Impact of Macrodiols on the Morphological Behavior of H12MDI/HDO-Based Polyurethane Elastomer
Abstract
:1. Introduction
2. Methodology
2.1. Materials and Synthesis
2.2. Characterization
3. Results and Discussion
3.1. Molecular Characterization
3.2. X-Ray Diffraction Studies (XRD)
3.3. Studies of Surface Morphology
3.3.1. Measurements of Contact Angle
3.3.2. Surface Free Energy
3.4. Morphology
3.4.1. Scanning Electron Microscopy (SEM)
3.4.2. Atomic Force Microscopy (AFM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammadi, A.; Daemi, H.; Barikani, M. Fast removal of malachite green dye using novel superparamagnetic sodium alginate-coated Fe3O4 nanoparticles. Int. J. Biol. Macromol. 2014, 69, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Kojio, K.; Kugumiya, S.; Uchiba, Y.; Mitsui, Y. Microphase separation of bulk and ultrathin films of polyurethane elastomers. In Macromolecular Symposia; WILEY-VCH GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 9–15. [Google Scholar]
- Zia, K.M.; Zuber, M.; Saif, M.J.; Jawaid, M.; Mahmood, K.; Shahid, M.; Anjum, M.N.; Ahmad, M.N. Chitin based polyurethanes using hydroxyl terminated polybutadiene, part III: Surface characteristics. Int. J. Biol. Macromol. 2013, 62, 670–676. [Google Scholar] [CrossRef]
- Eceiza, A.; Martin, M.; De La Caba, K.; Kortaberria, G.; Gabilondo, N.; Corcuera, M.; Mondragon, I. Thermoplastic polyurethane elastomers based on polycarbonate diols with different soft segment molecular weight and chemical structure: Mechanical and thermal properties. Polym. Eng. Sci. 2008, 48, 297–306. [Google Scholar] [CrossRef]
- Pilch-Pitera, B.; Król, P.; Pikus, S. Supramolecular structure of crosslinked polyurethane elastomers based on well-defined prepolymers. J. Appl. Polym. Sci. 2008, 110, 3292–3299. [Google Scholar] [CrossRef]
- Chun, B.C.; Cho, T.K.; Chung, Y.C. Blocking of soft segments with different chain lengths and its impact on the shape memory property of polyurethane copolymer. J. Appl. Polym. Sci. 2007, 103, 1435–1441. [Google Scholar] [CrossRef]
- Król, P.; Pilch-Pitera, B. Phase structure and thermal stability of crosslinked polyurethane elastomers based on well-defined prepolymers. J. Appl. Polym. Sci. 2007, 104, 1464–1474. [Google Scholar] [CrossRef]
- Kojio, K.; Nakamura, S.; Furukawa, M. Effect of side groups of polymer glycol on microphase-separated structure and mechanical properties of polyurethane elastomers. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 2054–2063. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Zhang, Y.; Sun, X.; Ye, H.; Li, W. Synthesis and characterization of polyurethane elastomers. J. Elastomers Plast. 2008, 40, 161–177. [Google Scholar] [CrossRef]
- Madhavan, K.; Reddy, B. Synthesis and characterization of poly (dimethylsiloxane-urethane) elastomers: Effect of hard segments of polyurethane on morphological and mechanical properties. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2980–2989. [Google Scholar] [CrossRef]
- Xia, H.; Song, M.; Zhang, Z.; Richardson, M. Microphase separation, stress relaxation, and creep behavior of polyurethane nanocomposites. J. Appl. Polym. Sci. 2007, 103, 2992–3002. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Luo, X.; Wang, X. Influence of processing conditions on dual-phase continuous blend system of thermoplastic polyurethane with ethylene-propylene-diene monomer elastomer. J. Appl. Polym. Sci. 2006, 102, 5472–5482. [Google Scholar] [CrossRef]
- Lagasse, R. Domain structure and time-dependent properties of a crosslinked urethane elastomer. J. Appl. Polym. Sci. 1977, 21, 2489–2503. [Google Scholar] [CrossRef]
- Hu, J.; Yang, Z.; Yeung, L.; Ji, F.; Liu, Y. Crosslinked polyurethanes with shape memory properties. Polym. Int. 2005, 54, 854–859. [Google Scholar] [CrossRef]
- Zia, K.M.; Barikani, M.; Zuber, M.; Bhatti, I.A.; Sheikh, M.A. Molecular engineering of chitin based polyurethane elastomers. Carbohydr. Polym. 2008, 74, 149–158. [Google Scholar] [CrossRef]
- Naheed, S.; Zuber, M.; Barikani, M. Synthesis and thermo-mechanical investigation of macrodiol-based shape memory polyurethane elastomers. Int. J. Mater. Res. 2017, 108, 515–522. [Google Scholar] [CrossRef]
- Zia, K.M.; Anjum, S.; Zuber, M.; Mujahid, M.; Jamil, T. Synthesis and molecular characterization of chitosan based polyurethane elastomers using aromatic diisocyanate. Int. J. Biol. Macromol. 2014, 66, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Naheed, S.; Zuber, M.; Barikani, M.; Bukhari, I.H.; Zia, K.M. Thermo-Mechanical Characteristics of Shape Memory Polyurethane Varying Molecular Weight of Polyol. Asian J. Chem. 2017, 29, 951–959. [Google Scholar] [CrossRef]
- Zia, K.M.; Bhatti, I.A.; Barikani, M.; Zuber, M.; Sheikh, M.A. XRD studies of chitin-based polyurethane elastomers. Int. J. Biol. Macromol. 2008, 43, 136–141. [Google Scholar] [CrossRef]
- Yamasaki, S.; Nishiguchi, D.; Kojio, K.; Furukawa, M. Effects of polymerization method on structure and properties of thermoplastic polyurethanes. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 800–814. [Google Scholar] [CrossRef]
- Lem, K.; Haw, J.; Curran, S.; Sund, S.; Brumlik, C.; Song, G.; Lee, D. Effect of hard segment molecular weight on concentrated solution and bulk properties of ether based thermoplastic polyurethanes. Nanosci. Nanoeng. 2013, 1, 71–88. [Google Scholar] [CrossRef]
- Zuber, M.; Tabasum, S.; Hussain, R.; Khan, M.B.; Bukhari, I.H. Blends of polyurethane-polymethyl methacrylate/TiO2-based composites. Korean J. Chem. Eng. 2013, 30, 1652–1658. [Google Scholar] [CrossRef]
- Wu, S. Calculation of interfacial tension in polymer systems. J. Polym. Sci. Part C Polym. Symp. 1971, 34, 19–30. [Google Scholar] [CrossRef]
- Ginde, R.M.; Gupta, R.K. In vitro chemical degradation of poly (glycolic acid) pellets and fibers. J. Appl. Polym. Sci. 1987, 33, 2411–2429. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Nan, Q.; Ye, X.; Sun, Y.; Zhang, F.; Wang, Z. Preparation and properties of optically active polyurethane/TiO2 nanocomposites derived from optically pure 1, 1′-binaphthyl. Eur. Polym. J. 2007, 43, 4151–4159. [Google Scholar] [CrossRef]
- Premkumar, S.; Chozhan, C.K.; Alagar, M. Studies on thermal, mechanical and morphological behaviour of caprolactam blocked methylenediphenyl diisocyanate toughened bismaleimide modified epoxy matrices. Eur. Polym. J. 2008, 44, 2599–2607. [Google Scholar] [CrossRef]
- Zuber, M.; Zia, K.M.; Iqbal, M.A.; Cheema, Z.T.; Ishaq, M.; Jamil, T. Synthesis and characterization of polyurethane based on aliphatic diisocyanate and stiff chain extenders. Korean J. Chem. Eng. 2015, 32, 184–190. [Google Scholar] [CrossRef]
- Čulin, J.; Andreis, M.; Šmit, I.; Veksli, Z.; Anžlovar, A.; Žigon, M. Motional heterogeneity and phase separation of functionalized polyester polyurethanes. Eur. Polym. J. 2004, 40, 1857–1866. [Google Scholar] [CrossRef]
- Klinedinst, D.B.; Yilgör, E.; Yilgör, I.; Beyer, F.L.; Wilkes, G.L. Structure–property behavior of segmented polyurethaneurea copolymers based on an ethylene–butylene soft segment. Polymer 2005, 46, 10191–10201. [Google Scholar] [CrossRef]
- Akram, N.; Zia, K.M.; Saeed, M.; Mansha, A.; Khan, W.G. Morphological studies of polyurethane based pressure sensitive adhesives by tapping mode atomic force microscopy. J. Polym. Res. 2018, 25, 1–11. [Google Scholar] [CrossRef]
- Naheed, S.; Zuber, M.; Barikani, M.; Salman, M. Molecular engineering and morphology of polyurethane elastomers containing various molecular weight of macrodiol. Mater. Sci. Eng. B 2021, 264, 114960. [Google Scholar] [CrossRef]
- Eceiza, A.; Kortaberria, G.; Mariet, C.; Gabilondo, N.; Corcuera, M.A.; Mondragon, I. Effect of Hard/Soft Segment Ratio on the Structure and Properties of Thermoplastic Polyurethane Elastomers Based on Polycarbonate Diol; Materials + Technologies’ Group. Escuela Universitaria Politécnica: Donostia, Spain, 2004; pp. 10–12. [Google Scholar]
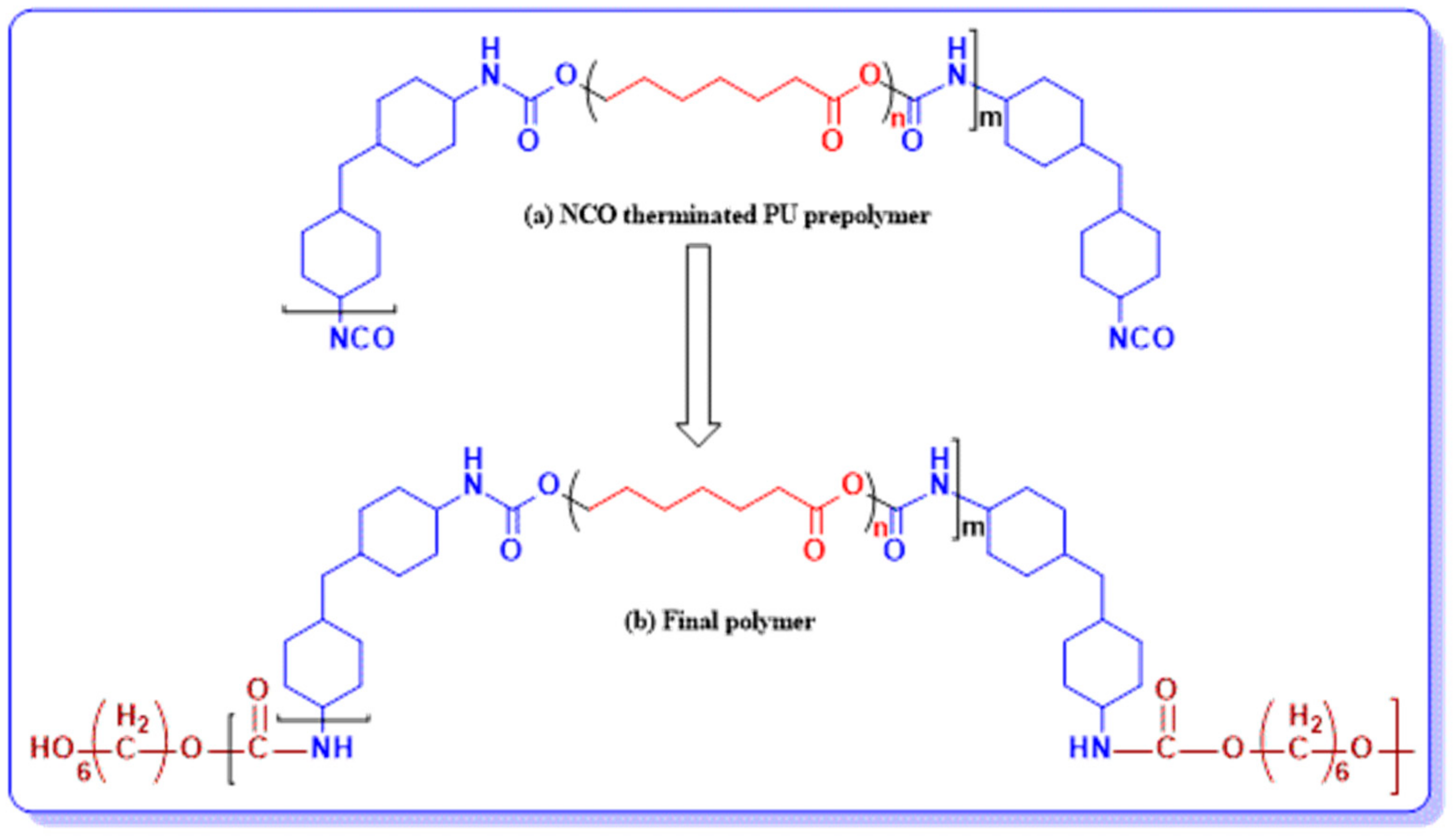

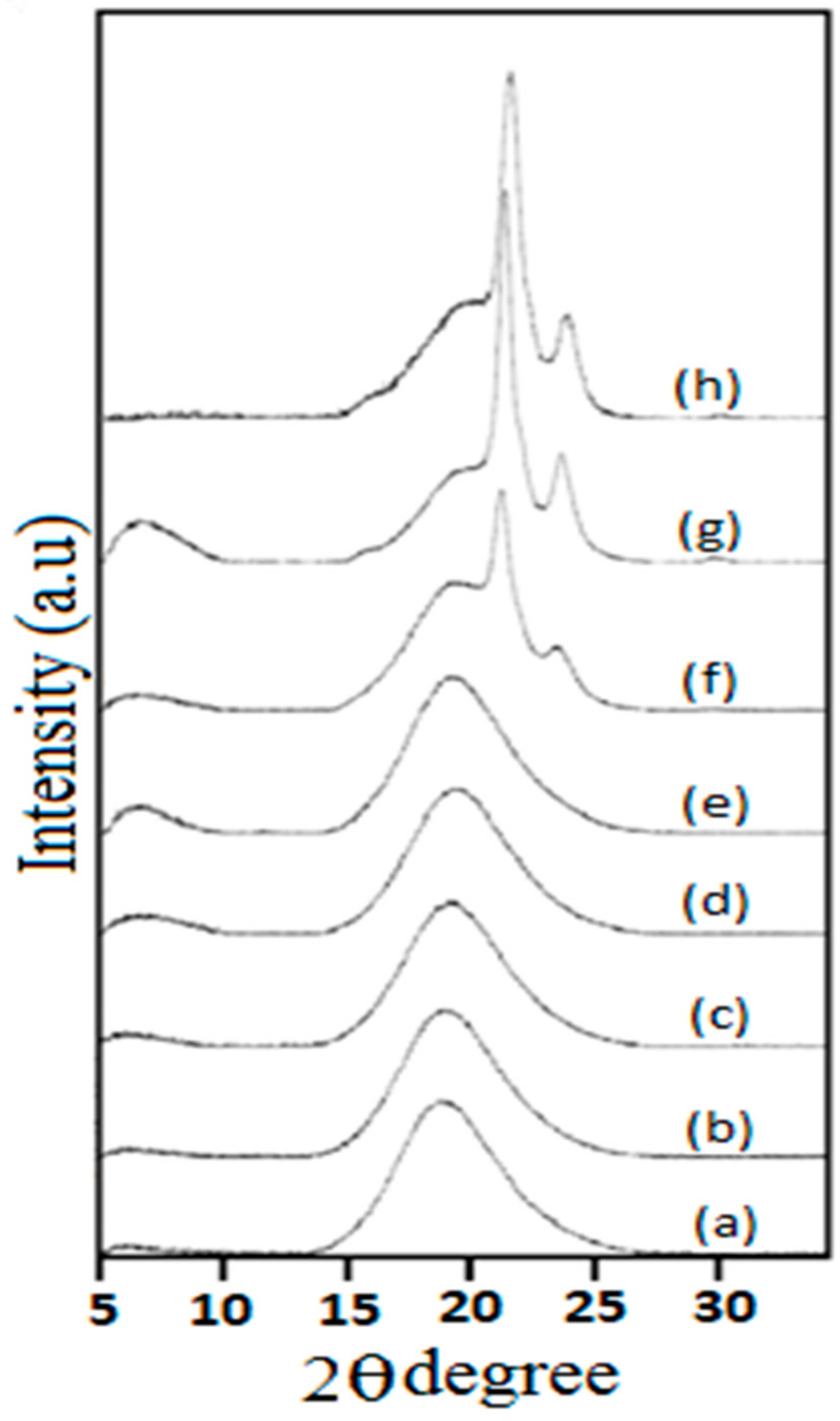

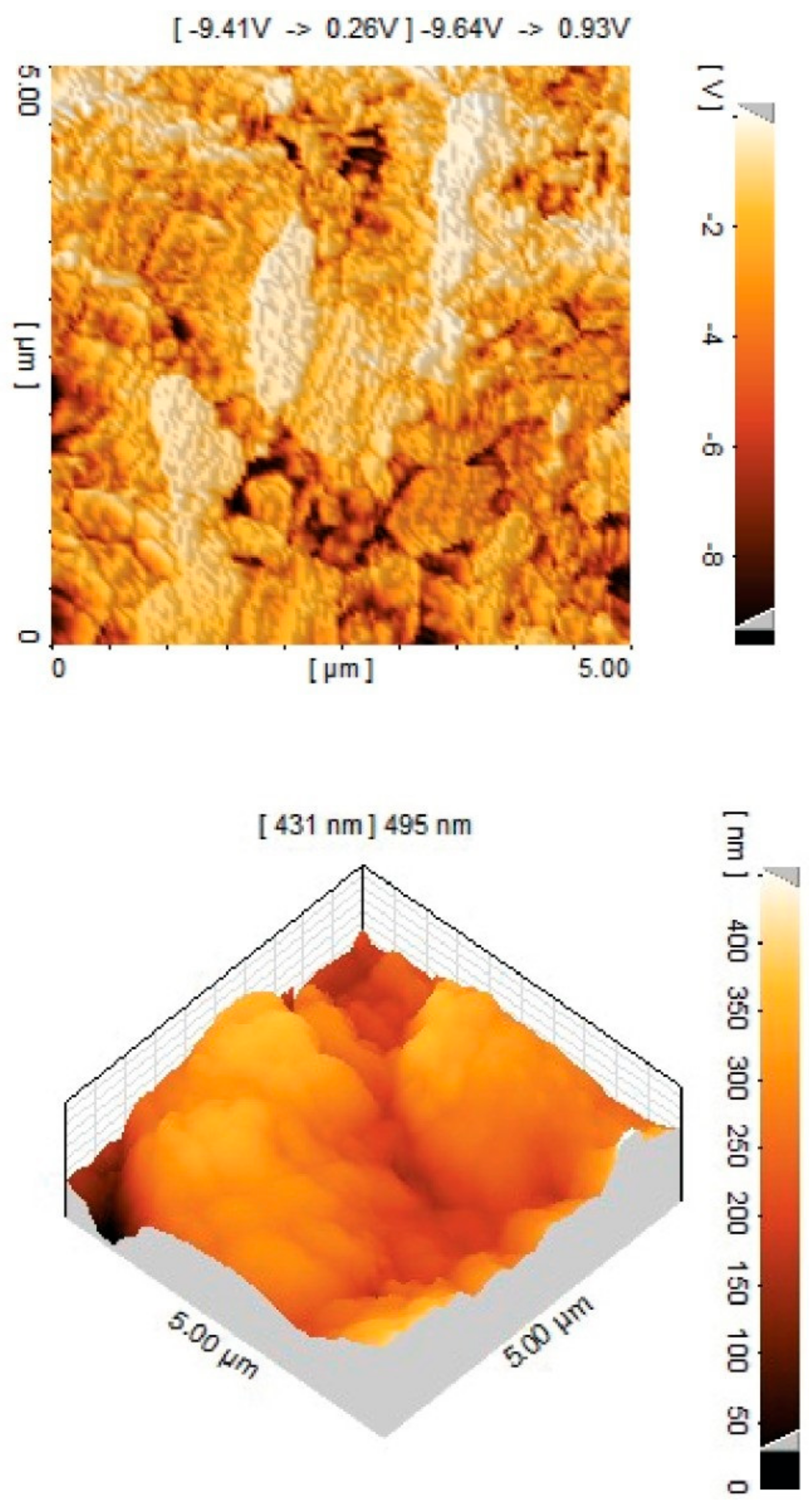
| Sr. No. | SamplesCode | PCL (MW) | Molar Ratio of (H12MDI/PCL/HDO) | Percentage of Soft Segments (%) SS |
|---|---|---|---|---|
| 1 | PUE1 | 400 | 2:0.8:1.2 | 34.86 |
| 2 | PUE2 | 750 | 2:0.8:1.2 | 50.08 |
| 3 | PUE3 | 1000 | 2:0.8:1.2 | 57.22 |
| 4 | PUE4 | 1250 | 2:0.8:1.2 | 62.58 |
| 5 | PUE5 | 1600 | 2:0.8:1.2 | 68.16 |
| 6 | PUE6 | 2000 | 2:0.8:1.2 | 72.79 |
| 7 | PUE7 | 3000 | 2:0.8:1.2 | 80.05 |
| 8 | PUE8 | 4000 | 2:0.8:1.2 | 84.25 |
| Samples | Degree of Crystallinity (%) |
|---|---|
| PUE1 | - |
| PUE2 | - |
| PUE3 | - |
| PUE4 | - |
| PUE5 | - |
| PUE6 | 17.2 |
| PUE7 | 22.5 |
| PUE8 | 33.4 |
| Sr. No. | Sample Code | Contact Angle (θ) with Water and Diiodomethane | |||
|---|---|---|---|---|---|
| DDW | DIM | ||||
| 1 | PUE 1 | 86.0 | 86.0 | 38.6 | 38.6 |
| 2 | PUE 2 | 86.9 | 86.9 | 44.6 | 44.6 |
| 3 | PUE 3 | 89.2 | 89.2 | 46.8 | 46.8 |
| 4 | PUE 4 | 91.4 | 91.4 | 48.1 | 48.1 |
| 5 | PUE 5 | 93.2 | 93.2 | 49.7 | 49.7 |
| 6 | PUE 6 | 94.2 | 94.2 | 50.5 | 50.5 |
| 7 | PUE 7 | 98.9 | 98.9 | 52 | 52 |
| 8 | PUE 8 | 108 | 108 | 54.9 | 54.9 |
| Sr. No. | Sample Code | Surface Free Energy (Wu’s Method) | ||
|---|---|---|---|---|
| Polar Portion (mN/m) | Disperse Portion (mN/m) | Total Surface Energy (mN/m) | ||
| 1 | PUE 1 | 2.53 | 44.42 | 46.95 |
| 2 | PUE 2 | 1.69 | 43.32 | 45.01 |
| 3 | PUE 3 | 1.42 | 36.92 | 38.34 |
| 4 | PUE 4 | 1.27 | 34.05 | 35.32 |
| 5 | PUE 5 | 0.37 | 33.88 | 34.25 |
| 6 | PUE 6 | 1.60 | 30.39 | 31.99 |
| 7 | PUE 7 | 1.13 | 29.53 | 30.66 |
| 8 | PUE 8 | 3.96 | 20.49 | 24.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naheed, S.; Zuber, M.; Salman, M.; Rasool, N.; Siddique, Z.; Shaik, M.R.; Sharaf, M.A.F.; Abdelgawad, A.; Sekou, D.; Awwad, E.M. Impact of Macrodiols on the Morphological Behavior of H12MDI/HDO-Based Polyurethane Elastomer. Polymers 2021, 13, 2060. https://doi.org/10.3390/polym13132060
Naheed S, Zuber M, Salman M, Rasool N, Siddique Z, Shaik MR, Sharaf MAF, Abdelgawad A, Sekou D, Awwad EM. Impact of Macrodiols on the Morphological Behavior of H12MDI/HDO-Based Polyurethane Elastomer. Polymers. 2021; 13(13):2060. https://doi.org/10.3390/polym13132060
Chicago/Turabian StyleNaheed, Shazia, Mohammad Zuber, Mahwish Salman, Nasir Rasool, Zumaira Siddique, Mohammed Rafi Shaik, Mohammed A. F. Sharaf, Abdelatty Abdelgawad, Doumbia Sekou, and Emad Mahrous Awwad. 2021. "Impact of Macrodiols on the Morphological Behavior of H12MDI/HDO-Based Polyurethane Elastomer" Polymers 13, no. 13: 2060. https://doi.org/10.3390/polym13132060








