Development of Peptide-Based Nanoparticles for Mitochondrial Plasmid DNA Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Peptides
2.2.2. Formulation of Peptide/pND1 Complexes
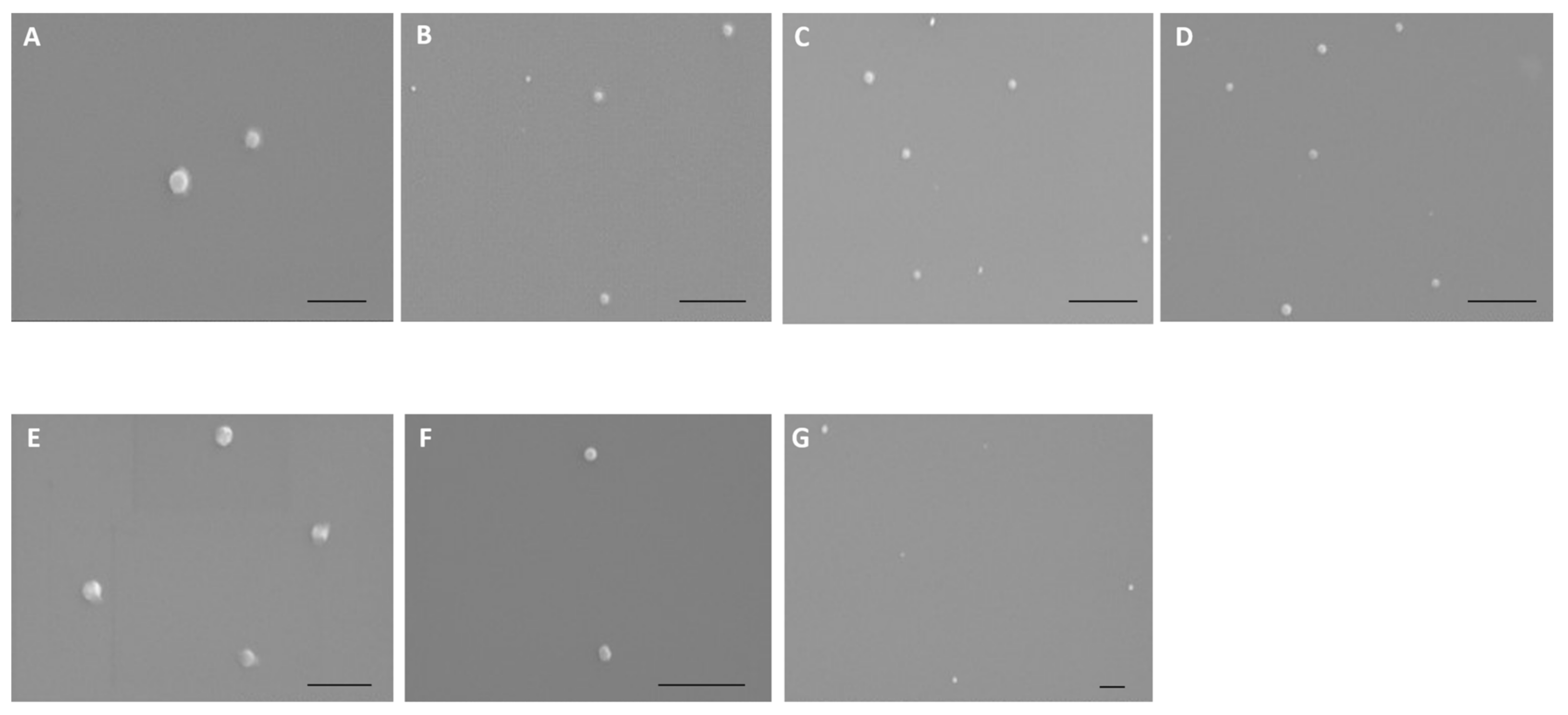
2.2.3. Scanning Electron Microscopy (SEM)
2.2.4. Particle Size and Zeta Potential Measurements
2.2.5. Cell Culture
2.2.6. Cytotoxicity Evaluation
2.2.7. Detection of Associated/Internalized pND1
2.2.8. Cellular Organelle-Associated Fluorescence
2.2.9. Fluorescence Confocal Microscopy
Live Cell Imaging
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Peptides
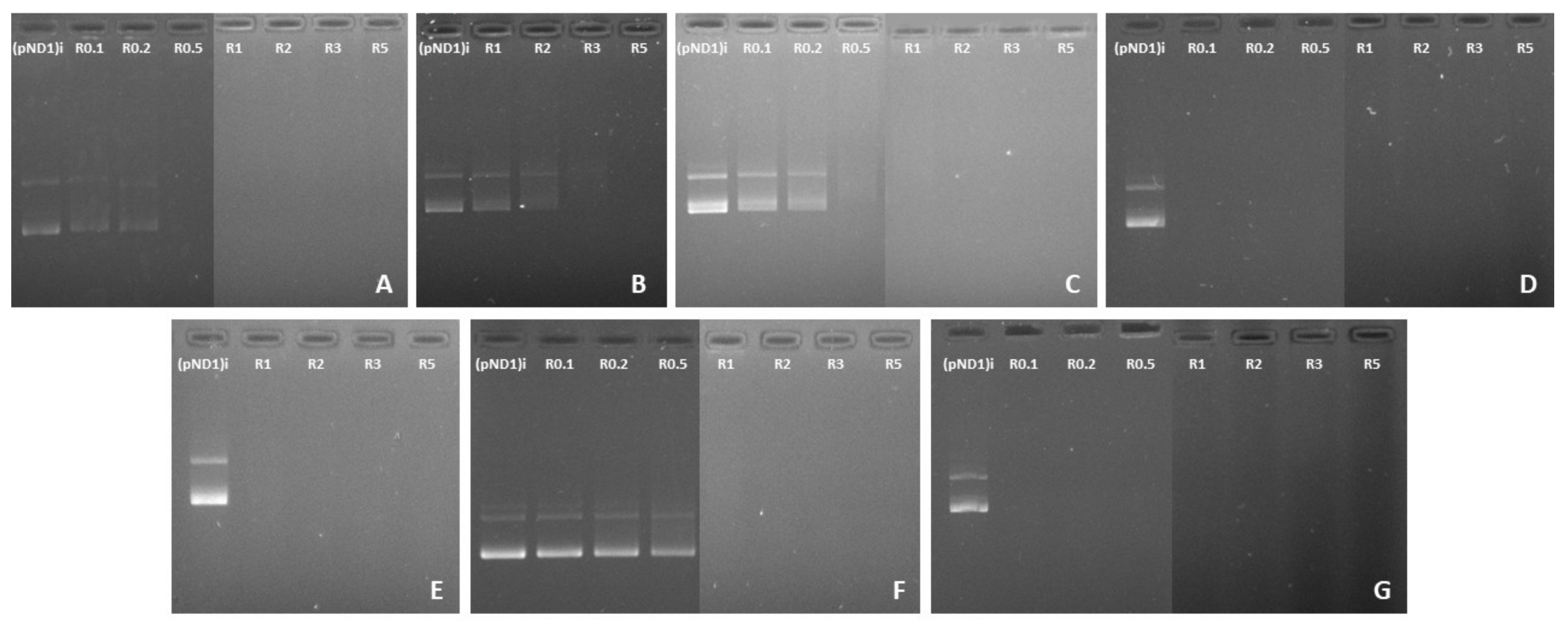
3.2. pND1 Complexation Capacity
3.3. Physicochemical Properties of Peptide/pND1 Complexes
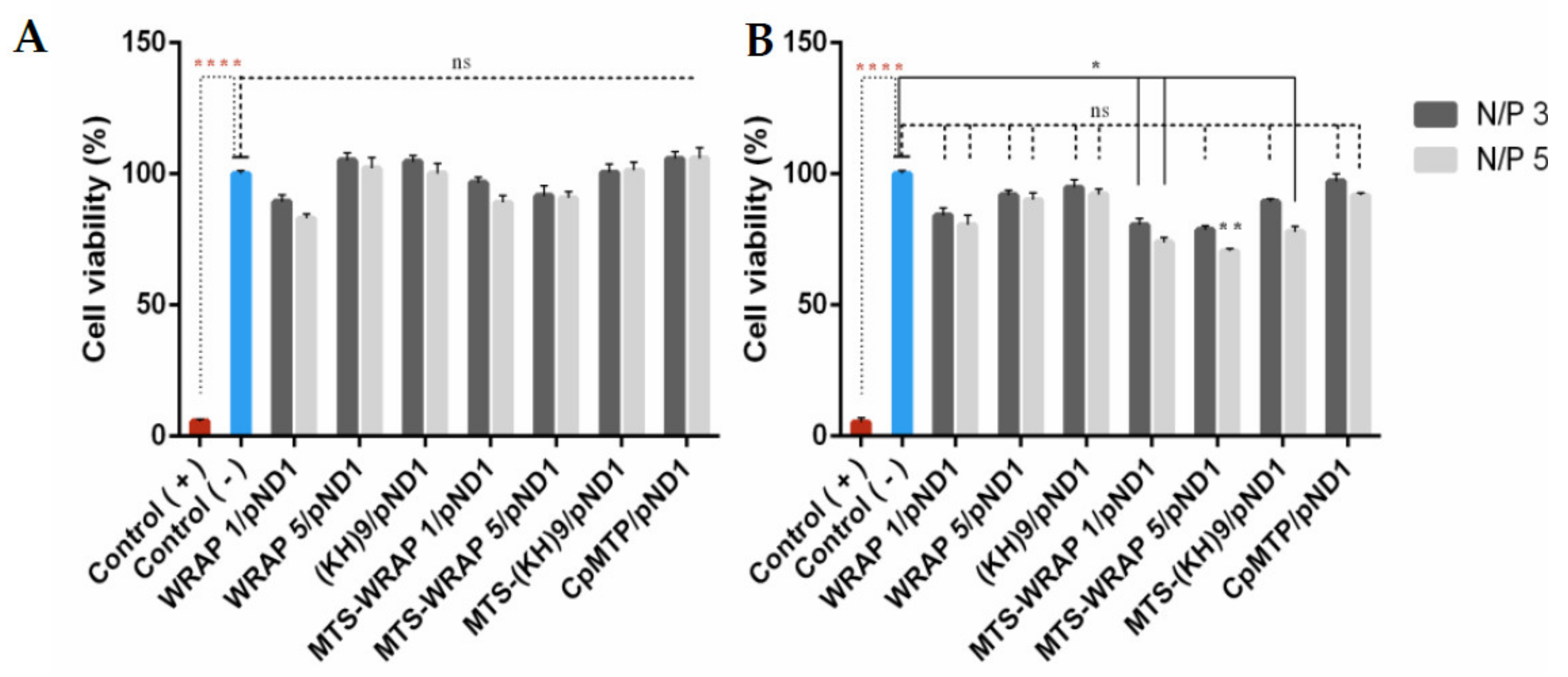
3.4. Cytotoxic Profile
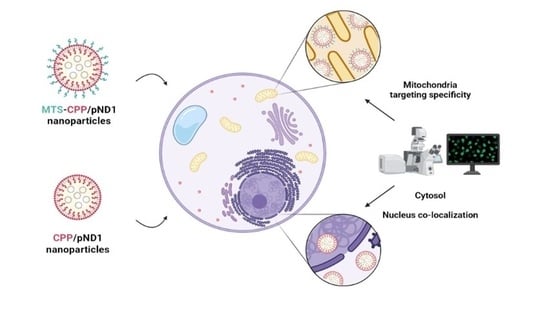
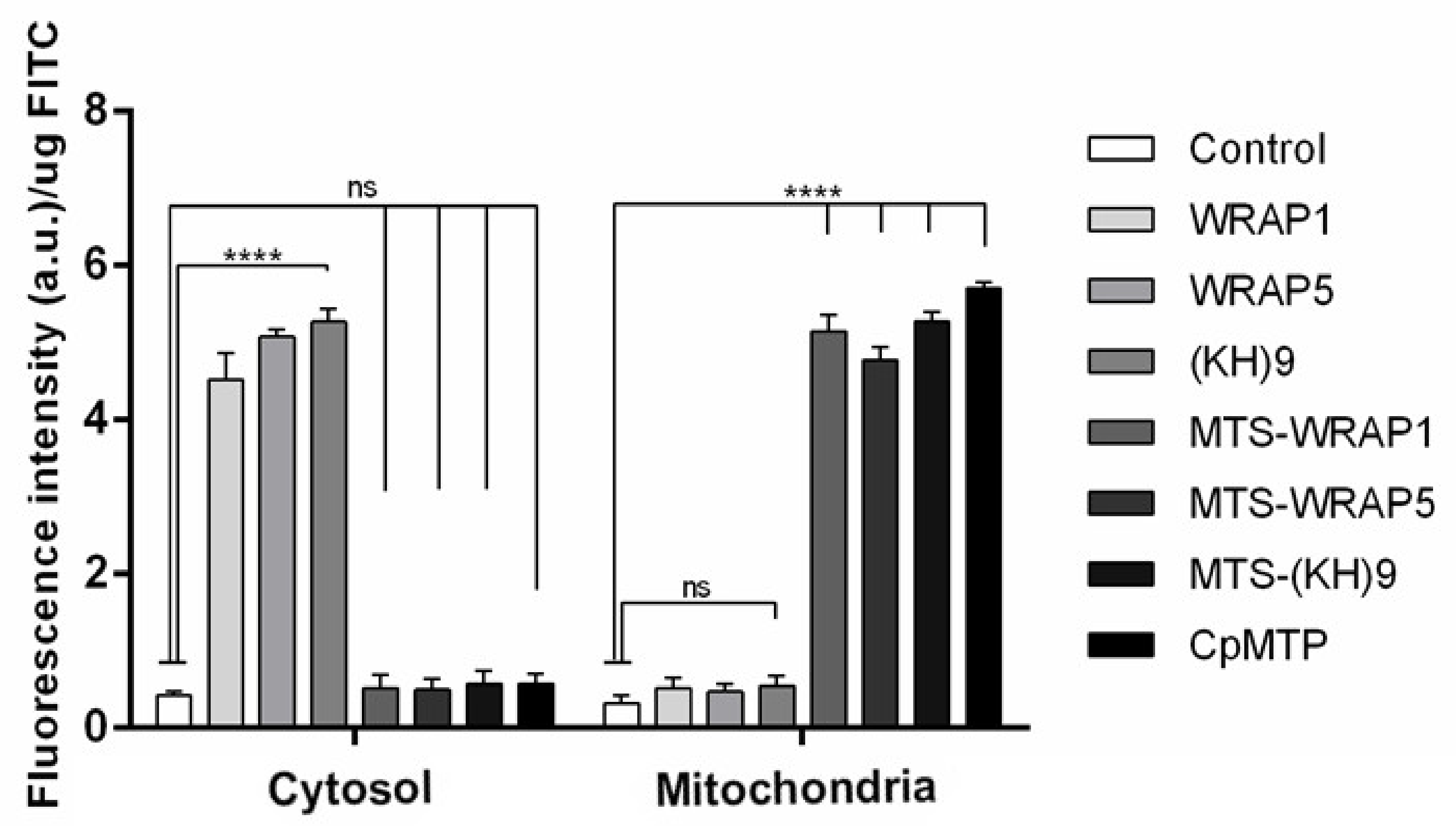
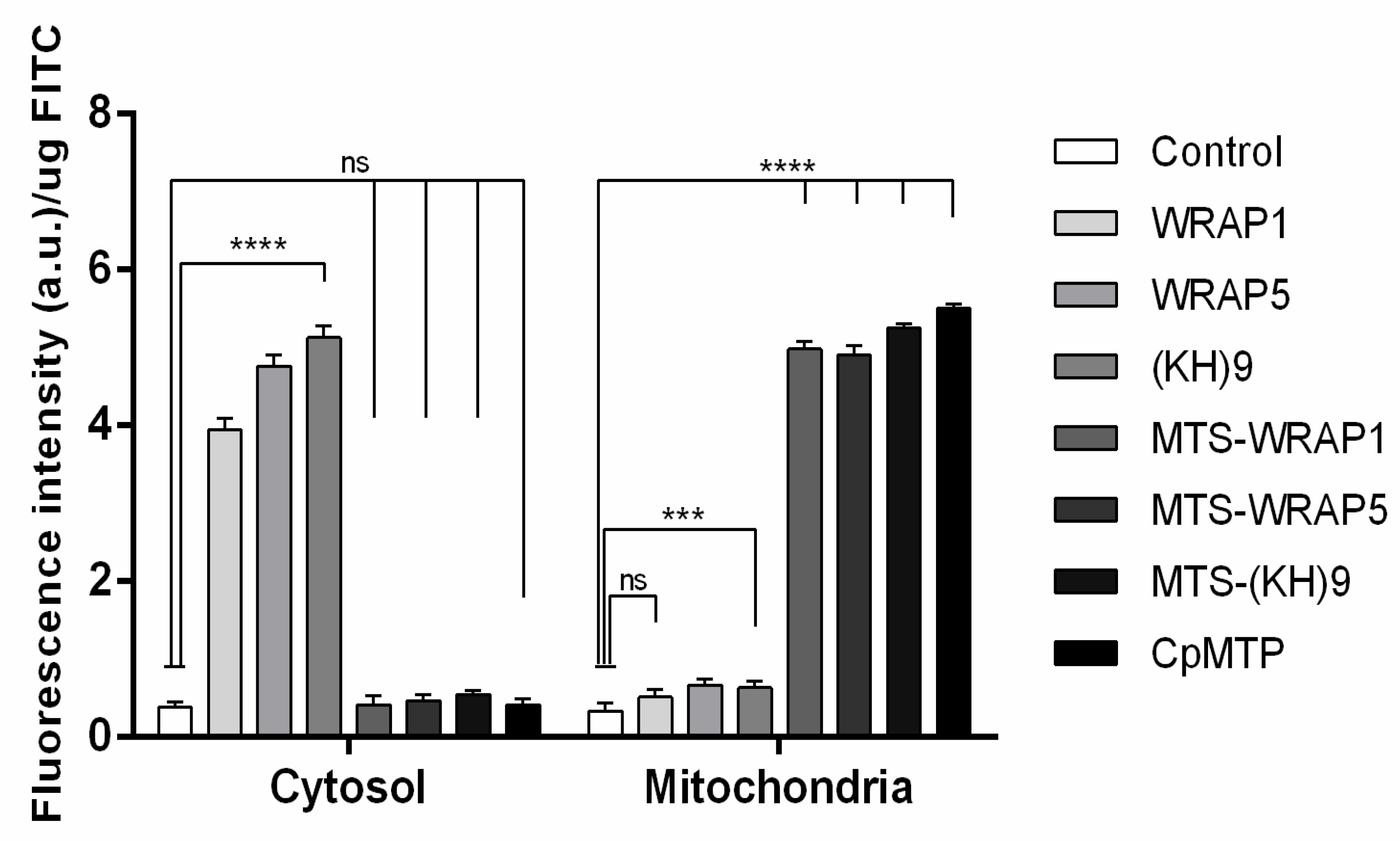
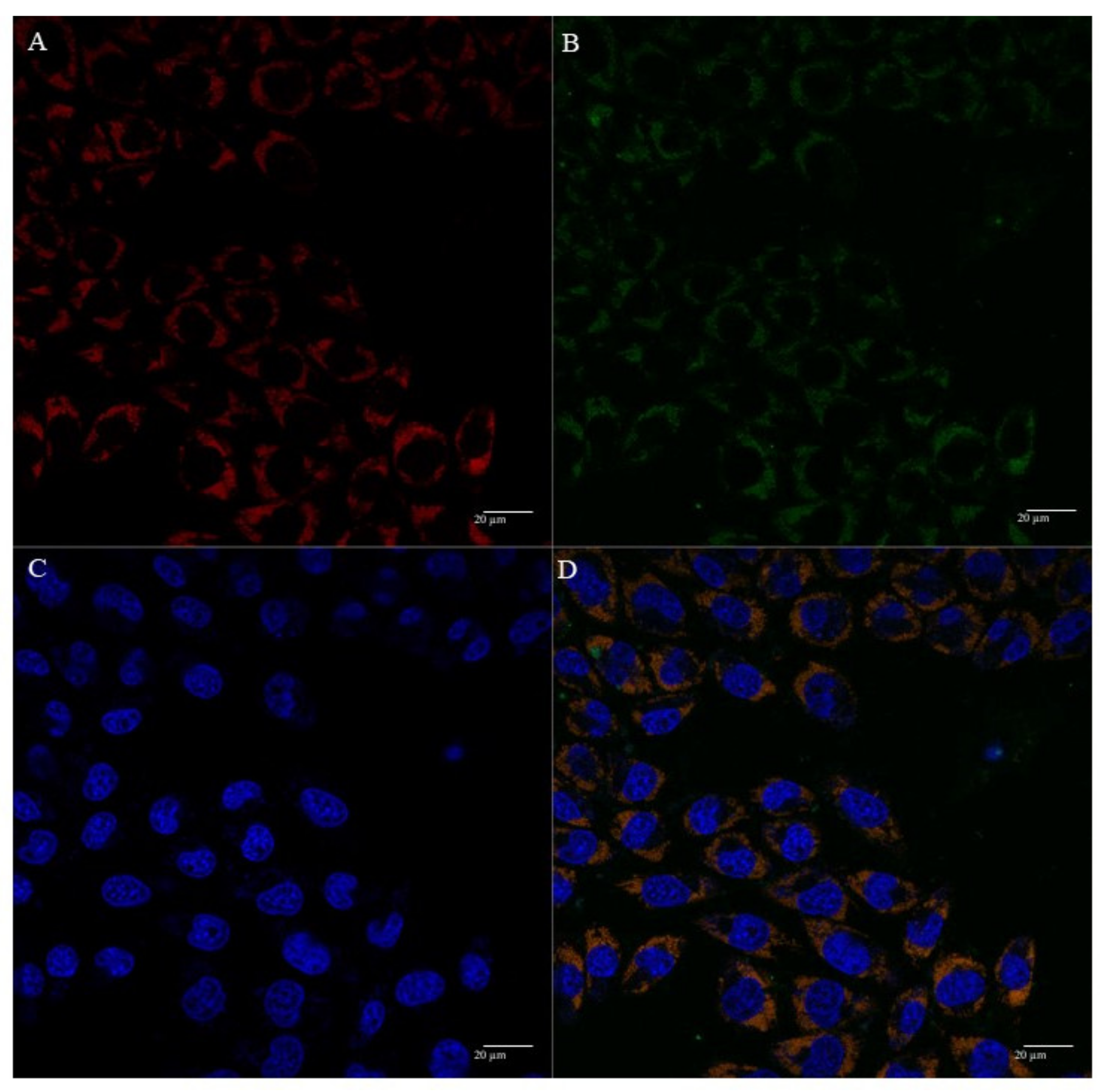
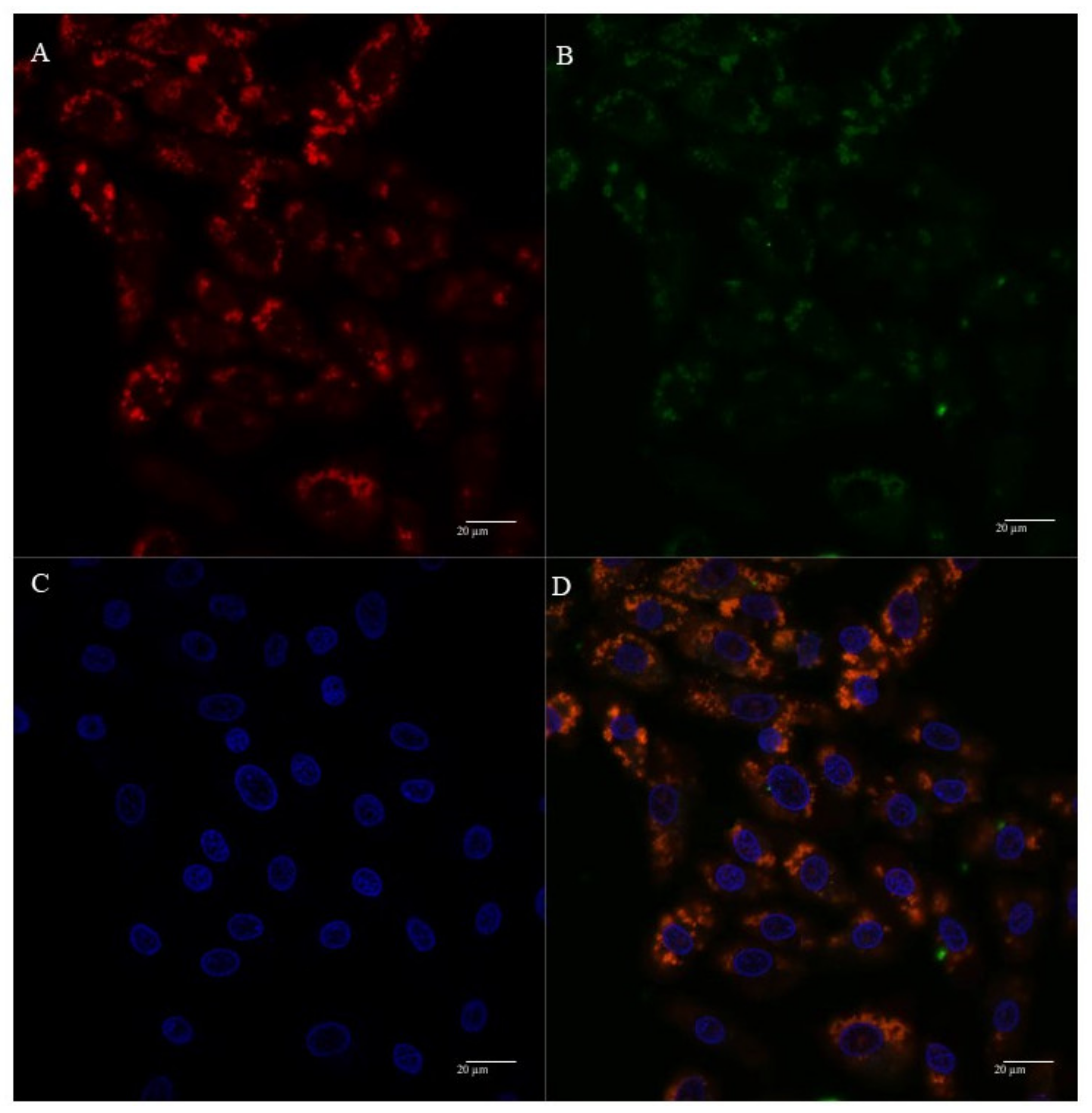
3.5. Mitochondria Targeting Ability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milenkovic, D.; Blaza, J.; Larsson, N.-G.; Hirst, J. The Enigma of the Respiratory Chain Supercomplex. Cell Metab. 2017, 25, 765–776. [Google Scholar] [CrossRef]
- Hertweck, K.L.; Dasgupta, S. The Landscape of mtDNA Modifications in Cancer: A Tale of Two Cities. Front. Oncol. 2017, 7, 262. [Google Scholar] [CrossRef] [PubMed]
- Popov, L. Mitochondrial biogenesis: An update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Javadov, S.; Kozlov, A.V.; Camara, A.K.S. Mitochondria in Health and Diseases. Cells 2020, 9, 1177. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Prasad, S.; Tripathi, A.; Pandey, A.N.; Ali, I.; Singh, A.K.; Shrivastav, T.G.; Chaube, S.K. Apoptosis in mammalian oocytes: A review. Apoptosis 2015, 20, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Tavassoly, I.; Parmar, J.; Shajahan-Haq, A.; Clarke, R.; Baumann, W.T.; Tyson, J.J. Dynamic Modeling of the Interaction Between Autophagy and Apoptosis in Mammalian Cells. CPT Pharmacomet. Syst. Pharmacol. 2015, 4, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska, K.; Szewczuk, M.; Kaźmierczak-Barańska, J.; Karwowski, B.T. The Similarities between Human Mitochondria and Bacteria in the Context of Structure, Genome, and Base Excision Repair System. Molecules 2020, 25, 2857. [Google Scholar] [CrossRef]
- Filograna, R.; Mennuni, M.; Alsina, D.; Larsson, N. Mitochondrial DNA copy number in human disease: The more the better? FEBS Lett. 2021, 595, 976–1002. [Google Scholar] [CrossRef]
- Monlleo-Neila, L.; Del Toro, M.; Bornstein, B.; Garcia-Arumi, E.; Sarrias, A.; Roig-Quilis, M.; Munell, F. Leigh syndrome and the mitochondrial m.13513G ˃ A mutation: Expanding the clinical spectrum. J. Child Neurol. 2013, 28, 1531–1534. [Google Scholar] [CrossRef]
- Zsurka, G.; Kunz, W.S. Mitochondrial dysfunction and seizures: The neuronal energy crisis. Lancet Neurol. 2015, 14, 956–966. [Google Scholar] [CrossRef]
- Rodenburg, R.J. Mitochondrial complex I-linked disease. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 938–945. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Martín-Jiménez, R.; Lurette, O.; Hebert-Chatelain, E. Damage in Mitochondrial DNA Associated with Parkinson’s Disease. DNA Cell Biol. 2020, 39, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Mingliang, G.; Zhang, Y.; Wang, P.; Wu, T.; Lin, J.; Yu, J.; Gu, M. Mitochondrial DNA Mutations Associated with Type 2 Diabetes Mellitus in Chinese Uyghur Population. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pinti, M.V.; Fink, G.K.; Hathaway, Q.; Durr, A.J.; Kunovac, A.; Hollander, J.M. Mitochondrial dysfunction in type 2 diabetes mellitus: An organ-based analysis. Am. J. Physiol. Metab. 2019, 316, E268–E285. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.S.; Alexandrov, L.B.; Gerstung, M.; Martin, S.; Nik-Zainal, S.; Ramakrishna, M.; Davies, H.R.; Papaemmanuil, E.; Gundem, G.; Shlien, A.; et al. Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. eLife 2014, 3. [Google Scholar] [CrossRef]
- Prag, H.A.; Murphy, M.P. mtDNA mutations help support cancer cells. Nat. Rev. Cancer 2020, 1, 941–942. [Google Scholar] [CrossRef]
- Coutinho, E.; Batista, C.; Sousa, F.; Queiroz, J.; Costa, D. Mitochondrial Gene Therapy: Advances in Mitochondrial Gene Cloning, Plasmid Production, and Nanosystems Targeted to Mitochondria. Mol. Pharm. 2017, 14, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Faria, R.; Albuquerque, T.; Neves, A.R.; Bhatt, H.; Biswas, S.; Cardoso, A.M.; de Lima, M.C.P.; Jurado, A.S.; Costa, D. Physicochemical characterization and targeting performance of triphenylphosphonium nano-polyplexes. J. Mol. Liq. 2020, 316, 113873. [Google Scholar] [CrossRef]
- Kawamura, E.; Maruyama, M.; Abe, J.; Sudo, A.; Takeda, A.; Takada, S.; Yokota, T.; Kinugawa, S.; Harashima, H.; Yamada, Y. Validation of Gene Therapy for Mutant Mitochondria by Delivering Mitochondrial RNA Using a MITO-Porter. Mol. Ther. Nucleic Acids 2020, 20, 687–698. [Google Scholar] [CrossRef]
- Costa, D.; Costa, C.; Caldeira, M.; Cortes, L.; Queiroz, J.A.; Cruz, C. Targeting of celular organelles by fluorescente plasmid DNA nanoparticles. Biomacromolecules 2017, 18, 2928–2936. [Google Scholar] [CrossRef]
- Li, Y.; Thambi, T.; Lee, D.S. Co-Delivery of Drugs and Genes Using Polymeric Nanoparticles for Synergistic Cancer Therapeutic Effects. Adv. Heal. Mater. 2018, 7, 1700886. [Google Scholar] [CrossRef]
- Faria, R.; Sousa, Â.; Neves, A.R.; Queiroz, J.; Costa, D. Methotrexate-plasmid DNA polyplexes for cancer therapy: Characterization, cancer cell targeting ability and tuned in vitro transfection. J. Mol. Liq. 2019, 292, 111391. [Google Scholar] [CrossRef]
- Chen, W.; Liu, J.; Wang, Y.; Jiang, C.; Yu, B.; Sun, Z.; Lu, L. AC5N2 Nanoparticle Based Direct Nucleus Delivery Platform for Synergistic Cancer Therapy. Angew. Chem. Int. Ed. 2019, 58, 6290–6294. [Google Scholar] [CrossRef]
- Wang, H.; Ding, S.; Zhang, Z.; Wang, L.; You, Y. Cationic micelle: A promising nanocarrier for gene delivery with high transfection efficiency. J. Gene Med. 2019, 21, e3101. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, K.; Zhao, J.; Gao, B.; Feng, Y. Polymeric nano-carriers for on-demand delivery of genes via specific responses to stimuli. J. Mater. Chem. B 2020, 8, 9621–9641. [Google Scholar] [CrossRef]
- Gómez-Aguado, I.; Rodríguez-Castejón, J.; Vicente-Pascual, M.; Rodríguez-Gascón, A.; Del Pozo-Rodríguez, A.; Aspiazu, M.; Ángeles, S. Nucleic Acid Delivery by Solid Lipid Nanoparticles Containing Switchable Lipids: Plasmid DNA vs. Messenger RNA. Molecules 2020, 25, 5995. [Google Scholar] [CrossRef]
- Neves, A.; Sousa, A.; Faria, R.; Albuquerque, T.; Queiroz, J.; Costa, D. Cancer gene therapy mediated by RALA/plasmid DNA vectors: Nitrogen to phosphate groups ratio (N/P) as a tool for tunable transfection efficiency and apoptosis. Colloids Surf. B Biointerfaces 2020, 185, 110610. [Google Scholar] [CrossRef]
- Sousa, Â.; Almeida, A.M.; Faria, R.; Konate, K.; Boisguerin, P.; Queiroz, J.; Costa, D. Optimization of peptide-plasmid DNA vectors formulation for gene delivery in cancer therapy exploring design of experiments. Colloids Surf. B Biointerfaces 2019, 183, 110417. [Google Scholar] [CrossRef]
- Deshayes, S.; Konate, K.; Dussot, M.; Chavey, B.; Vaissiére, A.; Van, T.N.N.; Aldrian, G.; Padari, K.; Pooga, M.; Vivès, E.; et al. Deciphering the internalization mechanism of WRAP:siRNA nanoparticles. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183252. [Google Scholar] [CrossRef] [PubMed]
- Konate, K.; Dussot, M.; Aldrian, G.; Vaissière, A.; Viguier, V.; Neira, I.F.; Couillaud, F.; Vivès, E.; Boisguerin, P.; Deshayes, S. Peptide-based nanoparticles to rapidly and efficiently “Wrap‘n roll” siRNA into cells. Bioconjugate Chem. 2019, 30, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Lombardi, L.; Galdiero, E.; Del Genio, V.; Galdiero, S. The world of cell penetrating: The future of medical applications. Futur. Med. Chem. 2020, 12, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Ruseska, I.; Zimmer, A. Internalization mechanisms of cell-penetrating peptides. Beilstein J. Nanotechnol. 2020, 11, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Derakhshankhah, H.; Jafari, S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed. Pharmacother. 2018, 108, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Gessner, I.; Neundorf, I. Nanoparticles Modified with Cell-Penetrating Peptides: Conjugation Mechanisms, Physicochemical Properties, and Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2020, 21, 2536. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Filipczak, N.; Torchilin, V.P. Cell penetrating peptides: A versatile vector for co-delivery of drug and genes in cancer. J. Control. Release 2021, 330, 1220–1228. [Google Scholar] [CrossRef]
- Leng, Q.; Goldgeier, L.; Zhu, J.; Cambell, P.; Ambulos, N.; Mixson, A. Histidine-lysine peptides as carriers of nucleic acids. Drug News Perspect. 2007, 20, 77–86. [Google Scholar] [CrossRef]
- Chuah, J.-A.; Matsugami, A.; Hayashi, F.; Numata, K. Self-Assembled Peptide-Based System for Mitochondrial-Targeted Gene Delivery: Functional and Structural Insights. Biomacromolecules 2016, 17, 3547–3557. [Google Scholar] [CrossRef]
- Jain, A.; Chugh, A. Mitochondrial transit peptide exhibits cell penetration ability and efficiently delivers macromolecules to mitochondria. FEBS Lett. 2016, 590, 2896–2905. [Google Scholar] [CrossRef]
- Cohen-Erez, I.; Harduf, N.; Rapaport, H. Oligonucleotide loaded polypeptide-peptide nanoparticles towards mitochondrial-targeted delivery. Polym. Adv. Technol. 2019, 30, 2506–2514. [Google Scholar] [CrossRef]
- Slone, J.; Huang, T. The special considerations of gene therapy for mitochondrial diseases. NPJ Genom. Med. 2020, 5, 1–7. [Google Scholar] [CrossRef]
- Park, D.; Lee, S.; Min, K.T. Techniques for investigating mitochondrial gene expression. BMB Rep. 2020, 53, 3–9. [Google Scholar] [CrossRef]
- Cerrato, C.P.; Kivijärvi, T.; Tozzi, R.; Lehto, T.; Gestin, M.; Langel, Ü. Intracellular delivery of therapeutic antisense oligonucleotides targeting mRNA coding mitochondrial proteins by cell-penetrating peptides. J. Mater. Chem. B 2020, 8, 10825–10836. [Google Scholar] [CrossRef]
- Su, S.; Cao, S.; Liu, J.; Xu, X. Mitochondria-specific delivery system for targeted regulation of mitochondrial gene expression. STAR Protoc. 2021, 2, 100275. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef]
- Costa, D.; Valente, A.J.; Queiroz, J.; Sousa, Â. Finding the ideal polyethylenimine-plasmid DNA system for co-delivery of payloads in cancer therapy. Colloids Surf. B Biointerfaces 2018, 170, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Characterization of nanoparticles intended for drug delivery. In Methods in Molecular Biology; Christine, V., Gilles, P., Eds.; Springer: Cham, Switzerland, 2011; Volume 697, 641p. [Google Scholar]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Zhang, P.-Y. Mitochondria targeting nano agents in cancer therapeutics. Oncol. Lett. 2016, 12, 4887–4890. [Google Scholar] [CrossRef] [PubMed]
- Ozsvari, B.; Sotgia, F.; Lisanti, M.P. Exploiting mitochondrial targeting signal(s), TPP and bis-TPP, for eradicating cancer stem cells (CSCs). Aging 2018, 10, 229–240. [Google Scholar] [CrossRef]
- Jang, Y.-H.; Lim, K.-I. Recent Advances in Mitochondria-Targeted Gene Delivery. Molecules 2018, 23, 2316. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.M.; Morais, C.M.; Cruz, A.R.; Silva, S.G.; Vale, M.L.D.; Marques, E.; de Lima, M.P.; Jurado, A.S. Gemini Surfactants Mediate Efficient Mitochondrial Gene Delivery and Expression. Mol. Pharm. 2015, 12, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, B.; Choi, Y.; Jin, H.; Lim, N.Y.; Park, J.; Kim, J.H.; Bae, J.; Jung, J.H. Non-peptidic guanidinium-functionalized silica nanoparticles as selective mitochondria-targeting drug nanocarriers. J. Mater. Chem. B 2018, 6, 5698–5707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-J.; Li, R.-F.; Xu, L.; Yin, H.; Chen, L.; Yuan, Y.; Zhong, W.; Lin, J. A Novel Self-Assembled Mitochondria-Targeting Protein Nanoparticle Acting as Theranostic Platform for Cancer. Small 2018, 15, e1803428. [Google Scholar] [CrossRef]


| Peptide | Peptide Sequence | Total Residues | Isotopic Mass (g/mol) | Positive Charges |
|---|---|---|---|---|
| MTS | NH2-MLSLRQSIRFFK-CONH2 | 12 | 1523.88 | 3 |
| WRAP1 | NH2-LLWRLWRLLWRLWRLL-CONH2 | 16 | 2290.42 | 5 |
| WRAP5 | NH2-LLRLLRWWWRLLRLL-CONH2 | 15 | 2104.34 | 5 |
| (KH)9 | NH2-KHKHKHKHKHKHKHKHKH-CONH2 | 18 | 2403.41 | 9 |
| CpMTP | NH2-ARLLWLLRGLTLGTAPRRA-CONH2 | 19 | 2132.32 | 4 |
| MTS-WRAP1 | NH2-MLSLRQSIRFFK-LLWRLWRLLWRLWRLL-CONH2 | 28 | 3797.27 | 8 |
| MTS-WRAP5 | NH2-MLSLRQSIRFFK-LLRLLRWWWRLLRLL-CONH2 | 27 | 3611.19 | 8 |
| MTS-(KH)9 | NH2-MLSLRQSIRFFK-KHKHKHKHKHKHKHKHKH-CONH2 | 30 | 3910.26 | 12 |
| System | Zeta (mV) | Size (nm) | PdI |
|---|---|---|---|
| CpMTP/pND1 N/P 0.5 | −3.50 ± 0.76 | 492.00 ± 29.58 | 0.60 ± 0.1 |
| CpMTP/pND1 N/P 1 | +2.00 ± 0.58 | 401.50 ± 25.12 | 0.52 ± 0.5 |
| CpMTP/pND1 N/P 2 | +3.50 ± 0.50 | 384.50 ± 14.26 | 0.24 ± 0.04 |
| CpMTP/pND1 N/P 3 | +5.83 ± 0.69 | 313.17 ± 10.34 | 0.30 ± 0.02 |
| CpMTP/pND1 N/P 5 | +12.67 ± 0.75 | 235.67 ± 12.60 | 0.21 ± 0.02 |
| WRAP1/pND1 N/P 3 | +25.17 ± 0.69 | 254.17 ± 13.90 | 0.34 ± 0.05 |
| WRAP1/pND1 N/P 5 | +32.67 ± 0.47 | 161.00 ± 8.82 | 0.30 ± 0.02 |
| WRAP5/pND1 N/P 0.5 | +1.17 ± 0.37 | 401.00 ± 19.15 | 0.54 ± 0.1 |
| WRAP5/pND1 N/P 1 | +2.00 ± 0.58 | 388.33 ± 14.75 | 0.31 ± 0.02 |
| WRAP5/pND1 N/P 2 | +10.83 ± 1.21 | 298.50 ± 12.96 | 0.33 ± 0.01 |
| WRAP5/pND1 N/P 3 | +14.17 ± 0.90 | 272.33 ± 10.70 | 0.24 ± 0.02 |
| WRAP5/pND1 N/P 5 | +20.67 ± 0.75 | 186.00 ± 9.82 | 0.32 ± 0.03 |
| (KH)9/pND1 N/P 0.1 | −2.50 ± 0.76 | 488.17 ± 22.34 | 0.62 ± 0.05 |
| (KH)9/pND1 N/P 0.2 | +1.50 ± 0.50 | 412.67 ± 18.60 | 0.53 ± 0.1 |
| (KH)9/pND1 N/P 0.5 | +2.67 ± 0.47 | 403.33 ± 15.75 | 0.41 ± 0.04 |
| (KH)9/pND1 N/P 1 | +4.50 ± 0.50 | 375.67 ± 14.25 | 0.24 ± 0.02 |
| (KH)9/pND1 N/P 2 | +6.00 ± 0.58 | 299.67 ± 11.25 | 0.33 ± 0.02 |
| (KH)9/pND1 N/P 3 | +11.83 ± 1.34 | 260.50 ± 9.76 | 0.21 ± 0.03 |
| (KH)9/pND1 N/P 5 | +22.33 ± 0.75 | 186.33 ± 9.49 | 0.34 ± 0.02 |
| MTS-WRAP1/pND1 N/P 1 | −1.83 ± 0.90 | 406.00 ± 18.63 | 0.42 ± 0.06 |
| MTS-WRAP1/pND1 N/P 2 | +1.33 ± 0.75 | 366.50 ± 14.38 | 0.30 ± 0.02 |
| MTS-WRAP1/pND1 N/P 3 | +6.50 ± 0.76 | 276.50 ± 13.12 | 0.21 ± 0.01 |
| MTS-WRAP1/pND1 N/P 5 | +11.50 ± 0.76 | 197.33 ± 8.49 | 0.20 ± 0.02 |
| MTS-WRAP5/pND1 N/P 1 | −2.17 ± 0.90 | 399.00 ± 12.41 | 0.44 ± 0.04 |
| MTS-WRAP5/pND1 N/P 2 | +7.17 ± 0.90 | 316.17 ± 10.21 | 0.27 ± 0.03 |
| MTS-WRAP5/pND1 N/P 3 | +10.83 ± 1.07 | 266.50 ± 8.22 | 0.31 ± 0.02 |
| MTS-WRAP5/pND1 N/P 5 | +19.33 ± 1.60 | 175.17 ± 10.86 | 0.32 ± 0.03 |
| MTS-(KH)9/pND1 N/P 0.1 | −3.33 ± 0.94 | 478.50 ± 22.43 | 0.61 ± 0.05 |
| MTS-(KH)9/pND1 N/P 0.2 | −1.00 ± 1.00 | 463.50 ± 20.63 | 0.63 ± 0.03 |
| MTS-(KH)9/pND1 N/P 0.5 | +1.83 ± 0.37 | 431.00 ± 18.63 | 0.52 ± 0.04 |
| MTS-(KH)9/pND1 N/P 1 | +3.17 ± 0.69 | 400.33 ± 14.25 | 0.44 ± 0.03 |
| MTS-(KH)9/pND1 N/P 2 | +5.67 ± 0.75 | 366.67 ± 11.49 | 0.30 ± 0.03 |
| MTS-(KH)9/pND1 N/P 3 | +8.50 ± 0.50 | 309.17 ± 9.34 | 0.22 ± 0.04 |
| MTS-(KH)9/pND1 N/P 5 | +14.67 ± 0.75 | 220.67 ± 9.29 | 0.24 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, R.; Vivés, E.; Boisguerin, P.; Sousa, A.; Costa, D. Development of Peptide-Based Nanoparticles for Mitochondrial Plasmid DNA Delivery. Polymers 2021, 13, 1836. https://doi.org/10.3390/polym13111836
Faria R, Vivés E, Boisguerin P, Sousa A, Costa D. Development of Peptide-Based Nanoparticles for Mitochondrial Plasmid DNA Delivery. Polymers. 2021; 13(11):1836. https://doi.org/10.3390/polym13111836
Chicago/Turabian StyleFaria, Rúben, Eric Vivés, Prisca Boisguerin, Angela Sousa, and Diana Costa. 2021. "Development of Peptide-Based Nanoparticles for Mitochondrial Plasmid DNA Delivery" Polymers 13, no. 11: 1836. https://doi.org/10.3390/polym13111836
APA StyleFaria, R., Vivés, E., Boisguerin, P., Sousa, A., & Costa, D. (2021). Development of Peptide-Based Nanoparticles for Mitochondrial Plasmid DNA Delivery. Polymers, 13(11), 1836. https://doi.org/10.3390/polym13111836