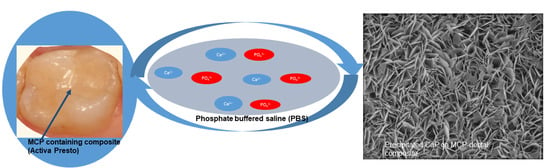
Experimental Dental Composites Containing a Novel Methacrylate-Functionalized Calcium Phosphate Component: Evaluation of Bioactivity and Physical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
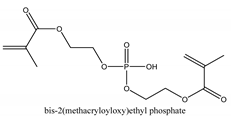
2.1.1. Methacrylate-Functionalized Calcium Phosphate (MCP)
2.1.2. Synthesis of Polymer Composites
2.2. Physico-Chemical Characterization
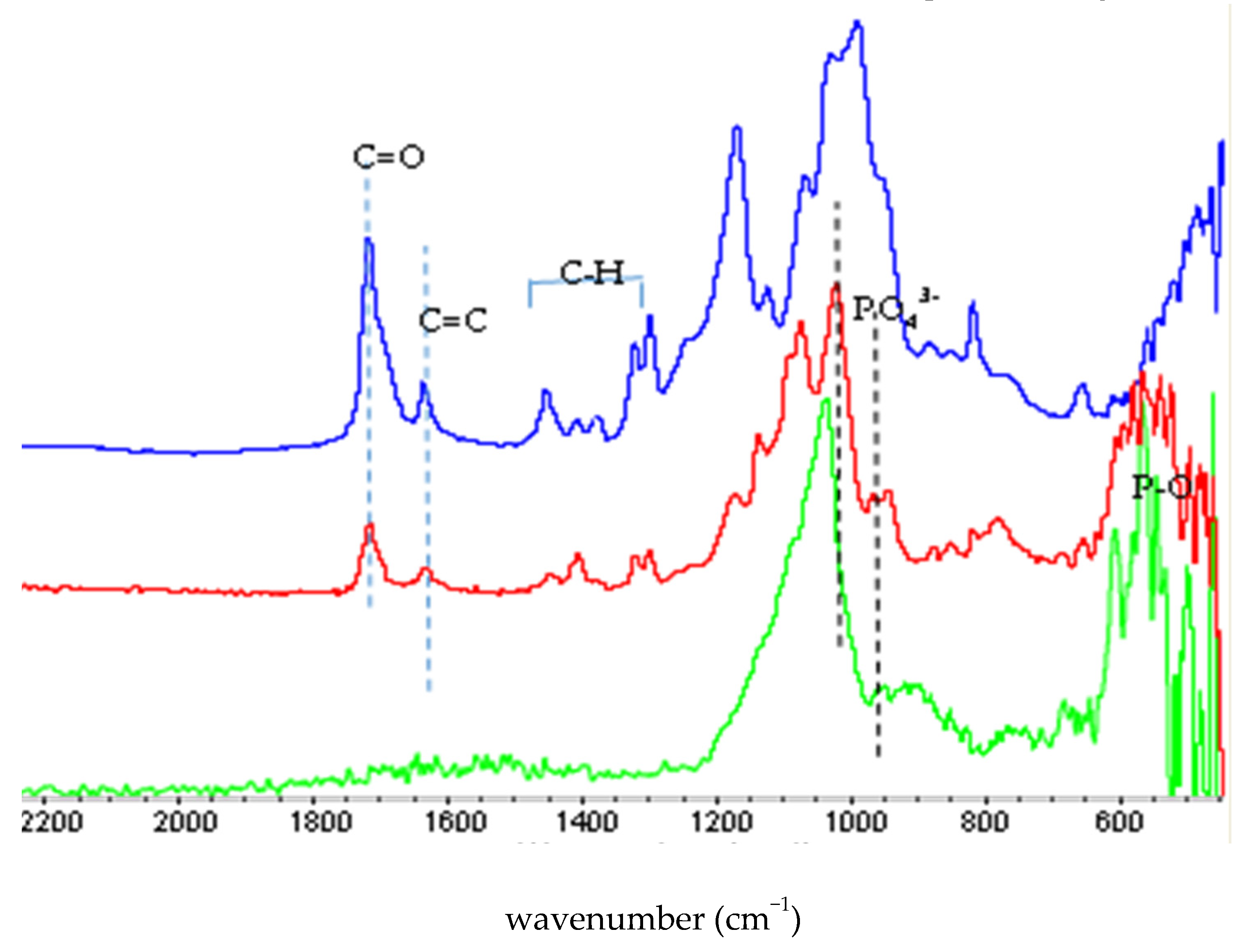
2.2.1. FTIR Characterization
2.2.2. Translucency Parameter
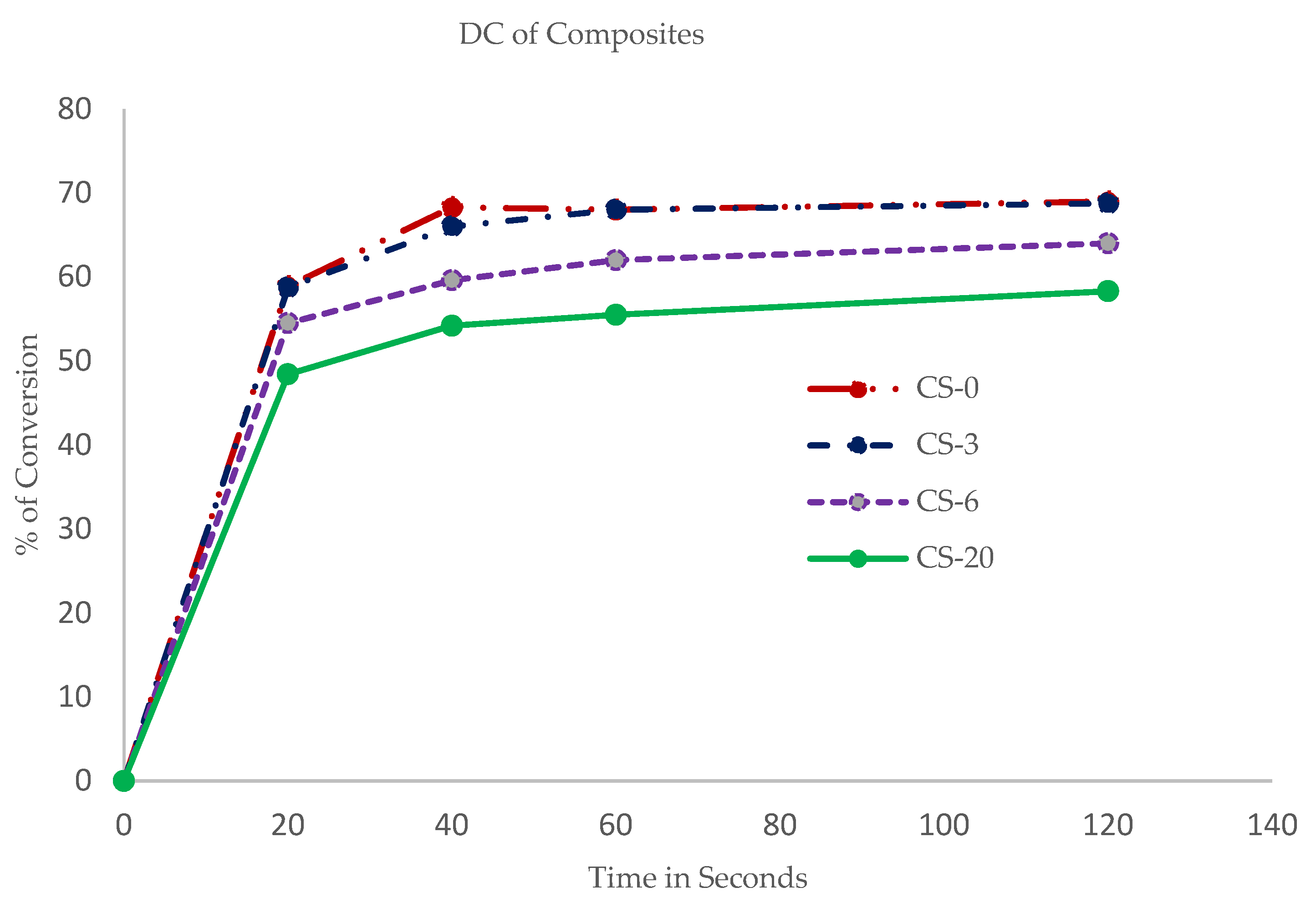
2.2.3. Degree of Conversion (DC)
2.2.4. Depth of Cure
2.2.5. Flexural Strength (FS) and Deflection at Break
2.2.6. Ion Release studies
2.2.7. Composite Disc Preparation and Bioactivity Studies
3. Results
3.1. Synthesis and Characterization of MCP
3.2. Physical Properties
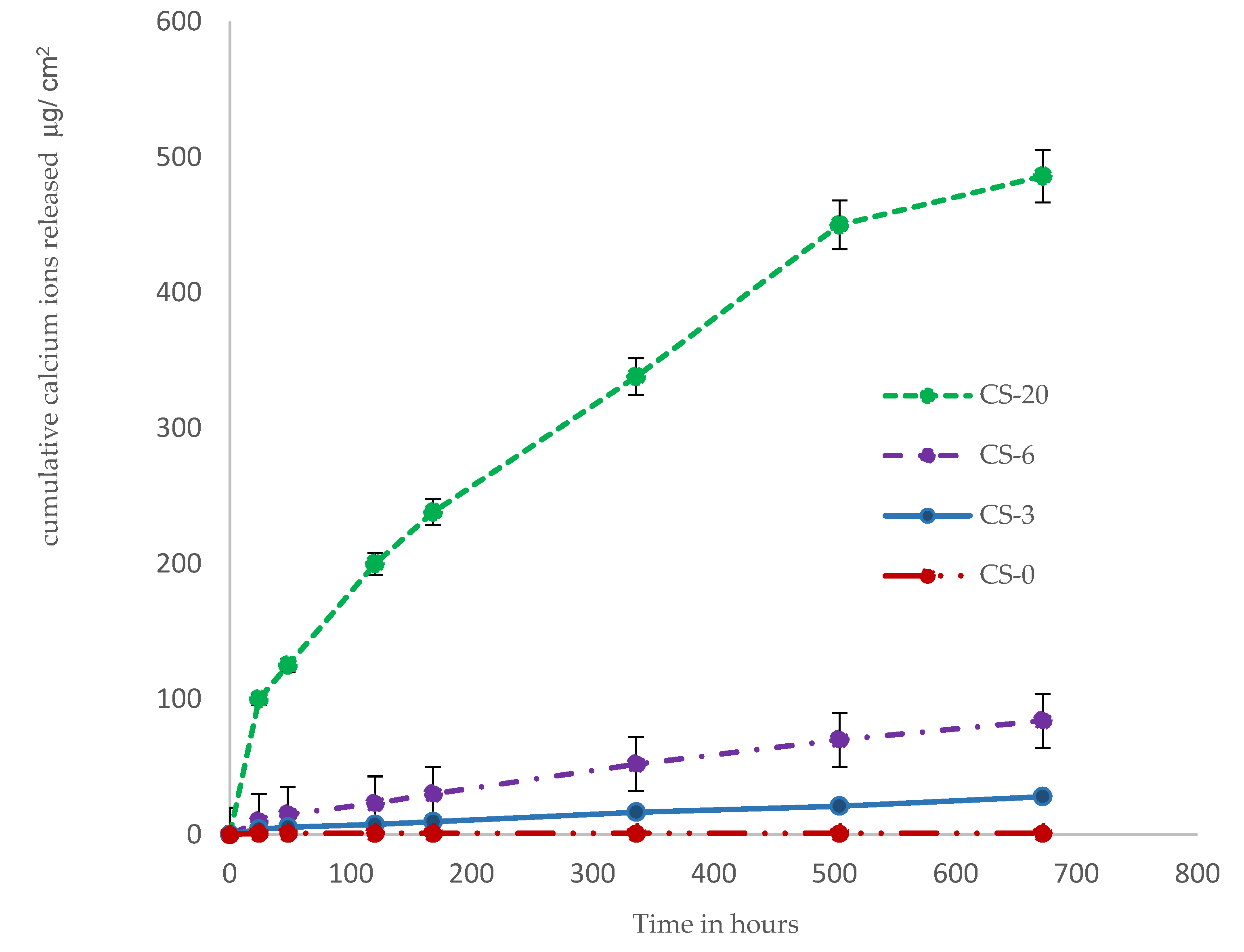
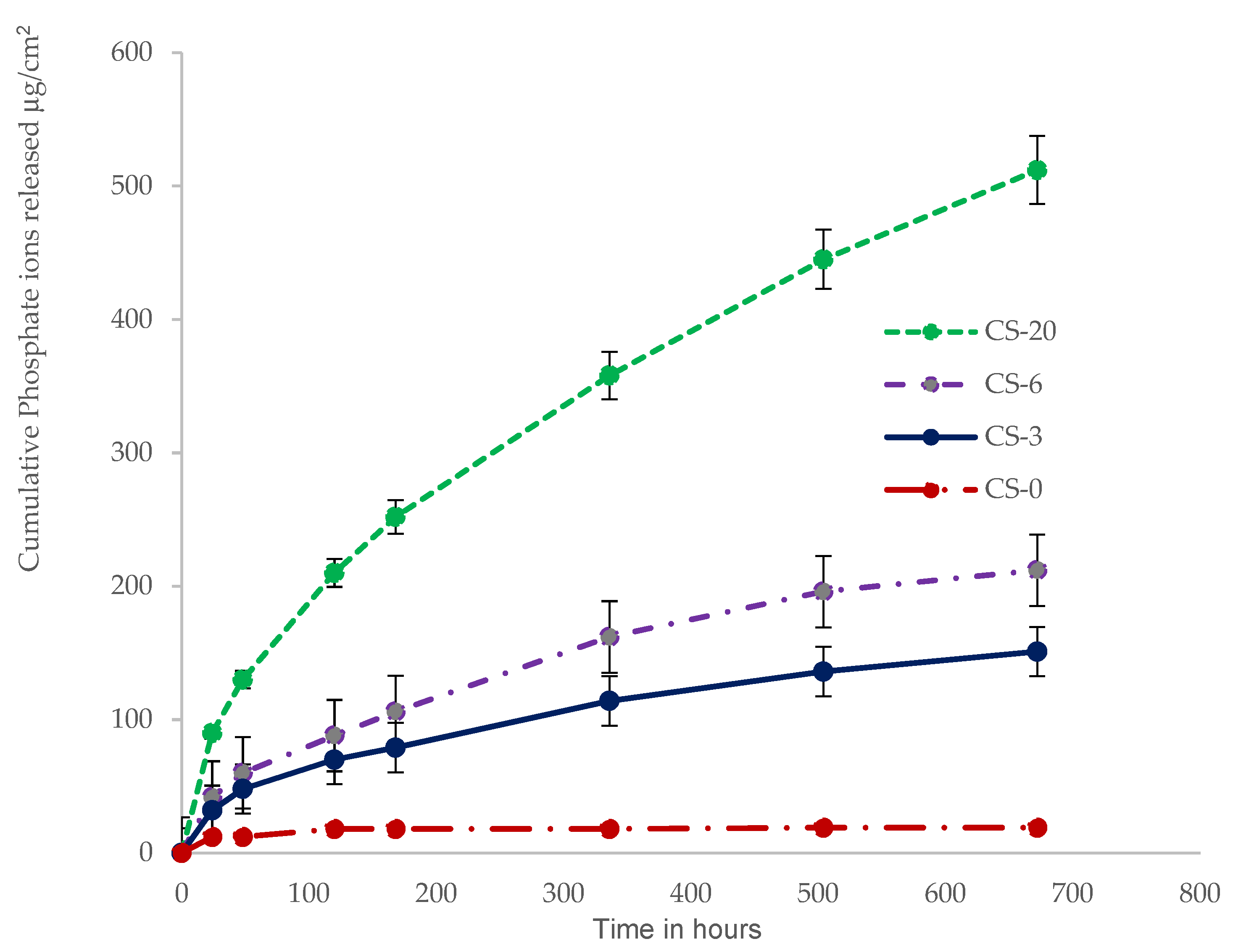
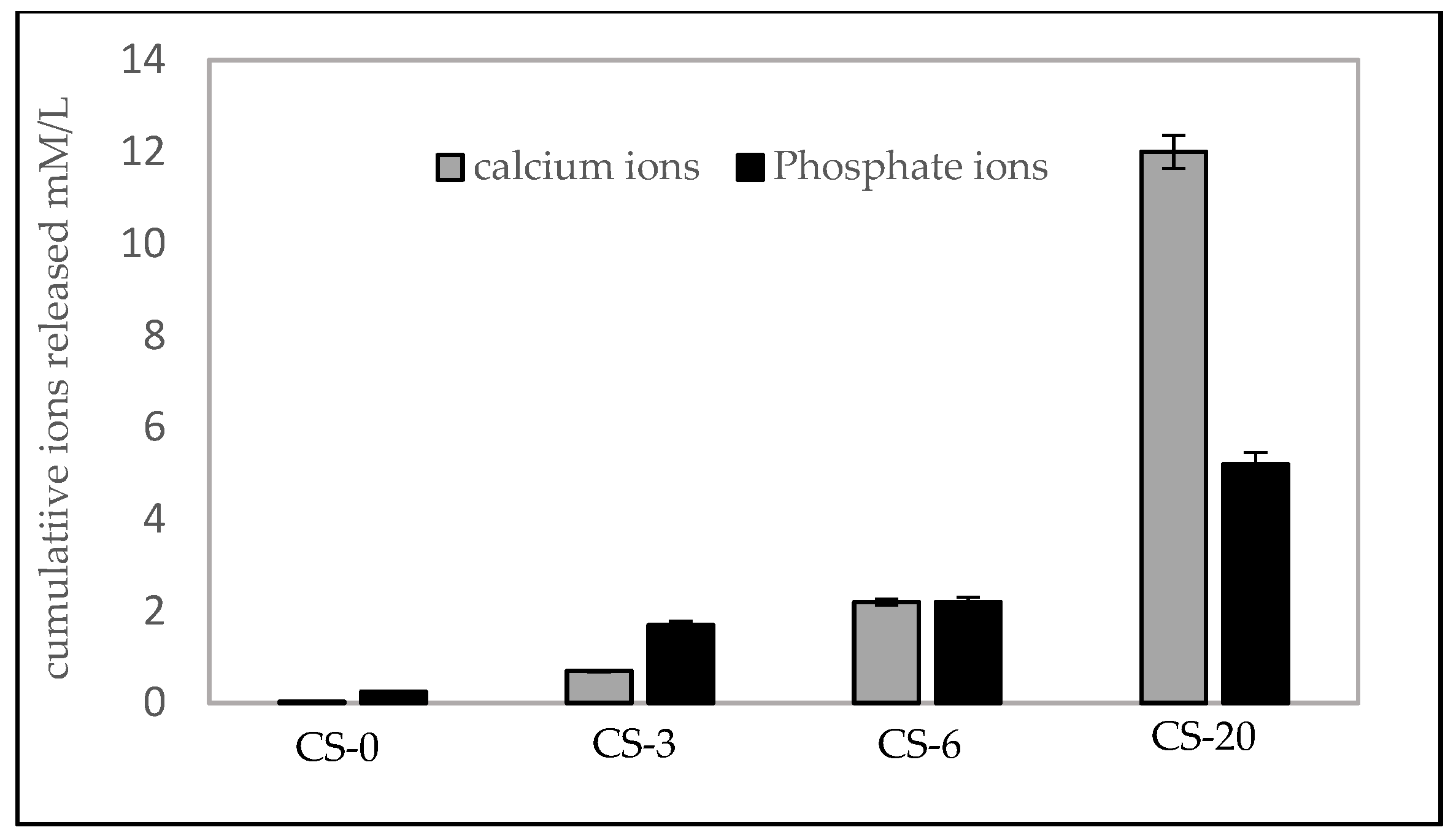
3.3. Calcium and Phosphate Ion Release
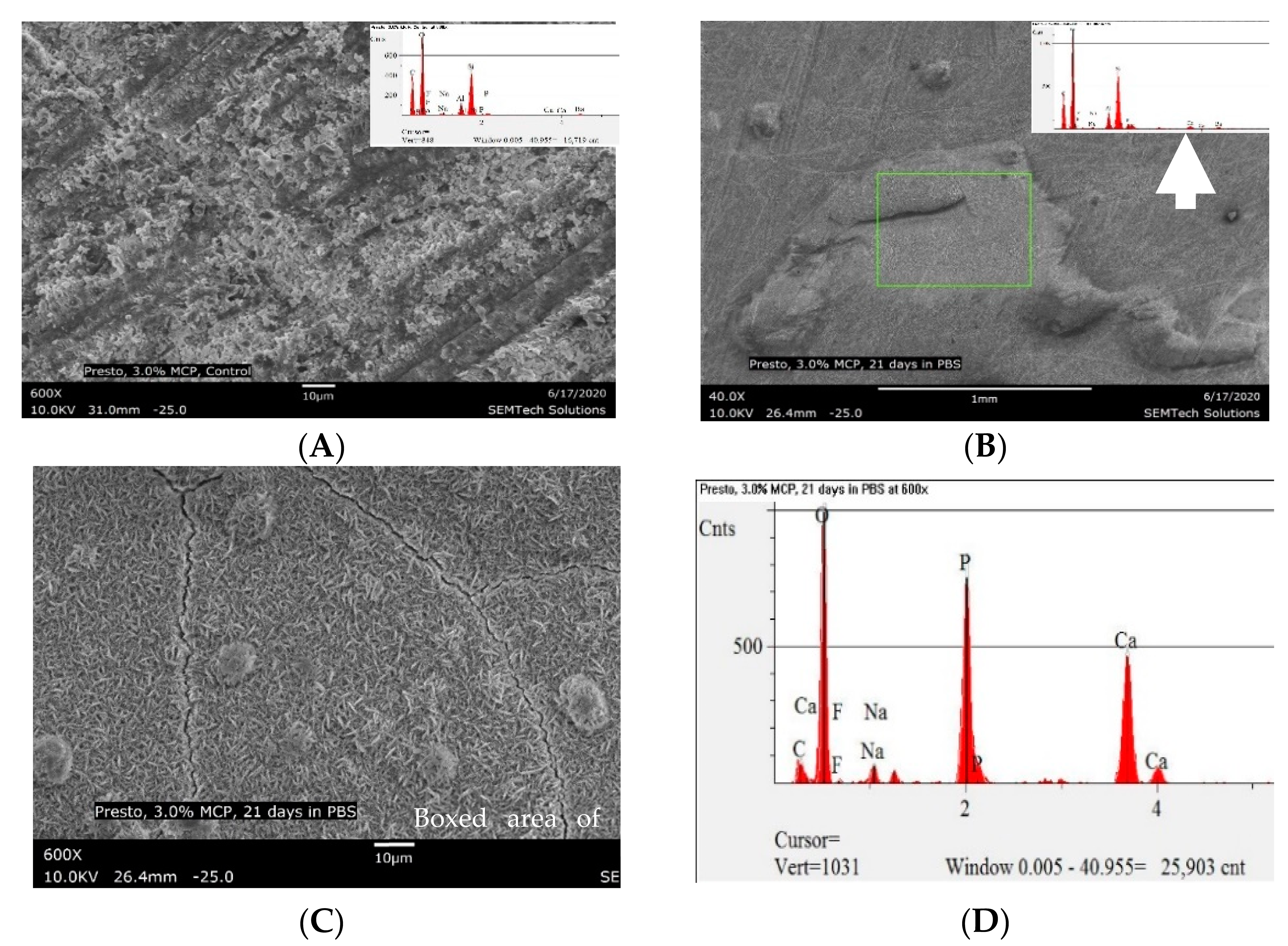
3.4. Bioactivity and Apatite Formation
4. Discussion
4.1. Methacrylated Calcium Phosphate
4.2. Physical Properties
4.3. Ion Realease and Bioactivity of Composites
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halgas, R.; Dusza, J.; Kaiferova, J.; Kovacsova, L.; Markovska, N. Nanoindentation testing of human enamel and dentin. Ceram. Silik. 2013, 57, 92–99. [Google Scholar]
- Linde, A.; Goldberg, M. Dentinogenesis. Crit. Rev. Oral Biol. Med. 1993, 4, 679–728. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Veis, A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou Neel, E.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathub, M.; Young, A.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef] [PubMed]
- White, D.J. The application of in vitro models to research on demineralization and remineralization of teeth. Adv. Dent. Res. 1995, 9, 175–193. [Google Scholar] [CrossRef]
- Featherstone, J.D.B. The continuum of dental caries- Evidence for a dynamic disease process. J. Dent. Res. 2004, 83, C39–C42. [Google Scholar] [CrossRef] [PubMed]
- Langhorst, S.E.; O’Donnell, J.N.R.; Skrtic, D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: Quantitative micro radiographic study. Dent. Mater. 2009, 25, 884–891. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.; Wang, S.; Tao, S.; Xiao, S.; Zhou, H.; Wang, P.; Cheng, L.; Zhou, X.; Weir, M.D.; Oates, T.W.; et al. Dental remineralization via Poly (amido amine) and restorative materials containing calcium phosphate nanoparticles. Int. J. Oral Sci. 2019, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.S.; Syed, M.R. A review of bioceramics-based dental restorative materials. Dent. Mater. J. 2019, 38, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V. Calcium orthophosphates in dentistry. J. Mater. Sci. Mater. Med. 2013, 24, 1335–1363. [Google Scholar] [CrossRef]
- Abedi-Amin, A.; Luzi, A.; Giovarruscio, M.; Paolone, G.; Darvizeh, A.; Agullo, V.V.; Sauro, S. Innovative root-end filling materials based on calcium-silicates and calcium-phosphates. J. Mater. Sci. Mater. Med. 2017, 28, 31. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Ohmae, H. Mechanical and biological properties of bioactive resin apatite composite resins. Biomaterials 1988, 9, 345–348. [Google Scholar] [CrossRef]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D. Amorphous Calcium Phosphate-Based Bioactive Polymeric Composites for Mineralized Tissue Regeneration. J. Res. Natl. Inst. Stand. Technol. 2003, 108, 167–182. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.N.R.; Schumacher, G.E.; Antonucci, J.M.; Skrtic, D. Structure-Composition-Property Relationships in Polymeric Amorphous Calcium Phosphate-Based Dental Composites. Materials 2009, 2, 1929–1954. [Google Scholar] [CrossRef]
- Mehdawi, I.; Neel, E.A.A.; Valappil, S.P.; Palmer, G.; Salih, V.; Pratten, J.; Spratt, D.A.; Young, A.M. Development of remineralizing antibacterial dental materials. Acta Biomater. 2009, 5, 2525–2529. [Google Scholar] [CrossRef]
- Aljabo, A.; Neel, E.A.A.; Knowles, J.C.; Young, A.M. Development of dental composites with reactive fillers that promote precipitation of antibacterial-hydroxyapatite layers. Mater. Sci. Eng. C 2016, 60, 285–292. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Zhu, X.X. Functional fillers for dental resin composites. Acta Biomater. 2021, 122, 50–67. [Google Scholar] [CrossRef]
- Santos, C.; Luklinska, Z.B.; Clarke, R.L.; Davy, K.W.M. Hydroxyapatite as a filler for dental composite materials: Mechanical properties and invitro bioactivity. J. Mater. Sci. Mater. Med. 2001, 12, 565–573. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Guedes, S.F.F.; Xu, H.H.K.; Rodrigues, L.K.A. Nanotechnology based restorative materials for dental Caries management. Trends Biotechnol. 2013, 31, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Moreau, J.L.; Sun, L.; Chow, L.C.; Xu, H.H.K. Mechanical and acid neutralizing properties and bacteria inhibition of amorphous calcium phosphate dental nanocomposite. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Natale, L.C.; Rodrigues, M.C.; Alania, Y.; Chiari, M.D.S.; Vilela, H.S.; Vieira, D.N.; Arana-Chavez, V.; Meier, M.M.; Vichi, F.M.; Braga, R.R. Development of calcium phosphate/ethylene glycol dimethacrylate particles for dental applications. J. Biomed. Mater. Res. Part B 2019, 107, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.C.; Chiari, M.D.S.; Alania, Y.; Natale, L.C.; Arana Chavez, V.E.; Meier, M.M.; Fadel, V.S.; Vichi, F.M.; Hewer, T.L.R.; Braga, R.R. Ion releasing dental restorative composites containing functionalized brushite nanoparticles for improved mechanical strength. Dent. Mater. 2018, 34, 746–755. [Google Scholar] [CrossRef]
- Chiari, M.D.S.; Rodrigues, M.C.; Pinto, M.F.C.; Vieira, D.N.; Vichi, F.M.; Vega, O.; Chrzanowski, W.; Nagoka, N.; Braga, R.R. Development of brushite particles synthesized in presence of acidic monomers for dental applications. Mater. Sci. Eng. C 2020, 116, 111178. [Google Scholar] [CrossRef]
- May, E.; Donly, K.J. Fluoride release and rerelease from a bioactive restorative material. Am. J. Dent. 2017, 30, 305. [Google Scholar]
- Saunders, K.G.; Mattevi, G.; Donly, K.J.; Anthony, R. Enamel demineralization adjacent to orthodontic brackets bonded with ACTIVA BioACTIVE-RESTORATIVE. APOS Trends Orthod. 2018, 8, 200–203. [Google Scholar] [CrossRef]
- Pameijer, C.H.; Garcia-Godoy, F.; Morrow, B.R.; Jefferies, S.R. Flexural strength and flexural fatigue properties of resin-modified glass ionomers. J. Clin. Dent. 2015, 26, 23–25. [Google Scholar]
- ElReash, A.A.; Hamama, H.; Abdo, W.; Wu, Q.; El-Din, A.Z.; Xioli, X. Biocompatibility of new bioactive resin composite versus calcium silicate cements: An animal study. BMC Oral Health 2019, 19, 194. [Google Scholar] [CrossRef] [Green Version]
- Maciak, M. Novel applications of a bioactive resin in perforations, root resorption and endodontic-periodontic lesions. Roots 2018, 4, 32. [Google Scholar]
- López-García, S.; Pecci-Lloret, M.P.; Pecci-Lloret, M.R.; Oñate-Sánchez, R.E.; García-Bernal, D.; Castelo-Baz, P.; Rodríguez-Lozano, F.J.; Guerrero-Gironés, J. In Vitro evaluation of the biological effects of Activa Kids bioactive restorative, Ionolux and Riva Light cure on Human dental pulpal stem cells. Materials 2019, 12, 3694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skaria, S.; Berk, K. Stabilized Calcium Phosphate and Methods of Forming Same. U.S. Patent 10,219,986, 5 March 2019. [Google Scholar]
- Skaria, S.; Berk, K. Radically Curable Urethane Dimethacrylates and Compositions Thereof for Tougher Dental Prosthetics. U.S. Patent 8,292,625, 23 October 2012. [Google Scholar]
- Johnston, W.M. Review of translucency determinations and applications to dental materials. J. Esthet. Restor. Dent. 2014, 26, 217–223. [Google Scholar] [CrossRef]
- Wang, X.; Huyang, G.; Palagummi, S.; Liu, X.; Skrtic, D.; Beauchamp, C.; Bowen, R.; Sun, J. High performance dental resin composites with hydrolytically stable monomers. Dent. Mater. 2018, 34, 248–257. [Google Scholar] [CrossRef]
- ISO Standard. ISO Standard 200. ISO 4049 Polymer based filling, restorative and luting materials. In International Organization for Standards, 3rd ed.; ISO Standard 200: Geneva, Switzerland, 2000; pp. 1–27. [Google Scholar]
- Raole, V.; Mashru, R. Quantification of Anions and Cations in Restorative Biochemic tissue salts. Public Health Prev. Med. 2018, 4, 129–147. [Google Scholar]
- ISO 23317. 2014 Implants for surgery. In In Vitro Evaluation for Apatite-Forming Ability of Implant Materials; ISO: Geneva, Switzerland, 2014. [Google Scholar]
- Ramli, R.A.; Adnan, R.; Abu Bakar, M.; Masudi, S.M. Synthesis and characterization of pure nanoporous hydroxyapatite. J. Phys. Sci. 2011, 22, 25–37. [Google Scholar]
- Homaeigohar, S.; Tsai, T.Y.; Zaire, E.S.; Elbahri, M.; Young, T.H.; Boccaccini, A.R. Bovine Serum Albumin (BSA)/polyacrylonitrile (PAN) biohybrid nanofibers coated with a biomineralized calcium deficient hydroxyapatite (HA) shell for wound dressing. Mater. Sci. Eng. C 2020, 16, 111248. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; Liu, D.W.; Skrtic, D. Amorphous calcium phosphate based composites: Effect of surfactants and poly(ethylene oxide) on filler and composite properties. J. Dispers. Sci. Technol. 2007, 28, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Self setting calcium orthophosphate formulations and their biomedical applications. Adv. Nano-Bio Mater. Dev. 2019, 3, 321–421. [Google Scholar]
- Properties of Activa Prestotm. White Pages. Available online: www.pulpdent.com (accessed on 20 February 2020).
- Ferracane, J.L.; Greener, W.H. The effect of resin formulations on the degree of conversion and mechanical properties of dental restorative resins. J. Biomed. Mater. Res. 1986, 20, 121–131. [Google Scholar] [CrossRef]
- Salgado, V.E.; Albuquerque, P.P.A.C.; Cavalcantec, L.M.; Pfeifer, C.S.; Moraes, R.R.; Schneider, L.P.J. Influence of photoinitiator system and nanofiller size on the optical properties and cure efficiency of model composites. Dent. Mater. 2014, 30, e264–e271. [Google Scholar] [CrossRef]
- Lee, Y.K. Translucency of human teeth and dental restorative materials and its clinical relevance. J. Biomed. Opt. 2015, 20, 0450016. [Google Scholar] [CrossRef]
- Salgado, V.E.; Rego, C.F.; Schneider, L.P.J.; Moraes, R.R.; Cavalcantec, L.M. Does translucency influence cure efficiency and color stability of resin-based composites. Dent. Mater. 2018, 34, 956–967. [Google Scholar] [CrossRef]
- Shortall, A.C.; Palin, W.M.; Burtscher, P. Refractive index mismatch and monomer reactivity influence composite curing depth. J. Dent. Res. 2008, 87, 84–88. [Google Scholar] [CrossRef]
- da Silva, E.M.; Poskus, L.T.; Guimaraes, J.G.A. Influence of Light-polymerization Modes on the Degree of Conversion and Mechanical Properties of Resin Composites: A Comparative Analysis between a Hybrid and a Nanofilled Composite. Oper. Dent. 2008, 33, 287–293. [Google Scholar] [CrossRef]
- Yap, A.U.J.; Pandya, M.; Toh, W.S. Depth of cure of contemporary bulk-fill resin-based composites. Dent. Mater. J. 2016, 35, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arola, D. Fatigue testing of biomaterials and their interfaces. Dent. Mater. 2017, 23, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Skrtic, D.; Antonnuci, J.M.; Eanes, E.D. Effect of the monomer and filler systems on the remineralizing potential of bioactive dental composites based on amorphous calcium phosphate. Polym. Adv. Technol. 2001, 12, 369–377. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hench, L. Bioactive Glass: Chronology, Characterization, and Genetic Control of Tissue Generation in Advances in Calcium Phosphate Biomaterials; Springer Series in Biomaterials Science and Engineering-2; Ben-Nissan, B., Ed.; Springer: New York, NY, USA, 2014; pp. 51–70. [Google Scholar]
- Cao, C.Y.; Mei, M.L.; Li, Q.; Lo, E.C.M.; Chu, C.H. Methods for biomimetic remineralization of human dentine: A systemic Review. Int. J. Mol. Sci. 2015, 16, 4615–4627. [Google Scholar] [CrossRef]
- He, L.; Hao, Y.; Li, Z.; Liu, H.; Shao, M.; Xu, X.; Liang, K.; Gao, Y.; Yuan, H.; Li, J.; et al. Biomineralization of dentin. J. Struct. Biol. 2019, 207, 115–122. [Google Scholar] [CrossRef]
- Gluseren, G.; Tansik, G.; Garifullin, R. Dentin phosphoprotein mimetic peptide nanofibers promote biomineralization. Macromol. Biosci. 2019, 19, 1800080. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, W.; Pashley, D.H.; Breschi, L.; Mao, J.; Chen, J.; Tay, F.R. Biomimetic remineralization of dentin. Dent. Mater. 2014, 30, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Veis, A.; Dorvee, J.R. Biomineralization mechanisms: A new paradigm for crystal nucleation in organic matrices. Calcif. Tissue Int. 2013, 93, 307–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, W.; Jiang, S.; Pan, H.; Tang, R. Amorphous phase mediated crystallization: Fundamentals of biomineralization. Crystals 2018, 8, 48. [Google Scholar] [CrossRef] [Green Version]
| Sample | TFC% | Resin Matrix % | MCP% | CQ% |
|---|---|---|---|---|
| Control Comp, 0% MCP (CS-0) | 69.5 | 30.0 | 0.0 | 0.08 |
| Comp + 3 wt.% MCP (CS-3) | 69.5 | 30.0 | 3.0 | 0.08 |
| Comp + 6 wt.% MCP (CS-6) | 70.4 | 29.0 | 6.0 | 0.08 |
| Comp + 20 wt.% MCP | 74.4 | 25.5 | 20.0 | 0.08 |
| Sample | Degree of Polymerization % | Translucency Parameter (TP) | Depth of Cure (mm) | Flexural Strength (MPa) | Deflection at Break (mm) |
|---|---|---|---|---|---|
| CS-0 | 69 (1.3) | 8.4 (0.26) | 2.62 (0.01) | 98.2 (2.8) | 0.58 (0.02) |
| CS-3 | 69 (2.2) | 8.6 (0.18) | 2.65 (0.01) | 106 (3.2) | 0.74 (0.03) |
| CS-6 | 64 (2.8) | 8.4 (0.24) | 2.58 (0.02) | 99.4 (2.9) | 0.75 (0.03) |
| CS-20 | 58 (3.2) | 5.5 (0.38) | 2.48 (0.02) | 92.6 (3.8) | 0.72 (0.02) |
| Material | Calcium (Ca) % | Phosphorous (P) % | Ca:P Ratio |
|---|---|---|---|
| 3 wt.% MCP (CS-3) before immersion in PBS (Figure 6A) | 0.73 | 0.65 | 1.12 |
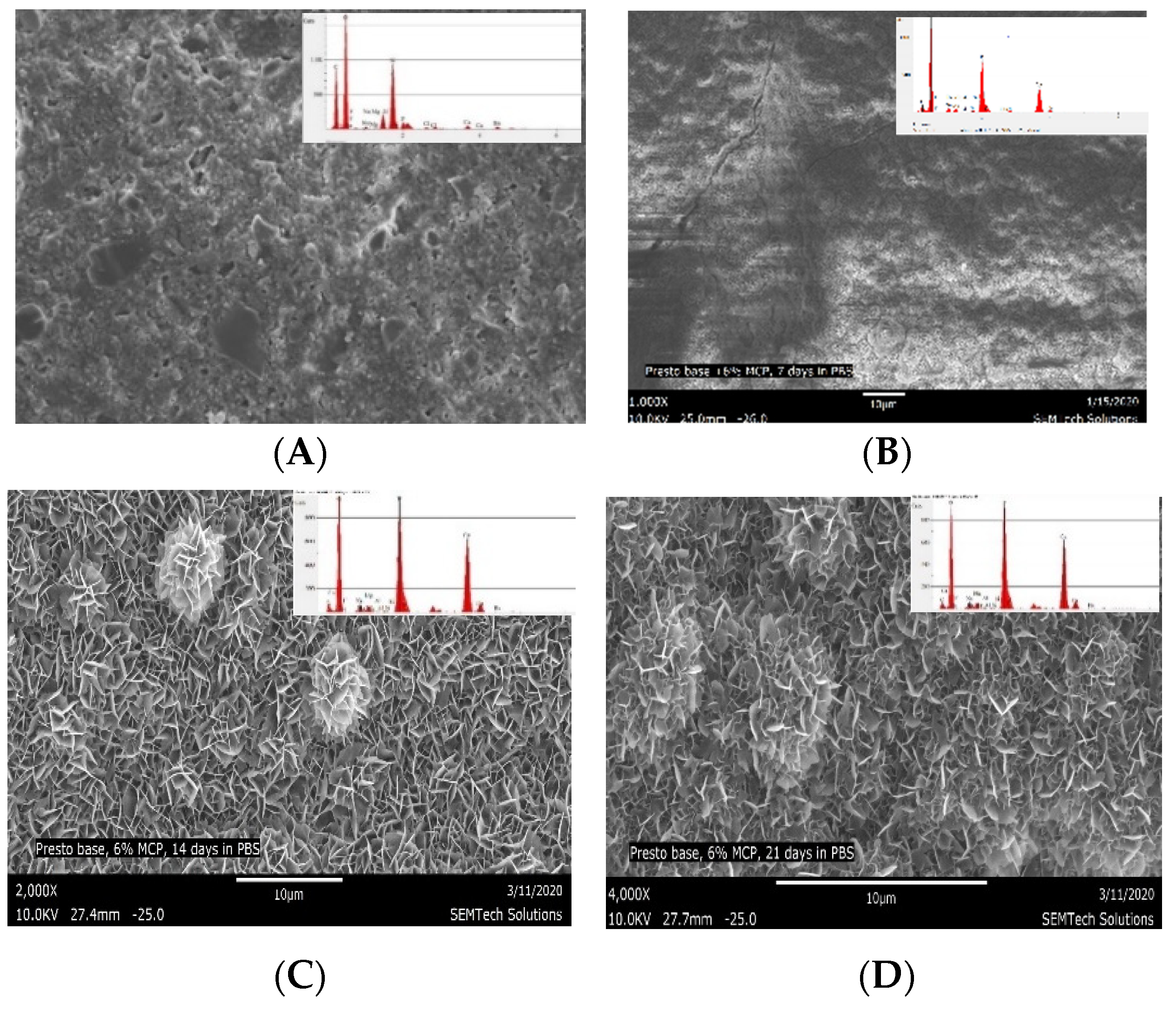
| CS-3 after immersion in PBS for 21 days. Light area indicates extensive CaP precipitation (See Figure 6B–D) | 31.26 | 19.42 | 1.61 |
| CS-3 after immersion in PBS for 21 days. Dark area indicates mild CaP precipitation (See Figure 6B) | 2.75 | 2.22 | 1.24 |
| Material | Calcium % | Phosphorous % | Ca/P Ratio |
|---|---|---|---|
| Composite with 6% MCP (CS-6) control (not immersed in PBS) (6000×) | 2.60 | 2.50 | 1.03 |
| CS-6 after 7 days immersion in PBS (6000×) | 29.81 | 21.79 | 1.37 |
| CS-6 after 14 days immersion in PBS (6000×) | 31.64 | 20.39 | 1.55 |
| CS-6 after 21 days immersion in PBS (6000×) | 30.52 | 19.63 | 1.56 |
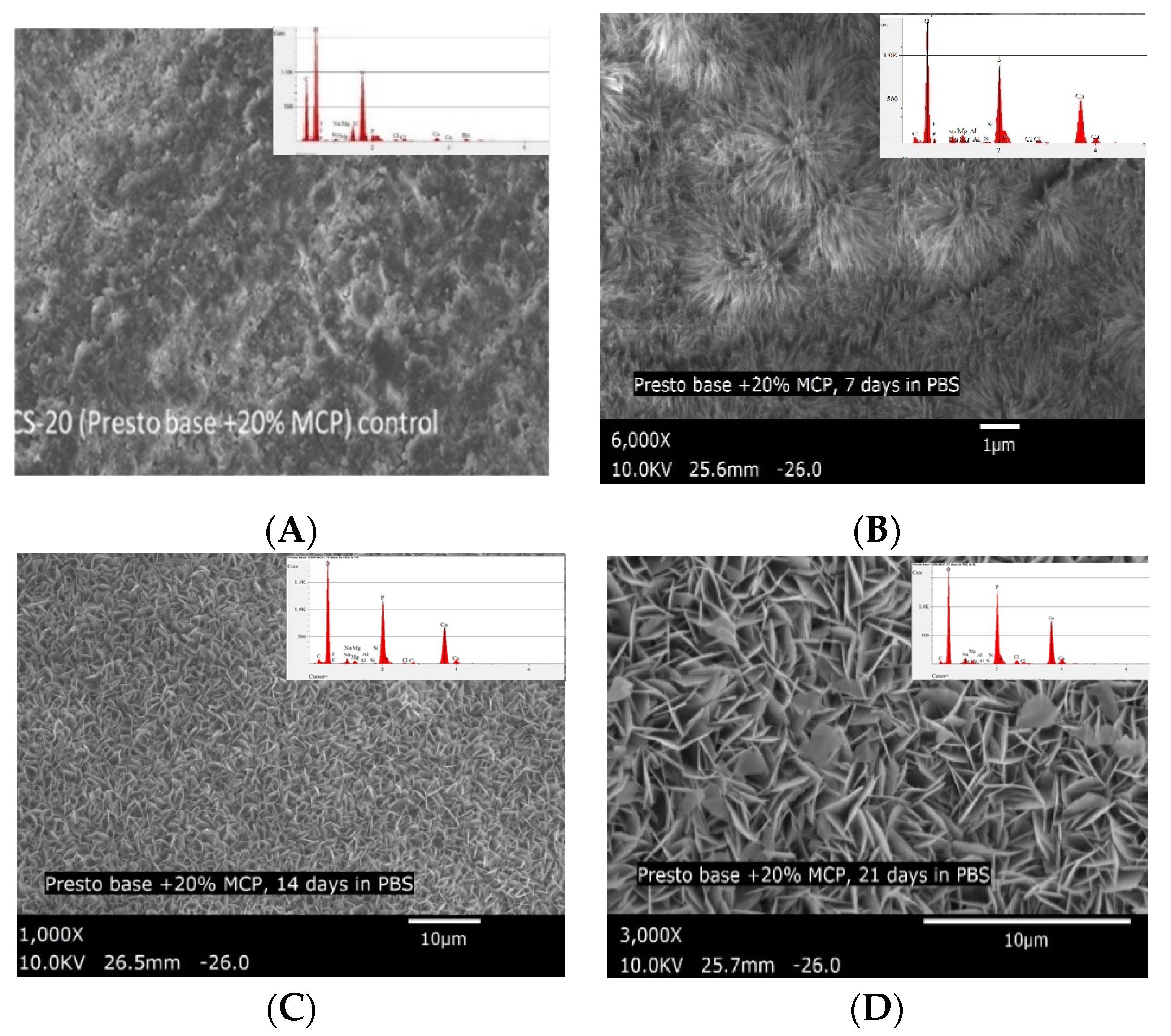
| Composite with 20% MCP (CS-20) control (not immersed in PBS) (6000×) | 8.40 | 6.90 | 1.22 |
| CS-20 after 7 days immersion in PBS (6000×) | 27.07 | 20.25 | 1.34 |
| CS-20 after 14 days immersion in PBS (6000×) | 30.12 | 19.62 | 1.54 |
| CS-20 after 21 days immersion in PBS (6000×) | 32.49 | 20.31 | 1.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skaria, S.; Berk, K.J. Experimental Dental Composites Containing a Novel Methacrylate-Functionalized Calcium Phosphate Component: Evaluation of Bioactivity and Physical Properties. Polymers 2021, 13, 2095. https://doi.org/10.3390/polym13132095
Skaria S, Berk KJ. Experimental Dental Composites Containing a Novel Methacrylate-Functionalized Calcium Phosphate Component: Evaluation of Bioactivity and Physical Properties. Polymers. 2021; 13(13):2095. https://doi.org/10.3390/polym13132095
Chicago/Turabian StyleSkaria, Sunny, and Kenneth J. Berk. 2021. "Experimental Dental Composites Containing a Novel Methacrylate-Functionalized Calcium Phosphate Component: Evaluation of Bioactivity and Physical Properties" Polymers 13, no. 13: 2095. https://doi.org/10.3390/polym13132095
APA StyleSkaria, S., & Berk, K. J. (2021). Experimental Dental Composites Containing a Novel Methacrylate-Functionalized Calcium Phosphate Component: Evaluation of Bioactivity and Physical Properties. Polymers, 13(13), 2095. https://doi.org/10.3390/polym13132095