Paclitaxel-Loaded Folate-Targeted Albumin-Alginate Nanoparticles Crosslinked with Ethylenediamine. Synthesis and In Vitro Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BSA/ALG Nanoparticles
2.3. Characterization of Nanoparticles
2.3.1. Composition of BSA/AlG Nanoparticles
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Determination of Folate-Conjugated to BSA/ALG Nanoparticles
2.3.4. Morphology, Size and Z-Potential of Nanoparticles
2.3.5. Preparation of PTX–Folate-Conjugated Nanoparticles
2.3.6. Estimation of PTX Content
2.3.7. In Vitro Drug Release Studies
2.3.8. Cell Culture Studies
Cellular Uptake of Nanoparticles
Fluorescence Microscopy
Cell Viability
2.4. Statistical Analysis
3. Results
3.1. Preparation of BSA/ALG Nanoparticles
3.1.1. Composition of Nanoparticles
3.1.2. Thermogravimetric Analysis (TGA)
3.1.3. Determination of Folate Conjugated to Nanoparticles
3.2. Characterization of Folate-Conjugated BSA/ALG Nanoparticles
3.2.1. Morphology, Size and Z-Potential of Nanoparticles
3.2.2. Estimation of PTX Content in Folate-Conjugate Nanoparticles
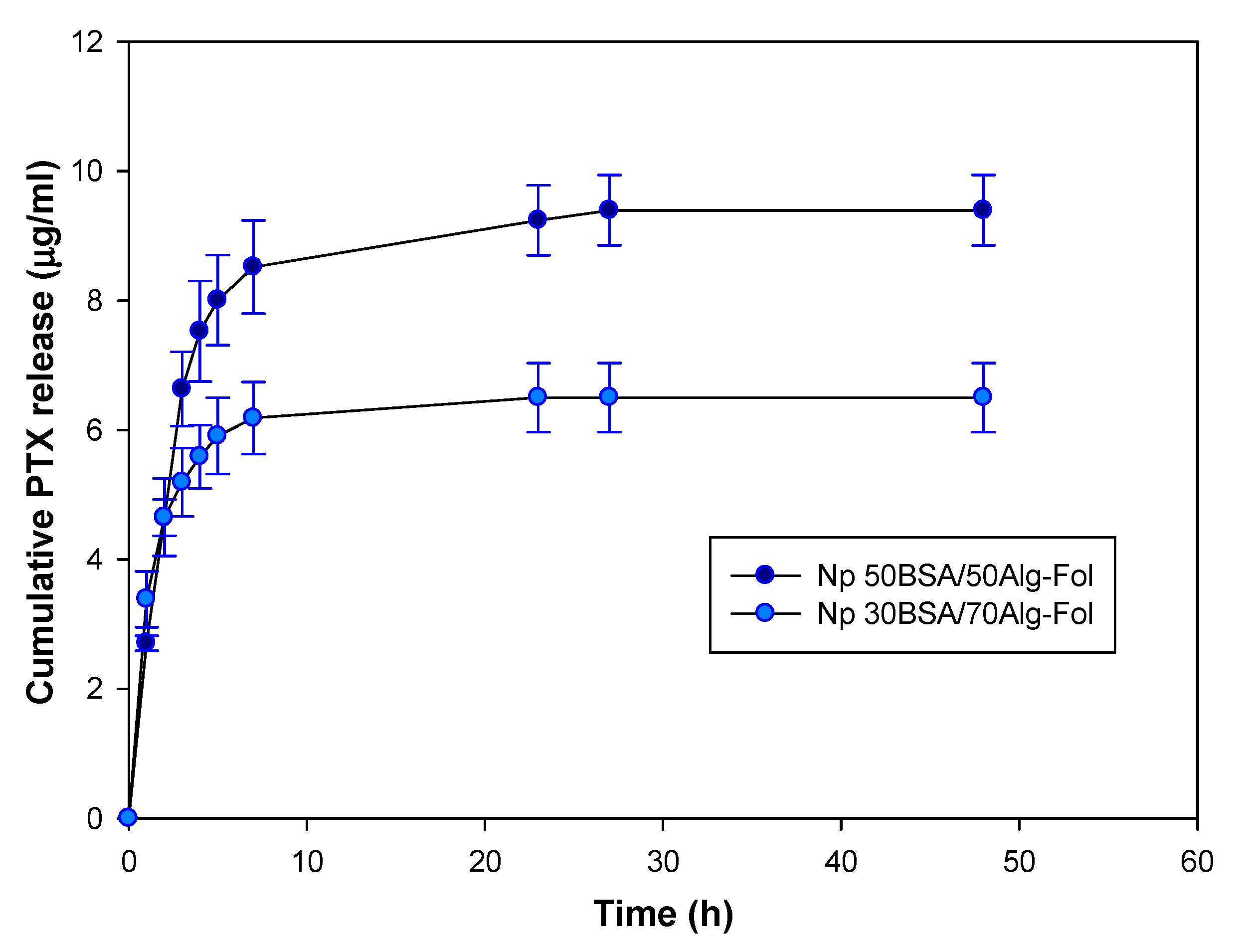
3.2.3. PTX Release from Folate-Conjugated Nanoparticles (Nps–Fol)
3.3. In Vitro Evaluation of Folate-Conjugated Nanoparticles in Tumour Cell Lines
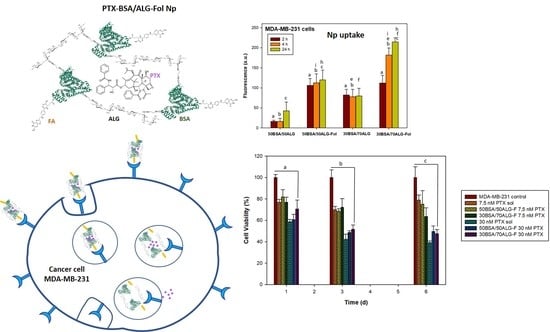
3.3.1. Cellular Uptake of Nanoparticles
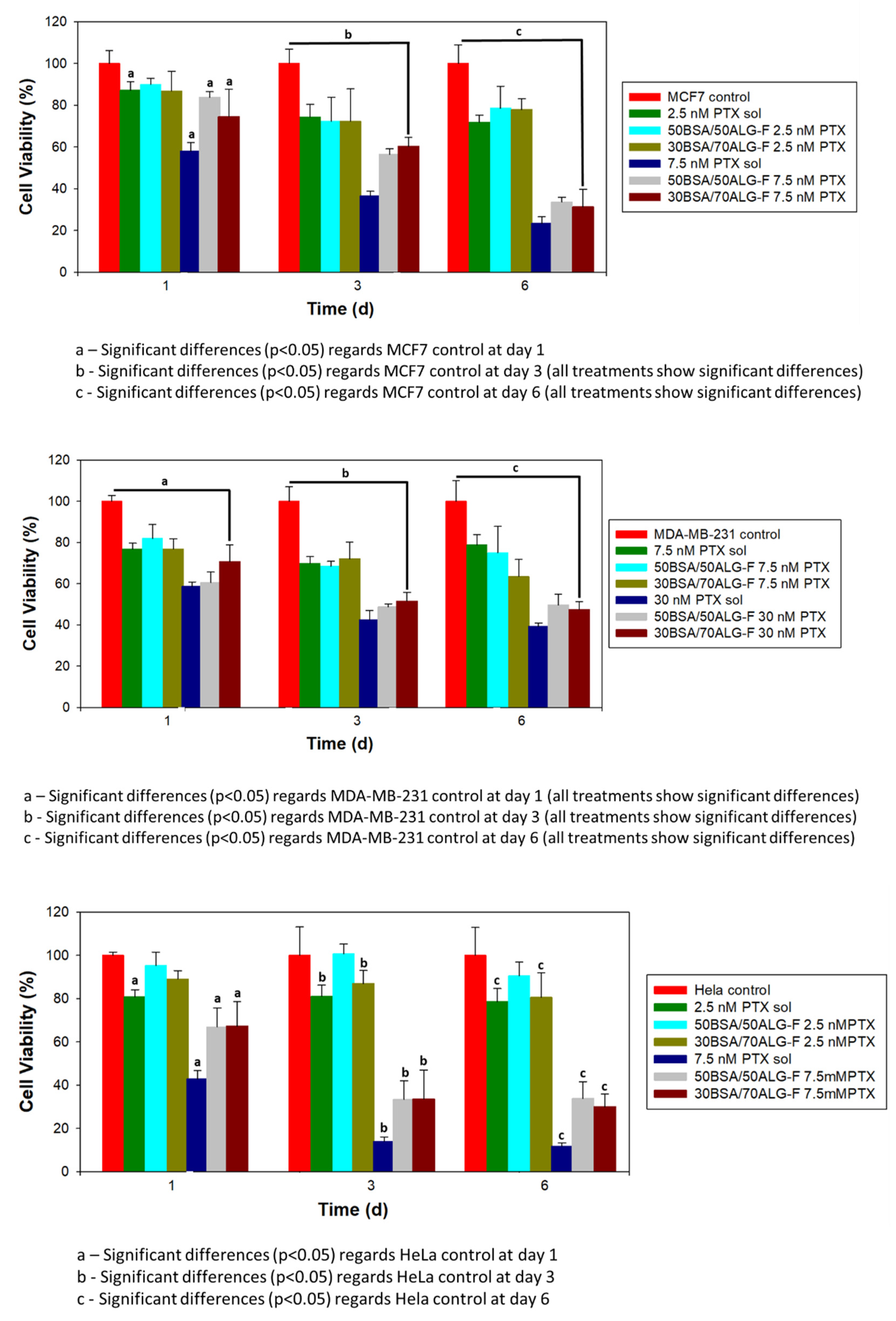
3.3.2. Cell Viability
4. Discussion
4.1. Composition, Characterization and PTX Release from Folate-Conjugated Nanoparticles
4.2. In Vitro Evaluation of Folate-Conjugated Nanoparticles in Tumor Cell Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, A.; Aftab, S.; Nisar, J.; Ashiq, M.N.; Iftikhar, F.J. Nanocarriers for targeted drug delivery. J. Drug Deliv. Sci. Technol. 2021, 62, 102426. [Google Scholar] [CrossRef]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Li, P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr. Polym. 2019, 222, 115004. [Google Scholar] [CrossRef]
- Martínez, A.M.; Benito, M.; Pérez, E.; Teijón, J.M.; Blanco, M.D. The role of anionic polysaccharides in the preparation of nanomedicines with anticancer applications. Curr. Pharm. Des. 2016, 22, 3364–3379. [Google Scholar] [CrossRef]
- Martínez, A.; Pérez, E.; Montero, N.; Teijón, C.; Olmo, R.; Blanco, M.D. Alginate-based drug carriers: Recent advances. In Alginic Acid: Chemical Structure, Uses and Health Benefits; Moore, A., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; pp. 15–43. ISBN 978-1-63463-224-9. [Google Scholar]
- Xu, Z.; Lam, M.T. Alginate application for heart and cardiovascular diseases. In Alginates and Their Biomedical Applications; Rehm, B., Moradali, M., Eds.; Springer Series in Biomaterials Science and Engineering; Springer: Singapore, 2018; Volume 11, pp. 185–212. ISBN 978-981-10-6910-9. [Google Scholar]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef]
- Chung, H.J.; Kim, H.J.; Hong, S.T. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomed. Nanotechnol. 2020, 23, 102089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lei, C.; Yang, Y.; Bu, X.; Ma, H.; Gong, H.; Liu, J.; Fang, X.; Hu, Z.; Fang, Q. Abraxane, the nanoparticle formulation of paclitaxel can induce drug resistance by up-regulation of P-gp. PLoS ONE 2015, 10, e0131429. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Gao, Y.; Wei, N.; Zhao, X.; Wang, C.; Li, Y.; Xiu, X.; Cui, J. Direct comparison of two albumin-based paclitaxel-loaded nanoparticle formulations: Is the crosslinked version more advantageous? Int. J. Pharm. 2014, 468, 15–25. [Google Scholar] [CrossRef]
- Paşcalău, V.; Tertis, M.; Pall, E.; Suciu, M.; Marinca, T.; Pustan, M.; Merie, V.; Rus, I.; Moldovan, C.; Topala, T.; et al. Bovine serum albumin gel/polyelectrolyte complex of hyaluronic acid and chitosan based microcarriers for Sorafenib targeted delivery. J. Appl. Polym. Sci. 2020. [Google Scholar] [CrossRef]
- Montero, N.; Pérez, E.; Benito, M.; Teijón, C.; Teijón, J.M.; Olmo, R.; Blanco, M.D. Biocompatibility studies of intravenously administered ionic-crosslinked chitosan-BSA nanoparticles as vehicles for antitumour drugs. Int. J. Pharm. 2019, 554, 337–351. [Google Scholar] [CrossRef]
- Martínez, A.M.; Benito, M.; Pérez, E.; Blanco, M.D. Recent advances of folate-targeted anticancer therapies and diagnostics: Current status and future prospectives. In Nanostructures for Cancer Therapy; Nanostructures in Therapeutic Medicine Series; Ficai, A., Grumezescu, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 329–350, ISBN 9780323461443/9780323461504. [Google Scholar]
- Gong, Y.C.; Xiong, X.Y.; Ge, X.J.; Li, Z.L.; Li, Y.P. Effect of the folate ligand density on the targeting property of folated-conjugated polymeric nanoparticles. Macromol. Biosci. 2019, 19, 1800348. [Google Scholar] [CrossRef] [PubMed]
- Vinothini, K.; Rajendran, N.K.; Ramu, A.; Elumalai, N.; Rajan, M. Folate receptor targeted delivery of paclitaxel to breast cancer cells via folic acid conjugated graphene oxide grafted methyl acrylate nanocarrier. Biomed. Pharmacother. 2019, 110, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Low, P.S. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J. Biol. Chem. 1994, 269, 3198–3204. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, S.; Mao, S.; Wei, D.; Song, X.; Lu, Y. Uptake of folate conjugated albumin nanoparticles to the SKOV3 cells. Int. J. Pharm. 2004, 287, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Iglesias, I.; Lozano, R.; Teijón, J.M.; Blanco, M.D. Synthesis and characterization of thiolated alginate-albumin nanoparticles stabilized by disulfide bonds. Evaluation as drug delivery systems. Carbohydr. Polym. 2011, 83, 1311–1321. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yang, T.; Cui, F.D.; Choi, M.K.; Cho, J.W.; Chung, S.J.; Shim, C.K.; Kim, D.D. Enhanced solubility and stability of PEGylated liposomal paclitaxel: In vitro and in vivo evaluation. Int. J. Pharm. 2007, 338, 317–326. [Google Scholar] [CrossRef] [PubMed]
- López-Gasco, P.; Iglesias, I.; Benedí, J.; Lozano, R.; Teijón, J.M.; Blanco, M.D. Paclitaxel-loaded polyester nanoparticles prepared by spray-drying technology: In vitro bioactivity evaluation. J. Microencapsul. 2011, 28, 417–429. [Google Scholar] [CrossRef]
- Ahuja, N.; Katare, O.P.; Singh, B. Studies on dissolution enhancement and mathematical modeling of drug release of a poorly water-soluble drug using water-soluble carriers. Eur. J. Pharm. Biopharm. 2007, 65, 26–38. [Google Scholar] [CrossRef]
- Martínez, A.; Olmo, R.; Iglesias, I.; Teijón, J.M.; Blanco, M.D. Folate-targeted nanoparticles based on albumin and albumin/alginate mixtures as controlled release systems of tamoxifen: Synthesis and in vitro characterization. Pharm. Res. 2014, 31, 182–193. [Google Scholar] [CrossRef]
- Barbosa, M.V.; Monteiro, L.O.F.; Carneiro, G.; Malagutti, A.R.; Vilela, J.M.C.; Andrade, M.S.; Oliveira, M.C.; Carvalho-Junior, A.D.; Leitea, E.A. Experimental design of a liposomal lipid system: A potential strategy for paclitaxel-based breast cancer treatment. Colloids Surf. B 2015, 136, 553–561. [Google Scholar] [CrossRef]
- Rizk, N.; Christoforou, N.; Lee, S. Optimization of anti-cancer drugs and a targeting molecule on multifunctional gold nanoparticles. Nanotechnology 2016, 27, 185704. [Google Scholar] [CrossRef]
- Pérez, E.; Fernández, A.; Olmo, R.; Teijón, J.M.; Blanco, M.D. pH and glutathion-responsive hydrogel for localized delivery of paclitaxel. Colloids Surf. B 2014, 116, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Benito-Miguel, M.; Iglesias, I.; Teijón, J.M.; Blanco, M.D. Tamoxifen-loaded thiolated alginate-albumin nanoparticles as antitumoral drug delivery systems. J. Biomed. Mater. Res. Part A 2012, 100A, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Fauzee, N.J.S.; Dong, Z.; Wang, Y. Taxanes: Promising anti-cancer drugs. Asian Pac. J. Cancer Prev. 2011, 12, 837–851. [Google Scholar] [PubMed]
- Kundranda, M.N.; Niu, J. Albumin-bound paclitaxel in solid tumors: Clinical development and future directions. Drug Des. Dev. Ther. 2015, 9, 3767–3777. [Google Scholar] [CrossRef] [Green Version]
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of “nano” paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef]
- Xu, X.; Wang, L.; Xu, H.-Q.Q.; Huang, X.-E.E.; Qian, Y.-D.D.; Xiang, J. Clinical comparison between paclitaxel liposome (Lipusu(R)) and paclitaxel for treatment of patients with metastatic gastric cancer. Asian Pac. J. Cancer Prev. 2013, 14, 2591–2594. [Google Scholar] [CrossRef] [Green Version]
- Madaan, A.; Singh, P.; Awasthi, A.; Verma, R.; Singh, A.T.; Jaggi, M.; Mishra, S.K.; Kulkarni, S.; Kulkarni, H. Efficiency and mechanism of intracellular paclitaxel delivery by novel nanopolymer-based tumor-targeted delivery system. NanoxelTM Clin. Transl. Oncol. 2013, 15, 26–32. [Google Scholar] [CrossRef]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Ke, J.; Zhou, X.E.; Yi, W.; Brunzelle, J.S.; Li, J.; Yong, E.L.; Xu, H.E.; Melcher, K. Structural basis for molecular recognition of folic acid by folate receptors. Nature 2013, 500, 486–489. [Google Scholar] [CrossRef] [Green Version]
- Leamon, C.P.; Vlahov, I.R.; Reddy, J.A.; Vetzel, M.; Santhapuram, H.K.; You, F.; Bloomfield, A.; Dorton, R.; Nelson, M.; Kleindl, P.; et al. Folate–vinca alkaloid conjugates for cancer therapy: A structure–activity relationship. Bioconjug. Chem. 2014, 25, 560–568. [Google Scholar] [CrossRef]
- Martínez, A.; Muñiz, E.; Teijón, C.; Iglesias, I.; Teijón, J.M.; Blanco, M.D. Targeting tamoxifen to breast cancer xenograft Tumours: Preclinical efficacy of folate-attached nanoparticles based on alginate-cysteine/disulphide-bond-reduced albumin. Pharm. Res. 2014, 31, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, L.; Angelopoulou, A.; Hatziantoniou, S.; Papadimitriou, M.; Apostolou, P.; Papasotiriou, I.; Avgoustakis, K. Folic acid-functionalized gold nanorods for controlled paclitaxel delivery: In vitro evaluation and cell studies. AAPS PharmSciTech 2019, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Viéville, J.; Tanty, M.; Delsuc, M.A. Polydispersity index of polymers revealed by DOSY NMR. J. Magn. Reson. 2011, 212, 169–173. [Google Scholar] [CrossRef]
- Muller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Perez, E.; Martinez, A.; Teijon, C.; Olmo, R.; Teijón, J.M.; Blanco, M.D. Improved antitumor effect of paclitaxel administered in vivo as pH and glutathione-sensitive nanohydrogels. Int. J. Pharm. 2015, 492, 10–19. [Google Scholar] [CrossRef]
- Paul, D.R. Elaborations on the Higuchi model for drug delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef]
- Machín, R.; Isasi, J.R.; Vélaz, I. Hydrogel matrices containing single and mixed natural cyclodextrins. Mechanisms of drug release. Eur. Polym. J. 2013, 49, 3912–3920. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of soluble release from porous hydrophilic polymers. Int. J. Pharmaceut. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharmaceut. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Jhaveri, M.S.; Rait, A.S.; Chung, K.-N.; Trepel, J.B.; Chang, E.H. Antisense oligonucleotides targeted to the human α folate receptor inhibit breast cancer cell growth and sensitize the cells to doxorubicin treatment. Mol. Cancer Ther. 2004, 3, 1505–1512. [Google Scholar] [PubMed]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadducci, A.; Cosio, S. Neoadjuvant chemotherapy in locally advanced cervical cancer: Review of the literature and perspectives of clinical research. Anticancer Res. 2020, 40, 4819–4828. [Google Scholar] [CrossRef] [PubMed]
- Banu, H.; Sethi, D.K.; Edgar, A.; Sheriff, A.; Rayees, N.; Renuka, N.; Faheem, S.M.; Premkumar, K.; Vasanthakumar, G. Doxorubicin loaded polymeric gold nanoparticles targeted to human folate receptor upon laser photothermal therapy potentiates chemotherapy in breast cancer cell lines. J. Photochem. Photobiol. B Biol. 2015, 149, 116–128. [Google Scholar] [CrossRef]
- Wilhelm, C.; Billotey, C.; Roger, J.; Pons, J.N.; Bacri, J.C.; Gazeau, F. Intracellular uptake of anionic superparamagnetic nanoparticles as a function of their surface coating. Biomaterials 2003, 24, 1001–1011. [Google Scholar] [CrossRef]
- Ke, C.Y.; Mathias, C.J.; Green, M.A. The folate receptor as a molecular target for tumor-selective radionuclide delivery. Nucl. Med. Biol. 2003, 30, 811–817. [Google Scholar] [CrossRef]


| Nanoparticles | Composition of Blend (w/w Ratio) | BSA Incorporated into Nanoparticles (%) | ALG Incorporated into Nanoparticles (%) | BSA/ALG Ratio in Nanoparticles |
|---|---|---|---|---|
| 30BSA/70ALG | 2BSA:1ALG | 52 ± 4 | 65 ± 5 | 1/1 |
| 40BSA/60ALG | 3BSA:1ALG | 13 ± 4 | 50 ± 2 | 0.25/1 |
| 50BSA/50ALG | 5BSA:1ALG | 25 ± 3 | 54 ± 4 | 0.5/1 |
| Nanoparticles | Mean Size (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|
| 30BSA/70ALG | 182 ± 82 | 1.4 ± 0.7 | −0.12 ± 0.04 a |
| 30BSA/70ALG–Fol | 189 ± 81 | 1.7 ± 0.3 | −69.3 ± 0.8 a |
| PTX-loaded 30BSA/70ALG–Fol | 290 ± 126 | 1.8 ± 0.4 | −67.3 ± 0.8 a |
| 50BSA/50ALG | 169 ± 28 | 1.5 ± 0.3 | −0.43 ± 0.06 b |
| 50BSA/50ALG–Fol | 268 ± 102 | 1.4 ± 0.3 | −66.2± 0.6 bc |
| PTX-loaded 50BSA/50ALG–Fol | 296 ± 57 | 1.2 ± 0.4 | −94.1± 0.4 bc |
| Drug Content in Nps–Fol | ||
|---|---|---|
| Nanoparticles | By Extraction with Ethanol (µg PTX/mg Np) | By Tryptic Hydrolysis of the Nanoparticles (µg PTX/mg Np) |
| 50BSA/50ALG–Fol | 3.56 ± 0.13 a | 3.28 ± 0.24 b |
| 30BSA/70ALG–Fol | 2.63 ± 0.19 a | 2.42 ± 0.36 b |
| Nanoparticles | Release Rate of PTX from Nanoparticle–Fol | |
|---|---|---|
| First Stage: 0–4 h | Second Stage: 5–27 h | |
| 50BSA/50ALG–Fol | 0.63 µg PTX/h per mg of Nps–Fol (r2 0.973) | 0.019 µg PTX/h per mg of Nps–Fol (r2 0.935) |
| 30BSA/70ALG–Fol | 0.43 µg PTX/h per mg of Nps–Fol (r2 0.823) | 0.008 µg PTX/h per mg of Nps–Fol (r2 0.877) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Relimpio, A.M.; Benito, M.; Pérez-Izquierdo, E.; Teijón, C.; Olmo, R.M.; Blanco, M.D. Paclitaxel-Loaded Folate-Targeted Albumin-Alginate Nanoparticles Crosslinked with Ethylenediamine. Synthesis and In Vitro Characterization. Polymers 2021, 13, 2083. https://doi.org/10.3390/polym13132083
Martínez-Relimpio AM, Benito M, Pérez-Izquierdo E, Teijón C, Olmo RM, Blanco MD. Paclitaxel-Loaded Folate-Targeted Albumin-Alginate Nanoparticles Crosslinked with Ethylenediamine. Synthesis and In Vitro Characterization. Polymers. 2021; 13(13):2083. https://doi.org/10.3390/polym13132083
Chicago/Turabian StyleMartínez-Relimpio, Ana María, Marta Benito, Elena Pérez-Izquierdo, César Teijón, Rosa María Olmo, and María Dolores Blanco. 2021. "Paclitaxel-Loaded Folate-Targeted Albumin-Alginate Nanoparticles Crosslinked with Ethylenediamine. Synthesis and In Vitro Characterization" Polymers 13, no. 13: 2083. https://doi.org/10.3390/polym13132083
APA StyleMartínez-Relimpio, A. M., Benito, M., Pérez-Izquierdo, E., Teijón, C., Olmo, R. M., & Blanco, M. D. (2021). Paclitaxel-Loaded Folate-Targeted Albumin-Alginate Nanoparticles Crosslinked with Ethylenediamine. Synthesis and In Vitro Characterization. Polymers, 13(13), 2083. https://doi.org/10.3390/polym13132083