google Scholar] [CrossRef]
- Qiu, G.; Huang, M.; Liu, J.; Wang, P.; Schneider, A.; Ren, K.; Oates, T.W.; Weir, M.D.; Xu, H.H.K.; Zhao, L. Antibacterial calcium phosphate cement with human periodontal ligament stem cell-microbeads to enhance bone regeneration and combat infection. J. Tissue Eng. Regen. Med. 2021, 15, 232–243. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Sun, L.; Ye, F.; Shen, X.; Zhao, Y. Claw-inspired microneedle patches with liquid metal encapsulation for accelerating incisional wound healing. Chem. Eng. J. 2021, 406, 126741. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Yang, B.; Song, J.; Jiang, Y.; Li, M.; Wei, J.; Qin, J.; Peng, W.; Lasaosa, F.L.; He, Y.; Mao, H.; et al. Injectable Adhesive Self-Healing Multicross-Linked Double-Network Hydrogel Facilitates Full-Thickness Skin Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 57782–57797. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, S.; Chen, C.; Zhang, D. A graphene hybrid supramolecular hydrogel with high stretchability, self-healable and photothermally responsive properties for wound healing. RSC Adv. 2021, 11, 6367–6373. [Google Scholar] [CrossRef]
- Liu, W.; Jing, X.; Xu, Z.; Teng, C. PEGDA/HA mineralized hydrogel loaded with Exendin4 promotes bone regeneration in rat models with bone defects by inducing osteogenesis. J. Biomater. Appl. 2021, 35, 1337–1346. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, C.; Wu, C.; Wu, H.; Zhu, C.; Ye, C.; Wang, S.; Zou, D. Fe3+-Induced Synchronous Formation of Composite Hydrogels for Effective Synergistic Tumor Therapy in NIR-I/II Biowindows. ACS Appl. Mater. Interfaces 2018, 10, 41947–41955. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Li, S.-J.; Zhang, C.; Ma, Y.-N.; Gao, P.; Gao, S.-Z.; Huang, X.-J. Tannic acid–thioctic acid hydrogel: A novel injectable supramolecular adhesive gel for wound healing. Green Chem. 2021, 23, 1794–1804. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, B.; Lu, X.; Wang, L.; Li, Y.; Ma, N.; Wei, H.; Zhang, X.; Wang, G. Readily producing a Silly Putty-like hydrogel with good self-healing, conductive and photothermal conversion properties based on dynamic coordinate bonds and hydrogen bonds. J. Mater. Chem. C 2020, 8, 6763–6770. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, J.; Shi, J.; Wu, W.; Debeli, D.K.; Pan, P.; Shan, G. Fast photothermal poly(NIPAM-co-β-cyclodextrin) supramolecular hydrogel with self-healing through host–guest interaction for intelligent light-controlled switches. Soft Matter 2020, 16, 10558–10566. [Google Scholar] [CrossRef]
- Guo, B.; Qu, J.; Zhao, X.; Zhang, M. Degradable conductive self-healing hydrogels based on dextran-graft-tetraaniline and N-carboxyethyl chitosan as injectable carriers for myoblast cell therapy and muscle regeneration. Acta Biomater. 2019, 84, 180–193. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, F.; Tang, L.; Wu, H.; Ni, Y.; Chen, L.; Huang, L.; Hu, X.; Lin, S.; Ding, C. Super-ductile, injectable, fast self-healing collagen-based hydrogels with multi-responsive and accelerated wound-repair properties. Chem. Eng. J. 2021, 405, 126756. [Google Scholar] [CrossRef]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 137. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Chen, Y.; Zhao, L.; Xu, J.; Zhao, R.; Serpe, M.J.; Hu, L. Bioinspired tissue-compliant hydrogels with multifunctions for synergistic surgery–photothermal therapy. J. Mater. Chem. B 2020, 8, 10117–10125. [Google Scholar] [CrossRef]
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, L.; Cheng, C.; Deng, Y.; Huang, J.; Fan, X.; Nie, C.; Zhao, W.; Zhao, C. Nonchemotherapic and Robust Dual-Responsive Nanoagents with On-Demand Bacterial Trapping, Ablation, and Release for Efficient Wound Disinfection. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Huang, P.; Jiang, G.; Zhang, M.; Yu, F.; Zhang, W.; Fu, G.; Wang, Y.; Li, W.; et al. A novel mineralized high strength hydrogel for enhancing cell adhesion and promoting skull bone regeneration in situ. Compos. Part B Eng. 2020, 197, 108183. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, S.; Ma, Z.; Kong, L.; Li, H.; Chan, H.F. Macrophages activated by akermanite/alginate composite hydrogel stimulate migration of bone marrow-derived mesenchymal stem cells. Biomed. Mater. 2021, 16, 045004. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ranjha, N.M.; Raza, M.R.; Hanif, M.; Majed, A.; Ameer, N. Enteric-coated Ca-alginate hydrogel beads: A promising tool for colon targeted drug delivery system. Polym. Bull. 2020, 1–15. [Google Scholar] [CrossRef]
- Li, J.; Yu, F.; Chen, G.; Liu, J.; Li, X.-L.; Cheng, B.; Mo, X.-M.; Chen, C.; Pan, J.-F. Moist-Retaining, Self-Recoverable, Bioadhesive, and Transparent in Situ Forming Hydrogels to Accelerate Wound Healing. ACS Appl. Mater. Interfaces 2019, 12, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Sheng, S.; Dong, X.; Zhang, Y.; Li, X.; Zhu, D.; Lv, F. A photo-triggered hydrogel for bidirectional regulation with imaging visualization. Soft Matter 2020, 16, 7598–7605. [Google Scholar] [CrossRef]
- Hou, L.; Shan, X.; Hao, L.; Feng, Q.; Zhang, Z. Copper sulfide nanoparticle-based localized drug delivery system as an effective cancer synergistic treatment and theranostic platform. Acta Biomater. 2017, 54, 307–320. [Google Scholar] [CrossRef]
- Liu, B.; Sun, J.; Zhu, J.; Li, B.; Ma, C.; Gu, X.; Liu, K.; Zhang, H.; Wang, F.; Su, J.; et al. Injectable and NIR-Responsive DNA–Inorganic Hybrid Hydrogels with Outstanding Photothermal Therapy. Adv. Mater. 2020, 32. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, G.; Xing, X.; Liu, J.; Cheng, Y.; Ye, T.; Wang, Q.; Xiao, X.; Li, Z.; Deng, H. Near-Infrared Light-Triggered Porous AuPd Alloy Nanoparticles to Produce Mild Localized Heat to Accelerate Bone Regeneration. J. Phys. Chem. Lett. 2019, 10, 4185–4191. [Google Scholar] [CrossRef]
- Su, J.; Lu, S.; Jiang, S.; Li, B.; Liu, B.; Sun, Q.; Li, J.; Wang, F.; Wei, Y. Engineered Protein Photo-Thermal Hydrogels for Outstanding In Situ Tongue Cancer Therapy. Adv. Mater. 2021, 33, 2100619. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, G.; Zhao, P.; Ou, C. Near-infrared light-controllable bufalin delivery from a black phosphorus-hybrid supramolecular hydrogel for synergistic photothermal-chemo tumor therapy. Nano Res. 2021, 1–11. [Google Scholar] [CrossRef]
- Yao, Q.; Lan, Q.-H.; Jiang, X.; Du, C.-C.; Zhai, Y.-Y.; Shen, X.; Xu, H.-L.; Xiao, J.; Kou, L.; Zhao, Y.-Z. Bioinspired biliverdin/silk fibroin hydrogel for antiglioma photothermal therapy and wound healing. Theranostics 2020, 10, 11719–11736. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-L.; Dai, X.; Yang, J.; Zhang, B.; Zhao, D.-H.; Li, C.-Q.; Yin, Z.-Y.; Zhao, Y.-D.; Liu, B. Injectable polypeptide-engineered hydrogel depot for amplifying the anti-tumor immune effect induced by chemo-photothermal therapy. J. Mater. Chem. B 2020, 8, 8623–8633. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.L.; Huynh, T.H.; Pöschko, P.; Fruergaard, A.S.; Olesen, M.T.J.; Chen, Y.; Birkedal, H.; Subbiahdoss, G.; Reimhult, E.; Thøgersen, J.; et al. Remotely Triggered Liquefaction of Hydrogel Materials. ACS Nano 2020, 14, 9145–9155. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Ruan, C.; Xie, H.; Li, Z.; Wang, H.; Chu, P.K.; Yu, X.-F. Black-Phosphorus-Incorporated Hydrogel as a Sprayable and Biodegradable Photothermal Platform for Postsurgical Treatment of Cancer. Adv. Sci. 2018, 5, 1700848. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Win, K.Y.; Yao, X.; Yang, W.; Han, M. Plasmonically Modulated Gold Nanostructures for Photothermal Ablation of Bacteria. Adv. Healthc. Mater. 2021, 10, e2001158. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Zhang, Z.; Gao, R.; Li, H.; Dong, Z.; Wang, Q.; Zhou, Q.; Wang, Y. Physiologically stable F127-GO supramolecular hydrogel with sustained drug release characteristic for chemotherapy and photothermal therapy. RSC Adv. 2018, 8, 1693–1699. [Google Scholar] [CrossRef] [Green Version]
- Nowroozi, N.; Faraji, S.; Nouralishahi, A.; Shahrousvand, M. Biological and structural properties of graphene oxide/curcumin nanocomposite incorporated chitosan as a scaffold for wound healing application. Life Sci. 2021, 264, 118640. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, J.; Chen, Y.; Li, W.; Han, X.; Wang, L. Engineering Microcapsules for Simultaneous Delivery of Combinational Therapeutics. Adv. Mater. Technol. 2020, 5, 5. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, S.; Chen, C.; Wang, D.; Liu, B.; Cai, D.; Wu, Z. Near infrared light-driven release of pesticide with magnetic collectability using gel-based nanocomposite. Chem. Eng. J. 2021, 411, 127881. [Google Scholar] [CrossRef]
- Li, L.; Fu, L.; Ai, X.; Zhang, J.; Zhou, J. Design and Fabrication of Temperature-Sensitive Nanogels with Controlled Drug Release Properties for Enhanced Photothermal Sterilization. Chem. A Eur. J. 2017, 23, 18180–18186. [Google Scholar] [CrossRef]
- Shou, X.; Liu, Y.; Wu, D.; Zhang, H.; Zhao, Y.; Sun, W.; Shen, X. Black phosphorus quantum dots doped multifunctional hydrogel particles for cancer immunotherapy. Chem. Eng. J. 2020, 408, 127349. [Google Scholar] [CrossRef]
- Han, X.-M.; Zheng, K.-W.; Wang, R.-L.; Yue, S.-F.; Chen, J.; Zhao, Z.-W.; Song, F.; Su, Y.; Ma, Q. Functionalization and optimization-strategy of graphene oxide-based nanomaterials for gene and drug delivery. Am. J. Transl. Res. 2020, 12, 1515–1534. [Google Scholar]
- Fan, L.; Zhang, X.; Liu, X.; Sun, B.; Li, L.; Zhao, Y. Responsive Hydrogel Microcarrier-Integrated Microneedles for Versatile and Controllable Drug Delivery. Adv. Healthc. Mater. 2021, 10, 2002249. [Google Scholar] [CrossRef] [PubMed]
- GhavamiNejad, A.; Samarikhalaj, M.; Aguilar, L.E.; Park, C.H.; Kim, C.S. pH/NIR Light-Controlled Multidrug Release via a Mussel-Inspired Nanocomposite Hydrogel for Chemo-Photothermal Cancer Therapy. Sci. Rep. 2016, 6, 33594. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; Shi, B.; Liu, D.; Liu, J.-H.; Zhao, D.; Yu, Z.-H.; Shen, X.-Q.; Gan, J.-M.; Shi, B.-L.; Qiu, Y.; et al. Conductive Hydrogel for a Photothermal-Responsive Stretchable Artificial Nerve and Coalescing with a Damaged Peripheral Nerve. ACS Nano 2020, 14, 16565–16575. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Shi, M.; Liang, Y.; Guo, B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Li, B.; Harlepp, S.; Gensbittel, V.; Wells, C.; Bringel, O.; Goetz, J.; Begin-Colin, S.; Tasso, M.; Begin, D.; Mertz, D. Near infra-red light responsive carbon nanotubes@mesoporous silica for photothermia and drug delivery to cancer cells. Mater. Today Chem. 2020, 17, 100308. [Google Scholar] [CrossRef]
- Deng, Z.; Hu, T.; Lei, Q.; He, J.; Ma, P.X.; Guo, B. Stimuli-Responsive Conductive Nanocomposite Hydrogels with High Stretchability, Self-Healing, Adhesiveness, and 3D Printability for Human Motion Sensing. ACS Appl. Mater. Interfaces 2019, 11, 6796–6808. [Google Scholar] [CrossRef]
- Lima-Sousa, R.; de Melo-Diogo, D.; Alves, C.G.; Cabral, C.S.; Miguel, S.P.; Mendonça, A.G.; Correia, I.J. Injectable in situ forming thermo-responsive graphene based hydrogels for cancer chemo-photothermal therapy and NIR light-enhanced antibacterial applications. Mater. Sci. Eng. C 2020, 117, 111294. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Nie, W.; Chen, L.; McCoul, D.; Liu, D.; Zhang, X.; Ji, Y.; Yu, B.; He, C. Self-Assembled Hydroxyapatite-Graphene Scaffold for Photothermal Cancer Therapy and Bone Regeneration. J. Biomed. Nanotechnol. 2018, 14, 2003–2017. [Google Scholar] [CrossRef]
- Liao, J.; Shi, K.; Jia, Y.; Wu, Y.; Qian, Z. Gold nanorods and nanohydroxyapatite hybrid hydrogel for preventing bone tumor recurrence via postoperative photothermal therapy and bone regeneration promotion. Bioact. Mater. 2021, 6, 2221–2230. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Soni, S.; Sachdev, A.; Vikas; Mishra, S. Near-infrared stimulated hydrogel patch for photothermal therapeutics and thermoresponsive drug delivery. J. Photochem. Photobiol. B Biol. 2020, 210, 111960. [Google Scholar] [CrossRef] [PubMed]
- Mirrahimi, M.; Beik, J.; Mirrahimi, M.; Alamzadeh, Z.; Teymouri, S.; Mahabadi, V.P.; Eslahi, N.; Tazehmahalleh, F.E.; Ghaznavi, H.; Shakeri-Zadeh, A.; et al. Triple combination of heat, drug and radiation using alginate hydrogel co-loaded with gold nanoparticles and cisplatin for locally synergistic cancer therapy. Int. J. Biol. Macromol. 2020, 158, 617–626. [Google Scholar] [CrossRef]
- Tong, L.; Liao, Q.; Zhao, Y.; Huang, H.; Gao, A.; Zhang, W.; Gao, X.; Wei, W.; Guan, M.; Chu, P.K.; et al. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials 2019, 193, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Fan, T.; Kim, J.H.; Xu, Y.; Wang, Y.; Liang, W.; Qiao, L.; Wu, Z.; Liu, Q.; Hu, W.; et al. Emetine-Loaded Black Phosphorus Hydrogel Sensitizes Tumor to Photothermal Therapy through Inhibition of Stress Granule Formation. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Guo, Z.; Xiao, Y.; Zhang, Y.; Sun, X.; Zhe, T.; Cao, Y.; Wang, L.; Lu, Q.; et al. Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections. Chem. Eng. J. 2020, 382, 122990. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, J.; Li, K.; Zhao, J.; Ma, H.; Wu, C.; Zhang, C.; Xie, Y.; Yang, F.; Zheng, X. A hydrogenated black TiO2 coating with excellent effects for photothermal therapy of bone tumor and bone regeneration. Mater. Sci. Eng. C 2019, 102, 458–470. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Dong, K.; Luo, J.; Zhang, Q.; Cheng, Y. Injectable and responsively degradable hydrogel for personalized photothermal therapy. Biomaterials 2016, 104, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Lu, S.; Hai, J.; Liang, K.; Li, T.; Sun, S.; Chen, F.; Yang, Z.; Wang, B. Confining Carbon Dots in Porous Wood: The Singlet Oxygen Enhancement Strategy for Photothermal Signal-Amplified Detection of Mn2+. ACS Sustain. Chem. Eng. 2020, 8, 17687–17696. [Google Scholar] [CrossRef]
- Cui, Q.; Yuan, H.; Bao, X.; Ma, G.; Wu, M.; Xing, C. Synergistic Photodynamic and Photothermal Antibacterial Therapy Based on a Conjugated Polymer Nanoparticle-Doped Hydrogel. ACS Appl. Bio Mater. 2020, 3, 4436–4443. [Google Scholar] [CrossRef]
- Lei, Z.; Zhu, W.; Xu, S.; Ding, J.; Wan, J.; Wu, P. Hydrophilic MoSe2 Nanosheets as Effective Photothermal Therapy Agents and Their Application in Smart Devices. ACS Appl. Mater. Interfaces 2016, 8, 20900–20908. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gu, X.; Sun, Q.; Jiang, S.; Sun, J.; Liu, K.; Wang, F.; Wei, Y. Injectable In Situ Induced Robust Hydrogel for Photothermal Therapy and Bone Fracture Repair. Adv. Funct. Mater. 2021, 31, 2010779. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Y.; Cao, Y.; Guo, Z.; Li, F.; Wang, L. Construction of Chitosan-Based Hydrogel Incorporated with Antimonene Nanosheets for Rapid Capture and Elimination of Bacteria. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Li, Q.; Wen, J.; Liu, C.; Jia, Y.; Wu, Y.; Shan, Y.; Qian, Z.; Liao, J. Graphene-Nanoparticle-Based Self-Healing Hydrogel in Preventing Postoperative Recurrence of Breast Cancer. ACS Biomater. Sci. Eng. 2019, 5, 768–779. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; Deng, Y.; Yuan, Z.; Shen, X.; Chen, M.; He, Y.; Peng, Z.; Hu, Y.; Cai, K. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J. Mater. Chem. B 2019, 7, 2534–2548. [Google Scholar] [CrossRef]
- Tong, C.; Zhong, X.; Yang, Y.; Liuc, X.; Zhonga, G.; Xiaoa, C.; Liuad, B.; Wangc, W.; Yangb, X. PB@PDA@Ag nanosystem for synergistically eradicating MRSA and accelerating diabetic wound healing assisted with laser irradiation. Biomaterials 2020, 243, 119936. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Huang, L.; Wu, Y.; Liao, J. Advances and trends of hydrogel therapy platform in localized tumor treatment: A review. J. Biomed. Mater. Res. Part A 2021, 109, 404–425. [Google Scholar] [CrossRef] [PubMed]
- Homsirikamol, C.; Suvanasuthi, S.; Viravaidya-Pasuwat, K. Inclusion of IR-820 into Soybean-Phosphatides-Based Nanoparticles for Near-Infrared-Triggered Release and Endolysosomal Escape in HaCaT Keratinocytes at Insignificant Cytotoxic Level. Int. J. Nanomed. 2020, 15, 8717–8737. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liang, J.; Yang, A.; Qian, Z.; Kong, D.; Lv, F. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials 2019, 209, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Y.; Wang, J.; Yuan, A.; Sun, M.; Wu, J.; Hu, Y. Self-assembled IR780-loaded transferrin nanoparticles as an imaging, targeting and PDT/PTT agent for cancer therapy. Sci. Rep. 2016, 6, 27421. [Google Scholar] [CrossRef]
- Yuan, P.; Luo, Y.; Luo, Y.; Ma, L. A “sandwich” cell culture platform with NIR-responsive dynamic stiffness to modulate macrophage phenotypes. Biomater. Sci. 2021, 9, 2553–2561. [Google Scholar] [CrossRef]
- Liu, C.; Ruan, C.; Shi, R.; Jiang, B.-P.; Ji, S.-C.; Shen, X.-C. A near infrared-modulated thermosensitive hydrogel for stabilization of indocyanine green and combinatorial anticancer phototherapy. Biomater. Sci. 2019, 7, 1705–1715. [Google Scholar] [CrossRef]
- Lu, J.; Cai, L.; Dai, Y.; Liu, Y.; Zuo, F.; Ni, C.; Shi, M.; Li, J. Polydopamine-based Nanoparticles for Photothermal Therapy/Chemotherapy and their Synergistic Therapy with Autophagy Inhibitor to Promote Antitumor Treatment. Chem. Rec. 2021, 21, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, N.; Yuan, W. NIR/Thermoresponsive Injectable Self-Healing Hydrogels Containing Polydopamine Nanoparticles for Efficient Synergistic Cancer Thermochemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 9118–9131. [Google Scholar] [CrossRef]
- Zhou, L.; Ge, J.; Wang, M.; Chen, M.; Cheng, W.; Ji, W.; Lei, B. Injectable muscle-adhesive antioxidant conductive photothermal bioactive nanomatrix for efficiently promoting full-thickness skeletal muscle regeneration. Bioact. Mater. 2021, 6, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Zhao, H.; Zhan, G.; Zhao, Y.; Yang, X. Injectable in Situ Forming Hydrogels of Thermosensitive Polypyrrole Nanoplatforms for Precisely Synergistic Photothermo-Chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 7995–8005. [Google Scholar] [CrossRef]
- Hsiao, C.-W.; Chuang, E.-Y.; Chen, H.-L.; Wan, D.; Korupalli, C.; Liao, Z.-X.; Chiu, Y.-L.; Chia, W.-T.; Lin, K.-J.; Sung, H.-W. Photothermal tumor ablation in mice with repeated therapy sessions using NIR-absorbing micellar hydrogels formed in situ. Biomaterials 2015, 56, 26–35. [Google Scholar] [CrossRef]
- He, X.Y.; Sun, A.; Li, T.; Qian, Y.J.; Qian, H.; Ling, Y.F.; Zhang, L.H.; Liu, Q.Y.; Peng, T.; Qian, Z. Mussel-inspired antimicrobial gelatin/chitosan tissue adhesive rapidly activated in situ by H2O2/ascorbic acid for infected wound closure. Carbohydr. Polym. 2020, 247, 116692. [Google Scholar] [CrossRef]
- Huang, S.; Liu, H.; Liao, K.; Hu, Q.; Guo, R.; Deng, K. Functionalized GO Nanovehicles with Nitric Oxide Release and Photothermal Activity-Based Hydrogels for Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 28952–28964. [Google Scholar] [CrossRef]
- Zhou, L.; Xi, Y.; Xue, Y.; Wang, M.; Liu, Y.; Guo, Y.; Lei, B. Injectable Self-Healing Antibacterial Bioactive Polypeptide-Based Hybrid Nanosystems for Efficiently Treating Multidrug Resistant Infection, Skin-Tumor Therapy, and Enhancing Wound Healing. Adv. Funct. Mater. 2019, 29, 29. [Google Scholar] [CrossRef]
- Xu, Q.; Chang, M.; Zhang, Y.; Wang, E.; Xing, M.; Gao, L.; Huan, Z.; Guo, F.; Chang, J. PDA/Cu Bioactive Hydrogel with “Hot Ions Effect” for Inhibition of Drug-Resistant Bacteria and Enhancement of Infectious Skin Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 31255–31269. [Google Scholar] [CrossRef]
- Yin, J.; Han, Q.; Zhang, J.; Liu, Y.; Gan, X.; Xie, K.; Xie, L.; Deng, Y. MXene-Based Hydrogels Endow Polyetheretherketone with Effective Osteogenicity and Combined Treatment of Osteosarcoma and Bacterial Infection. ACS Appl. Mater. Interfaces 2020, 12, 45891–45903. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: A review. Carbohydr. Polym. 2017, 174, 999–1017. [Google Scholar] [CrossRef]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Cen, D.; Wan, Z.; Fu, Y.; Pan, H.; Xu, J.; Wang, Y.; Wu, Y.; Li, X.; Cai, X. Implantable fibrous ‘patch’ enabling preclinical chemo-photothermal tumor therapy. Colloids Surf. B Biointerfaces 2020, 192, 111005. [Google Scholar] [CrossRef]
- Tong, X.; Qi, X.; Mao, R.; Pan, W.; Zhang, M.; Wu, X.; Chen, G.; Shen, J.; Deng, H.; Hu, R. Construction of functional curdlan hydrogels with bio-inspired polydopamine for synergistic periodontal antibacterial therapeutics. Carbohydr. Polym. 2020, 245, 116585. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, Q.; Huo, Y.; Liu, C.; Li, B.; Li, Y. Construction of a Mesoporous Polydopamine@GO/Cellulose Nanofibril Composite Hydrogel with an Encapsulation Structure for Controllable Drug Release and Toxicity Shielding. ACS Appl. Mater. Interfaces 2020, 12, 57410–57420. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Seo, J.-H.; Jeong, D.I.; Yang, M.; Lee, S.Y.; Lee, J.; Cho, H.-J. Fenton-like reaction, glutathione reduction, and photothermal ablation-built-in hydrogels crosslinked by cupric sulfate for loco-regional cancer therapy. Biomater. Sci. 2021, 9, 847–860. [Google Scholar] [CrossRef]
- Li, L.; Zeng, Z.; Chen, Z.; Gao, R.; Pan, L.; Deng, J.; Ye, X.; Zhang, J.; Zhang, S.; Mei, C.; et al. Microenvironment-Triggered Degradable Hydrogel for Imaging Diagnosis and Combined Treatment of Intraocular Choroidal Melanoma. ACS Nano 2020, 14, 15403–15416. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, Y.; Liu, X.; Li, B.; Han, Y.; Zheng, Y.; Yeung, K.W.K.; Li, C.; Cui, Z.; Liang, Y.; et al. Rapid bacteria trapping and killing of metal-organic frameworks strengthened photo-responsive hydrogel for rapid tissue repair of bacterial infected wounds. Chem. Eng. J. 2020, 396, 125194. [Google Scholar] [CrossRef]
- Tao, B.; Lina, C.; Yuana, Z.; Hea, Y.; Chena, M.; Lia, K.; Hua, J.; Yanga, Y.; Xiaa, Z.; Caiab, K. Near infrared light-triggered on-demand Cur release from Gel-PDA@Cur composite hydrogel for antibacterial wound healing. Chem. Eng. J. 2021, 403, 126182. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Zhou, Z.; Tan, L.; Wang, X.; Zheng, Y.; Han, Y.; Chen, D.-F.; Yeung, K.W.K.; Cui, Z.; et al. Lysozyme-Assisted Photothermal Eradication of Methicillin-Resistant Staphylococcus aureus Infection and Accelerated Tissue Repair with Natural Melanosome Nanostructures. ACS Nano 2019, 13, 11153–11167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Ma, W.S.; Wei, Y.X.; Xu, Y.H. Photothermal Effect-based Cytotoxic Ability of Melanin from Mytilus edulis Shells to Heal Wounds Infected with Drug-resistant Bacteria in vivo. Biomed. Environ. Sci. 2020, 33, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, S.; Weng, Z.; Zhang, W.; Wan, X.; Cui, T.; Ye, J.; Liao, L.; Wang, X. Jelly-Inspired Injectable Guided Tissue Regeneration Strategy with Shape Auto-Matched and Dual-Light-Defined Antibacterial/Osteogenic Pattern Switch Properties. ACS Appl. Mater. Interfaces 2020, 12, 54497–54506. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Mensaha, A.; Li, D.; Wang, Q.; Wei, Q. A plant-inspired long-lasting adhesive bilayer nanocomposite hydrogel based on redox-active Ag/Tannic acid-Cellulose nanofibers. Carbohydr. Polym. 2021, 255, 117508. [Google Scholar] [CrossRef]
- Qiao, Y.; He, J.; Chen, W.; Yu, Y.; Li, W.; Du, Z.; Xie, T.; Ye, Y.; Hua, S.Y.; Zhong, D.; et al. Light-Activatable Synergistic Therapy of Drug-Resistant Bacteria-Infected Cutaneous Chronic Wounds and Nonhealing Keratitis by Cupriferous Hollow Nanoshells. ACS Nano 2020, 14, 3299–3315. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Krisnawati, D.I.; Kristanto, H.; Jazidie, A.; Nuh, M.; Cheng, T.-M.; Kuo, T.-R. Nanomaterials for the Photothermal Killing of Bacteria. Nanomaterials 2020, 10, 1123. [Google Scholar] [CrossRef]
- Chen, S.; Tang, F.; Tang, L.; Li, L. Synthesis of Cu-Nanoparticle Hydrogel with Self-Healing and Photothermal Properties. ACS Appl. Mater. Interfaces 2017, 9, 20895–20903. [Google Scholar] [CrossRef]
- Kong, Y.; Hou, Z.; Zhou, L.; Zhang, P.; Ouyang, Y.; Wang, P.; Chen, Y.; Luo, X. Injectable Self-Healing Hydrogels Containing CuS Nanoparticles with Abilities of Hemostasis, Antibacterial activity, and Promoting Wound Healing. ACS Biomater. Sci. Eng. 2021, 7, 335–349. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Zhang, H.; Liu, X.; Shi, J.; Shi, H.; Yao, X.; Chu, P.K. A bifunctional hydrogel incorporated with CuS@MoS2 microspheres for disinfection and improved wound healing. Chem. Eng. J. 2020, 382, 122849. [Google Scholar] [CrossRef]
- Chambre, L.; Rosselle, L.; Barras, A.; Aydin, D.; Loczechin, A.; Gunbay, S.; Sanyal, R.; Skandrani, N.; Metzler-Nolte, N.; Bandow, J.E.; et al. Photothermally Active Cryogel Devices for Effective Release of Antimicrobial Peptides: On-Demand Treatment of Infections. ACS Appl. Mater. Interfaces 2020, 12, 56805–56814. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, B.; Li, M.; He, J.; Yin, Z.; Guo, B. Injectable Antimicrobial Conductive Hydrogels for Wound Disinfection and Infectious Wound Healing. Biomacromolecules 2020, 21, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Pang, Q.; Yuan, P.; Luo, Y.; Ma, L. Smart wound dressing for infection monitoring and NIR-triggered antibacterial treatment. Biomater. Sci. 2020, 8, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Howaili, F.; Özliseli, E.; Küçüktürkmen, B.; Razavi, S.M.; Sadeghizadeh, M.; Rosenholm, J.M. Stimuli-Responsive, Plasmonic Nanogel for Dual Delivery of Curcumin and Photothermal Therapy for Cancer Treatment. Front. Chem. 2021, 8, 602941. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Ge, J.; Wang, M.; Chen, M.; Niu, W.; Cheng, W.; Xue, Y.; Lin, C.; Lei, B. Bioactive Anti-inflammatory, Antibacterial, Antioxidative Silicon-Based Nanofibrous Dressing Enables Cutaneous Tumor Photothermo-Chemo Therapy and Infection-Induced Wound Healing. ACS Nano 2020, 14, 2904–2916. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhu, M.; Xu, G.; Liu, N.; Yu, C. A near-infrared light-responsive multifunctional nanocomposite hydrogel for efficient and synergistic antibacterial wound therapy and healing promotion. J. Mater. Chem. B 2020, 8, 3908–3917. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, Z.; Zhang, Y.; Wang, E.; Ma, B.; Xu, Q.; Ma, L.; Zhang, M.; Pei, G.; Chang, J. A novel “hot spring”-mimetic hydrogel with excellent angiogenic properties for chronic wound healing. Biomaterials 2021, 264, 120414. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Li, Z.; Zheng, Y.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Noninvasive rapid bacteria-killing and acceleration of wound healing through photothermal/photodynamic/copper ion synergistic action of a hybrid hydrogel. Biomater. Sci. 2018, 6, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Dang, W.; Yang, Z.; Chang, J.; Wu, C. MoS2 Nanoclusters-based biomaterials for disease- impaired wound therapy. Appl. Mater. Today 2020, 20, 100735. [Google Scholar] [CrossRef]
- Zhou, W.; Zi, L.; Cen, Y.; You, C.; Tian, M. Copper Sulfide Nanoparticles-Incorporated Hyaluronic Acid Injectable Hydrogel with Enhanced Angiogenesis to Promote Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 417. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Liu, Y.; Sun, L.; Zhao, Y. Black Phosphorus-Loaded Separable Microneedles as Responsive Oxygen Delivery Carriers for Wound Healing. ACS Nano 2020, 14, 5901–5908. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-P.; Su, C.-H.; Wang, S.-J.; Tsai, F.-J.; Chang, C.-T.; Liao, M.-C.; Yu, C.-C.; Tran, T.-T.V.; Lee, C.-N.; Chiu, W.-T.; et al. CO2 Delivery to Accelerate Incisional Wound Healing Following Single Irradiation of Near-Infrared Lamp on the Coordinated Colloids. ACS Nano 2017, 11, 5826–5835. [Google Scholar] [CrossRef]
- Xie, G.; Zhou, N.; Gao, Y.; Du, S.; Du, H.; Tao, J.; Zhang, L.; Zhu, J. On-demand release of CO2 from photothermal hydrogels for accelerating skin wound healing. Chem. Eng. J. 2021, 403, 126353. [Google Scholar] [CrossRef]
- Veith, A.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.; Vo, T.; Hoang, T.; Cho, S. Graphene Integrated Hydrogels Based Biomaterials in Photothermal Biomedicine. Nanomaterials 2021, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, X.; Guo, H.; Huang, H.; Huang, S.; Huang, S.; Xue, W.; Zhu, P.; Guo, R. Nitric oxide released injectable hydrogel combined with synergistic photothermal therapy for antibacterial and accelerated wound healing. Appl. Mater. Today 2020, 20, 100781. [Google Scholar] [CrossRef]
- Chen, J.; Shi, Z.-D.; Ji, X.; Morales, J.; Zhang, J.; Kaur, N.; Wang, S. Enhanced Osteogenesis of Human Mesenchymal Stem Cells by Periodic Heat Shock in Self-Assembling Peptide Hydrogel. Tissue Eng. Part A 2013, 19, 716–728. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds:A Stepwise Countermeasure for Osteosarcoma. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Shao, J.; Ruan, C.; Xie, H.; Chu, P.K.; Yu, X. Photochemical Activity of Black Phosphorus for Near-Infrared Light Controlled In Situ Biomineralization. Adv. Sci. 2020, 7, 2000439. [Google Scholar] [CrossRef]
- Miao, Y.; Shi, X.; Li, Q.; Hao, L.; Liu, L.; Liu, X.; Chen, Y.; Wang, Y. Engineering natural matrices with black phosphorus nanosheets to generate multi-functional therapeutic nanocomposite hydrogels. Biomater. Sci. 2019, 7, 4046–4059. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.-P.; Ge, Y.-W.; Liu, X.-L.; Ke, Q.-F.; Zhang, J.-W.; Zhu, Z.-A.; Guo, Y.-P. Ordered arrangement of hydrated GdPO4 nanorods in magnetic chitosan matrix promotes tumor photothermal therapy and bone regeneration against breast cancer bone metastases. Chem. Eng. J. 2020, 381, 122694. [Google Scholar] [CrossRef]
- Sanchez-Casanova, S.; Martin-Saavedra, F.M.; Escudero-Duch, C.; Uceda, M.I.F.; Prieto, M.; Arruebo, M.; Acebo, P.; Fabiilli, M.L.; Franceschi, R.T.; Vilaboa, N. Local delivery of bone morphogenetic protein-2 from near infrared-responsive hydrogels for bone tissue regeneration. Biomaterials 2020, 241, 119909. [Google Scholar] [CrossRef] [PubMed]
- Pensak, M.; Hong, S.; Dukas, A.; Tinsley, B.; Drissi, H.; Tang, A.; Côté, M.; Sugiyama, O.; Lichtler, A.; Rowe, D.; et al. The role of transduced bone marrow cells overexpressing BMP-2 in healing critical-sized defects in a mouse femur. Gene Ther. 2015, 22, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Feng, X.; Liang, H.; Wang, K.; Song, Y.; Tan, L.; Wang, B.; Luo, R.; Liao, Z.; Li, G.; et al. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Mater. Today 2020, 36, 48–62. [Google Scholar] [CrossRef]
- Pan, W.; Dai, C.; Li, Y.; Yin, Y.; Gong, L.; Machuki, J.O.; Yang, Y.; Qiu, S.; Guo, K.; Gao, F. PRP-chitosan thermoresponsive hydrogel combined with black phosphorus nanosheets as injectable biomaterial for biotherapy and phototherapy treatment of rheumatoid arthritis. Biomaterials 2020, 239, 119851. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Wu, F.-G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.W.K.; et al. Controlled-temperature photothermal and oxidative bacteria killing and acceleration of wound healing by polydopamine-assisted Au-hydroxyapatite nanorods. Acta Biomater. 2018, 77, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, Y.; Huang, Y.; He, J.; Han, Y.; Guo, B. Physical Double-Network Hydrogel Adhesives with Rapid Shape Adaptability, Fast Self-Healing, Antioxidant and NIR/pH Stimulus-Responsiveness for Multidrug-Resistant Bacterial Infection and Removable Wound Dressing. Adv. Funct. Mater. 2020, 30, 133. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, H.; Zhou, L.; Cheng, F.; Liu, Z.; Zhang, H.; Zhang, Q. Injectable redox and light responsive MnO2 hybrid hydrogel for simultaneous melanoma therapy and multidrug-resistant bacteria-infected wound healing. Biomaterials 2020, 260, 120314. [Google Scholar] [CrossRef]
- Yu, Y.; Li, P.; Zhu, C.; Ning, N.; Zhang, S.; Vancso, G.J. Multifunctional and Recyclable Photothermally Responsive Cryogels as Efficient Platforms for Wound Healing. Adv. Funct. Mater. 2019, 29, 29. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Wang, J.; Zhang, Y.; Dong, M.; Bu, T.; Li, L.; Liu, Y.; Wang, L. Multifunctional Injectable Hydrogel Dressings for Effectively Accelerating Wound Healing: Enhancing Biomineralization Strategy. Adv. Funct. Mater. 2021, 2100093. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Q.; Xu, Z.; Wang, Y.; Zhu, B.; Fan, L.; Gao, L. Aloe-Emodin/Carbon Nanoparticle Hybrid Gels with Light-Induced and Long-Term Antibacterial Activity. ACS Biomater. Sci. Eng. 2018, 4, 4391–4400. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhou, Q.; Chang, J.; Wu, C. Grape Seed-Inspired Smart Hydrogel Scaffolds for Melanoma Therapy and Wound Healing. ACS Nano 2019, 13, 4302–4311. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, H.; Shen, J. Synthesis and molecular dynamics simulation of CuS@GO–CS hydrogel for enhanced photothermal antibacterial effect. New J. Chem. 2021, 45, 6895–6903. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, F.; Hou, Z.; Chen, Y.; Luo, X. Injectable self-healing CuS nanoparticle complex hydrogels with antibacterial, anti-cancer, and wound healing properties. Chem. Eng. J. 2021, 409, 128224. [Google Scholar] [CrossRef]
- Martín-Saavedra, F.; Escudero-Duch, C.; Prieto, M.; Sánchez-Casanova, S.; López, D.; Arruebo, M.; Voellmy, R.; Santamaría, J.; Vilaboa, N. Pro-angiogenic near infrared-responsive hydrogels for deliberate transgene expression. Acta Biomater. 2018, 78, 123–136. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H.; Liu, H.; Jin, D.; Yin, M.; Lin, H.; Qu, X.; Liu, C. A reduced polydopamine nanoparticle-coupled sprayable PEG hydrogel adhesive with anti-infection activity for rapid wound sealing. Biomater. Sci. 2020, 8, 6946–6956. [Google Scholar] [CrossRef]
- Chu, X.; Zhang, P.; Wang, Y.; Sun, B.; Liu, Y.; Zhang, Q.; Feng, W.; Li, Z.; Li, K.; Zhou, N.; et al. Near-infrared carbon dot-based platform for bioimaging and photothermal/photodynamic/quaternary ammonium triple synergistic sterilization triggered by single NIR light source. Carbon 2021, 176, 126–138. [Google Scholar] [CrossRef]
- Deng, H.; Sun, J.; Yu, Z.; Guo, Z.; Xu, C. Low-intensity near-infrared light-triggered spatiotemporal antibiotics release and hyperthermia by natural polysaccharide-based hybrid hydrogel for synergistic wound disinfection. Mater. Sci. Eng. C 2021, 118, 111530. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, S.; Guo, L.; Wang, Y.; Feng, L. Intelligent Hybrid Hydrogels for Rapid In Situ Detection and Photothermal Therapy of Bacterial Infection. ACS Appl. Mater. Interfaces 2020, 12, 39685–39694. [Google Scholar] [CrossRef]
- Xu, C.; Akakuru, O.U.; Ma, X.; Zheng, J.; Zheng, J.; Wu, A. Nanoparticle-Based Wound Dressing: Recent Progress in the Detection and Therapy of Bacterial Infections. Bioconjugate Chem. 2020, 31, 1708–1723. [Google Scholar] [CrossRef]
- Wang, X.; Fang, J.; Zhu, W.; Zhong, C.; Ye, D.; Zhu, M.; Lu, X.; Zhao, Y.; Ren, F. Bioinspired Highly Anisotropic, Ultrastrong and Stiff, and Osteoconductive Mineralized Wood Hydrogel Composites for Bone Repair. Adv. Funct. Mater. 2021, 2010068. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, B.; Zheng, Y.; Han, Y.; Chen, D.-F.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z.; et al. Near-Infrared Light Triggered Phototherapy and Immunotherapy for Elimination of Methicillin-Resistant Staphylococcus aureus Biofilm Infection on Bone Implant. ACS Nano 2020, 14, 8157–8170. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zhang, P.; Lv, L.; Zhou, Y. NIR light-assisted phototherapies for bone-related diseases and bone tissue regeneration: A systematic review. Theranostics 2020, 10, 11837–11861. [Google Scholar] [CrossRef]
- Luo, S.; Wu, J.; Jia, Z.; Tang, P.; Sheng, J.; Xie, C.; Liu, C.; Gan, D.; Hu, D.; Zheng, W.; et al. An Injectable, Bifunctional Hydrogel with Photothermal Effects for Tumor Therapy and Bone Regeneration. Macromol. Biosci. 2019, 19, e1900047. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Niu, W.; Wang, M.; Chen, M.; Guo, Y.; Lei, B. Engineering a Biodegradable Multifunctional Antibacterial Bioactive Nanosystem for Enhancing Tumor Photothermo-Chemotherapy and Bone Regeneration. ACS Nano 2019, 14, 442–453. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, J. Photothermal effect of 3D printed hydroxyapatite composite scaffolds incorporated with graphene nanoplatelets. Ceram. Int. 2021, 47, 6336–6340. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Li, L.; Yu, F.; Li, J.; Liu, L.; Fang, B.; Xia, L. Photothermally triggered biomimetic drug delivery of Teriparatide via reduced graphene oxide loaded chitosan hydrogel for osteoporotic bone regeneration. Chem. Eng. J. 2021, 413, 127413. [Google Scholar] [CrossRef]
- Yang, C.; Gao, X.; Younis, M.R.; Blum, N.T.; Lei, S.; Zhang, D.; Luo, Y.; Huang, P.; Lin, J. Non-invasive monitoring of in vivo bone regeneration based on alkaline phosphatase-responsive scaffolds. Chem. Eng. J. 2021, 408, 127959. [Google Scholar] [CrossRef]


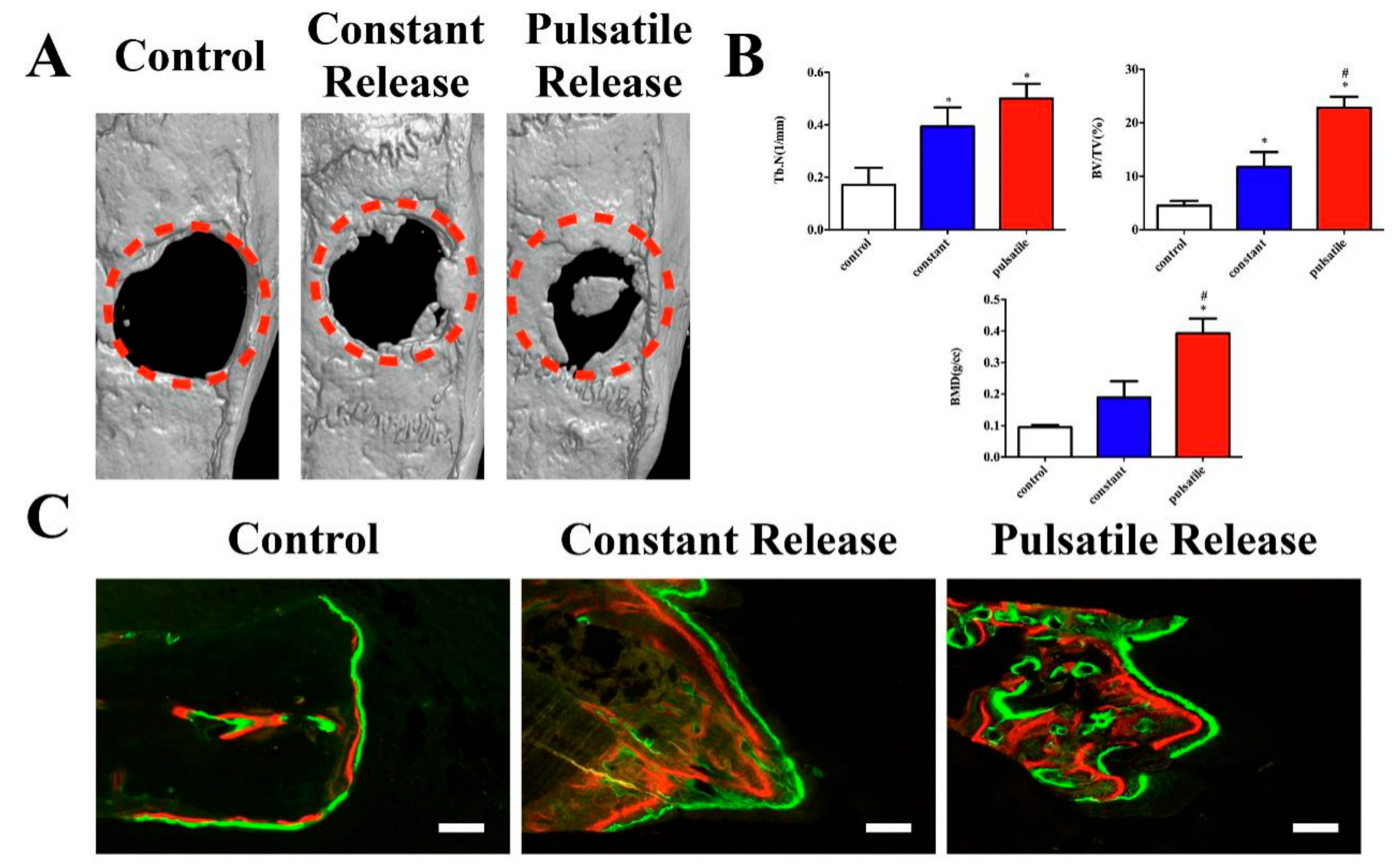
| Hydrogels | Photothermal Agents | Concentration | Intensity and Time | Temperature | Application | Reference |
|---|---|---|---|---|---|---|
| NOCS/OSA/FA | FA(Fe2SiO4) | 5 mg/mL | 0.36 W/cm2 10 min | 40 °C | Ion release Angiogenesis | [112] |
| CMCS/OSA/ CuS | CuS | 0.8 mg/mL | 1 W/cm2 5 min | 50 °C | Antibacterial Angiogenesis | [103] |
| CuS/HA | CuS | 0.2 mg/mL | 1 W/cm2 10 min | 53.1 °C | Wound healing Angiogenesis | [115] |
| NIPAAm/ AAm/CuS/ mSiO2 | CuS/mSiO2 NPs | 1.5 mg/mL | 2 W/cm2 8 min | 59.5 °C | Antibacterial effect | [113] |
| GelMA/BP | BP | 1 mg/mL | 1 W/cm2 5 min | 55.3 °C | Antibacterial effect Bone regeneration | [125] |
| BP/CS/PRP | BP | 0.05 mg/mL | 1 W/cm2 8 min | 45 °C | Drug release Antiarthritic | [130] |
| PDA/GC/Cip | PDA NPs | 4 mg/mL | 0.5 W/cm2 10 min | 46.8 °C | Antibacterial effect Drug release | [131] |
| Gel-PDA/Cur | PDA/Cur | 2 mg/mL | 1 W/cm2 10 min | 50.9 °C | Antibacterial effect Drug release | [95] |
| MPDA/GO/ CNF | MPDA/GO | 10 mg/mL | 2 W/cm2 10 min | 56 °C | Drug release | [90] |
| GelMA/oDex/BNN6@ZIF8/ PDA | PDA/ZIF8 | 1 mg/mL | 2 W/cm2 10 min | 50 °C | Antibacterial effect Angiogenesis | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tan, B.; Wu, Y.; Zhang, M.; Liao, J. A Review on Hydrogels with Photothermal Effect in Wound Healing and Bone Tissue Engineering. Polymers 2021, 13, 2100. https://doi.org/10.3390/polym13132100
Zhang X, Tan B, Wu Y, Zhang M, Liao J. A Review on Hydrogels with Photothermal Effect in Wound Healing and Bone Tissue Engineering. Polymers. 2021; 13(13):2100. https://doi.org/10.3390/polym13132100
Chicago/Turabian StyleZhang, Xu, Bowen Tan, Yanting Wu, Min Zhang, and Jinfeng Liao. 2021. "A Review on Hydrogels with Photothermal Effect in Wound Healing and Bone Tissue Engineering" Polymers 13, no. 13: 2100. https://doi.org/10.3390/polym13132100
APA StyleZhang, X., Tan, B., Wu, Y., Zhang, M., & Liao, J. (2021). A Review on Hydrogels with Photothermal Effect in Wound Healing and Bone Tissue Engineering. Polymers, 13(13), 2100. https://doi.org/10.3390/polym13132100







