Isolation and Production of Nanocrystalline Cellulose from Conocarpus Fiber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. NCC Preparation
2.3. Characterization
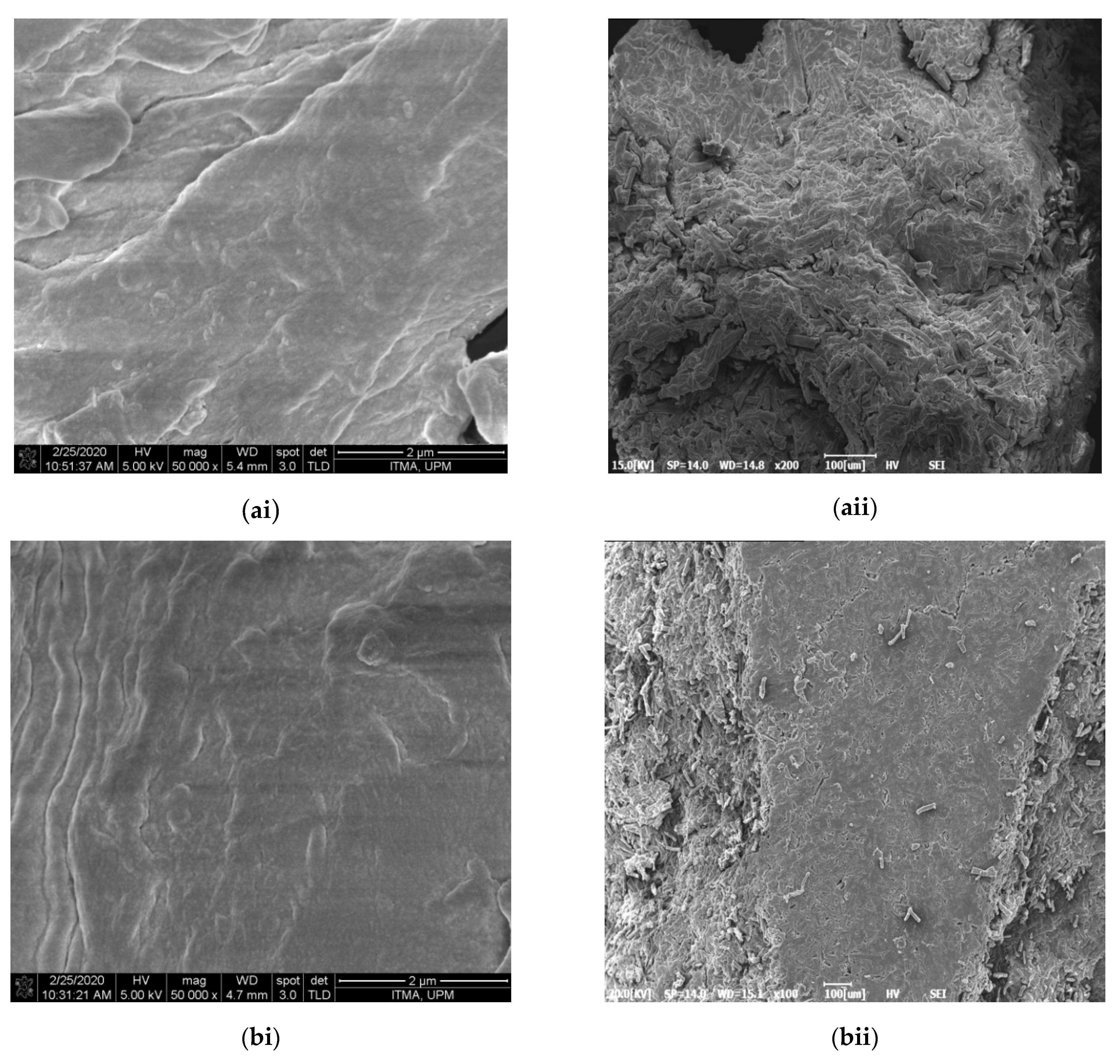
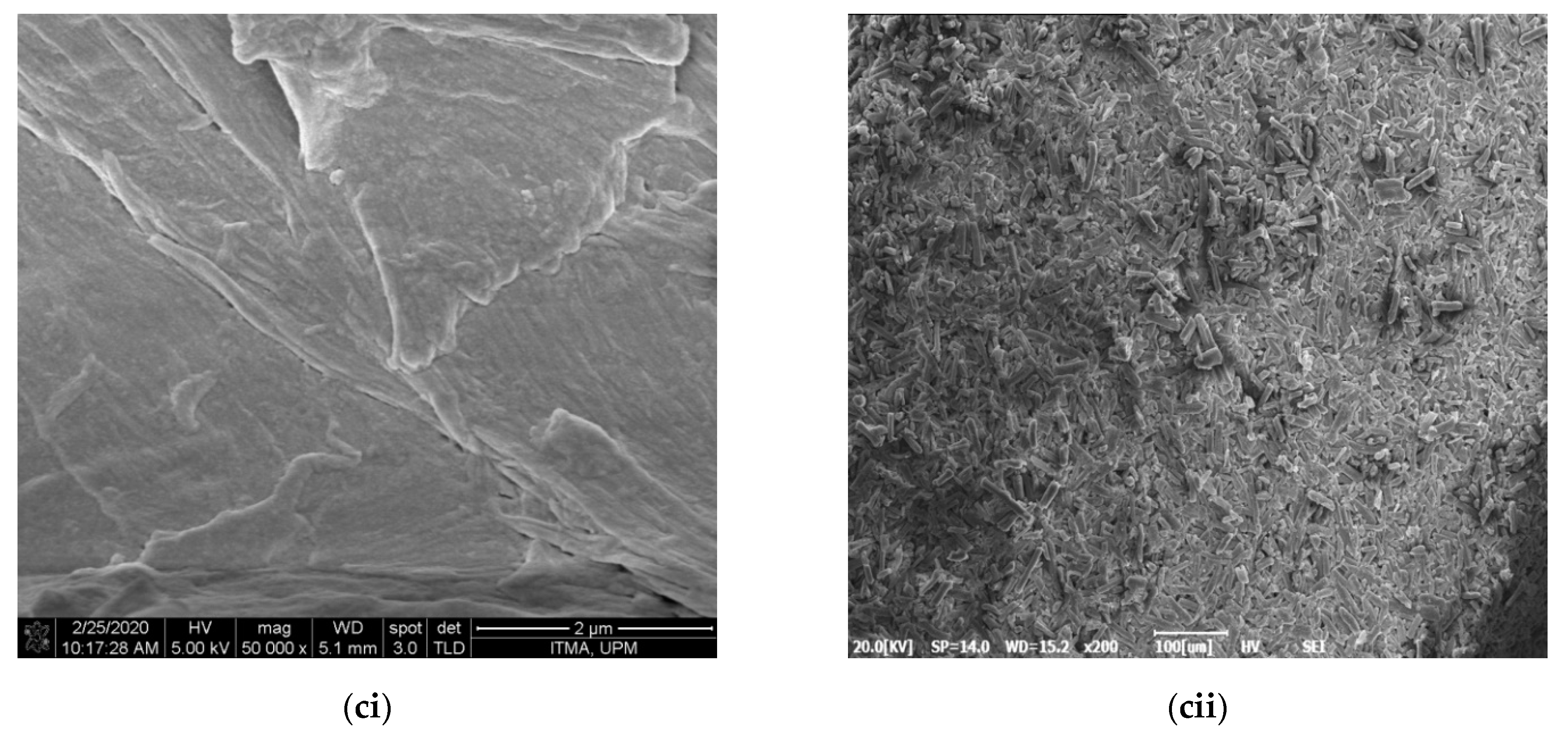
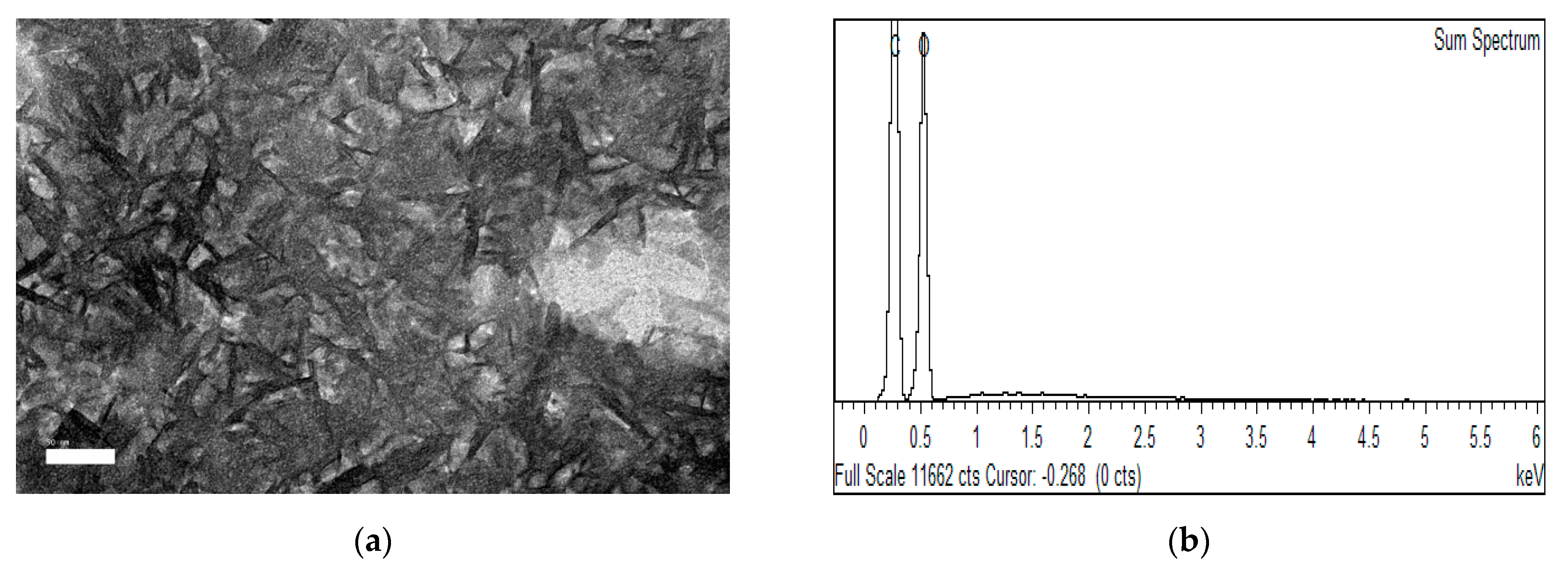
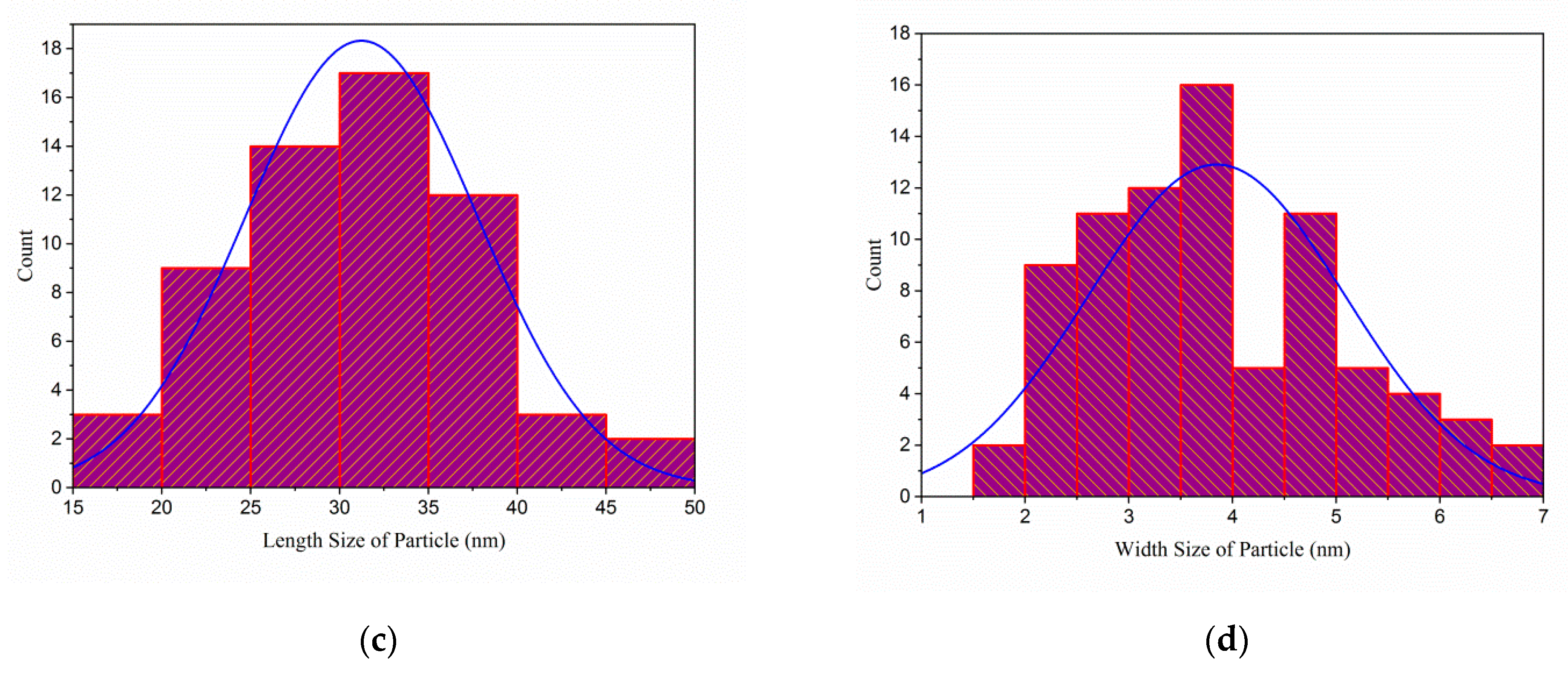
2.3.1. Morphology, Particle Size, Elements, Dispersion Behavior and Yield Analyses
2.3.2. Chemical Functionality Analysis
2.3.3. Crystalline Analysis
2.3.4. Thermal Stability Analysis
3. Results and Discussion
3.1. Morphology, Particle Size, Chemical Composition and Yield
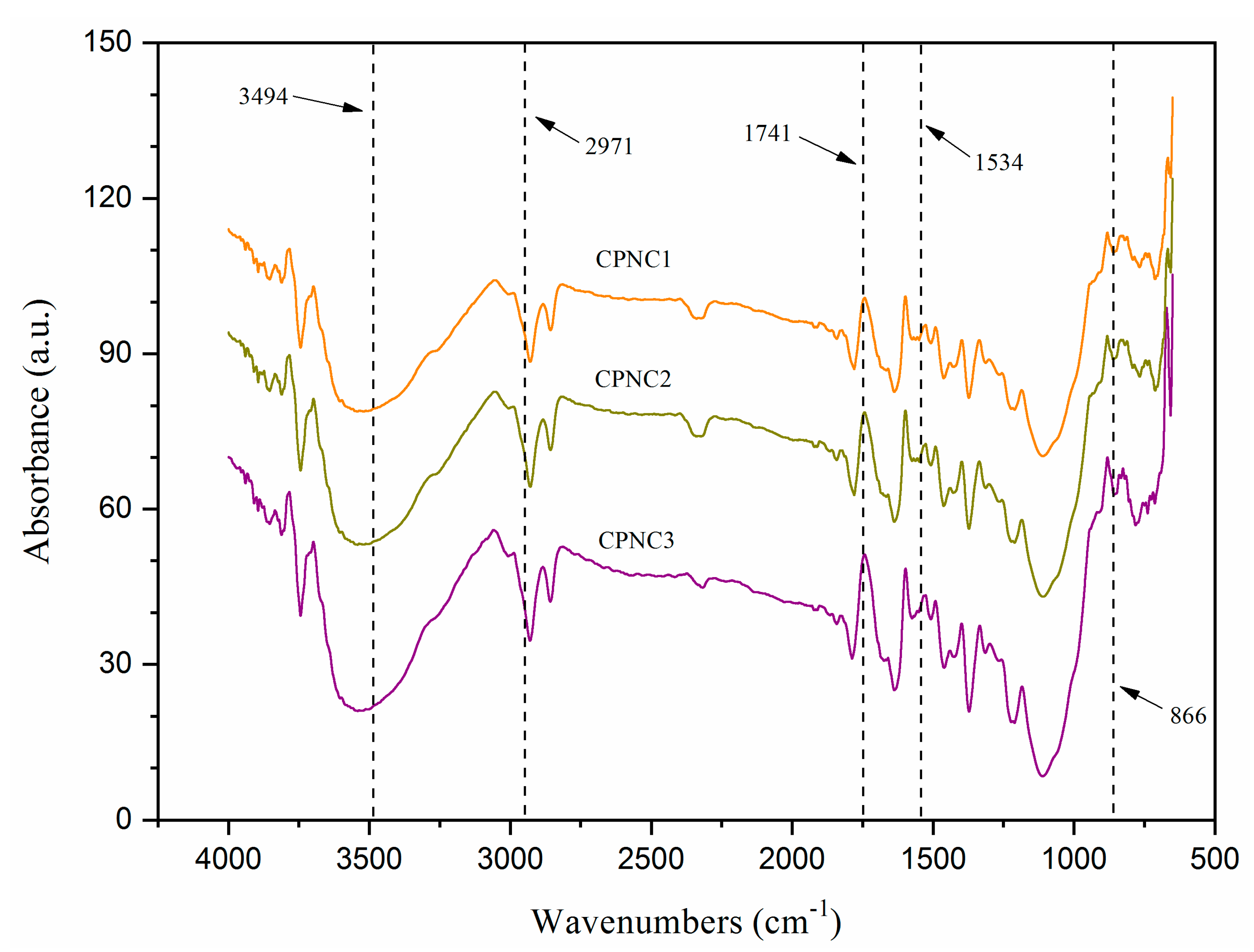
3.2. FTIR
3.3. XRD
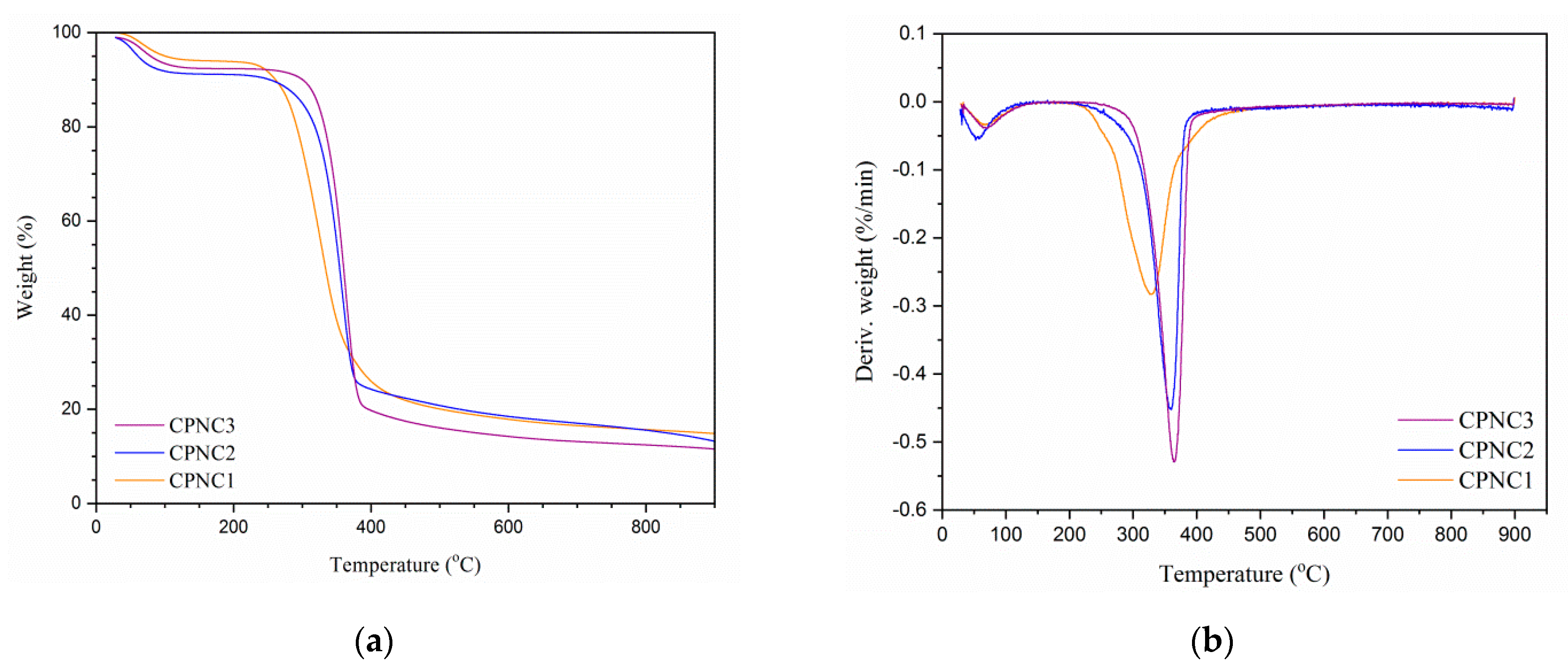
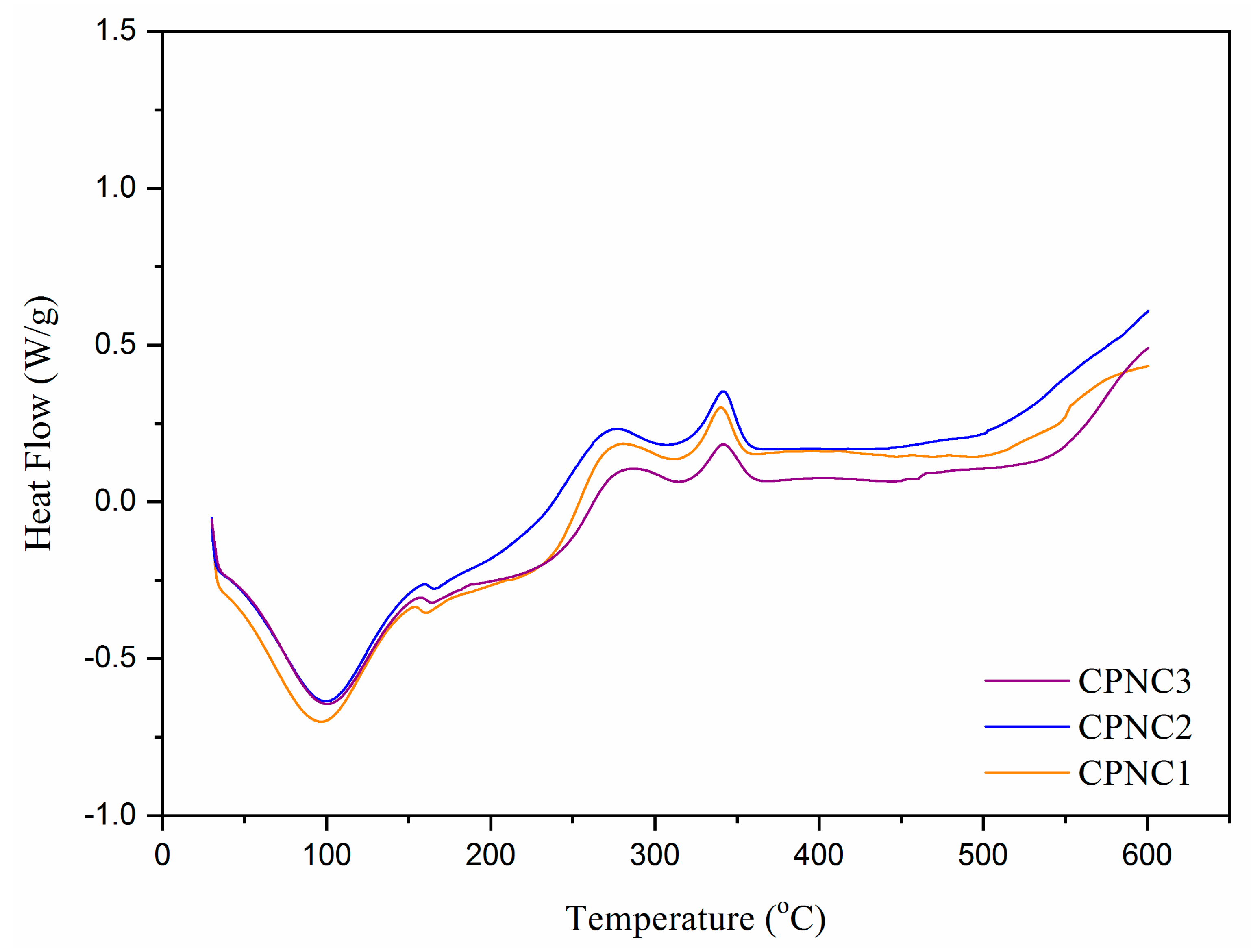
3.4. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fillat, U.; Wicklein, B.; Sampedro, R.M.; Ibarra, D.; Hitzky, E.R.; Valencia, C.; Sarrion, A.; Castro, E.; Eugenio, M.E. Assessing cellulose nanofiber production from olive tree pruning residue. Carbohydr. Polym. 2018, 179, 252–261. [Google Scholar] [CrossRef]
- Jiang, Q.; Xing, X.; Jing, Y.; Han, Y. Preparation of cellulose nanocrystals based on waste paper via different systems. Int. J. Biol. Macromol. 2020, 149, 1318–1322. [Google Scholar] [CrossRef]
- Nomura, S.; Kugo, Y.; Erata, T. 13C NMR and XRD studies on the enhancement of cellulose II crystallinity with low concentration NaOH post-treatments. Cellulose 2020, 27, 3553–3563. [Google Scholar] [CrossRef]
- Lizundia, E.; Puglia, D.; Nguyen, T.D.; Armentano, I. Cellulose nanocrystal based multifunctional nanohybrids. Prog. Mater. Sci. 2020, 112, 100668. [Google Scholar] [CrossRef]
- Verfaillie, A.; Blockx, J.; Praveenkumar, R.; Thielemans, W.; Muylaert, K. Harvesting of marine microalgae using cationic cellulose nanocrystals. Carbohydr. Polym. 2020, 240, 116165. [Google Scholar] [CrossRef]
- Rehman, S.; Abbas, G.; Shahid, M.; Saqib, M.; Farooq, A.B.U.; Hussain, M.; Farooq, A. Effect of salinity on cadmium tolerance ionic homeostasis and oxidative stress responses in conocarpus exposed to cadmium stress: Implications for phytoremediation. Ecotox. Environ. Saf. 2019, 171, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Tauqeer, H.M.; Rahman, M.; Hussain, S.; Abbas, F.; Iqbal, M. The potential of an energy crop “Conocarpus erectus” for lead phytoextraction and phytostabilization of chromium nickel and cadmium: An excellent option for the management of multi-metal contaminated soils. Ecotox. Environ. Saf. 2019, 173, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Rajabpour, A.; Mashahdi, A.R.A.; Ghorbani, M.R. Chemical compositions of leaf extracts from Conocarpus erectus L. Combretaceae and their bioactivities against Tribolium castaneum Herbst Coleoptera: Tenebrionidae. J. Asia Pac. Entomol. 2019, 22, 333–337. [Google Scholar]
- Santos, N.D.K.D.; Barros, B.R.S.; Aguiar, L.M.S.; Filho, I.J.C.; de Lorena, V.M.B.; de Melo, C.M.L.; Napoleão, T.H. Immunostimulatory and antioxidant activities of a lignin isolated from Conocarpus erectus leaves. Int. J. Biol. Macromol. 2020, 150, 169–177. [Google Scholar] [CrossRef]
- Wang, H.; Mugaanire, T.I.; Memon, H.; Jin, X. Preparation and characterization of cellulose films from ficus natalensis bark cloth fibers. Wood Fiber Sci. 2021, 53, 62–68. [Google Scholar] [CrossRef]
- Khattab, T.A.; Fouda, M.M.G.; Rehan, M.; Okla, M.K.; Alamri, S.A.; Alaraidh, I.A.; Allam, A.A. Novel halochromic cellulose nanowhiskers from rice straw: Visual detection of urea. Carbohydr. Polym. 2020, 231, 115740. [Google Scholar] [CrossRef] [PubMed]
- Kian, L.K.; Saba, N.; Jawaid, M.; Alothman, O.Y.; Fouad, H. Properties and characteristics of nanocrystalline cellulose isolated from olive fiber. Carbohydr. Polym. 2020, 241, 116423. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Liu, D.; Li, M.; Li, Q.; Liu, C.; Liu, L.; Qiao, H. Mesophase transition of cellulose nanocrystals aroused by the incorporation of two cellulose derivatives. Carbohydr. Polym. 2020, 233, 115843. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, S.; Grishkewich, N.; Berry, R.M.; Tam, K.C. Functional cellulose nanocrystals containing cationic and thermo-responsive polymer brushes. Carbohydr. Polym. 2020, 246, 116651. [Google Scholar] [CrossRef]
- Gao, A.; Chen, H.; Tang, J.; Xie, K.; Hou, A. Efficient extraction of cellulose nanocrystals from waste Calotropis gigantea fiber by SO42-/TiO2 nano-solid superacid catalyst combined with ball milling exfoliation. Ind. Crops Prod. 2020, 152, 112524. [Google Scholar] [CrossRef]
- Pirich, C.L.; Picheth, G.F.; Machado, J.P.E.; Sakakibara, C.N.; Martin, A.A.; de Freitas, R.A.; Sierakowski, M.R. Influence of mechanical pretreatment to isolate cellulose nanocrystals by sulfuric acid hydrolysis. Int. J. Biol. Macromol. 2019, 130, 622–626. [Google Scholar] [CrossRef]
- Sutliff, B.P.; Das, A.; Youngblood, J.; Bortner, M.J. High shear capillary rheometry of cellulose nanocrystals for industrially relevant processing. Carbohydr. Polym. 2020, 231, 115735. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Pinkl, S.; Altmutter, W.G. Application of surface chemical functionalized cellulose nanocrystals to improve the performance of UF adhesives used in wood based composites—MDF type. Carbohydr. Polym. 2019, 206, 11–20. [Google Scholar] [CrossRef]
- Jawaid, M.; Kian, L.K.; Fouad, H.; Saba, N.; Alothman, O.Y.; Hashem, M. Morphological, structural, and thermal analysis of three part of conocarpus cellulosic fibers. J. Mater. Res. Technol. 2021, 10, 24–33. [Google Scholar] [CrossRef]
- Benini, K.C.C.; Voorwald, H.J.C.; Cioffi, M.O.H.; Rezende, M.C.; Arantes, V. Preparation of nanocellulose from Imperata brasiliensis grass using Taguchi method. Carbohydr. Polym. 2018, 192, 337–346. [Google Scholar] [CrossRef]
- Omidi, S.; Pirhayati, M.; Kakanejadifard, A. Co-delivery of doxorubicin and curcumin by a pH-sensitive injectable and in situ hydrogel composed of chitosan graphene and cellulose nanowhisker. Carbohydr. Polym. 2020, 231, 115745. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, K.B.R.; Migliorini, F.L.; Facure, M.H.M.; Correa, D.S. Conductive electrospun nanofibers containing cellulose nanowhiskers and reduced graphene oxide for the electrochemical detection of mercuryII. Carbohydr. Polym. 2019, 207, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Pedro, F.W.; Kim, K.; Kim, K.; Tam, K.C. Phosphorylated-CNC/modified-chitosan nanocomplexes for the stabilization of Pickering emulsions. Carbohydr. Polym. 2019, 206, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Du, W.B.; Deng, A.; Guo, J.; Chen, J.; Li, H.; Gao, Y. An injectable self-healing hydrogel-cellulose nanocrystals conjugate with excellent mechanical strength and good biocompatibility. Carbohydr. Polym. 2019, 223, 115084. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhan, C.; Kong, F.; Li, W.; Yang, C.; Hsiao, B.S. Facile synthesis of TiO2/CNC nanocomposites for enhanced CrVI photoreduction: Synergistic roles of cellulose nanocrystals. Carbohydr. Polym. 2020, 233, 115838. [Google Scholar] [CrossRef]
- Xing, L.; Gu, J.; Zhang, W.; Tu, D.; Hu, C. Cellulose I and II nanocrystals produced by sulfuric acid hydrolysis of Tetra pak cellulose I. Carbohydr. Polym. 2018, 192, 184–192. [Google Scholar] [CrossRef]
- Lee, H.; You, J.; Jin, H.J.; Kwak, H.W. Chemical and physical reinforcement behavior of dialdehyde nanocellulose in PVA composite film: A comparison of nanofiber and nanocrystal. Carbohydr. Polym. 2020, 232, 115771. [Google Scholar] [CrossRef]
- Seta, F.T.; An, X.; Liu, L.; Zhang, H.; Yang, J.; Zhang, W.; Liu, H. Preparation and characterization of high yield cellulose nanocrystals CNC derived from ball mill pretreatment and maleic acid hydrolysis. Carbohydr. Polym. 2020, 234, 115942. [Google Scholar] [CrossRef]
- Chargot, M.S.; Chylińska, M.; Pieczywek, P.M.; Zdunek, A. Tailored nanocellulose structure depending on the origin: Example of apple parenchyma and carrot root celluloses. Carbohydr. Polym. 2019, 210, 186–195. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; Filho, M.S.M.S.; Rosa, M.F. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Negi, Y.S. Studies on cellulose nanocrystals isolated from groundnut shells. Carbohydr. Polym. 2017, 157, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Taflick, T.; Schwendler, L.A.; Rosa, S.M.L.; Bica, C.I.D.; Nachtigall, S.M.B. Cellulose nanocrystals from acacia bark–Influence of solvent extraction. Int. J. Biol. Macromol. 2017, 101, 553–561. [Google Scholar] [CrossRef] [PubMed]

| Samples | α-Cellulose (%) | Hemicellulose (%) | Lignin (%) | Yield (%) |
|---|---|---|---|---|
| CP-raw | 41.7 ± 0.32 | 28.5 ± 0.08 | 15.6 ± 0.02 | - |
| CP-NaOH | 69.3 ± 0.51 | 15.5 ± 0.19 | 9.3 ± 0.05 | 56.6 ± 0.37 |
| CP-NaClO2 | 70.2 ± 0.54 | 14.1 ± 0.17 | 7.5 ± 0.04 | 51.9 ± 0.41 |
| CPNC1 | 81.6 ± 0.67 | 6.1 ± 0.22 | 2.9 ± 0.11 | 17.6 ± 0.27 |
| CPNC2 | 82.3 ± 0.66 | 5.8 ± 0.24 | 2.6 ± 0.13 | 18.3 ± 0.29 |
| CPNC3 | 84.9 ± 0.64 | 5.3 ± 0.18 | 2.2 ± 0.09 | 18.9 ± 0.34 |
| Samples | OdT (°C) a | PdT (°C) b | MWL (%) c | RW (%) d |
|---|---|---|---|---|
| CPNC1 | 266.3 | 327.7 | 74.2 | 15.1 |
| CPNC2 | 304.2 | 359.0 | 73.8 | 14.3 |
| CPNC3 | 324.7 | 364.5 | 76.7 | 12.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Jawaid, M.; Kian, L.K.; Khan, A.A.P.; Asiri, A.M. Isolation and Production of Nanocrystalline Cellulose from Conocarpus Fiber. Polymers 2021, 13, 1835. https://doi.org/10.3390/polym13111835
Khan A, Jawaid M, Kian LK, Khan AAP, Asiri AM. Isolation and Production of Nanocrystalline Cellulose from Conocarpus Fiber. Polymers. 2021; 13(11):1835. https://doi.org/10.3390/polym13111835
Chicago/Turabian StyleKhan, Anish, Mohammad Jawaid, Lau Kia Kian, Aftab Aslam Parwaz Khan, and Abdullah M. Asiri. 2021. "Isolation and Production of Nanocrystalline Cellulose from Conocarpus Fiber" Polymers 13, no. 11: 1835. https://doi.org/10.3390/polym13111835
APA StyleKhan, A., Jawaid, M., Kian, L. K., Khan, A. A. P., & Asiri, A. M. (2021). Isolation and Production of Nanocrystalline Cellulose from Conocarpus Fiber. Polymers, 13(11), 1835. https://doi.org/10.3390/polym13111835









