Thermal Stability of Nanosilica-Modified Poly(vinyl chloride)
Abstract
:1. Introduction
1.1. PVC Composites with Mineral Additives
1.2. PVC Composites with Inorganic Nanofillers
1.3. Silica Nanofillers as Modifiers of PVC Composites
2. Materials and Methods
2.1. Materials
2.2. Processing
2.3. Measurements
2.3.1. Gelation Level by DSC
2.3.2. Thermal Stability
2.3.3. MFR Measurements
2.3.4. The Method of Color Measurement
3. Results
3.1. The DSC-Determined Gelation Level
3.2. Melt Flow Ratio of PVC and PVC with Nanosilica
3.3. The Relationship between MFR and DSC
3.4. Visual Observation of PVC Degradation
3.5. Determination of Color Parameters
3.6. The Thermal Stability Determined by Congo Red
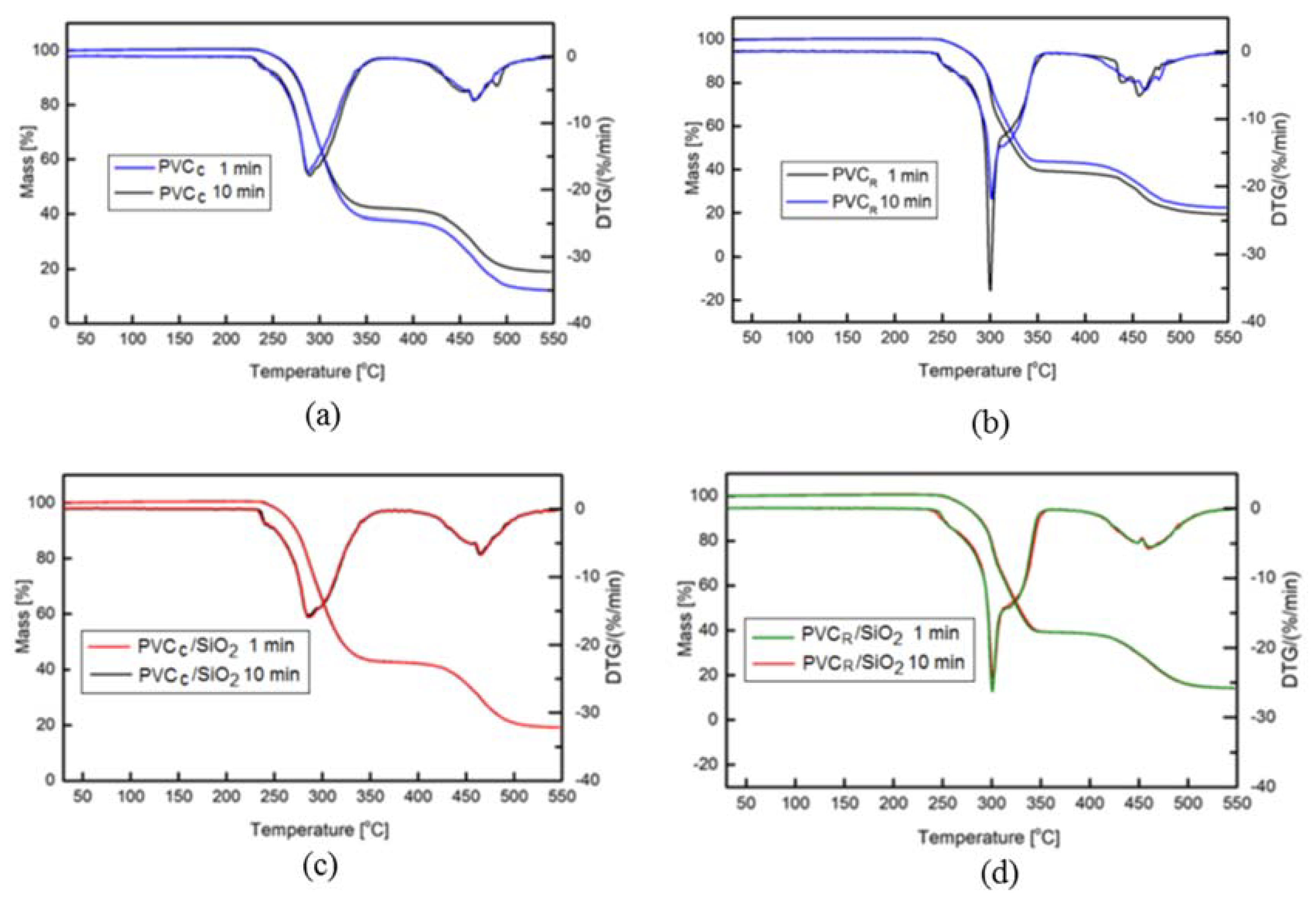
3.7. Thermogavimetric TGA Measurements
4. Summary
5. Conclusions
- Various thermal effects related to PVC degradation may be specified by means of Congo red test, color changes, and melt flow ratio measurements.
- The time-dependent discoloration of PVC samples signifies the progress of degradation related to dehydrochlorination. The use of SiO2 nanoparticles affects the time-related degradation and thus the time to observe the evolving of HCl.
- A significant role of processing conditions, particularly the rolling time, on the macromolecular degradation was stated by MFR measurements.
- The porosity of nanosilica plays an important role by immobilization of dehydrochlorination products, thus improving the thermal stability of PVC/SiO2 systems, an effect observed mainly at the beginning of thermal degradation due to limited volume of the pores.
- It has to be stressed that the stabilization role of SiO2 nanoparticles is related not only to PVC composition but also to type and conditions of its processing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hjertberg, T.; Sörvik, E.M. Thermal Degradation of PVC. In Degradation and Stabilisation of PVC, 1st ed.; Owen, E.D., Ed.; Elsevier Applied Science Publishers: London, UK; New York, NY, USA, 1984; pp. 21–80. [Google Scholar] [CrossRef]
- Folarin, O.M.; Sadiku, E.R. Thermal stabilizers for poly(vinyl chloride): A review. Int. J. Phys. Sci. 2011, 6, 4323–4330. [Google Scholar] [CrossRef]
- Bibi Effat, T.; Khan, M.I.; Rumana, S.; Shomaila, A. Thermal Degradation and Stabilization of Polyvinyl Chloride. Int. J. Res. 2014, 1, 732–750. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Šimon, P. Kinetics of polymer degradation involving the splitting off of small molecules: Part 7—Thermo-oxidative dehydrochlorination of PVC. Polym. Degrad. Stab. 1992, 36, 85–89. [Google Scholar] [CrossRef]
- Bacaloglu, R.; Fisch, M. Degradation and stbilization of poly(vinyl chloride). I. Kinetics of thermal degradation of poly(vinyl chloride). Polym. Degrad. Stab. 1994, 45, 301–313. [Google Scholar] [CrossRef]
- McNeill, I.C.; Memetea, L.; Cole, W.J. A study of the products of PVC thermal degradation. Polym. Degrad. Stab. 1995, 49, 181–191. [Google Scholar] [CrossRef]
- Starnes, W.H. Structural and mechanistic aspects of the thermal degradation of poly(vinyl chloride). Prog. Polym. Sci. 2002, 27, 2133–2170. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Huang, X.; Yi, L. Zinc Maleate and Calcium Stearate As a Complex Thermal Stabilizer for Poly(vinyl chloride). J. Vinyl Addit. Technol. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Ambrogi, V.; Carfagna, C.; Cerruti, P.; Marturano, V. Additives in Polymers. In Modification of Polymer Properties; Jasso-Gastinel, C.F., Kenny, J.M., Eds.; William Andrew: Norwich, NY, USA, 2017; pp. 87–108. [Google Scholar] [CrossRef]
- Wypych, G. Principles of Stabilization. In PVC Degradation and Stabilization, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2015; p. 79. [Google Scholar]
- Habicher, W.D.; Bauer, I.; Pospíšil, J. Organic phosphites as polymer stabilizers. Macromol. Symp. 2005, 225, 147–164. [Google Scholar] [CrossRef]
- Doworkin, R.D. PVC Stabilizers of the past, present, and future. J. Vinyl Addit. Technol. 1989, 11, 15–22. [Google Scholar] [CrossRef]
- Wang, G.; Yang, S.; Xia, J. Thermal degradation kinetics of calcium stearate/PVC composite overlay panel. Results Mater. 2020, 8, 100123–100130. [Google Scholar] [CrossRef]
- Ye, F.; Guo, X.J.; Zhan, H.H.; Lin, J.X.; Lou, W.C.; Ma, X.T.; Wang, X. The synergistic effect of zinc urate with calcium stearate and commercial assistant stabilizers for stabilizing poly(vinyl chloride). Polym. Degrad. Stab. 2018, 156, 193–201. [Google Scholar] [CrossRef]
- Han, W.; Zhang, M.; Li, D.; Dong, T.; Ai, B.; Dou, J.; Sun, H. Design and Synthesis of a New Mannitol Stearate Ester-Based Aluminum Alkoxide as a Novel Tri-Functional Additive for Poly(Vinyl Chloride) and Its Synergistic Effect with Zinc Stearate. Polymers 2019, 11, 1031. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Tang, Z.; Liu, X.; Lin, W.; Feng, M.; Lin, X.; Gu, Y.; Li, Y.; Yi, G. A facile strategy improving poly(vinyl chloride) stability by introducing unsaturated maleic acid-sorbitol ester and zinc maleic acid-sorbitol ester complex. Polym. Degrad. Stab. 2020, 181, 109343–109354. [Google Scholar] [CrossRef]
- Brostow, W.; Hnatchuk, N.; Kim, T. Preventing thermal degradation of PVC insulation by mixtures of cross-linking agents and antioxidants. J. Appl. Polym. Sci. 2020, 137, 44816–44823. [Google Scholar] [CrossRef]
- Pagacz, J.; Pielichowski, K. Preparation and characterization of PVC/Montmorillonite Nanocomposites A review. J. Vinyl Addit. Technol. 2009, 15, 61–76. [Google Scholar] [CrossRef]
- Palin, L.; Rombolá, G.; Milanesio, M.; Boccaleri, E. The use of POSS-based nanoadditives for cable-grade PVC: Effects on its thermal stability. Polymers 2019, 11, 1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Zhao, M.; Guo, J. Thermal Stabilizers of Poly (Vinyl Chloride)/Calcium Carbonate (PVC/CaCO3) Composites Thermal Stabilities of Poly (Vinyl Chloride)/Calcium. J. Macromol. Sci. 2006, 45, 1135–1140. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, H.; Zhang, S. Preparation of MgAl LDHs Intercalated with Amines and Effect on Thermal Behavior for Poly(vinyl chloride). Adv. Mater. Phys. Chem. 2014, 4, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Wang, J.; Evans, D.G.; Li, D. Layered and intercalated hydrotalcite-like materials as thermal stabilizers in PVC resin. J. Phys. Chem. Solids 2006, 67, 998–1001. [Google Scholar] [CrossRef]
- Gong, F.; Feng, M.; Zhao, C.; Zhang, S.; Yang, M. Thermal properties of poly(vinyl chloride)/montmorillonite nanocomposites. Polym. Degrad. Stab. 2004, 84, 289–294. [Google Scholar] [CrossRef]
- Zheng, X.; Gilbert, M. An Investigation into the Thermal Stability of PVC/ Montmorillonite Composites. J. Vinyl Addit. Technol. 2011, 17, 77–84. [Google Scholar] [CrossRef]
- Wan, C.; Zhang, Y.; Zhang, Y. Effect of alkyl quaternary ammonium on processing discoloration of melt-intercalated PVC-montmorillonite composites. Polym. Test. 2004, 23, 299–306. [Google Scholar] [CrossRef]
- Turhan, Y.; Doǧan, M.; Alkan, M. Poly(vinyl chloride)/kaolinite nanocomposites: Characterization and thermal and optical properties. Ind. Eng. Chem. Res. 2010, 49, 1503–1513. [Google Scholar] [CrossRef]
- Liu, C.; Luo, Y.F.; Jia, Z.X.; Zhong, B.C.; Li, S.Q.; Guo, B.C.; Jia, D.M. Enhancement of mechanical properties of poly(vinyl chloride) with Polymethyl methacrylate-grafted halloysite nanotube. Express Polym. Lett. 2011, 5, 591–603. [Google Scholar] [CrossRef]
- Xie, X.L.; Liu, Q.X.; Li, R.K.Y.; Zhou, X.P.; Zhang, Q.X.; Yu, Z.Z.; Mai, Y.W. Rheological and mechanical properties of PVC/CaCO3 nanocomposites prepared by in situ polymerization. Polymer 2004, 45, 6665–6673. [Google Scholar] [CrossRef]
- Chen, C.H.O.; Teng, C.C.; Su, S.F.; Wu, W.C.; Yang, C.H. Effects of microscale calcium carbonate and nanoscale calcium carbonate on the fusion, thermal, and mechanical characterizations of rigid poly(vinyl chloride)/calcium carbonate composites. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 451–460. [Google Scholar] [CrossRef]
- Shimpi, N.G.; Verma, J.; Mishra, S. Preparation, characterization and properties of poly(vinyl chloride)/CaSO4 nanocomposites. Polym. Plast. Technol. Eng. 2009, 48, 997–1001. [Google Scholar] [CrossRef]
- Chen, G.; Tian, M.; Guo, S. A study on the morphology and mechanical properties of PVC/nano-SiO2 composites. J. Macromol. Sci. Part B Phys. 2006, 45, 709–725. [Google Scholar] [CrossRef]
- Gulsen Albayrak Ari, I.A. A Study on Fusion and Rheological Behaviors of PVC/SiO2 Microcomposites and Nanocomposites: The Effects of SiO2 Particle Size. Polym. Eng. Sci. 2011, 51, 1575–1579. [Google Scholar] [CrossRef]
- Habashy, M.M.; Abd-Elhady, A.M.; Elsad, R.A.; Izzularab, M.A. Performance of PVC/SiO2 nanocomposites under thermal ageing. Appl. Nanosci. 2019. [Google Scholar] [CrossRef] [Green Version]
- Klapiszewski, L.; Pawlak, F.; Tomaszewska, J.; Jesionowski, T. Preparation and characterization of novel pvc/silica-lignin composites. Polymers 2015, 7, 1767–1788. [Google Scholar] [CrossRef] [Green Version]
- Ye, F.; Ye, Q.; Zhan, H.; Ge, Y.; Ma, X.; Xu, Y.; Wang, X. Synthesis and study of zinc orotate and its synergistic effect with commercial stabilizers for stabilizing poly(vinyl chloride). Polymers 2019, 11, 194. [Google Scholar] [CrossRef] [Green Version]
- Michel, A. PVC Thermal Stability Assesment Using Tests in the Presence of Stabilizers. J. Vinyl Technol. 1985, 7, 77–91. [Google Scholar] [CrossRef]
- Simonov-Emelianov, I.D.; Markov, A.V.; Prokopov, N.I.; Gritskova, I.A.; Munkin, N.I.; Hexel, L. Effect of self-heating during processing of rigid and plasticized poly(vinyl chloride) on its thermal stability. Polimery 2013, 58, 703–706. [Google Scholar] [CrossRef]
- Peprnicek, T.; Duchet, J.; Kovarova, L.; Malac, J.; Gerard, J.F.; Simonik, J. Poly(vinyl chloride)/clay nanocomposites: X-ray diffraction, thermal and rheological behavior. Polym. Degrad. Stab. 2006, 91, 1855–1860. [Google Scholar] [CrossRef]
- Lanham, B.J.; Titow, W.V. Extrusion of PVC-General Aspects. In PVC Technology, 4th ed.; Titow, V.W., Ed.; Springer: Dordrecht, The Netherlands, 1984; pp. 673–721. [Google Scholar]
- Potente, H.; Schultheis, S.M. Bestimmung des Geliergrades von PVC mit der DSC. Kunststoffe 1987, 77, 401–404. [Google Scholar]
- Zajchowski, S.; Piszczek, K.; Tomaszewska, J. Gelation of rigid PVC during processing. Polimery 2001, 46, 232–243. [Google Scholar] [CrossRef]
- Gilbert, M.; Hemsley, D.; Miadonye, A. Assesment of fusion in PVC compounds. Plast. Rubber Process. Appl. 1983, 3, 343–351. [Google Scholar]
- Kareem Alobad, Z.; Alraheem, H.S. Effect of additives on the Tg,Tp and Gelation Degree of the PVC Products. Int. J. Civ. Eng. Technol. 2018, 9, 2036–2043. [Google Scholar]
- Tomaszewska, J.; Sterzyński, T.; Piszczek, K. Rigid Poly(vinyl chloride) Gelation in a Brabender Measuring Mixer. III. Transformation in the Torque Maximum. J. Appl. Polym. Sci. 2007, 106, 3158–3164. [Google Scholar] [CrossRef]
- Fillot, L.-A.; Hajji, P.; Gauthier, C.; Maneselli-Varlot, K. U-PVC Gelation Level Assessment, Part 1: Comparison of Different Techniques. J. Vinyl Addit. Technol. 2006, 12, 98–107. [Google Scholar] [CrossRef]
- Wood-Imitating Composites of Poly(vinyl chloride) Resistant to Microorganisms and Method for Producing them. Polish Patent P. 228448, 15 November 2017.
- Elakesh, E.O.; Hull, T.R.; Price, D.; Carty, P. Thermal decomposition of chlorinated poly(vinyl chloride) (CPVC). J. Vinyl Technol. 2003, 9, 116–126. [Google Scholar] [CrossRef]
- Gilbert, M. Poly(vinyl chloride)(PVC)-based nanocomposites—Advances in Polymer Nanocomposites. In Advances in Polymer Nanocomposites. Types and Applications; Woodhead Publishing Series in Composites Science and Engineering; Gao, F., Ed.; Elsevier: Cambridge, UK, 2012; pp. 216–237. [Google Scholar]
- Abramowicz, A.; Obloj-Muzaj, M.; Zielecka, M. Poly(Vinyl Chloride) Nanocomposites Prepared in the Suspension Polymerization Process. Part II. PVC Filled with Silica Nanofiller. J. Mater. Sci. Eng. B 2014, 4, 210–219. [Google Scholar] [CrossRef] [Green Version]



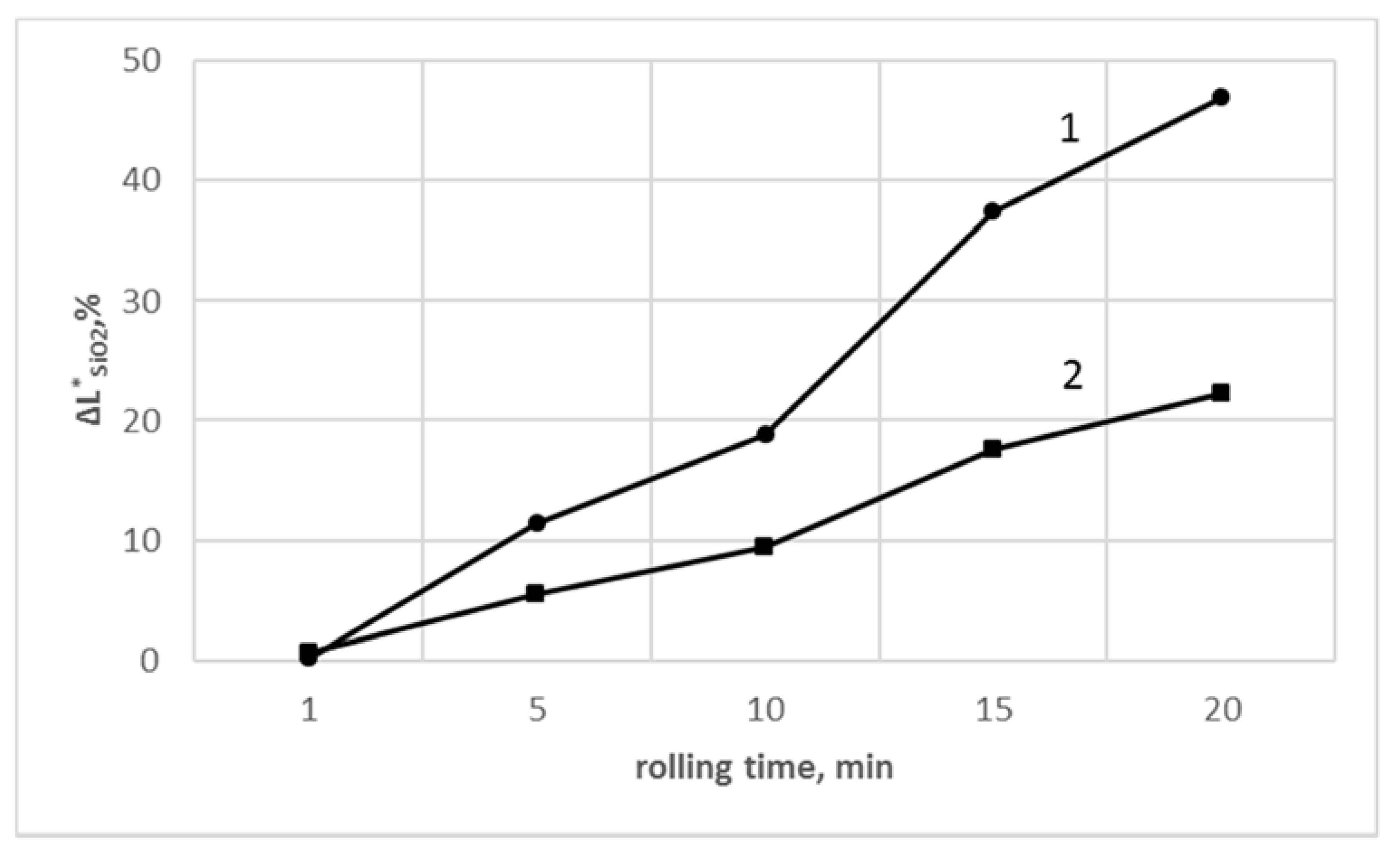
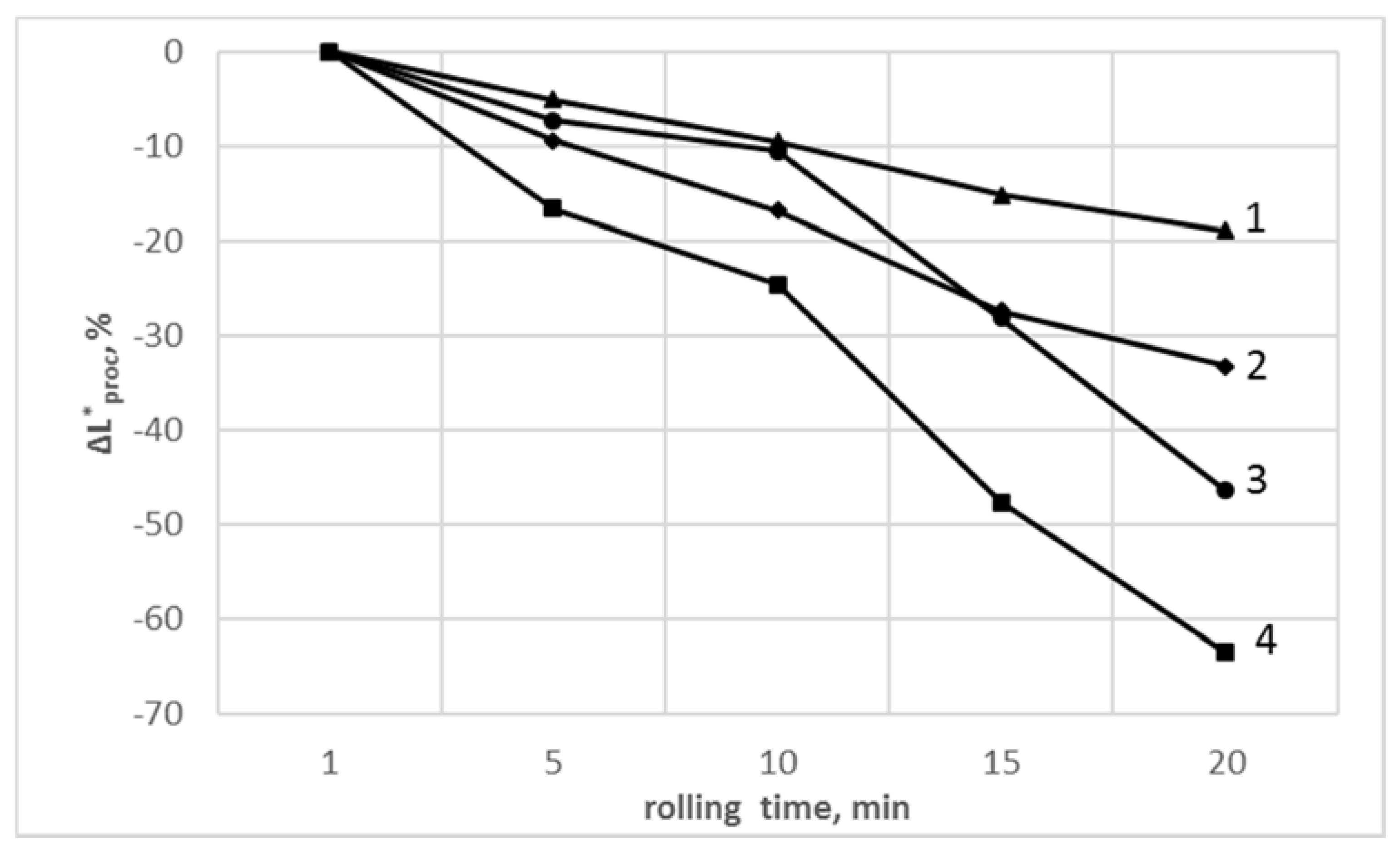
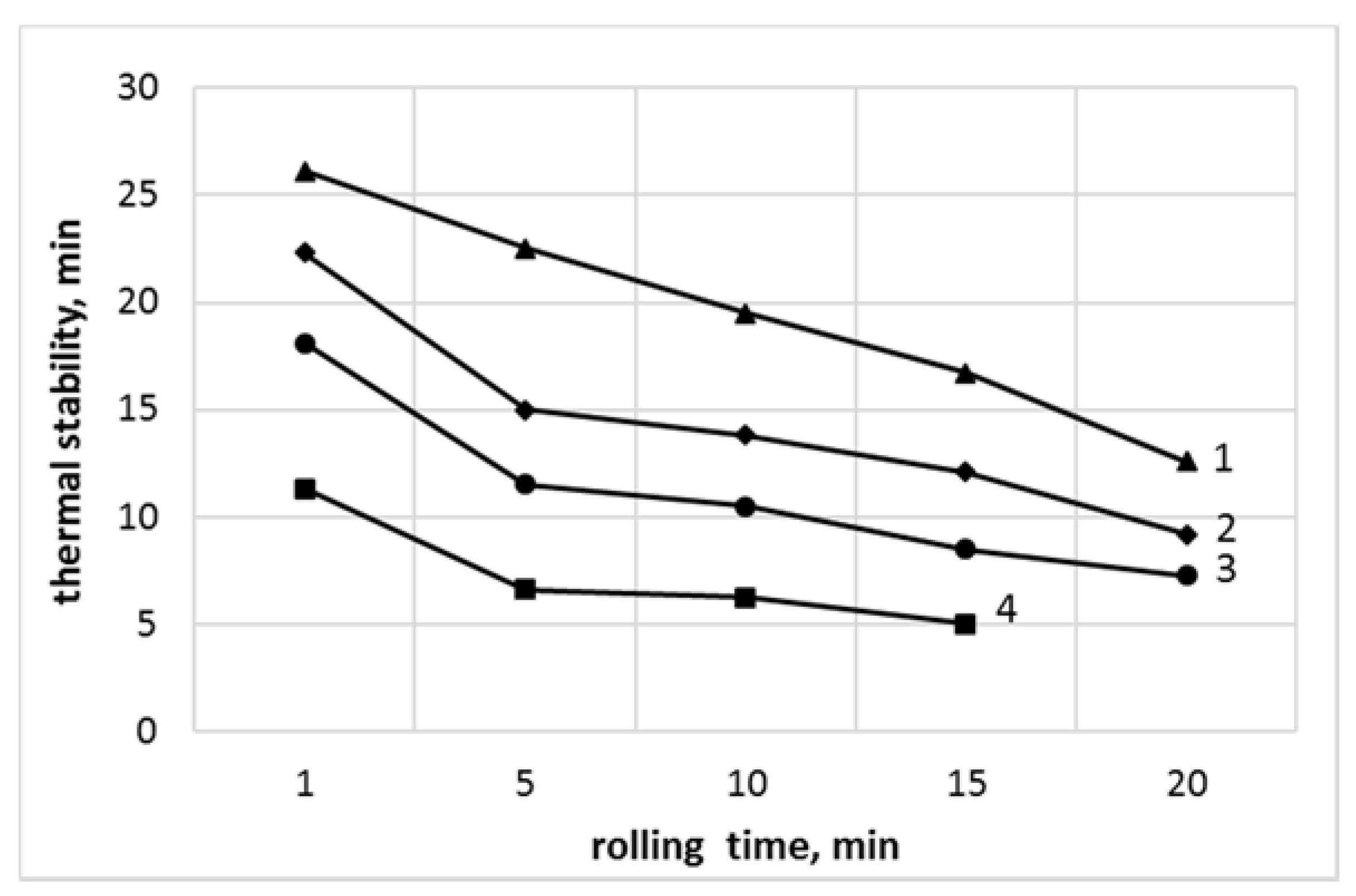
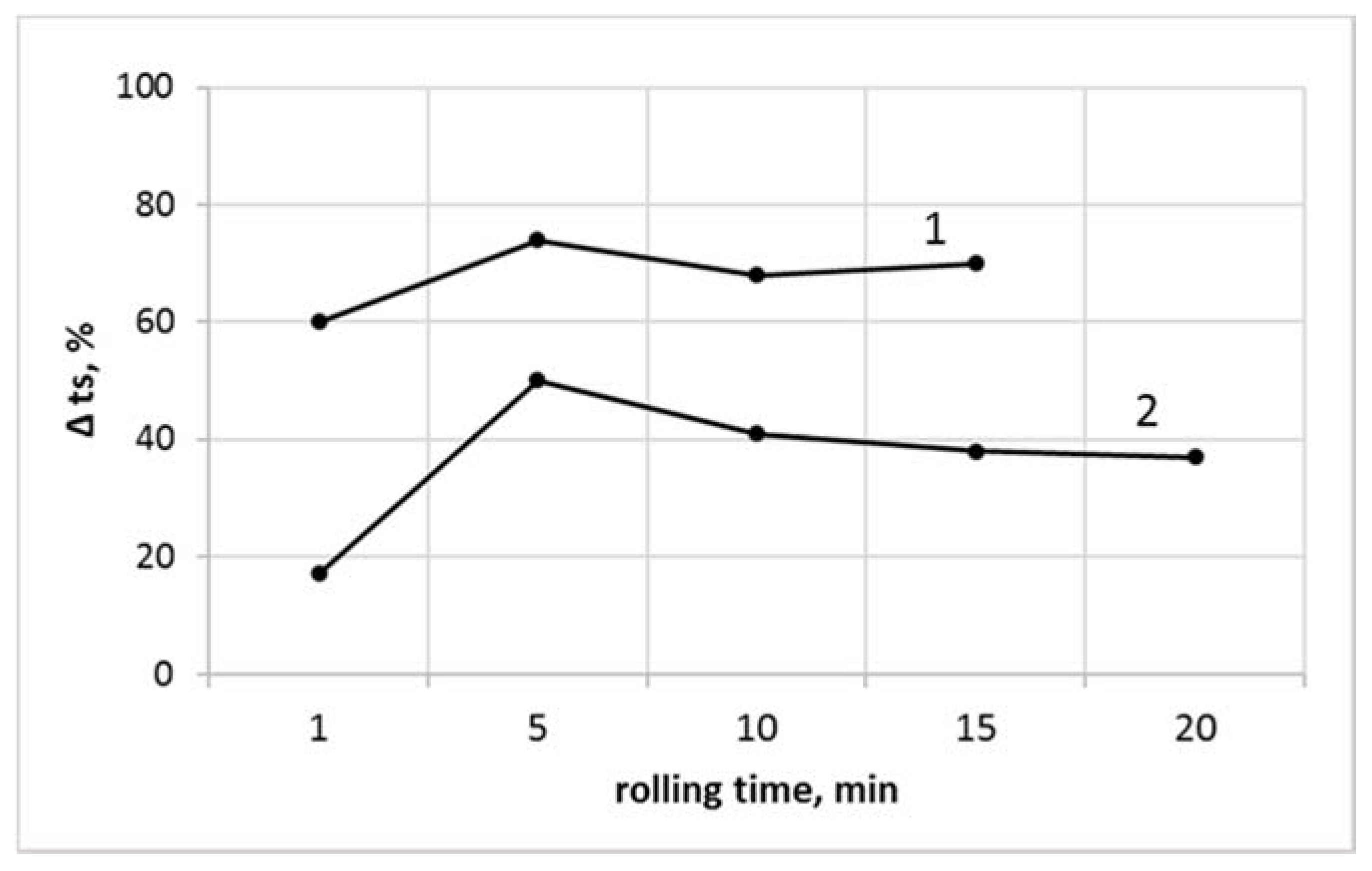
| Rolling Time, min | PVCR | PVCR/SiO2 | PVCC | PVCC/SiO2 |
|---|---|---|---|---|
| 1 | 91 | 98 | 88 | 84 |
| 5 | 96 | 98 | 94 | 93 |
| 10 | 97 | 97 | 98 | 95 |
| 15 | 98 | 97 | 99 | 97 |
| 20 | 98 | 97 | - | 98 |
| Sample | PVCC | PVCC/SiO2 | ||||
|---|---|---|---|---|---|---|
| Rolling time | L* | a* | b* | L* | a* | b* |
| 1 min | 87.33 | −2.85 | 9.27 | 87.49 | −2.81 | 5.30 |
| 5 min | 72.8 | 0.71 | 18.955 | 81.10 | −2.10 | 9.83 |
| 10 min | 65.85 | 5.03 | 26.43 | 78.26 | −1.13 | 6.21 |
| 15 min | 45.72 | 19.06 | 13.51 | 62.83 | 3.34 | 8.35 |
| 20 min | 31.87 | 6.22 | 0.5 | 46.84 | 5.99 | 12.55 |
| Sample | PVCR | PVCR/SiO2 | ||||
|---|---|---|---|---|---|---|
| Rolling time | L* | a* | b* | L* | a* | b* |
| 1 min | 85.51 | −1.19 | 0.09 | 86.09 | −2.00 | 0.16 |
| 5 min | 77.48 | −2.34 | 5.54 | 81.72 | −2.775 | 4.86 |
| 10 min | 71.16 | −2.58 | 8.49 | 77.89 | 0.69 | 9.03 |
| 15 min | 62.09 | −0.91 | 12.6 | 72.99 | 1.08 | 16.04 |
| 20 min | 57.08 | 4.88 | 20.61 | 69.77 | 4.55 | 8.51 |
| Sample | T1, °C | TDTG, °C |
|---|---|---|
| Reference PVC | ||
| PVCR, 1 min | 255.9 | 300.0 |
| PVCR, 10 min | 254.3 | 301.9 |
| PVCR/SiO2, 1 min | 258.2 | 300.7 |
| PVCR/SiO2 10 min | 257.5 | 301.6 |
| Commercial PVC | ||
| PVCC, 1 min | 245.6 | 289.4 |
| PVCC, 10 min | 243.9 | 288.1 |
| PVCC/SiO2, 1 min | 246.6 | 285.1 |
| PVCC/SiO2, 10 min | 245.6 | 285.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaszewska, J.; Sterzyński, T.; Walczak, D. Thermal Stability of Nanosilica-Modified Poly(vinyl chloride). Polymers 2021, 13, 2057. https://doi.org/10.3390/polym13132057
Tomaszewska J, Sterzyński T, Walczak D. Thermal Stability of Nanosilica-Modified Poly(vinyl chloride). Polymers. 2021; 13(13):2057. https://doi.org/10.3390/polym13132057
Chicago/Turabian StyleTomaszewska, Jolanta, Tomasz Sterzyński, and Damian Walczak. 2021. "Thermal Stability of Nanosilica-Modified Poly(vinyl chloride)" Polymers 13, no. 13: 2057. https://doi.org/10.3390/polym13132057
APA StyleTomaszewska, J., Sterzyński, T., & Walczak, D. (2021). Thermal Stability of Nanosilica-Modified Poly(vinyl chloride). Polymers, 13(13), 2057. https://doi.org/10.3390/polym13132057








