Recent Developments in Inonotus obliquus (Chaga mushroom) Polysaccharides: Isolation, Structural Characteristics, Biological Activities and Application
Abstract
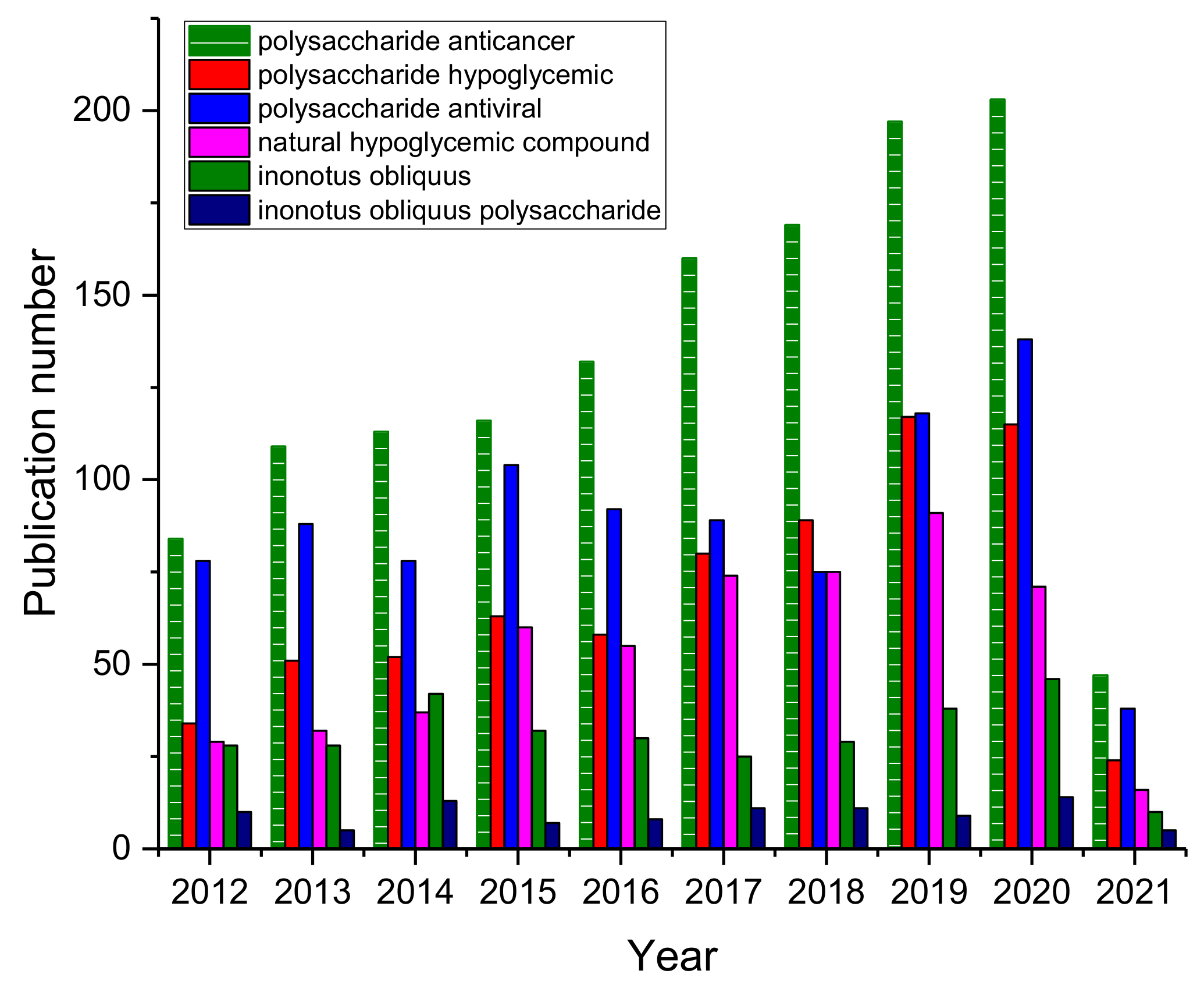
1. Introduction
2. Extraction and Purification Methods
2.1. Extraction of IOPS
2.2. Purification Method of IOPS
3. Physiochemical and Structural Features
3.1. Monosaccharide Composition and Glycosidic Bond Connection
3.2. High-Level Structure of Polysaccharides
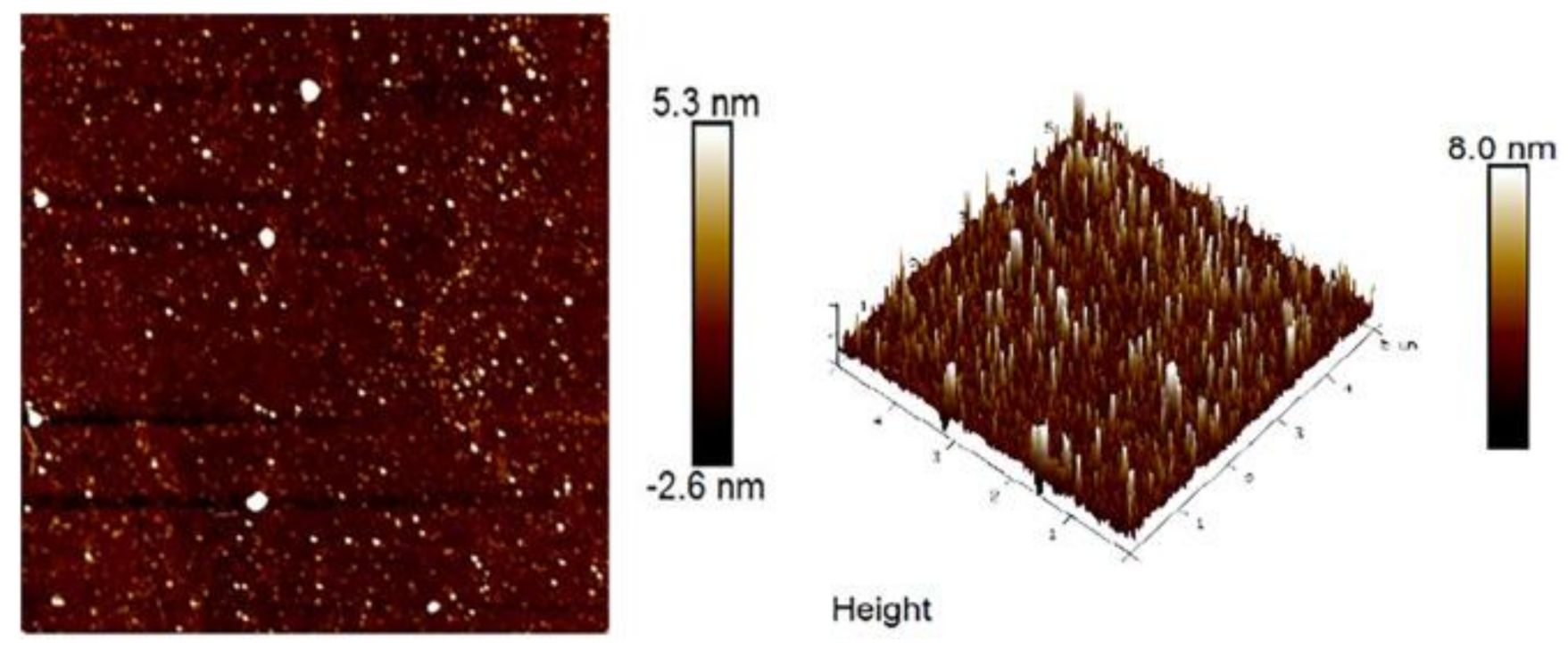
3.3. Surface Morphology
4. Biological Activity
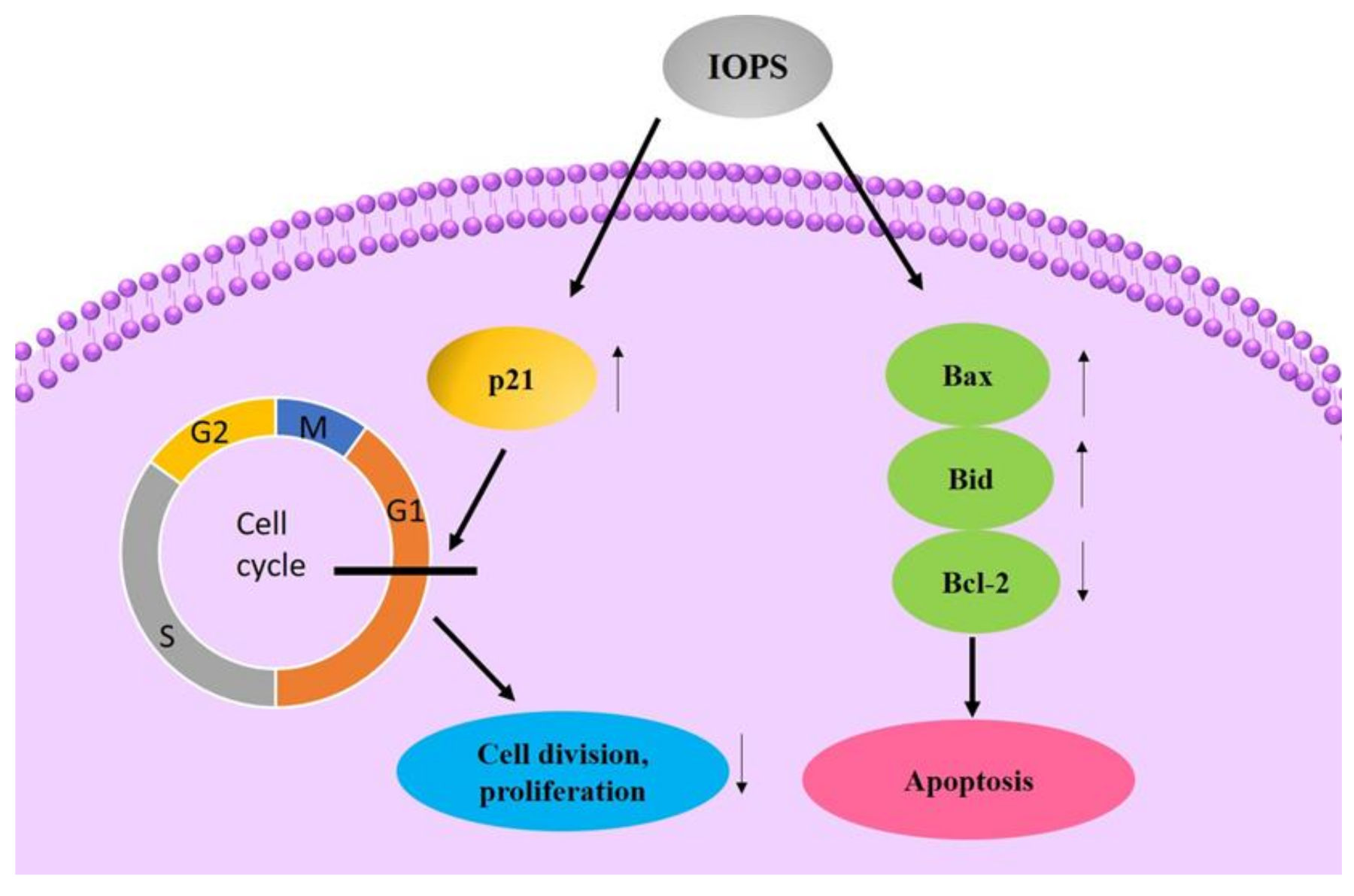
4.1. Antitumor Activity
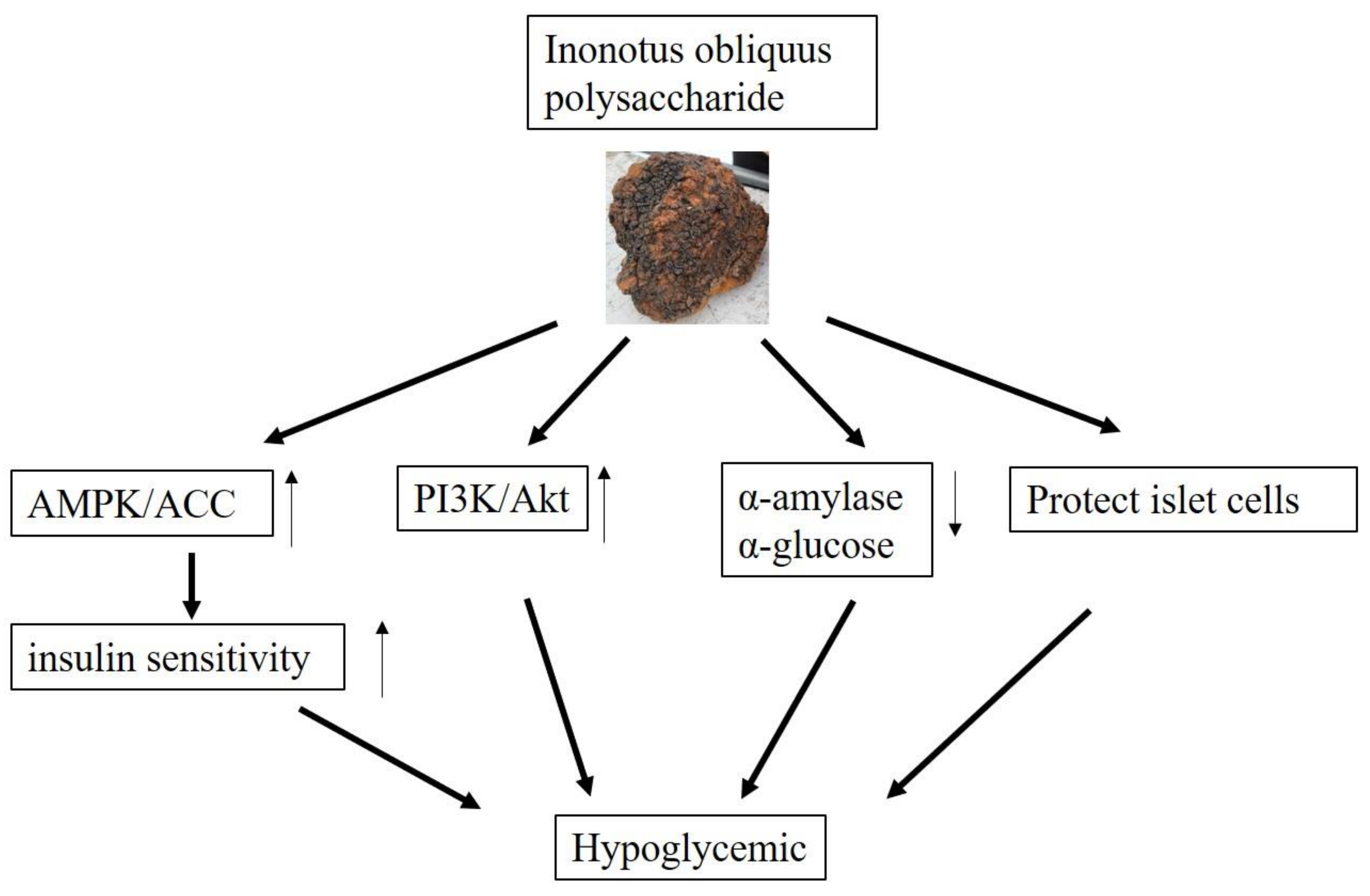
4.2. Hypoglycemic and Hypolipidemic Activities
4.3. Antioxidant Activity
4.4. Anti-Fatigue Activity
4.5. Antiviral Effect
4.6. Immunomodulatory Activity
4.7. Other Bioactivities
5. Toxicity of IOPS
6. Application
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020; U.S. Dept of Health and Human Services: Washington, DC, USA, 2020. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures 2021. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html (accessed on 15 March 2021).
- Hazhir, N.; Chekin, F.; Raoof, J.B.; Fathi, S. Anticancer activity of Doxorubicin conjugated to polymer/carbon based-nanohybrid against MCF-7 breast and HT-29 colon cancer cells. Int. J. Nano Dimens. 2021, 12, 11–19. [Google Scholar]
- Malik, Z.; Parveen, R.; Parveen, B.; Zahiruddin, S.; Aasif Khan, M.; Khan, A.; Massey, S.; Ahmad, S.; Husain, S.A. Anticancer potential of andrographolide from Andrographis paniculata (Burm.f.) Nees and its mechanisms of action. J. Ethnopharmacol. 2021, 272, 113936. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Xiang, Y.; Sheng, R.; Tomas, H.; Rodrigues, J.; Gu, Z.; Zhang, H.; Gong, Q.; Luo, K. Polysaccharide-based nanomedicines for cancer immunotherapy: A review. Bioact. Mater. 2021, 6, 3358–3382. [Google Scholar] [CrossRef] [PubMed]
- Zapora, E.; Wolkowycki, M.; Bakier, S.; Zjawiony, J.K. Phellinus igniarius: A Pharmacologically Active Polypore Mushroom. Nat. Prod. Commun. 2016, 11, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Roh, H.S.; Baek, K.H.; Lee, S.; Lee, S.; Song, S.S.; Kim, K.H. Bioactivity-based analysis and chemical characterization of cytotoxic constituents from Chaga mushroom (Inonotus obliquus) that induce apoptosis in human lung adenocarcinoma cells. J. Ethnopharmacol. 2018, 224, 63–75. [Google Scholar] [CrossRef]
- Ma, L.S.; Chen, H.X.; Dong, P.; Lu, X.M. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013, 139, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Song, D.; Mu, H.B.; Zhang, W.X.; Sun, F.F.; Duan, J.Y. Investigation of three lignin complexes with antioxidant and immunological capacities from Inonotus obliquus. Int. J. Biol. Macromol. 2016, 86, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Saar, M. Fungi in Khanty folk medicine. J. Ethnopharmacol. 1991, 31, 175–179. [Google Scholar] [CrossRef]
- Zheng, W.F.; Miao, K.J.; Liu, Y.B.; Zhao, Y.X.; Zhang, M.M.; Pan, S.Y.; Dai, Y.C. Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl. Microbiol. Biotechnol. 2010, 87, 1237–1254. [Google Scholar] [CrossRef]
- Lee, M.W.; Hur, H.; Chang, K.C.; Lee, T.S.; Ka, K.H.; Jankovsky, L. Introduction to Distribution and Ecology of Sterile Conks of Inonotus obliquus. Mycobiology 2008, 36, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Babitskaya, V.G.; Shcherba, V.V.; Ikonnikova, N.V. Melanin complex of the fungus Inonotus obliquus. Appl. Biochem. Microbiol. 2000, 36, 377–381. [Google Scholar] [CrossRef]
- Yiyong, C. Study on Purification, Structure and Anti-Tumor Mechanism of Inonotus Obliquus Polysaccharide; Jiangnan University: Wuxi, China, 2010. [Google Scholar]
- Chen, Y.Y.; Gu, X.H.; Huang, S.Q.; Li, J.W.; Wang, X.; Tang, J. Optimization of ultrasonic/microwave assisted extraction (UMAE) of polysaccharides from Inonotus obliquus and evaluation of its anti-tumor activities. Int. J. Biol. Macromol. 2010, 46, 429–435. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Huang, Y.R.; Cui, Z.M.; Liu, J.J. Purification, characterization and biological activity of a novel polysaccharide from Inonotus obliquus. Int. J. Biol. Macromol. 2015, 79, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Zheng, W.F. Deciphering the antitumoral potential of the bioactive metabolites from medicinal mushroom Inonotus obliquus. J. Ethnopharmacol. 2021, 265, 113321. [Google Scholar] [CrossRef]
- Yu, J.; Xiang, H.Y.; Xie, Q.H. The difference of regulatory effect of two Inonotus obliquus extracts on high-fat diet mice in relation to the fatty acid elongation function of gut microbiota. Food Sci. Nutr. 2021, 9, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Tanye, X. Optimization of Extraction of Polysaccharides from Inonotus obliquus by Response Surface Methodology and Antioxidant Activity. Food Res. Dev. 2021, 42, 143–148. [Google Scholar]
- Lu, X.H.; Wang, M.Y.; Zhao, Z.Z.; Hu, J.R.; Zhang, J.S.; Liu, P. Effect of Different Pretreatment of Birch Sawdust on the Production of Active Polysaccharides by Inonotus obliquus Under Submerged Fermentation and Its Structural Mechanism. Appl. Biochem. Biotech. 2021, 1–13. [Google Scholar] [CrossRef]
- Li, J.W.; Qu, C.; Li, F.F.; Chen, Y.F.; Zheng, J.J.; Xiao, Y.; Jin, Q.X.; Jin, G.H.; Huang, X.Z.; Jin, D. Inonotus obliquus Polysaccharide Ameliorates Azoxymethane/Dextran Sulfate Sodium-Induced Colitis-Associated Cancer in Mice via Activation of the NLRP3 Inflammasome. Front. Pharm. 2021, 11, 2505. [Google Scholar] [CrossRef]
- Xu, L.; Yu, Y.F.; Sang, R.; Ge, B.J.; Wang, M.; Zhou, H.Y.; Zhang, X.M. Inonotus obliquus polysaccharide protects against adverse pregnancy caused by Toxoplasma gondii infection through regulating Th17/Treg balance via TLR4/NF-kappa B pathway. Int. J. Biol. Macromol. 2020, 146, 832–840. [Google Scholar] [CrossRef]
- Wold, C.W.; Gerwick, W.H.; Wangensteen, H.; Inngjerdingen, K.T. Bioactive triterpenoids and water-soluble melanin from Inonotus obliquus (Chaga) with immunomodulatory activity. J. Funct. Foods 2020, 71. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, F.; Teng, C.; Qu, J. Optimization for the extraction of polyphenols from Inonotus obliquus and its antioxidation activity. Prep. Biochem. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Du, N.; Wu, K.; Zhang, J.; Wang, L.; Pan, X.; Zhu, Y.; Wu, X.; Liu, J.; Chen, Y.; Ye, Y.; et al. Inonotsuoxide B regulates M1 to M2 macrophage polarization through sirtuin-1/endoplasmic reticulum stress axis. Int. Immunopharmacol. 2021, 96, 107603. [Google Scholar] [CrossRef] [PubMed]
- Maza, P.A.M.A.; Lee, J.-H.; Kim, Y.-S.; Sun, G.-M.; Sung, Y.-J.; Ponomarenko, L.P.; Stonik, V.A.; Ryu, M.; Kwak, J.-Y. Inotodiol From Inonotus obliquus Chaga Mushroom Induces Atypical Maturation in Dendritic Cells. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Zou, C.-X.; Dong, S.-H.; Hou, Z.-L.; Yao, G.-D.; Lin, B.; Huang, X.-X.; Song, S.-J. Modified lanostane-type triterpenoids with neuroprotective effects from the fungus Inonotus obliquus. Bioorganic Chem. 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, S.C.; Hwang, A.Y.; Cho, H.; Hwang, K.T. Composition of Triterpenoids in Inonotus obliquus and Their Anti-Proliferative Activity on Cancer Cell Lines. Molecules 2020, 25, 4066. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yan, Z.-F.; Li, C.-T. Effects of Exogenous Elicitors on Triterpenoids Accumulation and Expression of Farnesyl Diphosphate Synthase Gene inInonotus obliquus. Biotechnol. Bioproc. Eng. 2020, 25, 580–588. [Google Scholar] [CrossRef]
- Nosik, D.N.; Nosik, N.N.; Teplyakova, T.V.; Lobach, O.A.; Kiseleva, I.A.; Kondrashina, N.G.; Bochkova, M.S.; Ananko, G.G. Antiviral activity of extracts of basidiomycetes and humic compounds substances against Human Immunodeficiency Virus (Retroviridae: Orthoretrovirinae: Lentivirus: Human immunodeficiency virus 1) and Herpes Simplex Virus (Herpesviridae: Simplexvirus: Human alphaherpesvirus 1). Vopr. Virusol. 2020, 65, 276–283. [Google Scholar]
- Teplyakova, T.V.; Ilyicheva, T.N.; Kosogova, T.A.; Wasser, S.P. Medicinal Mushrooms against Influenza Viruses. Int. J. Med. Mushrooms 2021, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sysoeva, M.A.; Khabibrakhmanova, V.R.; Urazlina, L.N. Nutrient Medium for Cultivation of Chaga Fungus. RU2019124646, 31 July 2019. [Google Scholar]
- Teplyakova, T.V.; Ilyicheva, T.N.; Markovich, N.A. Prospects for the Development of Anti-Influenza Drugs Based on Medicinal Mushrooms (Review). Appl. Biochem. Microbiol. 2020, 56, 489–496. [Google Scholar] [CrossRef]
- Haixia, C. Relationship between structure and effect of active polysaccharide. Sci. Focus 2013, 8, 53–55. [Google Scholar]
- Wold, C.W.; Kjeldsen, C.; Corthay, A.; Rise, F.; Christensen, B.E.; Duus, J.O.; Inngjerdingen, K.T. Structural characterization of bioactive heteropolysaccharides from the medicinal fungus Inonotus obliquus (Chaga). Carbohydr. Polym. 2018, 185, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Zhao, Z.Z.; Zhou, X.; Hu, J.R.; Xue, J.; Liu, X.; Zhang, J.S.; Liu, P.; Tong, S.S. Simultaneous Use of Stimulatory Agents to Enhance the Production and Hypoglycaemic Activity of Polysaccharides from Inonotus obliquus by Submerged Fermentation. Molecules 2019, 24, 4400. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Chen, Z.; Gao, X.; Yuan, G.; Pan, Y.; Chen, H. Effects of simulated gastrointestinal digestion in vitro on the chemical properties, antioxidant activity, alpha-amylase and alpha-glucosidase inhibitory activity of polysaccharides from Inonotus obliquus. Food Res. Int. 2018, 103, 280–288. [Google Scholar] [CrossRef]
- Xue, J.; Tong, S.S.; Wang, Z.R.; Liu, P. Chemical Characterization and Hypoglycaemic Activities In Vitro of Two Polysaccharides from Inonotus obliquus by Submerged Culture. Molecules 2018, 23, 3261. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, C.; Li, S.; Li, W.; Yuan, G.; Pan, Y.; Chen, H. Anti-diabetic effects of Inonotus obliquus polysaccharides in streptozotocin-induced type 2 diabetic mice and potential mechanism via PI3K-Akt signal pathway. Biomed. Pharm. 2017, 95, 1669–1677. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Zhao, Y.; Cui, H.F.; Cao, C.Y.; Guo, J.Y.; Liu, S. Comparison of Hypoglycemic Activity of Fermented Mushroom of Inonotus obliquus Rich in Vanadium and Wild-Growing I. obliquus. Biol. Trace Elem. Res. 2011, 144, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Rybczynska-Tkaczyk, K.; Tobiasz, J.; Yelnytska-Stawasz, V.; Pomianek, T.; Gminski, J. Biological and anticancer properties of Inonotus obliquus extracts. Process Biochem. 2018, 73, 180–187. [Google Scholar] [CrossRef]
- Gery, A.; Dubreule, C.; Andre, V.; Rioult, J.P.; Bouchart, V.; Heutte, N.; de Pecoulas, P.E.; Krivomaz, T.; Garon, D. Chaga (Inonotus obliquus), a Future Potential Medicinal Fungus in Oncology? A Chemical Study and a Comparison of the Cytotoxicity Against Human Lung Adenocarcinoma Cells (A549) and Human Bronchial Epithelial Cells (BEAS-2B). Integr. Cancer 2018, 17, 832–843. [Google Scholar] [CrossRef]
- Smith, A.; Javed, S.; Barad, A.; Myhre, V.; Li, W.M.; Reimer, K.; Massicotte, H.B.; Tackaberry, L.E.; Payne, G.W.; Egger, K.N.; et al. Growth-Inhibitory and Immunomodulatory Activities of Wild Mushrooms from North-Central British Columbia (Canada). Int. J. Med. Mushrooms 2017, 19, 485–497. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, K.R.; Lee, S.; Lee, H.J.; Yang, H.S.; Yeo, J.; Park, J.M.; Choi, B.H.; Hong, E.K. Polysaccharides Isolated from Liquid Culture Broth of Inonotus obliquus Inhibit the Invasion of Human Non-small Cell Lung Carcinoma Cells. Biotechnol. Bioprocess Eng. 2017, 22, 45–51. [Google Scholar] [CrossRef]
- Fan, L.P.; Ding, S.D.; Ai, L.Z.; Deng, K.Q. Antitumor and immunomodulatory activity of water-soluble polysaccharide from Inonotus obliquus. Carbohyd. Polym. 2012, 90, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Fan, C.; Liu, S.C.; Zang, Z.F.; Jiao, L.L.; Zhang, L.P. Chemical composition and antitumor activity of polysaccharide from Inonotus obliquus. J. Med. Plants Res. 2011, 5, 1251–1260. [Google Scholar]
- Lemieszek, M.K.; Langner, E.; Kaczor, J.; Kandefer-Szerszen, M.; Sanecka, B.; Mazurkiewicz, W.; Rzeski, W. Anticancer Effects of Fraction Isolated from Fruiting Bodies of Chaga Medicinal Mushroom, Inonotus obliquus (Pers.:Fr.) Pilat (Aphyllophoromycetideae): In Vitro Studies. Int. J. Med. Mushrooms 2011, 13, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z. Study on Inonotus obliquus Polysaccharide Induced Apoptosis of Human Ovarian Cancer Cells. Edible Fungi China 2010, 29, 38–39. [Google Scholar]
- Meuninck, J. Foraging Mushrooms Oregon: Finding, Identifying, and Preparing Edible Wild Mushrooms; Falcon Guides: Michigan, MI, USA, 2017; p. 111. [Google Scholar]
- Tingjuan, Q.; Yubo, Z.; Yi, Z. Preliminary Study on the Physical and Chemical Properties and Biological Activity of Polysaccharide from Inonotus obliquus. Smart Health 2018, 4, 50–53. [Google Scholar]
- Li, Z.G.; Mei, J.J.; Jiang, L.P.; Geng, C.Y.; Li, Q.J.; Yao, X.F.; Cao, J. Chaga Medicinal Mushroom Inonotus obliquus (Agaricomycetes) Polysaccharides Suppress Tacrine-Induced Apoptosis by Reactive Oxygen Species-Scavenging and Mitochondrial Pathway in HepG2 Cells. Int. J. Med. Mushrooms 2019, 21, 583–593. [Google Scholar] [CrossRef]
- Han, Y.Q.; Nan, S.J.; Fan, J.; Chen, Q.H.; Zhang, Y.Z. Inonotus obliquus polysaccharides protect against Alzheimer’s disease by regulating Nrf2 signaling and exerting antioxidative and antiapoptotic effects. Int. J. Biol. Macromol. 2019, 131, 769–778. [Google Scholar] [CrossRef]
- Wang, C.; Santhanam, R.K.; Gao, X.; Chen, Z.; Chen, Y.; Wang, C.; Xu, L.; Chen, H. Preparation, characterization of polysaccharides fractions from Inonotus obliquus and their effects on alpha-amylase, alpha-glucosidase activity and H2O2 induced oxidative damage in hepatic L02 cells. J. Funct. Foods 2018, 48, 179–189. [Google Scholar] [CrossRef]
- Wang, C.; Gao, X.; Santhanam, R.K.; Chen, Z.; Chen, Y.; Xu, L.; Wang, C.; Ferri, N.; Chen, H. Effects of polysaccharides from Inonotus obliquus and its chromium (III) complex on advanced glycation end-products formation, alpha-amylase, alpha-glucosidase activity and H2O2-induced oxidative damage in hepatic L02 cells. Food Chem. Toxicol. 2018, 116, 335–345. [Google Scholar] [CrossRef]
- Jingbo, W. Optimization of Extraction Process of Inonotus obliquus Polysaccharides and Evaluation of Antioxidant Activity. Food Nutr. China 2017, 23, 46–49. [Google Scholar]
- Hu, Y.; Shi, S.Y.; Lu, L.; Teng, C.Y.; Yu, S.M.; Wang, X.; Yu, M.; Liang, J.S.; Qu, J.J. Effects of Selenizing Modification on Characteristics and Antioxidant Activities of Inonotus obliquus Polysaccharide. Macromol. Res. 2017, 25, 222–230. [Google Scholar] [CrossRef]
- Zheng, W.F.; Zhang, M.M.; Zhao, Y.X.; Miao, K.J.; Pan, S.Y.; Cao, F.; Dai, Y.C. Analysis of Antioxidant Metabolites by Solvent Extraction from Sclerotia of Inonotus obliquus (Chaga). Phytochem. Anal. 2011, 22, 95–102. [Google Scholar] [CrossRef]
- Xu, X.Q.; Wu, Y.D.; Chen, H. Comparative antioxidative characteristics of polysaccharide-enriched extracts from natural sclerotia and cultured mycelia in submerged fermentation of Inonotus obliquus. Food Chem. 2011, 127, 74–79. [Google Scholar] [CrossRef]
- Chen, H.; Yan, M.C.; Zhu, J.W.; Xu, X.Q. Enhancement of exo-polysaccharide production and antioxidant activity in submerged cultures of Inonotus obliquus by lignocellulose decomposition. J. Ind. MicroBiol. Biotechnol. 2011, 38, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pang, C.; Yang, C.J.; Zheng, Y.T.; Xu, H.Y.; Lu, Z.M.; Xu, Z.H. Antihyperglycemic and Antilipidperoxidative Effects of Polysaccharides Extracted from Medicinal Mushroom Chaga, Inonotus obliquus (Pers.: Fr.) Pilat (Aphyllophoromycetideae) on Alloxan-Diabetes Mice. Int. J. Med. Mushrooms 2010, 12, 235–244. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Wei, T.; Chen, Q. Stimulatory Effects of Oleci Acid and Fungal Elicitor on Betulinic Acid Production by Submerged Cultivation of Medicinal Mushroom Inonotus obliquus. J. Fungi (Baselswitzerland) 2021, 7, 266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, R.; Liu, C.; Li, S.; Hou, W. Development of ultrasound-assisted centrifugal extraction combined with two countercurrent chromatography systems for the simultaneous extraction and isolation of phytochemicals. J. Sep. Sci. 2021. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, X.; Tong, L.; Lv, Y.; Yi, H.; Gong, P.; Tian, X.; Cui, Q.; Liu, T.; Zhang, L. Effect of Inonotus obliquus (Fr.) Pilat extract on the regulation of glycolipid metabolism via PI3K/Akt and AMPK/ACC pathways in mice. J. Ethnopharmacol. 2021, 273, 113963. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; De-Shui, Y.U.; Liu, J.N.; Dai, X.D.; Han, Z.H. Extraction of Polysaccharide from Inonotus obliquus Fruit Bodies. Biotechnology 2012. [Google Scholar] [CrossRef]
- Liu, Z.D.; Yu, D.S.; Li, L.; Liu, X.X.; Zhang, H.A.; Sun, W.B.; Lin, C.C.; Chen, J.F.; Chen, Z.; Wang, W.H.; et al. Three-Phase Partitioning for the Extraction and Purification of Polysaccharides from the Immunomodulatory Medicinal Mushroom Inonotus obliquus. Molecules 2019, 24, 403. [Google Scholar] [CrossRef]
- Joo, J.I.; Kim, D.H.; Yun, J.W. Extract of Chaga Mushroom (Inonotus obliquus) stimulates 3t3-l1 adipocyte differentiation. Phytother. Res. 2010, 24, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Park, H.W.; Kim, J.H.; Lee, J.Y.; Moon, S.H.; Shin, C.S. Anti-cancer effect and structural characterization of endo-polysaccharide from cultivated mycelia of Inonotus obliquus. Life Sci. 2006, 79, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.L.; Chen, H.X.; Dong, P.; Zhang, X.; Zhang, M. Effects of Ultrasonic Treatment on the Physicochemical Properties and DPPH Radical Scavenging Activity of Polysaccharides from Mushroom Inonotus obliquus. J. Food Sci. 2010, 75, C322–C327. [Google Scholar] [CrossRef]
- Semedo, M.C.; Karmali, A.; Fonseca, L. A high throughput colorimetric assay of beta-1,3-D-glucans by Congo red dye. J. MicroBiol. Meth. 2015, 109, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, C.; Li, W.W.; Pan, Y.X.; Yuan, G.Q.; Chen, H.X. Ball milling improves extractability and antioxidant properties of the active constituents of mushroom Inonotus obliquus powders. Int. J. Food Sci. Tech. 2016, 51, 2193–2200. [Google Scholar] [CrossRef]
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- Shichun, Z.; Zhaocheng, Y.; Deyi, Z.; Kangjian, Z.; Jinming, G. Optimization of Extraction Technology for Polysaccharides in Inonotus Obliquus. Chin. J. Mod. Appl. Pharm. 2014, 31, 167–172. [Google Scholar]
- Xintong, J.; Siyue, Z.; Jie, Z. Research on the optimization of exaction parameter for polysaccharide of Inonotus obliquus and its antioxidant activity. J. Nat. Sci. Heilongjiang Univ. 2017, 34, 584–594. [Google Scholar]
- Yaoling, H.; Tengfei, X.; Zhongxiang, J. Studies on the Purification Methods of Crude polysaccharides of Inonotus Obliquus. J. Henan Univ. (Med. Sci.) 2012, 31, 229–232. [Google Scholar]
- Qing, L. Study on Extraction, Purification and Anti-Tumor Activity of Polysaccharide from Inonotus Obliquus. Ph.D. Thesis, Qingdao University of Science and Technology, Qingdao, China, 2014. [Google Scholar]
- Yang, X.; Wei, S.; Lu, X.; Qiao, X.; Simal-Gandara, J.; Capanoglu, E.; Wozniak, L.; Zou, L.; Cao, H.; Xiao, J.; et al. A neutral polysaccharide with a triple helix structure from ginger: Characterization and immunomodulatory activity. Food Chem. 2021, 350. [Google Scholar] [CrossRef]
- Huang, Q.; He, W.; Khudoyberdiev, I.; Ye, C.-L. Characterization of polysaccharides from Tetrastigma hemsleyanum Diels et Gilg Roots and their effects on antioxidant activity and H2O2-induced oxidative damage in RAW 264.7 cells. BMC Chem. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Q.; Zhang, L.M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohyd. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Maity, P.; Sen, I.K.; Chakraborty, I.; Mondal, S.; Bar, H.; Bhanja, S.K.; Mandal, S.; Maity, G.N. Biologically active polysaccharide from edible mushrooms: A review. Int. J. Biol. Macromol. 2021, 172, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H. Advances in the extraction, purification, structural-property relationships and bioactive molecular mechanism of Flammulina velutipes polysaccharides: A review. Int. J. Biol. Macromol. 2021, 167, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Kang, J.; Xu, Z.; Guo, Q.; Zhang, L.; Ning, H.; Cui, S.W. Triple-helix polysaccharides: Formation mechanisms and analytical methods. Carbohyd. Polym. 2021, 262, 117962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Guo, J.Y.; Cheng, H.; Li, L.; Liu, Y.; Shi, Y.; Xu, J.; Yu, H.T. Spatial structure and anti-fatigue of polysaccharide from Inonotus obliquus. Int. J. Biol. Macromol. 2020, 151, 855–860. [Google Scholar] [CrossRef]
- Chen, Q.C.; Mao, R.; Zhao, J.J.; Bi, X.Y.; Li, Z.Y.; Huang, Z.; Zhang, Y.F.; Zhou, J.G.; Zhao, H.; Cai, J.Q. From the completion of neoadjuvant chemotherapy to surgery for colorectal cancer liver metastasis: What is the optimal timing? Cancer Med. 2020. [Google Scholar] [CrossRef]
- Chung, M.J.; Chung, C.K.; Jeong, Y.; Ham, S.S. Anticancer activity of subfractions containing pure compounds of Chaga mushroom (Inonotus obliquus) extract in human cancer cells and in Balbc/c mice bearing Sarcoma-180 cells. Nutr. Res. Pract. 2010, 4, 177–182. [Google Scholar] [CrossRef]
- Heavey, S.; O’Byrne, K.J.; Gately, K. Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC. Cancer Treat. Rev. 2014, 40, 445–456. [Google Scholar] [CrossRef]
- Kopru, B.; Ebiloglu, T.; Kaya, E.; Alagoz, E.; Zor, M.; Emer, M.O.; Gurdal, M.; Bedir, S.; Arslan, N. What is the Prostate Specific Antigen Cut-off Value to Detect Metastases in Ga-68 Prostate Specific Membrane Antigen Ligand Positron Emission Tomography/Computer Tomography Imaging for Intermediate and High-risk Prostate Cancer? Uroonkoloji Buelteni Bull. Urooncology 2020, 19, 151–156. [Google Scholar] [CrossRef]
- Mensah, K.B.; Mensah, A.B.B.; Bangalee, V.; Oosthuizen, F. Awareness is the first step: What Ghanaian community pharmacists know about cancer. J. Oncol. Pharm. Pract. 2020, 1078155220955211. [Google Scholar] [CrossRef]
- Sciacovelli, M.; Schmidt, C.; Maher, E.R.; Frezza, C. Metabolic Drivers in Hereditary Cancer Syndromes. Annu Rev. Cancer Biol. 2020, 4, 77–97. [Google Scholar] [CrossRef]
- Subotic, S.; Wyler, S.; Bachmann, A. Surgical Treatment of Localised Renal Cancer. Eur. Urol. Suppl. 2012, 11, 60–65. [Google Scholar] [CrossRef]
- Wang, H.D.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Joseph, M.M.; Aravind, S.R.; George, S.K.; Pillai, K.R.; Mini, S.; Sreelekha, T.T. Antitumor activity of galactoxyloglucan-gold nanoparticles against murine ascites and solid carcinoma. Colloid Surf. B 2014, 116, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; You, Q.; Wang, J.; Liu, L.; Wang, Y.; Song, Y.; Cheng, Y.; Wang, S.; Tan, F.; Li, N. Theranostic Nanoplatform: Triple-Modal Imaging-Guided Synergistic Cancer Therapy Based on Liposome-Conjugated Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 1963–1975. [Google Scholar] [CrossRef] [PubMed]
- Song, F.Q.; Liu, Y.; Kong, X.S.; Chang, W.; Song, G. Progress on Understanding the Anticancer Mechanisms of Medicinal Mushroom: Inonotus Obliquus. Asian Pac. J. Cancer Prev. 2013, 14, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Huili, Z.; Song, Y.; Yu, L. Extraction of Inonotus obliquus polysaccharide and the effect of anti-proliferation on SMMC7721 cells of lung cancer. Edible Fungi China 2006, 25, 31–33. [Google Scholar]
- Lee, K.R.; Lee, J.S.; Lee, S.; Son, Y.K.; Kim, G.R.; Sim, Y.C.; Song, J.E.; Ha, S.J.; Hong, E.K. Polysaccharide isolated from the liquid culture broth of Inonotus obliquus suppresses invasion of B16-F10 melanoma cells via AKT/NF-kappa B signaling pathway. Mol. Med. Rep. 2016, 14, 4429–4435. [Google Scholar] [CrossRef]
- Lee, K.R.; Lee, J.S.; Song, J.E.; Ha, S.J.; Hong, E.K. Inonotus obliquus-derived polysaccharide inhibits the migration and invasion of human non-small cell lung carcinoma cells via suppression of MMP-2 and MMP-9. Int. J. Oncol. 2014, 45, 2533–2540. [Google Scholar] [CrossRef]
- Yi-yong, C.; Xiao-hong, G.; Jian, T. Study on Anti-Tumor Activities of Polysaccharides from Inonotus obliquus. J. Food Sci. Biotechnol. 2011, 30, 65–69. [Google Scholar]
- Yan, L. Research on Deep Cultivation of Inonotus Obliquus and Extraction Technology and Anti-Tumor Activity of Its Polysaccharide. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2009. [Google Scholar]
- Jiang, S.P.; Shi, F.L.; Lin, H.; Ying, Y.; Luo, L.Y.; Huang, D.Q.; Luo, Z.J. Inonotus obliquus polysaccharides induces apoptosis of lung cancer cells and alters energy metabolism via the LKB1/AMPK axis. Int. J. Biol. Macromol. 2020, 151, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Su, B.H.; Yan, X.Z.; Li, Y.Z.; Zhang, J.S.; Xia, X.Y. Effects of Inonotus obliquus Polysaccharides on Proliferation, Invasion, Migration, and Apoptosis of Osteosarcoma Cells. Anal. Cell Pathol. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Min, Y. The inhibitory Effect of Water Extract of Inonotus Obliquus on Human Hepatocarcinoma Cell Line HepG2. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2019. [Google Scholar]
- Gang, L. Preliminary Study on the Physical and Chemical Properties and Biological Activity of Polysaccharide from Inonotus obliquus. Master’s Thesis, Northeast Normal University, Changchun, China, 2008. [Google Scholar]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Li, J.E.; Wang, Z.J.; Gao, J.; Nie, S.P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. 2016, 56, S60–S84. [Google Scholar] [CrossRef]
- He, P.; Zhang, Y.; Li, N. The phytochemistry and pharmacology of medicinal fungi of the genus Phellinus: A review. Food Funct. 2021, 12, 1856–1881. [Google Scholar] [CrossRef] [PubMed]
- Alejandrina Virgen-Carrillo, C.; Martinez Moreno, A.G.; Valdes Miramontes, E.H. Potential Hypoglycemic Effect of Pomegranate Juice and Its Mechanism of Action: A Systematic Review. J. Med. Food 2020, 23, 1–11. [Google Scholar] [CrossRef]
- Guo, M.; Shao, S.; Wang, D.; Zhao, D.; Wang, M. Recent progress in polysaccharides from Panax ginseng C. A. Meyer. Food Funct. 2021, 12, 494–518. [Google Scholar] [CrossRef]
- Shen, J.; Hu, M.; Tan, W.; Ding, J.; Jiang, B.; Xu, L.; Hamulati, H.; He, C.; Sun, Y.; Xiao, P. Traditional uses, phytochemistry, pharmacology, and toxicology of Coreopsis tinctoria Nutt.: A review. J. Ethnopharmacol. 2021, 269. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Ji, Y.; Peng, L.; Liu, Q.; Cao, H.; Yang, Y.; He, X.; Zeng, N. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Codonopsis pilosula: A review. Carbohyd. Polym. 2021, 261. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.-H.; Sun, K.-F.; Guo, Y. Effect of molecular weight on hypolipidemic and hypoglycemic activities of fermented Auriculaia auricula supernatant. Food Sci. Technol. 2020, 40, 106–112. [Google Scholar] [CrossRef]
- Chen, H.X.; Lu, X.M.; Qu, Z.S.; Wang, Z.S.; Zhang, L.P. Glycosidase Inhibitory Activity and Antioxidant Properties of a Polysaccharide from the Mushroom Inonotus Obliquus. J. Food Biochem. 2010, 34, 178–191. [Google Scholar] [CrossRef]
- Liu, P.; Xue, J.; Tong, S.S.; Dong, W.X.; Wu, P.P. Structure Characterization and Hypoglycaemic Activities of Two Polysaccharides from Inonotus obliquus. Molecules 2018, 23, 1948. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Kim, D.S.; Park, K.C. Antioxidant effect of Inonotus obliquus. J. Ethnopharmacol. 2005, 96, 79–85. [Google Scholar] [CrossRef]
- Azeem, U.; Shri, R.; Dhingra, G.S. In Vitro Antioxidant Efficacy of Some Selected Medicinal Mushrooms from India. Int. J. Med. Mushrooms 2020, 22, 641–649. [Google Scholar] [CrossRef]
- Xu, X.Q.; Zhao, W.; Shen, M.W. Antioxidant activity of liquid cultured Inonotus obliquus polyphenols using tween-20 as a stimulatory agent: Correlation of the activity and the phenolic profiles. J. Taiwan Inst. Chem. E 2016, 69, 41–47. [Google Scholar] [CrossRef]
- Ma, L.S.; Chen, H.X.; Zhang, Y.; Zhang, N.; Fu, L.L. Chemical modification and antioxidant activities of polysaccharide from mushroom Inonotus obliquus. Carbohyd. Polym. 2012, 89, 371–378. [Google Scholar] [CrossRef]
- Hu, Y.; Sheng, Y.; Yu, M.; Li, K.K.; Ren, G.M.; Xu, X.H.; Qu, J.J. Antioxidant activity of Inonotus obliquus polysaccharide and its amelioration for chronic pancreatitis in mice. Int. J. Biol. Macromol. 2016, 87, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, H.X.; Ma, L.S.; Zhang, Y. Physical modifications of polysaccharide from Inonotus obliquus and the antioxidant properties. Int. J. Biol. Macromol. 2013, 54, 209–215. [Google Scholar] [CrossRef]
- Ma, L.S.; Chen, H.X.; Zhu, W.C.; Wang, Z.S. Effect of different drying methods on physicochemical properties and antioxidant activities of polysaccharides extracted from mushroom Inonotus obliquus. Food Res. Int. 2013, 50, 633–640. [Google Scholar] [CrossRef]
- Chen, H.; Xu, X.Q.; Zhu, Y. Optimization of Hydroxyl Radical Scavenging Activity of Exopolysaccharides from Inonotus obliquus in Submerged Fermentation Using Response Surface Methodology. J. MicroBiol. Biotechn. 2010, 20, 835–843. [Google Scholar]
- Xu, X.Q.; Wu, P.; Wang, T.Z.; Yan, L.L.; Lin, M.M.; Chen, C. Synergistic effects of surfactant-assisted biodegradation of wheat straw and production of polysaccharides by Inonotus obliquus under submerged fermentation. Bioresour. Technol. 2019, 278, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Du, X.J.; Mu, H.M.; Zhou, S.; Zhang, Y.; Zhu, X.L. Chemical analysis and antioxidant activity of polysaccharides extracted from Inonotus obliquus sclerotia. Int. J. Biol. Macromol. 2013, 62, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.W.; Dai, Y.C. Progress report on the study of wood-decaying fungi in China. Chin. Sci. Bull. 2012, 57, 4328–4335. [Google Scholar] [CrossRef]
- Xiang, Y.L.; Xu, X.Q.; Li, J. Chemical properties and antioxidant activity of exopolysaccharides fractions from mycelial culture of Inonotus obliquus in a ground corn stover medium. Food Chem. 2012, 134, 1899–1905. [Google Scholar] [CrossRef]
- Mu, H.B.; Zhang, A.M.; Zhang, W.X.; Cui, G.T.; Wang, S.C.; Duan, J.Y. Antioxidative Properties of Crude Polysaccharides from Inonotus obliquus. Int. J. Mol. Sci. 2012, 13, 9194–9206. [Google Scholar] [CrossRef]
- Huang, S.Q.; Ding, S.D.; Fan, L.P. Antioxidant activities of five polysaccharides from Inonotus obliquus. Int. J. Biol. Macromol. 2012, 50, 1183–1187. [Google Scholar] [CrossRef]
- Sim, Y.C.; Lee, J.S.; Lee, S.; Son, Y.K.; Park, J.E.; Song, J.E.; Ha, S.J.; Hong, E.K. Effects of polysaccharides isolated from Inonotus obliquus against hydrogen peroxide-induced oxidative damage in RINm5F pancreatic beta-cells. Mol. Med. Rep. 2016, 14, 4263–4270. [Google Scholar] [CrossRef]
- Xu, X.Q.; Hu, Y.; Quan, L.L. Production of bioactive polysaccharides by Inonotus obliquus under submerged fermentation supplemented with lignocellulosic biomass and their antioxidant activity. Bioprocess Biosyst. Eng. 2014, 37, 2483–2492. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhong, X.H.; Yang, S.Y.; Zheng, Z.H. Effect of Inonotus Obliquus Polysaccharides on physical fatigue in mice. J. Tradit. Chin. Med. 2015, 35, 468–472. [Google Scholar]
- Koonin, E.V.; Senkevich, T.G.; Dolja, V.V. The ancient Virus World and evolution of cells. Biol. Direct 2006, 1, 29. [Google Scholar] [CrossRef]
- University, J.H. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available online: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (accessed on 18 March 2021).
- Van, Q.; Nayak, B.N.; Reimer, M.; Jones, P.J.H.; Fulcher, R.G.; Rempel, C.B. Anti-inflammatory effect of Inonotus obliquus, Polygala senega L., and Viburnum trilobum in a cell screening assay. J. Ethnopharmacol. 2009, 125, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The Antiviral, Anti-Inflammatory Effects of Natural Medicinal Herbs and Mushrooms and SARS-CoV-2 Infection. Nutrients 2020, 12, 2573. [Google Scholar] [CrossRef]
- Tian, J.; Hu, X.L.; Liu, D.F.; Wu, H.X.; Qu, L.D. Identification of Inonotus obliquus polysaccharide with broad-spectrum antiviral activity against multi-feline viruses. Int. J. Biol. Macromol. 2017, 95, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.H.; Yu, X.T.; Li, T.; Wu, H.L.; Jiao, C.W.; Cai, M.H.; Li, X.M.; Xie, Y.Z.; Wang, Y.; Peng, T. Aqueous Extract from a Chaga Medicinal Mushroom, Inonotus obliquus (Higher Basidiomyetes), Prevents Herpes Simplex Virus Entry Through Inhibition of Viral-Induced Membrane Fusion. Int. J. Med. Mushrooms 2013, 15, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Watanabe, O.; Maruyama, S. Inhibition of HIV-1 protease by water-soluble lignin-like substance from an edible mushroom, Fuscoporia obliqua. Biosci. Biotechnol. Biochem. 1998, 62, 575–577. [Google Scholar] [CrossRef]
- Won, D.P.; Lee, J.S.; Kwon, D.S.; Lee, K.E.; Shin, W.C.; Hong, E.K. Immunostimulating activity by polysaccharides isolated from fruiting body of Inonotus obliquus. Mol. Cells 2011, 31, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Jinjuan, Z. The effect of Inonotus Obliquus Polysaccharides on Cell Inflammation Modeland and Its Possible Mechanism Based on the Signaling Pathway of NLRP3 Inflammasome. Master’s Thesis, Yanbian University, Yanbian, China, 2019. [Google Scholar]
- Xiao, Y.; Zheng, J.J.; Chen, Y.F.; Qu, C.; Bo, L.; Li, J.W.; Jin, D. Inonotus obliquus polysaccharides ameliorated inflammation of LPS-stimulated RAW264.7 macrophages involving inhibited NLRP3 inflammasome. Eur J. Immunol. 2019, 49, 230. [Google Scholar]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.S. Effect of Inonotus obliquus enriched diet on hematology, immune response, and disease protection in kelp grouper, Epinephelus bruneus against Vibrio harveyi. Aquaculture 2012, 344, 48–53. [Google Scholar] [CrossRef]
- Hu, Y.; Teng, C.Y.; Yu, S.M.; Wang, X.; Liang, J.S.; Bai, X.; Dong, L.Y.; Song, T.; Yu, M.; Qu, J.J. Inonotus obliquus polysaccharide regulates gut microbiota of chronic pancreatitis in mice. AMB Express 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Zheng, J.J.; Qu, C.; Xiao, Y.; Li, F.F.; Jin, Q.X.; Li, H.H.; Meng, F.P.; Jin, G.H.; Jin, D. Inonotus obliquus polysaccharide ameliorates dextran sulphate sodium induced colitis involving modulation of Th1/Th2 and Th17/Treg balance. Artif. Cells Nanomed. Biotechnol. 2019, 47, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sang, R.; Yu, Y.F.; Li, J.X.; Ge, B.J.; Zhang, X.M. The polysaccharide from Inonotus obliquus protects mice from Toxoplasma gondii-induced liver injury. Int. J. Biol. Macromol. 2019, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, Q.; Guan, X.; Xie, Y. Inonotus Obliquus Polysaccharide Extract Useful for Preparing Drug for Treating Atopic Dermatitis, Food Allergy, Allergic Rhinitis or Asthma, Comprises Mannose, Glucose, Galactose and Xylose. CN112409503-A, 26 February 2021. [Google Scholar]
- Gao, X.D.; Santhanam, R.K.; Xue, Z.H.; Jia, Y.A.; Wang, Y.J.; Lu, Y.P.; Phisalaphong, M.; Chen, H.X. Antioxidant, alpha-amylase and alpha-glucosidase activity of various solvent fractions of I. obliquus and the preventive role of active fraction against H2O2 induced damage in hepatic L02 cells as fungisome. J. Food Sci. 2020, 85, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Z.Q.; Pan, Y.X.; Gao, X.D.; Chen, H.X. Anti-diabetic effects of Inonotus obliquus polysaccharides-chromium (III) complex in type 2 diabetic mice and its sub-acute toxicity evaluation in normal mice. Food Chem. Toxicol. 2017, 108, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Skóra, B.; Pomianek, T.; Gmiński, J. Inonotus obliquus–from folk medicine to clinical use. J. Tradit. Complementary Med. 2020. [Google Scholar] [CrossRef]
- Chen, H.J.; Liou, B.K.; Hsu, K.C.; Chen, C.S.; Chuang, P.T. Implementation of food safety management systems that meets ISO 22000:2018 and HACCP: A case study of capsule biotechnology products of chaga mushroom. J. Food Sci. 2021, 86, 40–54. [Google Scholar] [CrossRef]
- Guo, S.; Li, G.; Li, Y.; Guo, X.; Nan, X. Inonotus Obliquus Hypoglycemic Beverage Comprises Extracts of Inonotus Obliquus, Kudzu Vine Root, Fructus Lycii and Ginseng. CN109673790A, 26 April 2019. [Google Scholar]
- Liu, F.; Yang, F.; Zhao, H. Healthcare Food Used e.g., for Preventing and Treating Diabetes, Comprises Inonotus Obliquus Polysaccharide, Ganoderma Lucidum Peptide Polysaccharide, and Tremella Polysaccharide. CN104366193A, 25 February 2015. [Google Scholar]
- Xu, L.; Lu, X.; Guo, L.; Ji, J. Functional Yogurt Used for Improving Immunity, Comprises Fresh Milk, Inonotus Obliquus Extracting Solution, Ganoderma Lucidum Spore Powder Polysaccharide, Tremella Concentrated Slurry, Xylitol and Fermentation Agent. CN108902307A, 30 November 2018. [Google Scholar]
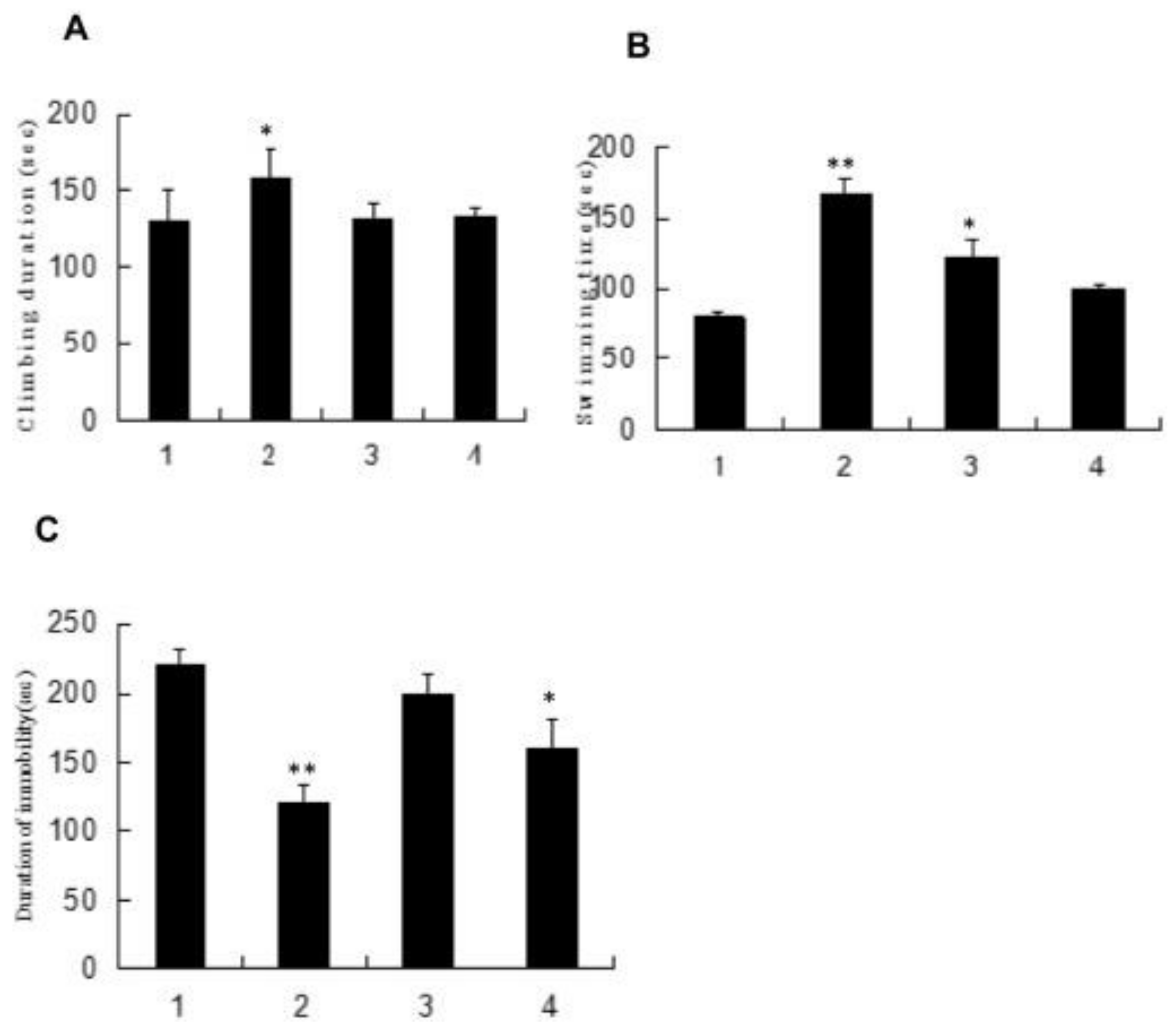
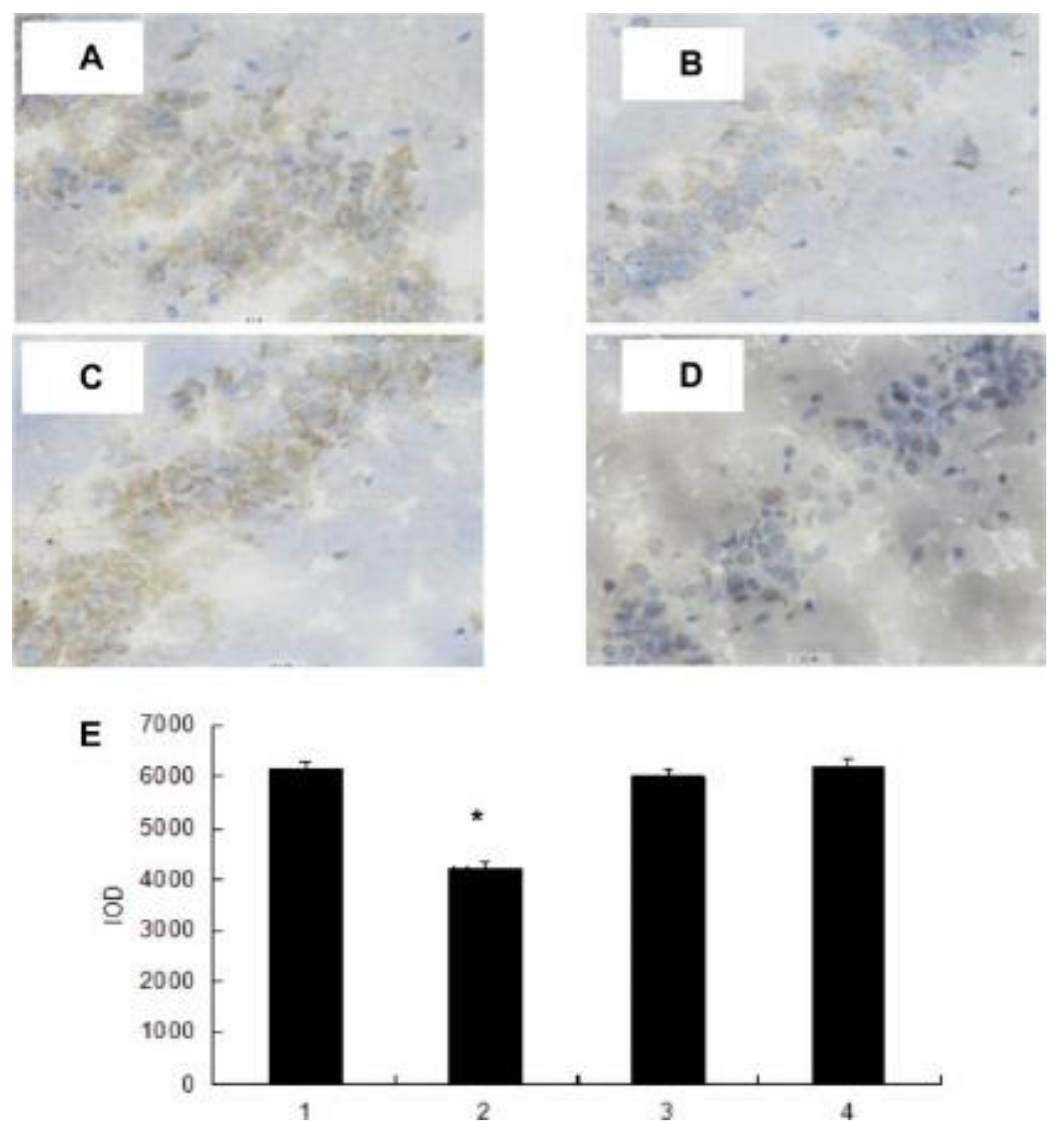
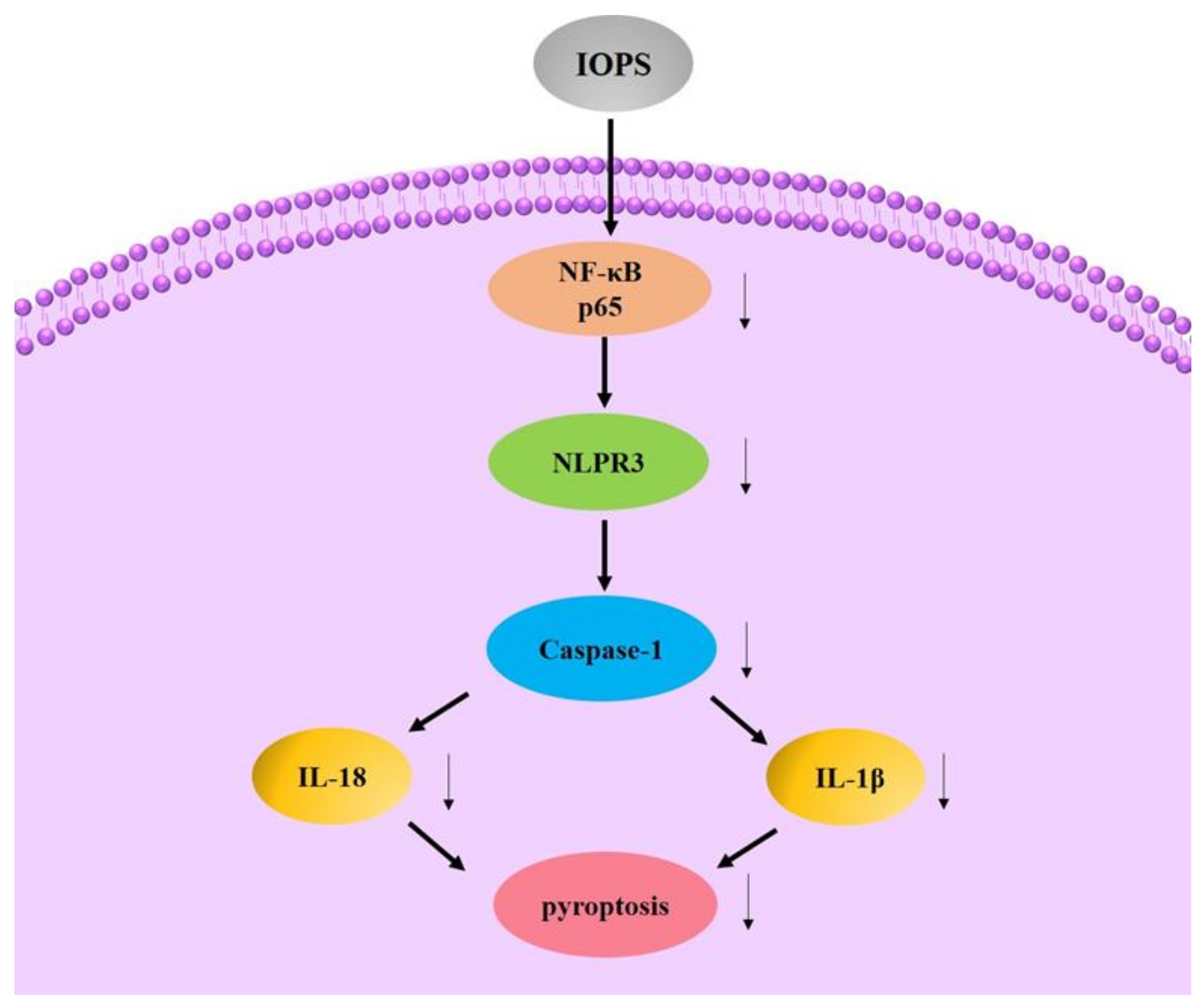
| Component | Biological Activities | References |
|---|---|---|
| Polysaccharide | Anticancer, anti-inflammatory, antiviral, antioxidant, immunomodulatory, hypoglycemic, hypolipidemic, hepatoprotective, etc. | [18,19,20,21,22,23,24] |
| Polyphenol | Antioxidant | [25] |
| Triterpenoid | Anticancer, anti-inflammatory, antiviral, and antioxidant | [26,27,28,29,30] |
| Melanin | Antioxidant, anti-inflammatory, antiviral, hypolipidemic and immunomodulatory | [24,31,32,33,34] |
| Composition | Percentage Content (Mean ± SD, %) |
|---|---|
| Moisture | 3.5 ± 0.36 |
| Crude protein | 2.4 ± 0.44 |
| Crude fat | 1.7 ± 0.25 |
| Ash | 10.4 ± 0.44 |
| Crude fiber | 67.5 ± 0.95 |
| Reducing sugar | 4.2 ± 0.30 |
| Polysaccharide | 10.3 ± 0.40 |
| Extraction Method | Extracting Temperature (°C) | Extracting Time | Solid/Water Ratio (w/v) | Polysaccharide Yield (%) | References |
|---|---|---|---|---|---|
| Water Extraction | 80 | 1.5 h | 1:40 | 2.53 | [65] |
| Water Extraction | 85 | 4.77 h | 1:43 | 16.86 ± 0.48 | [74] |
| UMAE | 19 min | 1:20 | 3.25 | [15] | |
| UMAE | 52 | 31 min | 3.81 ± 0.19 | [20] | |
| 30% Ethanol Extraction | 95 | 2.5 h | 1:30 | 5993 | [73] |
| 0.6 mol/L NaOH | 26 h | 1:28 | 28.64 ± 5.19 (alkali-soluble crude polysaccharides) | [74] | |
| Ultrasonic Method | 50 | 25 min | 1:40 | [56] |
| Cancer/Tumor Type | Concentration/Dose | Inhibition Rate | Mechanism | References |
|---|---|---|---|---|
| Hepatic carcinoma cell line (SMMC7721) | 1.0–16.0 μg/mL | 43.6–69.2% | N/A | [95] |
| Human non-small cell lung cancer cells (A549) | 100 μg/mL | 26% | NF-κB nuclear translocation (−), JNK/AKT phosphorylation (−), AKT/NF -κB (−), MMP expression level (−), Invasion (−) | [45,96,97] |
| Ovarian cancer cells (SKOV3) | 160 μg/mL | 23.94% | p53 gene mRNA expression (+), Bcl-xl gene mRNA expression (−), regulating cell apoptosis | [49] |
| Human cervical cancer cells (Hela) | 10–1000 μg/mL | 4.78–46.54% | N/A | [76] |
| Mouse bone marrow tumor cells (SP2) | 10–1000 μg/mL | 2.89–44.90% | N/A | [76] |
| Human Chang’s liver cells (Chang) | 10–1000 μg/mL | 3.56–22.56% | N/A | [76] |
| Human T lymphocytic leukemia cells (Jurkat) | 200 μg/mL 50–100 mg/kg·d | 71.84% 43.52–57.48% | Tumor cell mitochondria release Cytc(+), caspase-3 (+), endogenous (+), DNA cleavage | [15,98] |
| Human B lymphocyte tumor cells (Daudi) | 200 μg/mL | 75.14% | N/A | [15] |
| Kunming mouse S180 tumor | 200 mg/kg·d | 41.07% (intracellular polysaccharide) 36.73% (extracellular polysaccharide) | N/A | [99] |
| Human cervical cancer cells (Hela) | 200 μg/mL | 57.7% | N/A | [103] |
| Osteosarcoma cells (MG-63 and U2OS) | 320 μg/mL | 22.3% (MG-63) 23.64% (U2OS) | Akt/mTOR (−), NF-κB (−) | [101] |
| Application | Function | Brand |
|---|---|---|
| Health product | Hypoglycemic | Komsomlski |
| Health product | Enhance immunity | Wonder Land Herbs |
| Health product | Hypoglycemic | Sorlife |
| Tea | Enhance immunity | Johncan production |
| Tea | Enhance immunity, antioxidant | Atomic nature |
| Tincture | Florida Herbs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Jia, Y.; Xue, Z.; Li, N.; Liu, J.; Chen, H. Recent Developments in Inonotus obliquus (Chaga mushroom) Polysaccharides: Isolation, Structural Characteristics, Biological Activities and Application. Polymers 2021, 13, 1441. https://doi.org/10.3390/polym13091441
Lu Y, Jia Y, Xue Z, Li N, Liu J, Chen H. Recent Developments in Inonotus obliquus (Chaga mushroom) Polysaccharides: Isolation, Structural Characteristics, Biological Activities and Application. Polymers. 2021; 13(9):1441. https://doi.org/10.3390/polym13091441
Chicago/Turabian StyleLu, Yangpeng, Yanan Jia, Zihan Xue, Nannan Li, Junyu Liu, and Haixia Chen. 2021. "Recent Developments in Inonotus obliquus (Chaga mushroom) Polysaccharides: Isolation, Structural Characteristics, Biological Activities and Application" Polymers 13, no. 9: 1441. https://doi.org/10.3390/polym13091441
APA StyleLu, Y., Jia, Y., Xue, Z., Li, N., Liu, J., & Chen, H. (2021). Recent Developments in Inonotus obliquus (Chaga mushroom) Polysaccharides: Isolation, Structural Characteristics, Biological Activities and Application. Polymers, 13(9), 1441. https://doi.org/10.3390/polym13091441








