An In-Vitro Evaluation of the Characteristics of Zein-Based Films for the Release of Lactobionic Acid and the Effects of Oleic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Zein-Based LBA Films
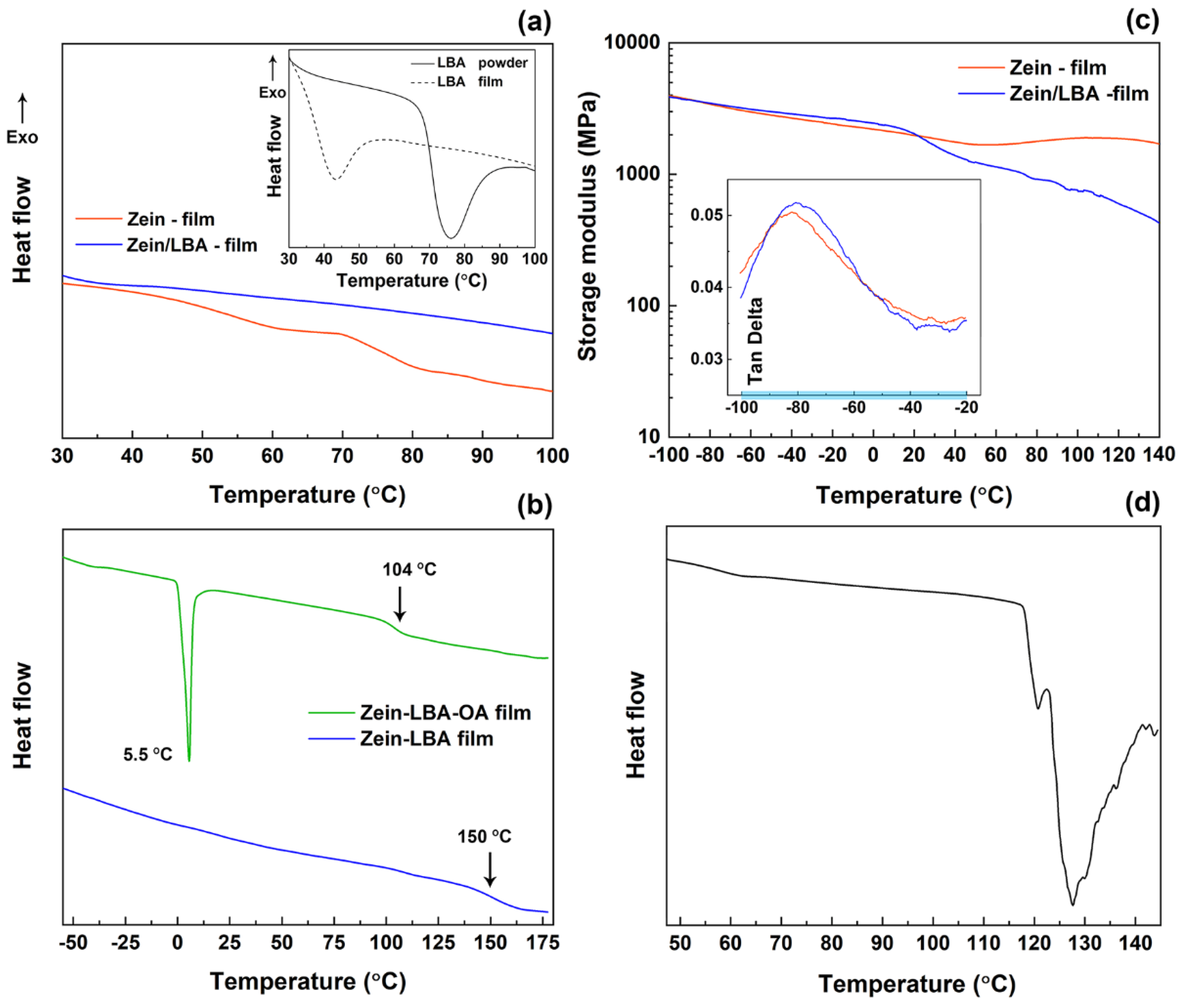
2.2. Differential Scanning Calorimetry
2.3. Dynamic Mechanical Analysis
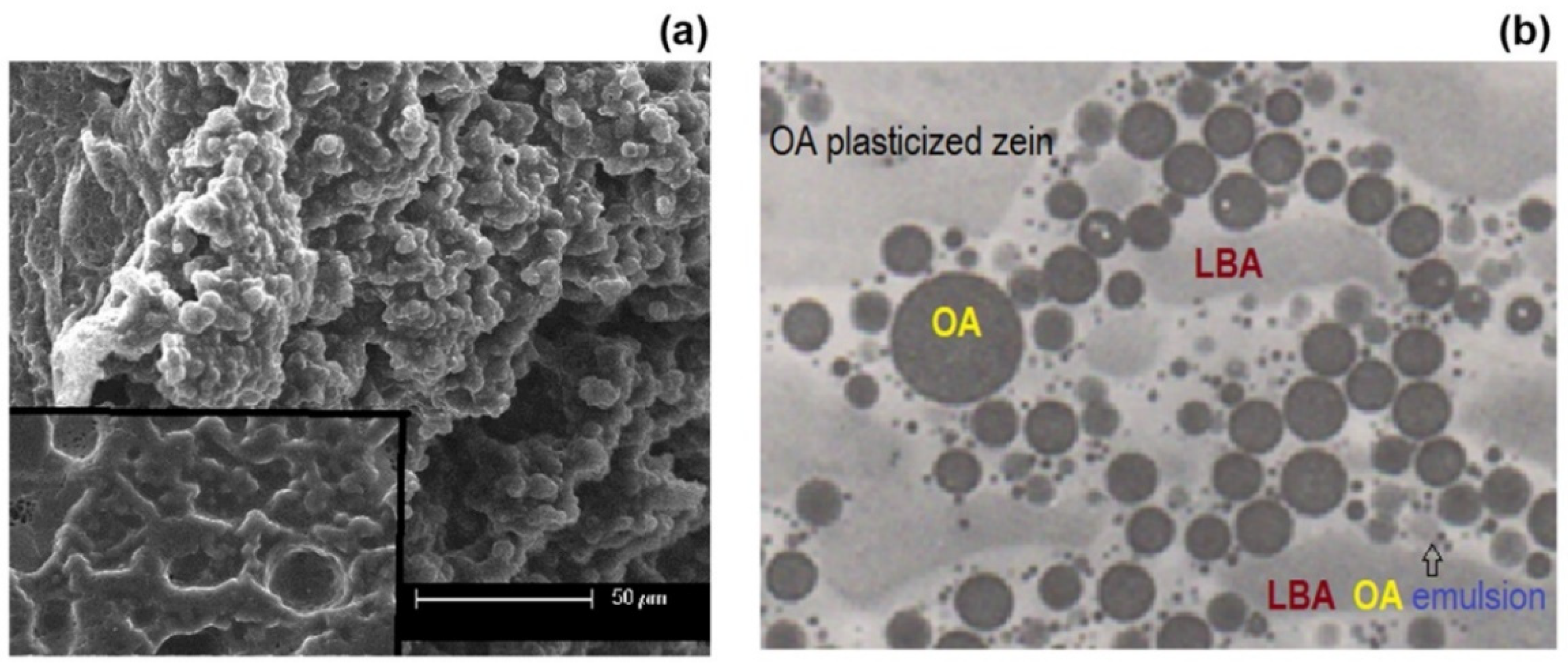
2.4. Scanning Electron Microscopy
2.5. Release of LBA from Zein-Based Films
2.6. Antioxidant Capability of Zein-LBA Films
2.7. Antimicrobial Activity of Released LBA
2.8. Statistical Analyses
3. Results and Discussion
3.1. Morphology and Physical State of Mixtures
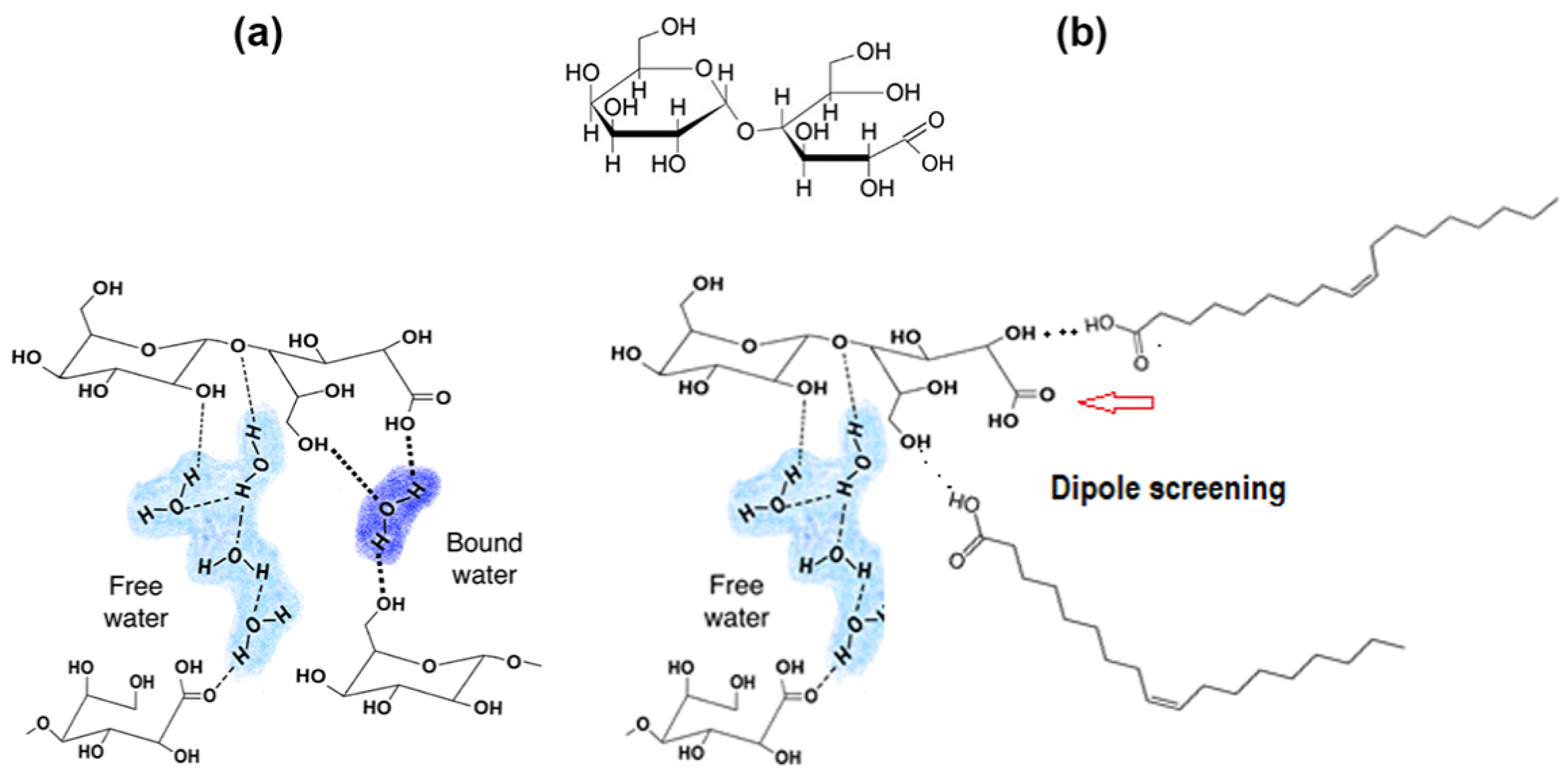
3.2. Release of LBA from the Zein-Based Films
3.3. Antioxidant Activity of Zein-LBA Films
3.4. Antimicrobial Activity of Zein-LBA Films against E. coli and S. epidermidis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Giorgi, S.; Raddadi, N.; Fabbri, A.; Toschi, T.G.; Fava, F. Potential use of ricotta cheese whey for the production of lactobionic acid by Pseudomonas taetrolens strains. New Biotechnol. 2018, 42, 71–76. [Google Scholar] [CrossRef]
- Gutiérrez, L.-F.; Hamoudi, S.; Belkacemi, K. Lactobionic acid: A high value-added lactose derivative for food and pharmaceutical applications. Int. Dairy J. 2012, 26, 103–111. [Google Scholar] [CrossRef]
- Tasic-Kostov, M.; Pavlovic, D.; Lukic, M.; Jaksic, I.; Arsić, I.; Savic, S. Lactobionic acid as antioxidant and moisturizing active in alkyl polyglucoside-based topical emulsions: The colloidal structure, stability and efficacy evaluation. Int. J. Cosmet. Sci. 2012, 34, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Rendueles, M.; Diaz, M. Bio-production of lactobionic acid: Current status, applications and future prospects. Biotechnol. Adv. 2013, 31, 1275–1291. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, T.; Marques, C.; Dagostin, J.L.A.; Masson, M.L. Lactobionic Acid as a Potential Food Ingredient: Recent Studies and Applications. J. Food Sci. 2019, 84, 1672–1681. [Google Scholar] [CrossRef]
- Mascia, L.; Coroli, A.; Mele, E. Probing the Thermal Transitions of Lactobionic Acid and Effects of Sample History by DSC Analysis. J. Pharm. Sci. 2019, 108, 3781–3784. [Google Scholar] [CrossRef]
- Alonso, S. Exploiting the bioengineering versatility of lactobionic acid in targeted nanosystems and biomaterials. J. Control. Release 2018, 287, 216–234. [Google Scholar] [CrossRef]
- Godse, R.; Rathod, M.; De, A.; Shinde, U. Intravitreal galactose conjugated polymeric nanoparticles of etoposide for retinoblastoma. J. Drug Deliv. Sci. Technol. 2021, 61, 102259. [Google Scholar] [CrossRef]
- Habib, S.M.; Rehman, J.-U.; Maharjan, R.; Kanwal, T.; Althagafi, I.I.; Saifullah, S.; Ullah, S.; Simjee, S.U.; Shah, M.R. Synthesis of lactobionic acid based bola-amphiphiles and its application as nano-carrier for curcumin delivery to cancer cell cultures in-vitro. Int. J. Pharm. 2020, 590, 119897. [Google Scholar] [CrossRef]
- Li, S.; Saw, P.E.; Lin, C.; Nie, Y.; Tao, W.; Farokhzad, O.C.; Zhang, L.; Xu, X. Redox-responsive polyprodrug nanoparticles for targeted siRNA delivery and synergistic liver cancer therapy. Biomaterials 2020, 234, 119760. [Google Scholar] [CrossRef]
- Upadhyay, P.; Bhattacharjee, M.; Bhattacharya, S.; Ahir, M.; Adhikary, A.; Patra, P. Silymarin-Loaded, Lactobionic Acid-Conjugated Porous PLGA Nanoparticles Induce Apoptosis in Liver Cancer Cells. ACS Appl. Bio. Mater. 2020, 3, 7178–7192. [Google Scholar] [CrossRef]
- Abdelmoneem, M.A.; Elnaggar, M.A.; Hammady, R.S.; Kamel, S.M.; Helmy, M.W.; Abdulkader, M.A.; Zaky, A.; Fang, J.-Y.; Elkhodairy, K.A.; Elzoghby, A.O. Dual-Targeted Lactoferrin Shell-Oily Core Nanocapsules for Synergistic Targeted/Herbal Therapy of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2019, 11, 26731–26744. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, J.; Xu, Y.; Shao, H.; Gu, J. Surface-modified PLGA nanoparticles with PEG/LA-chitosan for targeted delivery of arsenic trioxide for liver cancer treatment: Inhibition effects enhanced and side effects reduced. Colloids Surf. B Biointerfaces 2019, 180, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, L.; Wei, B.; Yan, G.; Wang, J.; Tang, R. Bromelain-immobilized and lactobionic acid-modified chitosan nanoparticles for enhanced drug penetration in tumor tissues. Int. J. Biol. Macromol. 2018, 115, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, M.; Yu, A.; Ji, J.; Zhai, G. Insight into the role of dual-ligand modification in low molecular weight heparin based nanocarrier for targeted delivery of doxorubicin. Int. J. Pharm. 2017, 523, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.-M.; Padua, G.W.; Wei, L.S. Properties and Microstructure of Zein Sheets Plasticized with Palmitic and Stearic Acids. Cereal Chem. J. 1997, 74, 83–90. [Google Scholar] [CrossRef]
- Xu, H.; Chai, Y.; Zhang, G. Synergistic Effect of Oleic Acid and Glycerol on Zein Film Plasticization. J. Agric. Food Chem. 2012, 60, 10075–10081. [Google Scholar] [CrossRef] [PubMed]
- Paramawati, R.; Yoshino, T.; Isobe, S. Properties of Plasticized-Zein Film as Affected by Plasticizer Treatments. Food Sci. Technol. Res. 2001, 7, 191–194. [Google Scholar] [CrossRef]
- Paliwal, R.; Palakurthi, S. Zein in controlled drug delivery and tissue engineering. J. Control. Release 2014, 189, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Hayat, U.; Bilal, M.; Iqbal, H.M.; Wang, J.-Y. Zein-based micro- and nano-constructs and biologically therapeutic cues with multi-functionalities for oral drug delivery systems. J. Drug Deliv. Sci. Technol. 2020, 58, 101818. [Google Scholar] [CrossRef]
- Tran, P.H.; Duan, W.; Lee, B.-J.; Tran, T.T. The use of zein in the controlled release of poorly water-soluble drugs. Int. J. Pharm. 2019, 566, 557–564. [Google Scholar] [CrossRef]
- Yong, Z.; Lili, C.; Feng, L.; Nianqiu, S.; Chunlei, L.; Xianghui, Y.; Yan, C.; Wei, K. Design, fabrication and biomedical applications of zein-based nano/micro-carrier systems. Int. J. Pharm. 2016, 513, 191–210. [Google Scholar] [CrossRef]
- Mascia, L.; Zhang, W.; Gatto, F.; Scarpellini, A.; Pompa, P.P.; Mele, E. In Situ Generation of ZnO Nanoparticles within a Polyethyleneimine Matrix for Antibacterial Zein Fibers. ACS Appl. Polym. Mater. 2019, 1, 1707–1716. [Google Scholar] [CrossRef]
- Esmaeili, H.; Karami, A.; Maggi, F. Essential oil composition, total phenolic and flavonoids contents, and antioxidant activity of Oliveria decumbens Vent. (Apiaceae) at different phenological stages. J. Clean. Prod. 2018, 198, 91–95. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- De Almeida, C.B.; Corradini, E.; Forato, L.A.; Fujihara, R.; Filho, J.F.L. Microstructure and thermal and functional properties of biodegradable films produced using zein. Polímeros 2018, 28, 30–37. [Google Scholar] [CrossRef]
- Cedeño, F.O.; Prieto, M.M.; Espina, A.; García, J.R. Measurements of temperature and melting heat of some pure fatty acids and their binary and ternary mixtures by differential scanning calorimetry. Thermochim. Acta 2001, 369, 39–50. [Google Scholar] [CrossRef]
- Singh, N.; Georget, D.M.R.; Belton, P.S.; Barker, S.A. Zein−Iodine Complex Studied by FTIR Spectroscopy and Dielectric and Dynamic Rheometry in Films and Precipitates. J. Agric. Food Chem. 2009, 57, 4334–4341. [Google Scholar] [CrossRef] [PubMed]
- Mascia, L.; Kouparitsas, Y.; Nocita, D.; Bao, X. Antiplasticization of Polymer Materials: Structural Aspects and Effects on Mechanical and Diffusion-Controlled Properties. Polymers 2020, 12, 769. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, B.; Oromiehi, A.R. Studies on glass transition temperature of mono and bilayer protein films plasticized by glycerol and olive oil. J. Appl. Polym. Sci. 2008, 109, 2848–2854. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Bouman, J.; Belton, P.; Venema, P.; Van Der Linden, E.; De Vries, R.; Qi, S. Controlled Release from Zein Matrices: Interplay of Drug Hydrophobicity and pH. Pharm. Res. 2016, 33, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Podaralla, S.; Perumal, O. Influence of Formulation Factors on the Preparation of Zein Nanoparticles. AAPS PharmSciTech 2012, 13, 919–927. [Google Scholar] [CrossRef]
- Drzymala, J. Chemistry of the Oleic Acid-H2O-NaCl System VS pH at 25 °C. In Surfactants in Solution; Mittal, K.L., Ed.; Springer: Boston, MA, USA, 1989. [Google Scholar]
- Mascia, L.; Capra, C.; Lavorgna, M. Organic-Inorganic Hybrid Fillers for The Controlled Release of Antioxidants. Macromol. Symp. 2007, 247, 129–139. [Google Scholar] [CrossRef]
- Mascia, L.; Prezzi, L.; Wilcox, G.D.; Lavorgna, M. Molybdate doping of networks in epoxy–silica hybrids: Domain structuring and corrosion inhibition. Prog. Org. Coatings 2006, 56, 13–22. [Google Scholar] [CrossRef]
- Herpels, J.; Mascia, L. Effects of styrene-acrylonitrile/butadiene ratio on the toughness of polycarbonate/ABS blends. Eur. Polym. J. 1990, 26, 997–1003. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free. Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Cobas, A.C.; García-Bermejo, A.B.; Montilla, A.; Olano, A.; Corzo, N. Synthesis, characterization and functional properties of galactosylated derivatives of chitosan through amide formation. Food Hydrocoll. 2013, 33, 245–255. [Google Scholar] [CrossRef]
- Soliman, E.A.; Khalil, A.A.; Deraz, S.; El-Fawal, G.; Elrahman, S.A. Synthesis, characterization and antibacterial activity of biodegradable films prepared from Schiff bases of zein. J. Food Sci. Technol. 2012, 51, 2425–2434. [Google Scholar] [CrossRef]
- Kang, S.; Kong, F.; Shi, X.; Han, H.; Li, M.; Guan, B.; Yang, M.; Cao, X.; Tao, D.; Zheng, Y.; et al. Antibacterial activity and mechanism of lactobionic acid against Pseudomonas fluorescens and Methicillin-resistant Staphylococcus aureus and its application on whole milk. Food Control. 2020, 108, 106876. [Google Scholar] [CrossRef]
- Kang, S.; Kong, F.; Liang, X.; Li, M.; Yang, N.; Cao, X.; Yang, M.; Tao, D.; Yue, X.; Zheng, Y. Label-Free Quantitative Proteomics Reveals the Multitargeted Antibacterial Mechanisms of Lactobionic Acid against Methicillin-Resistant Staphylococcus aureus (MRSA) using SWATH-MS Technology. J. Agric. Food Chem. 2019, 67, 12322–12332. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhong, Q. Lactobionic acid enhances the synergistic effect of nisin and thymol against Listeria monocytogenes Scott A in tryptic soy broth and milk. Int. J. Food Microbiol. 2017, 260, 36–41. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coroli, A.; Romano, R.; Saccani, A.; Raddadi, N.; Mele, E.; Mascia, L. An In-Vitro Evaluation of the Characteristics of Zein-Based Films for the Release of Lactobionic Acid and the Effects of Oleic Acid. Polymers 2021, 13, 1826. https://doi.org/10.3390/polym13111826
Coroli A, Romano R, Saccani A, Raddadi N, Mele E, Mascia L. An In-Vitro Evaluation of the Characteristics of Zein-Based Films for the Release of Lactobionic Acid and the Effects of Oleic Acid. Polymers. 2021; 13(11):1826. https://doi.org/10.3390/polym13111826
Chicago/Turabian StyleCoroli, Alessandro, Roberta Romano, Andrea Saccani, Noura Raddadi, Elisa Mele, and Leno Mascia. 2021. "An In-Vitro Evaluation of the Characteristics of Zein-Based Films for the Release of Lactobionic Acid and the Effects of Oleic Acid" Polymers 13, no. 11: 1826. https://doi.org/10.3390/polym13111826
APA StyleCoroli, A., Romano, R., Saccani, A., Raddadi, N., Mele, E., & Mascia, L. (2021). An In-Vitro Evaluation of the Characteristics of Zein-Based Films for the Release of Lactobionic Acid and the Effects of Oleic Acid. Polymers, 13(11), 1826. https://doi.org/10.3390/polym13111826






