Purification of Hollow Sporopollenin Microcapsules from Sunflower and Chamomile Pollen Grains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatment of Sunflower Pollen
2.2.1. Extraction of the Pollenkitt of Sunflower Pollen
2.2.2. Elimination of Sporoplasm, Production of Hollow Sunflower Pollen with Acids
2.3. Treatment of Chamomile Dried Plant and Flower
2.3.1. Purification of Pollen from Dried Chamomile
2.3.2. Extraction of Pollenkitt of Chamomile Pollen
2.3.3. Elimination of Sporoplasm, Production of Hollow Chamomile Pollen with Acids
2.4. Analytical Measurements
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.2. Elemental CHN Analysis
2.4.3. Scanning Electron Microscopy (SEM) Analysis
2.4.4. Confocal Laser Scanning Microscopy (CLSM)
2.4.5. Determination of Pollen Density and Number of Grains per Unit of Mass
2.4.6. Thermal Gravimetric Analysis (TGA)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Extraction of the Pollenkitt: Sunflower Pollen
3.2. Extraction of Pollenkitt: Chamomile Pollen
3.3. Comparison of Different Acid Treatments on the Purification of Pollen
3.4. Optimization of the Treatment with H3PO4
3.5. Thermogravimetric Characterization of Hollow Pollen Platforms
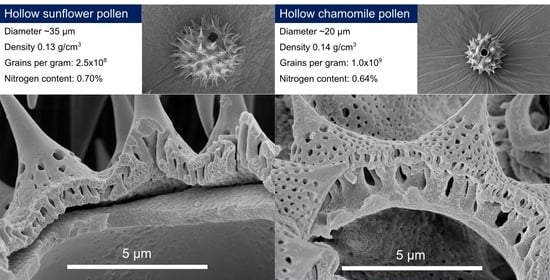
3.6. Comparison between Hollow Sporopollenin Microcapsules from Sunflower and Chamomile
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, J.; Zhang, Z.; Cao, J. Pollen wall development: The associated enzymes and metabolic pathways. Plant Biol. 2013, 15, 249–263. [Google Scholar] [CrossRef]
- Mackenzie, G.; Beckett, S.; Atkin, S.; Diego-Taboada, A. Pollen and spore shells—Nature’s microcapsules. In Microencapsulation in the Food Industry; Academic Press: Cambridge, MA, USA, 2014; ISBN 9780124045682. [Google Scholar]
- Punt, W.; Hoen, P.P.; Blackmore, S.; Nilsson, S.; Le Thomas, A. Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 2007, 143, 1–81. [Google Scholar] [CrossRef]
- Knox, R.B. The Pollen Grain. In Embryology of Angiosperms; Johri, B.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 197–271. ISBN 9783642693045. [Google Scholar]
- Qu, Z.; Meredith, J.C. The atypically high modulus of pollen exine. J. R. Soc. Interface 2018, 15, 20180533. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, G.; Boa, A.N.; Diego-Taboada, A.; Atkin, S.L.; Sathyapalan, T. Sporopollenin, The Least Known Yet Toughest Natural Biopolymer. Front. Mater. 2015, 2, 66. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Phyo, P.; Jacobowitz, J.; Hong, M.; Weng, J. The molecular structure of plant sporopollenin. Nat. Plants 2019, 5, 41–46. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, L.; Chen, G.; Shao, C.; Liu, Y.; Lu, P.; Rong, F.; Zhao, Y. Pollen-inspired microparticles with strong adhesion for drug delivery. Appl. Mater. Today 2018, 13, 303–309. [Google Scholar] [CrossRef]
- Traidl-Hoffmann, C.; Kasche, A.; Menzel, A.; Jakob, T.; Thiel, M.; Ring, J.; Behrendt, H. Impact of Pollen on Human Health: More Than Allergen Carriers? Int. Arch. Allergy Immunol. 2003, 131, 1–13. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Potroz, M.G.; Park, J.H.; Seo, J.; Lee, J.H.; Cho, N.-J. Extraction of sporopollenin exine capsules from sunflower pollen grains. RSC Adv. 2016, 6, 16533–16539. [Google Scholar] [CrossRef]
- Fan, T.; Park, S.; Shi, Q.; Zhang, X.; Liu, Q.; Song, Y.; Chin, H.; Shahrudin, M.; Ibrahim, B.; Mokrzecka, N.; et al. Transformation of hard pollen into soft matter. Nat. Commun. 2020, 11, 1449. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Cruz, P.; Uddin, M.J.; Atwe, S.U.; Abidi, N.; Gill, H.S. Chemical Treatment Method for Obtaining Clean and Intact Pollen Shells of Different Species. ACS Biomater. Sci. Eng. 2018, 4, 2319–2329. [Google Scholar] [CrossRef] [PubMed]
- Diego-Taboada, A.; Maillet, L.; Banoub, J.H.; Lorch, M.; Rigby, A.S.; Boa, A.N.; Atkin, S.L.; Mackenzie, G. Protein free microcapsules obtained from plant spores as a model for drug delivery: Ibuprofen encapsulation, release and taste masking. J. Mater. Chem. B 2013, 1, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Dyab, A.K.F.; Mohamed, M.A.; Meligi, N.M.; Mohamed, S.K. Encapsulation of erythromycin and bacitracin antibiotics into natural sporopollenin microcapsules: Antibacterial, cytotoxicity, in vitro and in vivo release studies for enhanced bioavailability. RSC Adv. 2018, 8, 33432–33444. [Google Scholar] [CrossRef] [Green Version]
- Wakil, A.; MacKenzie, G.; Diego-Taboada, A.; Bell, J.G.; Atkin, S.L. Enhanced bioavailability of eicosapentaenoic acid from fish oil after encapsulation within plant spore exines as microcapsules. Lipids 2010, 45, 645–649. [Google Scholar] [CrossRef]
- Hamad, S.A.; Dyab, A.F.K.; Stoyanov, S.D.; Paunov, V.N. Encapsulation of living cells into sporopollenin microcapsules. J. Mater. Chem. 2011, 21, 18018–18023. [Google Scholar] [CrossRef]
- Potroz, M.G.; Mundargi, R.C.; Gillissen, J.J.; Tan, E.-L.; Meker, S.; Park, J.H.; Jung, H.; Park, S.; Cho, D.; Bang, S.-I.; et al. Plant-Based Hollow Microcapsules for Oral Delivery Applications: Toward Optimized Loading and Controlled Release. Adv. Funct. Mater. 2017, 27, 1700270. [Google Scholar] [CrossRef]
- Fan, T.; Park, J.H.; Pham, Q.A.; Tan, E.-L.; Mundargi, R.C.; Potroz, M.G.; Jung, H.; Cho, N.-J. Extraction of cage-like sporopollenin exine capsules from dandelion pollen grains. Sci. Rep. 2018, 8, 6565. [Google Scholar] [CrossRef]
- Maric, T.; Nasir, M.Z.M.; Rosli, N.F.; Budanović, M.; Webster, R.D.; Cho, N.-J.; Pumera, M. Microrobots Derived from Variety Plant Pollen Grains for Efficient Environmental Clean Up and as an Anti-Cancer Drug Carrier. Adv. Funct. Mater. 2020, 30, 2000112. [Google Scholar] [CrossRef]
- Elmacı, G. Magnetic Hollow Biocomposites Prepared from Lycopodium clavatum Pollens as Efficient Recyclable Catalyst. ChemistrySelect 2020, 5, 2225–2231. [Google Scholar] [CrossRef]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, D.L.; Blumberg, J.B. A Review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phyther. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef]
- Halbritter, H. Matricaria chamomilla. Available online: https://www.paldat.org/pub/Matricaria_chamomilla/301539;jsessionid=9C42510DC1E00F411213816DA650466B (accessed on 22 March 2021).
- Halbritter, H.; Heig, H.; Svojtka, N. Helianthus annuus. Available online: https://www.paldat.org/pub/Helianthus_annuus/304619 (accessed on 2 April 2021).
- Bashir, M.E.H.; Ward, J.M.; Cummings, M.; Karrar, E.E.; Root, M.; Mohamed, A.B.A.; Naclerio, R.M.; Preuss, D. Dual Function of Novel Pollen Coat (Surface) Proteins: IgE-binding Capacity and Proteolytic Activity Disrupting the Airway Epithelial Barrier. PLoS ONE 2013, 8, e53337. [Google Scholar] [CrossRef]
- Sánchez Juárez, D.L.; Martínez Montes, G.; Rubio Rosas, E. Chemical Characterization of Yellow and Orange Pollen (Helianthus annuus) and its Effect in the Growth of Hydroxyapatite. Mater. Sci. Forum 2013, 755, 163–169. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Potroz, M.G.; Park, J.H.; Seo, J.; Tan, E.-L.; Lee, J.H.; Cho, N.-J. Eco-friendly streamlined process for sporopollenin exine capsule extraction. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Delaplane, K.S.; Dag, A.; Danka, R.G.; Freitas, B.M.; Garibaldi, L.A.; Goodwin, R.M.; Hormaza, J.I. Standard methods for pollination research with Apis mellifera. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Lin, H.; Gomez, I.; Meredith, J.C. Pollenkitt wetting mechanism enables species-specific tunable pollen adhesion. Langmuir 2013, 29, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Pappas, C.S.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M.G. New Method for Pollen Identication by FT-IR Spectroscopy. Appl. Spectrosc. 2003, 57, 23–27. [Google Scholar] [CrossRef]
- Buta, E.; Cantor, M.; Tefan, R.; Pop, R.; Mitre, I.; Buta, M.; Sestra, R.E. FT-IR Characterization of Pollen Biochemistry, Viability, and Germination Capacity in Saintpaulia H. Wendl. Genotypes. J. Spectrosc. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Anjos, O.; Santos, A.J.A.; Dias, T.; Estevinho, L.M. Application of FTIR-ATR spectroscopy on the bee pollen characterization. J. Apic. Res. 2017, 8839, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Grube, M.; Bekers, M.; Upite, D.; Kaminska, E. Infrared spectra of some fructans. Spectroscopy 2002, 16, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Schulte, F.; Lingott, J.; Panne, U.; Kneipp, J. Chemical Characterization and Classification of Pollen. Anal. Chem. 2008, 80, 9551–9556. [Google Scholar] [CrossRef]
- Bağcıoğlu, M.; Zimmermann, B.; Kohler, A. A multiscale vibrational spectroscopic approach for identification and biochemical characterization of pollen. PLoS ONE 2015, 10, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Farrán, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernáiz, M.J.; Linhardt, R.J. Green Solvents in Carbohydrate Chemistry: From Raw Materials to Fine Chemicals. Chem. Rev. 2015, 115, 6811–6853. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Gill, H.S. Ragweed pollen as an oral vaccine delivery system: Mechanistic insights. J. Control. Release 2017, 268, 416–426. [Google Scholar] [CrossRef]
- Ares, A.M.; Valverde, S.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Extraction and determination of bioactive compounds from bee pollen. J. Pharm. Biomed. Anal. 2018, 147, 110–124. [Google Scholar] [CrossRef]
- Schulz, S.; Arsene, C.; Tauber, M.; McNeil, J.N. Composition of lipids from sunflower pollen (Helianthus annuus). Phytochemistry 2000, 54, 325–336. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Kinugasa, S.; Tanabe, K.; Tamura, T. SDBSWeb: National Institute of Advanced Industrial Science and Technology. Available online: https://sdbs.db.aist.go.jp (accessed on 22 June 2021).
- Shearer, G.L. An Evaluation of Fourier Transform Infrared Spectroscopy for the Characterzation of Organic Compounds in Art and Archaeology. Ph.D. Thesis, University College London, London, UK, 1989. [Google Scholar]
- Kędzierska-Matysek, M.; Matwijczuk, A.; Florek, M.; Barłowska, J.; Wolanciuk, A.; Matwijczuk, A.; Chruściel, E.; Walkowiak, R.; Karcz, D.; Gładyszewska, B. Application of FTIR spectroscopy for analysis of the quality of honey. BIO Web Conf. 2018, 10, 02008. [Google Scholar] [CrossRef] [Green Version]
- Fang, K.; Wang, Y.; Jinxing, L.; Yu, T.; Zhang, L.; Baluška, F.; Šamaj, J.; Lin, J. Isolation of de-exined pollen and cytological studies of the pollen intines of Pinus bungeana Zucc. Ex Endl. and Picea wilsonii Mast. Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 332–340. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables Along a Fraction Process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolson, S.W.; Human, H. Chemical composition of the “low quality” pollen of sunflower (Helianthus annuus, Asteraceae). Apidologie 2013, 44, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, E.; Mercado, J.A.; Quesada, M.A.; Heredia, A. Pollen sporopollenin: Degradation and structural elucidation. Sex. Plant Reprod. 1999, 12, 171–178. [Google Scholar] [CrossRef]
- Ghosh, N.; Sircar, G.; Saha, B.; Pandey, N.; Bhattacharya, S.G. Search for allergens from the pollen proteome of sunflower (Helianthus annuus L.): A major sensitizer for respiratory allergy patients. PLoS ONE 2015, 10, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Hicks, D.M.; Ouvrard, P.; Baldock, K.C.R.; Baude, M.; Goddard, M.A.; Kunin, W.E.; Mitschunas, N.; Memmott, J.; Morse, H.; Nikolitsi, M.; et al. Food for Pollinators: Quantifying the Nectar and Pollen Resources of Urban Flower Meadows. PLoS ONE 2016, 11, e0158117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential oil quality and purity evaluation via FT-IR spectroscopy and pattern recognition techniques. Appl. Sci. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Dinant, S.; Wolff, N.; De Marco, F.; Vilaine, F.; Gissot, L.; Aubry, E.; Sandt, C.; Bellini, C.; Le Hir, R. Synchrotron FTIR and Raman spectroscopy provide unique spectral fingerprints for Arabidopsis floral stem vascular tissues. J. Exp. Bot. 2019, 70, 937–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chukanov, N.V. Infrared Spectra of Mineral Species; Springer: Dordrecht, The Netherlands, 2014; Volume 1, ISBN 9789400771277. [Google Scholar]
- Süfer, Ö.; Bozok, F. Characterization of essential oil from Matricaria sevanensis by microwave-assisted distillation. J. Therm. Anal. Calorim. 2020, 140, 253–261. [Google Scholar] [CrossRef]
- Taraj, K.; Malollari, I.; Andoni, A.; Ciko, L.; Lazo, P.; Ylli, F.; Osmeni, A.; Como, A. Eco-extraction of albanian chamomile essential oils by liquid Co2 at different temperatures and characterisation by FTIR spectroscopy. J. Environ. Prot. Ecol. 2017, 18, 117–124. [Google Scholar]
- Kasai, T.; Furukawa, K.; Sakamura, S.J. Infrared and Mass Spectra of α-Amino Acid Amides. J. Fac. Agric. Hokkaido Univ. 1979, 59, 279–283. [Google Scholar]
- Chen, X.; Li, H.; Sun, S.; Cao, X.; Sun, R. Effect of hydrothermal pretreatment on the structural changes of alkaline ethanol lignin from wheat straw. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Aloisi, I.; Cai, G.; Serafini-Fracassini, D.; Del Duca, S. Polyamines in Pollen: From Microsporogenesis to Fertilization. Front. Plant Sci. 2016, 7, E305–E307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, Y.; Yasuda, H.; Ohba, Y.; Harada, H. Isolation and characterization of a novel nuclear protein from pollen mother cells of lily. Plant. Physiol. 1990, 94, 1467–1471. [Google Scholar] [CrossRef]
- Gouda, N.M.N.; Naika, S.; Gowda, N.M.N.; Naikar, S.B.; Reddy, G.K.N. Perchlorate Ion Complexes. Adv. Inorg. Chem. 1984, 28, 255–299. [Google Scholar] [CrossRef]
- Lutzke, A.; Morey, K.J.; Medford, J.I.; Kipper, M.J. Detailed characterization of Pinus ponderosa sporopollenin by infrared spectroscopy. Phytochemistry 2020, 170, 112195. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E. Phosphorus. In Water Quality: An Introduction; Springer: Cham, Switzerland, 2015; pp. 243–261. ISBN 9783319174457. [Google Scholar]
- Pöhlker, C.; Huffman, J.A.; Pöschl, U. Autofluorescence of atmospheric bioaerosols—Fluorescent biomolecules and potential interferences. Atmos. Meas. Tech. 2012, 5, 37–71. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Seo, J.; Jackman, J.A.; Cho, N.-J. Inflated Sporopollenin Exine Capsules Obtained from Thin-Walled Pollen. Sci. Rep. 2016, 6, 28017. [Google Scholar] [CrossRef] [Green Version]
- She, D.; Xu, F.; Geng, Z.C.; Sun, R.C.; Jones, G.L.; Baird, M.S. Physicochemical characterization of extracted lignin from sweet sorghum stem. Ind. Crop. Prod. 2010, 32, 21–28. [Google Scholar] [CrossRef]
- Jardine, P.E.; Fraser, W.T.; Lomax, B.H.; Gosling, W.D. The impact of oxidation on spore and pollen chemistry. J. Micropalaeontol. 2015, 34, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Soni, B.; Hassan, E.B.; Mahmoud, B. Chemical isolation and characterization of different cellulose nanofibers from cotton stalks. Carbohydr. Polym. 2015, 134, 581–589. [Google Scholar] [CrossRef]
- Ház, A.; Jablonský, M.; Orságová, A. Determination of Temperature Regions in Thermal Degradation of Lignin. In Proceedings of the 4 th International Conference on Renewable Energy Sources, Tatranské Matliare, Slovakia, 21–23 May 2013; pp. 1–6. [Google Scholar]
- Gubbuk, I.H.; Gürfidan, L.; Erdemir, S.; Yilmaz, M. Surface modification of sporopollenin with calixarene derivative: Characterization and application for metal removal. Water Air Soil Pollut. 2012, 223, 2623–2632. [Google Scholar] [CrossRef]
- Palazzo, I.; Mezzetta, A.; Guazzelli, L.; Sartini, S.; Pomelli, C.S.; Parker, W.O.; Chiappe, C. Chiral ionic liquids supported on natural sporopollenin microcapsules. RSC Adv. 2018, 8, 21174–21183. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ageitos, J.M.; Robla, S.; Valverde-Fraga, L.; Garcia-Fuentes, M.; Csaba, N. Purification of Hollow Sporopollenin Microcapsules from Sunflower and Chamomile Pollen Grains. Polymers 2021, 13, 2094. https://doi.org/10.3390/polym13132094
Ageitos JM, Robla S, Valverde-Fraga L, Garcia-Fuentes M, Csaba N. Purification of Hollow Sporopollenin Microcapsules from Sunflower and Chamomile Pollen Grains. Polymers. 2021; 13(13):2094. https://doi.org/10.3390/polym13132094
Chicago/Turabian StyleAgeitos, Jose Manuel, Sandra Robla, Lorena Valverde-Fraga, Marcos Garcia-Fuentes, and Noemi Csaba. 2021. "Purification of Hollow Sporopollenin Microcapsules from Sunflower and Chamomile Pollen Grains" Polymers 13, no. 13: 2094. https://doi.org/10.3390/polym13132094
APA StyleAgeitos, J. M., Robla, S., Valverde-Fraga, L., Garcia-Fuentes, M., & Csaba, N. (2021). Purification of Hollow Sporopollenin Microcapsules from Sunflower and Chamomile Pollen Grains. Polymers, 13(13), 2094. https://doi.org/10.3390/polym13132094