Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems
Abstract
:1. Introduction
2. Thermo-Responsive Polysaccharides and Their Drug Delivery Applications
3. Thermo-Responsive Proteins/Polypeptides and Their Drug Delivery Applications
4. Future Prospects of Thermo-Responsive Biopolymers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 43, 3–12. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymers 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications—A review. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Chang, R.; Wang, X.; Li, X.; An, H.; Qin, J. Self-activated healing hydrogels with reversible temperature responsiveness. ACS Appl. Mater. Interfaces 2016, 8, 25544–25551. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, C.L.; Kan, C.W. Thermoresponsive hydrogels and their biomedical applications: Special insight into their applications in textile based transdermal therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, K.J.; Mikos, A.G. Biodegradable thermoresponsive polymers: Applications in drug delivery and tissue engineering. Polymers 2020, 211, 123063. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Castro-Alpízar, J.; Lopretti-Correa, M.; Vega-Baudrit, J. Exploration of Bioengineered Scaffolds Composed of Thermo-Responsive Polymers for Drug Delivery in Wound Healing. Int. J. Mol. Sci. 2021, 22, 1408. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, C.L. Review of stimuli-responsive polymers in drug delivery and textile application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasparakis, G.; Tsitsilianis, C. LCST polymers: Thermoresponsive nanostructured assemblies towards bioapplications. Polymers 2020, 211, 123146. [Google Scholar] [CrossRef]
- Niskanen, J.; Tenhu, H. How to manipulate the upper critical solution temperature (UCST)? Polym. Chem. 2017, 8, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Cook, M.T.; Haddow, P.; Kirton, S.B.; McAuley, W.J. Polymers Exhibiting Lower Critical Solution Temperatures as a Route to Thermoreversible Gelators for Healthcare. Adv. Funct. Mater. 2021, 31, 2008123. [Google Scholar] [CrossRef]
- Op ’t Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F.A.D.T.G. Dressings: Strategic and molecular advances. Tissue Eng. Part B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Sarwan, T.; Kumar, P.; Choonara, Y.E.; Pillay, V. Hybrid Thermo-Responsive Polymer Systems and Their Biomedical Applications. Front. Mater. 2020, 7, 73. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Sun, H.; Chen, S. Assessment of release kinetics, stability and polymer interaction of poloxamer 407-based thermosensitive gel of interleukin-1 receptor antagonist. Pharm. Dev. Technol. 2014, 19, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, C.L.; Kan, C.W.; Wang, W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 11658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coughlan, D.; Corrigan, O. Drug–polymer interactions and their effect on thermoresponsive poly(N-isopropylacrylamide) drug delivery systems. Int. J. Pharm. 2006, 313, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, S.J.; Gorelov, A.V.; Rochev, Y.A.; McGillicuddy, F.; Dawson, K.A.; Gallagher, W.M.; Keenan, A.K. Extended delivery of the antimitotic agent colchicine from thermoresponsive N-isopropylacrylamide-based copolymer films to human vascular smooth muscle cells. J. Biomed. Mater. Res. 2003, 67, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, S.; Rinoldi, C.; Nakielski, P.; Ziai, Y.; Urbanek, O.; Li, X.; Kowalewski, T.A.; Ding, B.; Pierini, F. Ultraviolet light-assisted electrospinning of core-shell fully cross-lnked P(NIPAAm-co-NIPMAAm) hydrogel-based nanofibers for thermally induced drug delivery self-regulation. Avd. Mater. Interfaces 2020, 7, 2000247. [Google Scholar] [CrossRef]
- Gan, J.; Guan, X.X.; Zheng, J.; Guo, H.; Wu, K.; Liang, L.; Lu, M. Biodegradable, thermoresponsive PNIPAM-based hydrogel scaffolds for the sustained release of levofloxacin. RSC Adv. 2016, 6, 32967–32978. [Google Scholar] [CrossRef]
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef]
- Safakas, K.; Saravanou, S.-F.; Iatridi, Z.; Tsitsilianis, C. Alginate-g-PNIPAM-Based Thermo/Shear-Responsive Injectable Hydrogels: Tailoring the Rheological Properties by Adjusting the LCST of the Grafting Chains. Int. J. Mol. Sci. 2021, 22, 3824. [Google Scholar] [CrossRef]
- Alexandridis, P.; Athanassiou, V.; Hatton, T.A. Pluronic-P105 PEO-PPO-PEO Block Copolymer in Aqueous Urea Solutions: Micelle Formation, Structure, and Microenvironment. Langmuir 1995, 11, 2442–2450. [Google Scholar] [CrossRef]
- Wang, W.; Wat, E.; Hui, C.-L.; Chan, B.; Ng, F.S.F.; Kan, C.-W.; Wang, X.; Hu, H.; Wong, E.C.W.; Lau, C.; et al. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci. Rep. 2016, 6, 24112. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-J.; Wang, T.-P.; Lue, S.-I.; Wang, L.-F. Pentablock copolymers of pluronic F127 and modified poly(2-dimethyl amino)ethyl methacrylate for internalization mechanism and gene transfection studies. Int. J. Nanomed. 2013, 8, 2011–2027. [Google Scholar]
- Liu, Y.; Fu, S.; Lin, L.; Cao, Y.; Xie, X.; Yu, H.; Chen, M.; Li, H. Redox-sensitive Pluronic F127-tocopherol micelles: Synthesis, characterization, and cytotoxicity evaluation. Int. J. Nanomed. 2017, 12, 2635–2644. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Hui, P.C.-L.; Wat, E.; Kan, C.-W.; Leung, P.-C.; Wang, W. Drug delivery system of dual-responsive PF127 hydrogel with polysaccharide-based nano-conjugate for textile-based transdermal therapy. Carbohydr. Polym. 2020, 236, 116074. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, C.L.; Siu, W.S.; Kan, C.W.; Leung, P.-C.; Wanxue, C.; Chiou, J.-C. Influence of pH-responsive compounds synthesized from chitosan and hyaluronic acid on dual-responsive (pH/temperature) hydrogel drug delivery systems of Cortex Moutan. Int. J. Biol. Macromol. 2021, 168, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Altomare, L.; Bonetti, L.; Campiglio, C.E.; De Nardo, L.; Draghi, L.; Tana, F.; Farè, S. Biopolymer-based strategies in the design of smart medical devices and artificial organs. Int. J. Artif. Organs 2018, 41, 337–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y. Solvent isotope effect on sol–gel transition of methylcellulose studied by DSC. Polym. Bull. 2014, 71, 1441–1448. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, W.; Liu, H.; Xu, H.; Zhong, Y.; Zhang, L.; Chen, Z.; Sui, X.; Mao, Z. Aggregation behaviors of thermo-responsive methylcellulose in water: A molecular dynamics simulation study. J. Mol. Graph. Model. 2020, 97, 107554. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, L.; De Nardo, L.; Fare’, S. Thermo-Responsive Methylcellulose Hydrogels: From Design to Applications as Smart Biomaterials. Tissue Eng. Part B Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Aprodu, A.; Mantaj, J.; Raimi-Abraham, B.; Vllasaliu, D. Evaluation of a Methylcellulose and Hyaluronic Acid Hydrogel as a Vehicle for Rectal Delivery of Biologics. Pharmer 2019, 11, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskens, O.; Villani, G.; Amin, S. Rheological Investigation of Thermoresponsive Alginate-Methylcellulose Gels for Epidermal Growth Factor Formulation. Cosmetics 2020, 8, 3. [Google Scholar] [CrossRef]
- Shimoyama, T.; Uraki, M.; Takahashi, A.; Kobayashi MTakahata, M.; Makino, Y.; Itoh, K.; Kobayashi, M. Effect of drug on physical characteristics of oral methylcellulose/alginate formulation. J. Pharm. Sci. Technol. Japan. 2014, 74, 73–83. [Google Scholar]
- Bhowmik, M.; Bain, M.K.; Ghosh, L.K.; Chattopadhyay, D. Effect of salts on gelation and drug release profiles of methylcellulose-based ophthalmic thermo-reversible in situ gels. Pharm. Dev. Technol. 2010, 16, 385–391. [Google Scholar] [CrossRef]
- Payne, C.; Dolan, E.; O’Sullivan, J.; Cryan, S.-A.; Kelly, H.M. A methylcellulose and collagen based temperature responsive hydrogel promotes encapsulated stem cell viability and proliferation in vitro. Drug Deliv. Transl. Res. 2017, 7, 132–146. [Google Scholar] [CrossRef]
- Tomsic, M.; Prossnigg, F.; Glatter, O. A thermoreversible double gel: Characterization of a methylcellulose and kappa-carrageenan mixed system in water by SAXS, DSC and rheology. J. Colloid Interface Sci. 2008, 322, 41–50. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Li, Y.; Lei, M.; Du, Y.; Kennedy, J.F.; Knill, C.J. Production and characterisation of novel injectable chitosan/methylcellulose/salt blend hydrogels with potential application as tissue engineering scaffolds. Carbohydr. Polym. 2010, 82, 833–841. [Google Scholar] [CrossRef]
- Contessi, N.; Altomare, L.; Filipponi, A.; Farè, S. Thermo-responsive properties of methylcellulose hydrogels for cell sheet engineering. Mater. Lett. 2017, 207, 157–160. [Google Scholar] [CrossRef]
- Brun-Graeppi, A.K.A.S.; Richard, C.; Bessodes, M.; Scherman, D.; Narita, T.; Ducouret, G.; Merten, O.-W. Study on the sol–gel transition of xyloglucan hydrogels. Carbohydr. Polym. 2010, 80, 555–562. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Tyagi, V.; Lohiya, G.; Nerkar, P. Thermally reversible xyloglucan gels as vehicles for nasal drug delivery. Drug Deliv. 2012, 19, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Talantikite, M.; Stimpson, T.C.; Gourlay, A.; Le-Gall, S.; Moreau, C.; Cranston, E.D.; Moran-Mirabal, J.M.; Cathala, B. Bioinspired Thermoresponsive Xyloglucan–Cellulose Nanocrystal Hydrogels. Biomacromolecules 2021, 22, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Suisha, F.; Kawasaki, N.; Miyazaki, S.; Shirakawa, M.; Yamatoya, K.; Sasaki, M.; Attwood, D. Xyloglucan gels as sustained release vehicles for the intraperitoneal administration of mitomycin C. Int. J. Pharm. 1998, 172, 27–32. [Google Scholar] [CrossRef]
- Kawasaki, N.; Ohkura, R.; Miyazaki, S.; Uno, Y.; Sugimoto, S.; Attwood, D. Thermally reversible xyloglucan gels as vehicles for oral drug delivery. Int. J. Pharm. 1999, 181, 227–234. [Google Scholar] [CrossRef]
- Miyazaki, S.; Suzuki, S.; Kawasaki, N.; Endo, K.; Takahashi, A.; Attwood, D. In situ gelling xyloglucan formulations for sustained release ocular delivery of pilocarpine hydrochloride. Int. J. Pharm. 2001, 229, 29–36. [Google Scholar] [CrossRef]
- Dalvi, A.V.; Ravi, P.R.; Uppuluri, C.T.; Mahajan, R.R.; Katke, S.V.; Deshpande, V.S. Thermosensitive nasal in situ gelling systems of rufinamide formulated using modified tamarind seed xyloglucan for direct nose-to-brain delivery: Design, physical characterization, and in vivo evaluation. J. Pharm. Investig. 2021, 51, 199–211. [Google Scholar] [CrossRef]
- Nisbet, D.R.; Rodda, A.E.; Horne, M.K.; Forsythe, J.S.; Finkelstein, D.I. Implantation of functionalized thermally gelling xyloglucan hydrogel within the brain: Associated neurite infiltration and inflammatory response. Tissue Eng. Part A 2020, 16, 2833–2842. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Bruggeman, K.F.; Kauhausen, J.A.; Rodriguez, A.L.; Nisbet, D.R.; Parish, C.L. Functionalized composite scaffolds improve the engraftment of transplanted dopaminergic progenitors in a mouse model of Parkinson’s disease. Biomaterials 2016, 74, 89–98. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Cheng, S.; Pozzoli, M.; Messerotti, E.; Traini, D.; Young, P.; Kourmatzis, A.; Ong, H.X. Smart thermosensitive chitosan hydrogel for nasal delivery of ibuprofen to treat neurological disorders. Expert Opin. Drug Deliv. 2019, 16, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, A.; Farooq, M.A.; Parveen, A. Thermosensitive Chitosan-Based Injectable Hydrogel as an Efficient Anticancer Drug Carrier. ACS Omega 2020, 5, 20450–20460. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Chen, X.G.; Kong, M.; Liu, C.S.; Cha, D.S.; Kennedy, J.F. Effect of molecular weight and degree of chitosan deacetylation on the preparation and characteristics of chitosan thermosensitive hydrogel as a delivery system. Carbohydr. Polym. 2008, 73, 265–273. [Google Scholar] [CrossRef]
- Hastings, C.L.; Kelly, H.M.; Murphy, M.J.; Barry, F.P.; O’Brien, F.J.; Duffy, G.P. Development of a thermoresponsive chitosan gel combined with human mesenchymal stem cells and desferrioxamine as a multimodal pro-angiogenic therapeutic for the treatment of critical limb ischaemia. J. Control. Release 2012, 161, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Wei, W.; Wang, L.-Y.; Su, Z.-G.; Ma, G.-H. A thermosensitive hydrogel based on quaternized chitosan and poly(ethylene glycol) for nasal drug delivery system. Biomaterials 2007, 28, 2220–2232. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Lin, Y.-C.; Chen, C.-Y.; Hsieh, Y.-Y.; Liaw, C.-K.; Huang, S.-W.; Tsuang, Y.-H.; Chen, C.-H.; Lin, F.-H. Thermosensitive Chitosan–Gelatin–Glycerol Phosphate Hydrogels as Collagenase Carrier for Tendon–Bone Healing in a Rabbit Model. Polymers 2020, 12, 436. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Xiao, L.; Du, Y. Preparation and properties of a novel thermosensitive N-trimethyl chitosan hydrogel. Polym. Bull. 2009, 63, 531–545. [Google Scholar] [CrossRef]
- Sá-Lima, H.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Stimuli-responsive chitosan-starch injectable hydrogels combined with encapsulated adipose-derived stromal cells for articular cartilage regeneration. Soft Matter 2010, 6, 5184–5195. [Google Scholar] [CrossRef] [Green Version]
- Crompton, K.E.; Goud, J.D.; Bellamkonda, R.V.; Gengenbach, T.R.; Finkelstein, D.I.; Horne, M.K.; Forsythe, J.S. Polylysine-functionalised thermoresponsive chitosan hydrogel for neural tissue engineering. Biomaterials 2007, 28, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liang, Y.; Zhang, D.; Sun, X.; Liang, L.; Li, J.; Liu, Y.-N. Gelatin-Based Hydrogels Blended with Gellan as an Injectable Wound Dressing. ACS Omega 2018, 3, 4766–4775. [Google Scholar] [CrossRef] [Green Version]
- te Nijenhuis, K. On the nature of crosslinks in thermoreversible gels. Polym. Bull. 2007, 58, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.-H.; Ko, Y.-C.; Chang, Y.-F.; Huang, S.-H.; Liu, C.J.-L. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Chavez, E.; Tsai, K.-L.; Yang, K.-C.; Kuo, W.-T.; Yang, Y.-P.; Chiou, S.-H.; Lin, F.-H. Effects of thermosensitive chitosan-gelatin based hydrogel containing glutathione on Cisd2-deficient chondrocytes under oxidative stress. Carbohydr. Polym. 2017, 173, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Yang, S.-H.; Su, W.-Y.; Chen, Y.-C.; Yang, K.-C.; Cheng, W.T.-K.; Wu, S.-C.; Lin, F.-H. Thermosensitive Chitosan–Gelatin–Glycerol Phosphate Hydrogels as a Cell Carrier for Nucleus Pulposus Regeneration: An In Vitro Study. Tissue Eng. Part A 2010, 16, 695–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Zhao, L.; Cong, M.; Liu, L.; Yan, G.; Li, Z.; Li, B.; Yu, W.; Sun, H.; Yang, B. Injectable thermosensitive chitosan/gelatin-based hydrogel carried erythropoietin to effectively enhance maxillary sinus floor augmentation in vivo. Dent. Mater. 2020, 36, e229–e240. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Zhao, Y.-M.; Sun, H.-H.; Jin, T.; Wang, Q.-T.; Zhou, W.; Wu, Z.-F.; Jin, Y. Novel glycidyl methacrylated dextran (Dex-GMA)/gelatin hydrogel scaffolds containing microspheres loaded with bone morphogenetic proteins: Formulation and characteristics. J. Control. Release 2007, 118, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Roehm, K.D.; Madihally, S.V. Bioprinted chitosan-gelatin thermosensitive hydrogels using an inexpensive 3D printer. Biofabrication 2017, 10, 015002. [Google Scholar] [CrossRef] [PubMed]
- Martín, L.; Castro, E.; Ribeiro, A.; Alonso, M.; Rodríguez-Cabello, J.C. Temperature-Triggered Self-Assembly of Elastin-Like Block Co-Recombinamers: The Controlled Formation of Micelles and Vesicles in an Aqueous Medium. Biomacromolecules 2012, 13, 293–298. [Google Scholar] [CrossRef]
- Le, D.C.T.; Suguwara-Narutaki, A. Elastin-like polypeptides as building motifs toward designing functional nanobiomaterials. Mol. Syst. Des. Eng. 2019, 4, 545–565. [Google Scholar] [CrossRef]
- Luo, T.; David, M.A.; Dunshee, L.C.; Scott, R.A.; Urello, M.A.; Price, C.; Kiick, K.L. Thermoresponsive Elastin-b-Collagen-Like Peptide Bioconjugate Nanovesicles for Targeted Drug Delivery to Collagen-Containing Matrices. Biomacromolecules 2017, 18, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.S.; Raucher, D. Elastin-like polypeptide for improved drug delivery for anticancer therapy: Preclinical studies and future applications. Expert Opin. Drug Deliv. 2015, 12, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Li, H. In situ phase transition of elastin-like polypeptide chains regulates thermoresponsive properties of elastomeric protein-based hydrogels. Biomacromolecules 2020, 21, 2258–2267. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chinoy, Z.S.; Pecastaings, G.; Bathany, K.; Garanger, E.; Lecommandoux, S. Design of Polysaccharide-b-Elastin-Like Polypeptide Bioconjugates and Their Thermoresponsive Self-Assembly. Biomacromolecules 2020, 21, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hu, H.; Yang, Z.; He, L.; Kong, Y.; Fei, B.; Xin, J.H. Smart hydrogel-functionalized textile system with moisture management property for skin application. Smart Mater. Struct. 2014, 23, 125027. [Google Scholar] [CrossRef]
- Massella, D.; Argenziano, M.; Ferri, A.; Guan, J.; Giraud, S.; Cavalli, R.; Barresi, A.A.; Salaün, F. Bio-Functional Textiles: Combining Pharmaceutical Nanocarriers with Fibrous Materials for Innovative Dermatological Therapies. Pharmaceutics 2019, 11, 403. [Google Scholar] [CrossRef] [Green Version]
- Massella, D.; Ancona, A.; Garino, N.; Cauda, V.; Guan, J.; Salaun, F.; Barresi, A.A.; Ferri, A. Preparation of bio-functional textiles by surface functionalization of cellulose fabrics with caffeine loaded nanoparticles. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 460, p. 012044. [Google Scholar]
- Massella, D.; Leone, F.; Peila, R.; Barresi, A.A.; Ferri, A. Functionalization of Cotton Fabrics with Polycaprolactone Nanoparticles for Transdermal Release of Melatonin. J. Funct. Biomater. 2017, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Yoo, K.H.; Kwon, T.-R.; Oh, C.T.; Ko, K.C.; No, Y.H.; Oh, W.J.; Kim, B.J. Improvement of a slimming cream’s efficacy using a novel fabric as a transdermal drug delivery system: An in vivo and in vitro study. Exp. Ther. Med. 2020, 19, 3282–3288. [Google Scholar] [CrossRef] [Green Version]
- Atanasova, D.; Staneva, D.; Grabchev, I. Textile Materials Modified with Stimuli-Responsive Drug Carrier for Skin Topical and Transdermal Delivery. Materials 2021, 14, 930. [Google Scholar] [CrossRef]
- Petrusic, S.; Jovancic, P.; Lewandowski, M.; Giraud, S.; Grujic, S.; Ostojic, S.; Bugarski, B.; Koncar, V. Properties and drug release profile of poly(N-isopropylacrylamide) microgels functionalized with maleic anhydride and alginate. J. Mater. Sci. 2013, 48, 7935–7948. [Google Scholar] [CrossRef]
- Csóka, G.; Gelencsér, A.; Makó, A.; Marton, S.; Zelkó, R.; Klebovich, I.; Antal, I. Potential application of Metolose in a thermoresponsive transdermal therapeutic system. Int. J. Pharm. 2007, 338, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, G.; Nichifor, M.; Mihai, D.; Oproiu, L. Bioactive cotton fabrics containing chitosan and biologically active substances extracted from plants. Mater. Sci. Eng. C 2013, 33, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knipe, M.; Peppas, N.A. Multi-responsive hydrogels for drug delivery and tissue engineering. Regen. Biomater. 2014, 1, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathi, M.; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Biol. Macromol. 2019, 128, 957–964. [Google Scholar] [CrossRef]
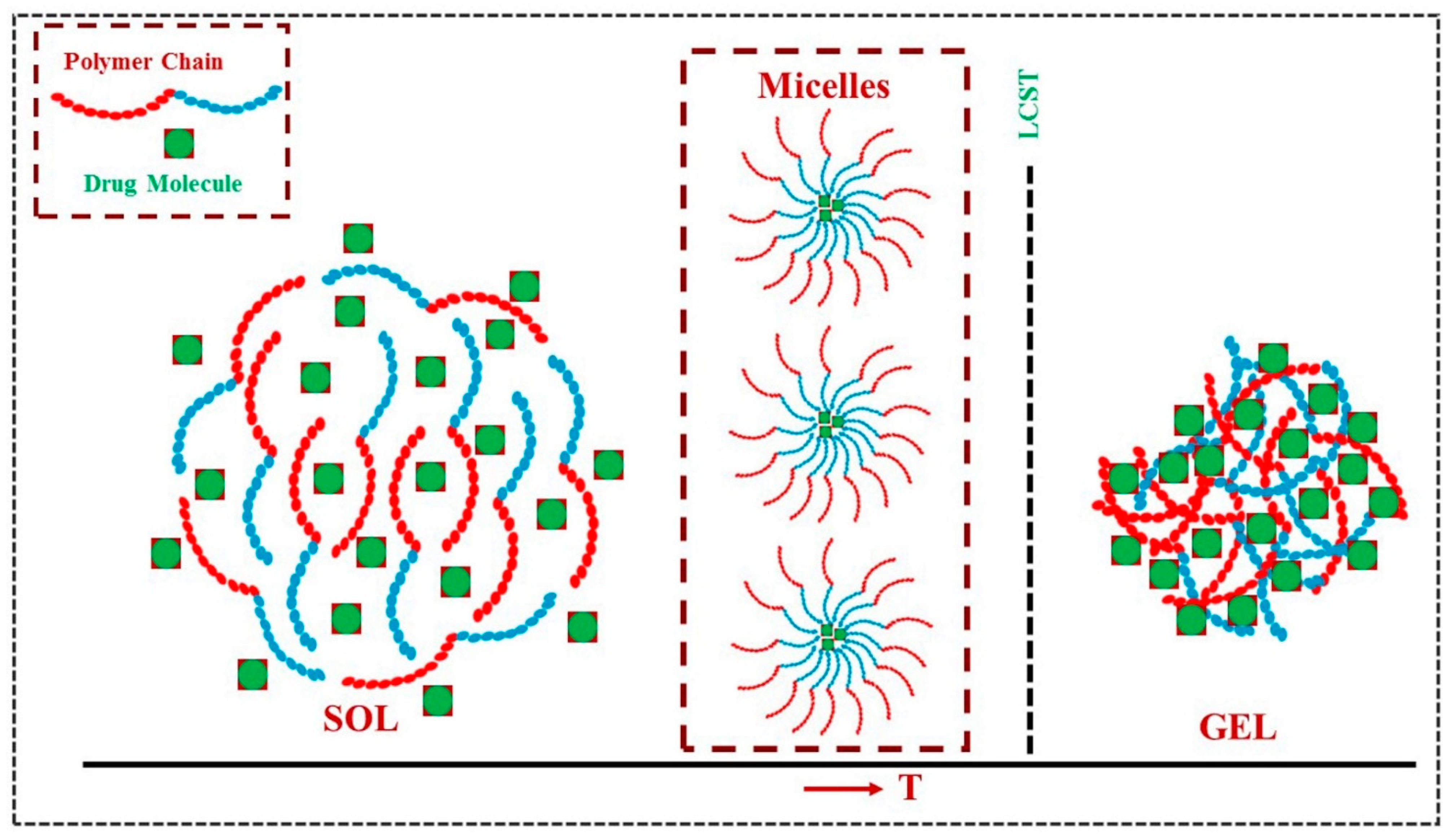
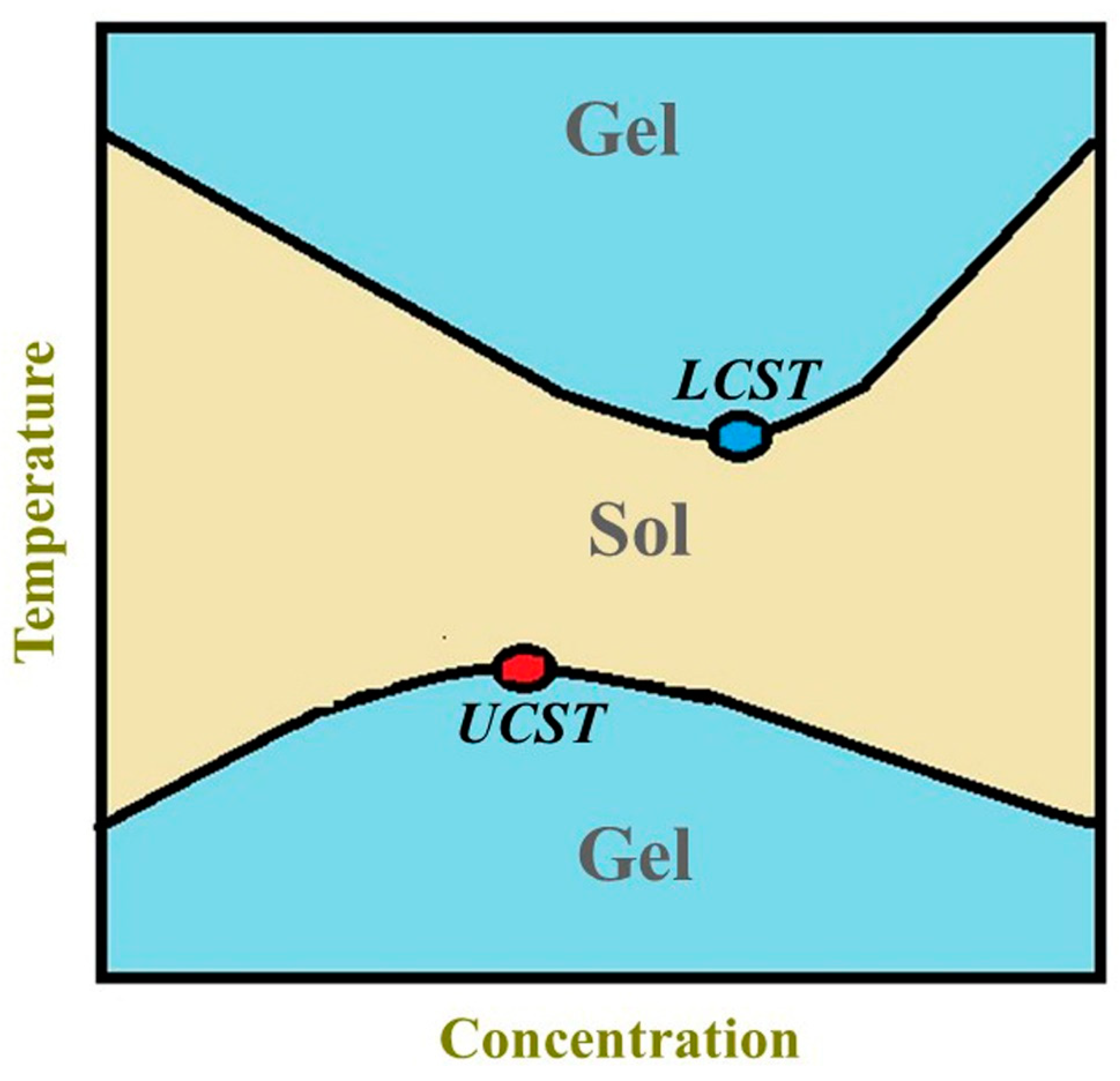
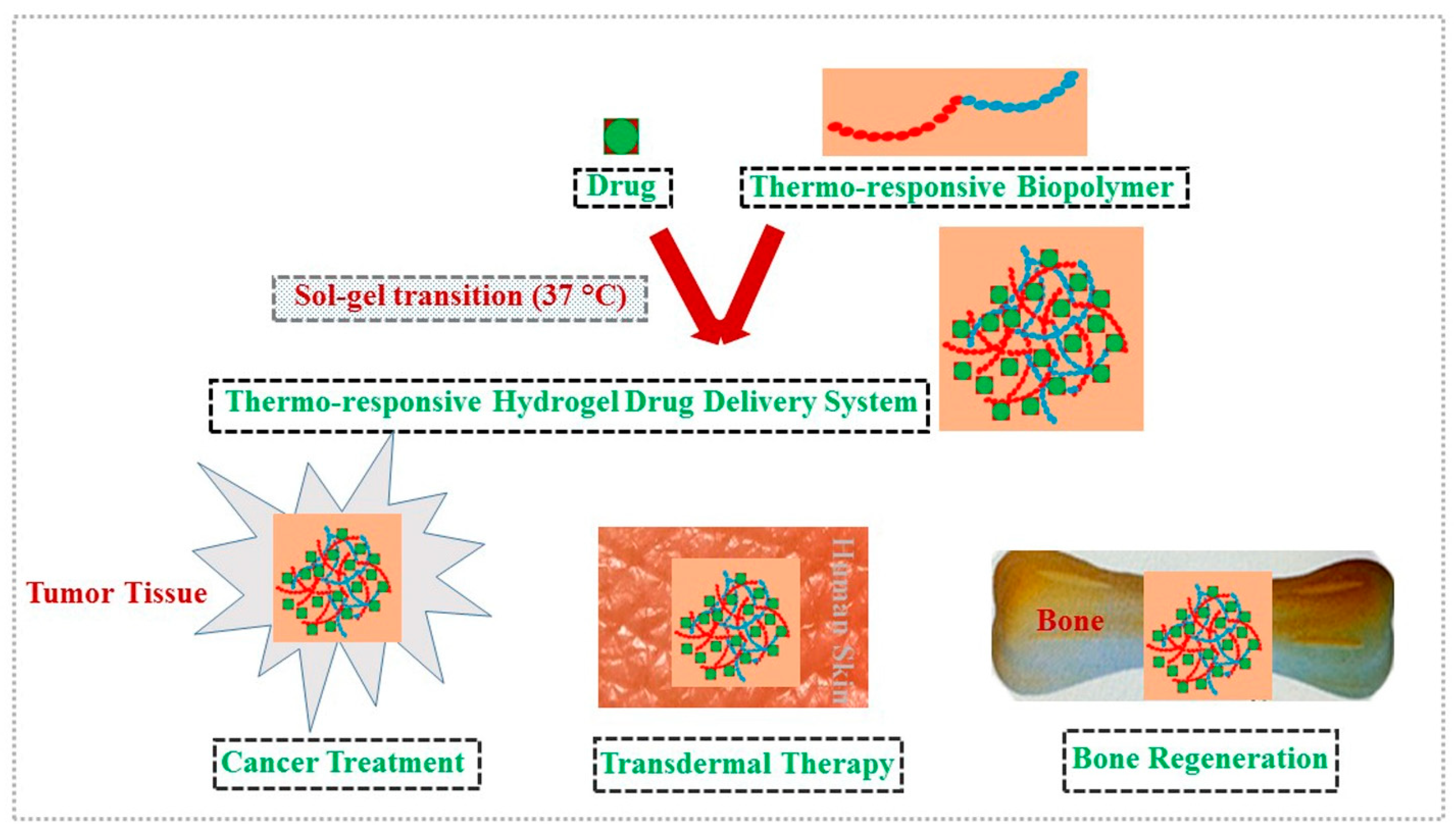

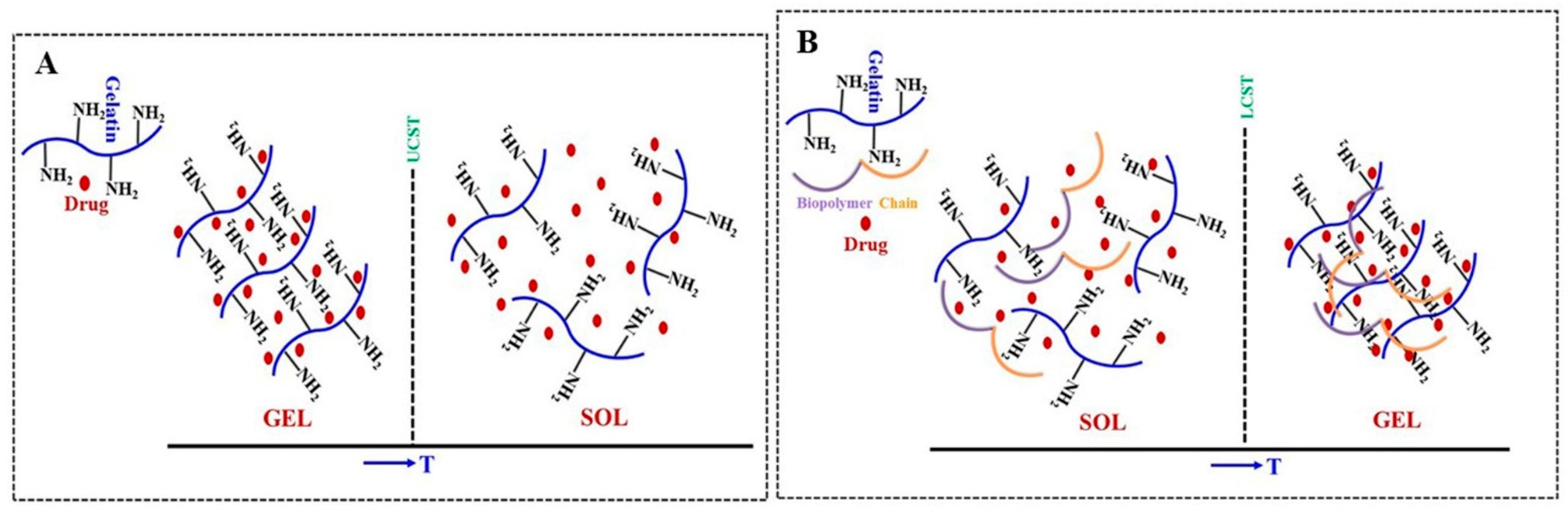
| Polysaccharide | Properties | Structural Units (Monosaccharides) | Origin/Source (Natural) | Sol–Gel Transition Type | Biomedical Applications | |
|---|---|---|---|---|---|---|
| Drug Delivery [Ref] | Tissue Engineering [Ref] | |||||
| Cellulose (Methyl Cellulose) | (i) biodegradable, nontoxic, and biocompatible (ii) hydrogel strength is high | O-methylated D-glucopyranose and D-glucopyranose units | Green plants and many varieties of algae | LCST | [35,36,37,39] | [41,42] |
| Xyloglucan | (i) biodegradable, injectable, nontoxic, and biocompatible (ii) hydrogel strength is average | glucan units substituted with xylose | Primary cell wall of many higher plants | LCST | [44,45,48,49] | [50,51] |
| Chitosan | (i) biodegradable, nontoxic, and biocompatible (ii) hydrogel strength is medium or low | D-glucosamine and N-acetyl-D-glucosamine | Exoskeleton of crustaceans | LCST | [52,53,55,57] | [59,60] |
| Protein/Polypeptide | Properties | Structural Units (Monosaccharides) | Origin/Source (Natural) | Sol–Gel Transition Type | Biomedical Applications | |
|---|---|---|---|---|---|---|
| Drug Delivery [Ref] | Tissue Engineering [Ref] | |||||
| Gelatin (protein) | (i) biodegradable, injectable, nontoxic, and biocompatible (ii) hydrogel strength is poor | Glycine, proline, alanine, and other amino acids | Animal tissues such as beef bones, cartilage, tendons, and pig skin | LCST A | [63,64,65] | [66,67,68] |
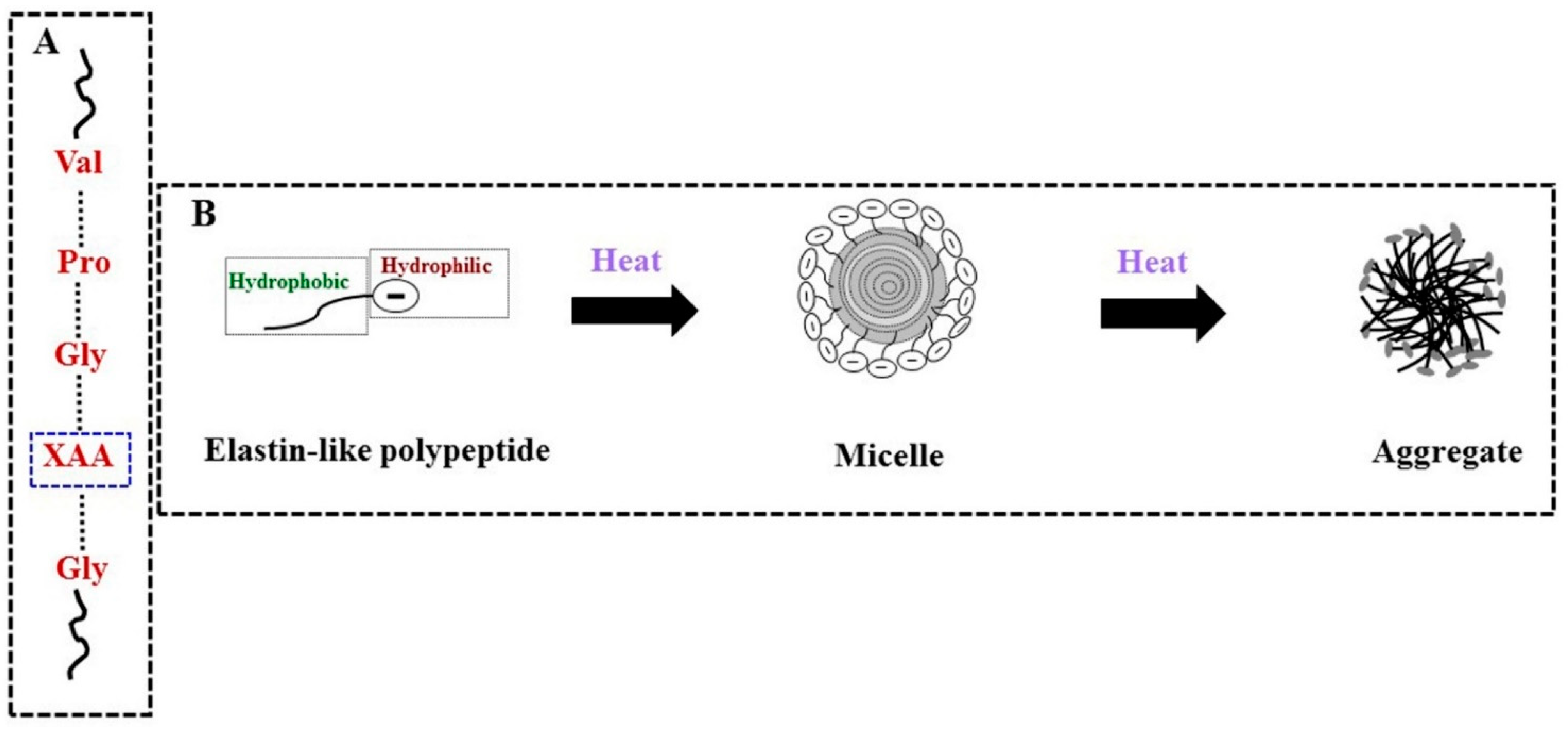
| Elastin-like polypeptides | (i) biodegradable and biocompatible (ii) mechanical strength is poor | Valine, proline, glycine, and other amino acids | Human tropoelastin | LCST | [72,73] | [70,74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, S.; Hui, P.C.-l. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 2086. https://doi.org/10.3390/polym13132086
Chatterjee S, Hui PC-l. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers. 2021; 13(13):2086. https://doi.org/10.3390/polym13132086
Chicago/Turabian StyleChatterjee, Sudipta, and Patrick Chi-leung Hui. 2021. "Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems" Polymers 13, no. 13: 2086. https://doi.org/10.3390/polym13132086
APA StyleChatterjee, S., & Hui, P. C.-l. (2021). Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers, 13(13), 2086. https://doi.org/10.3390/polym13132086








