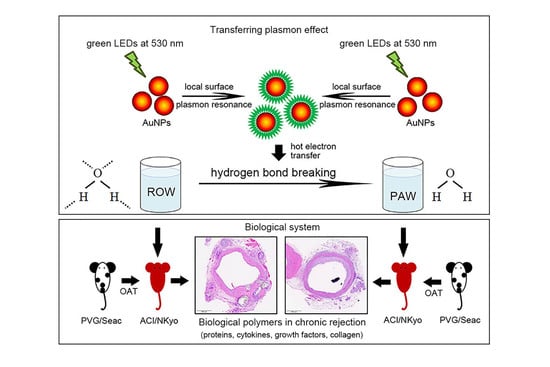
Transferring Plasmon Effect on a Biological System: Expression of Biological Polymers in Chronic Rejection and Inflammatory Rat Model
Abstract
1. Introduction
2. Materials and Methods
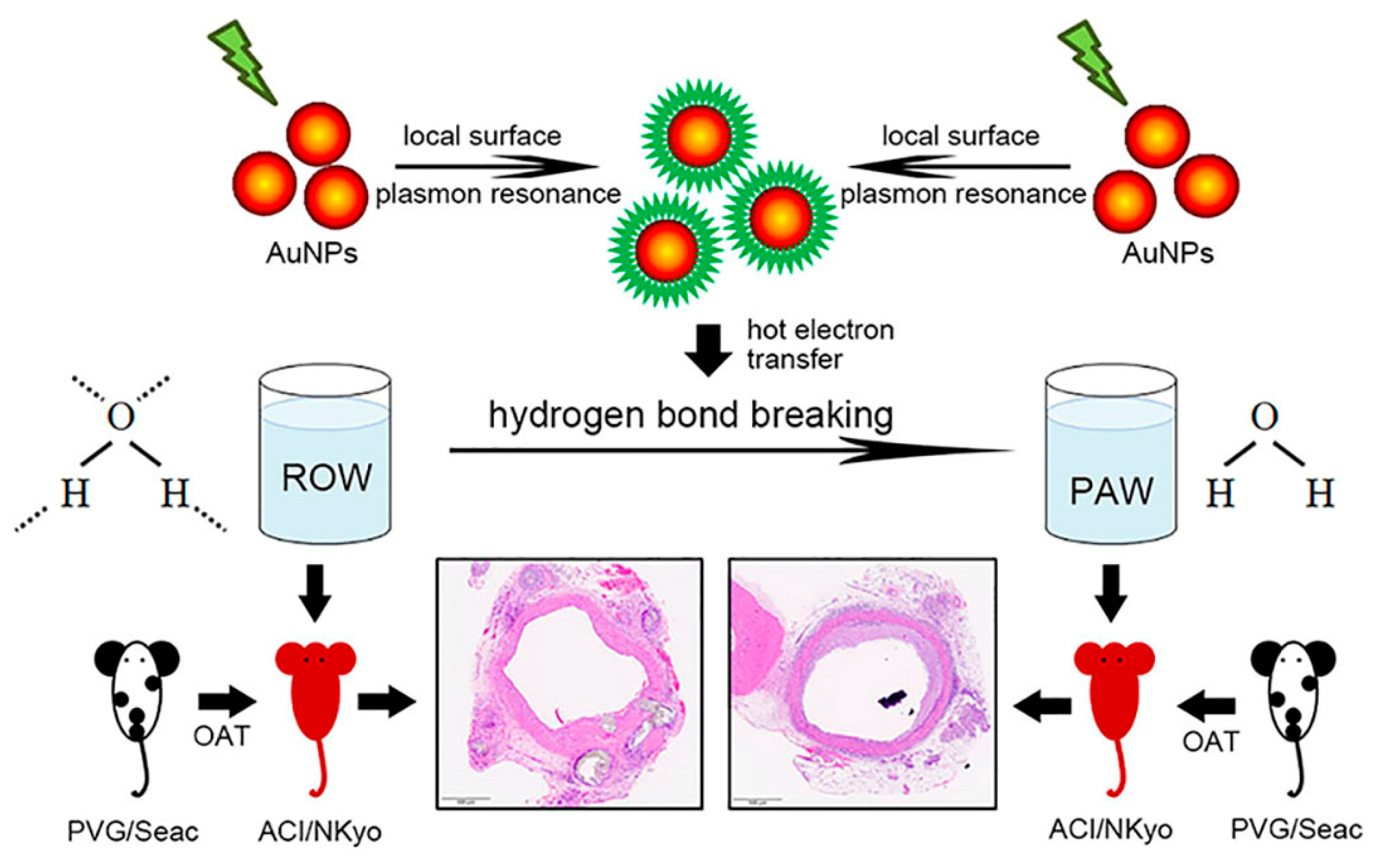
2.1. Preparation and Quality Examination of Plasmon-Activated Water
2.2. Animal Grouping and OAT Experiment
2.3. Orthotopic Aortic Transplantation
2.4. Biochemical Measurements and Enzyme-Linked Immunosorbent Assays
2.5. Morphological Analysis
2.6. Flow Cytometry
2.7. Early EPC Colony-Forming Assay
2.8. Statistical Analyses
3. Results
3.1. Administration of PAW Did Not Affect the Biochemical Characteristics in OAT ACI/NKyo Rats
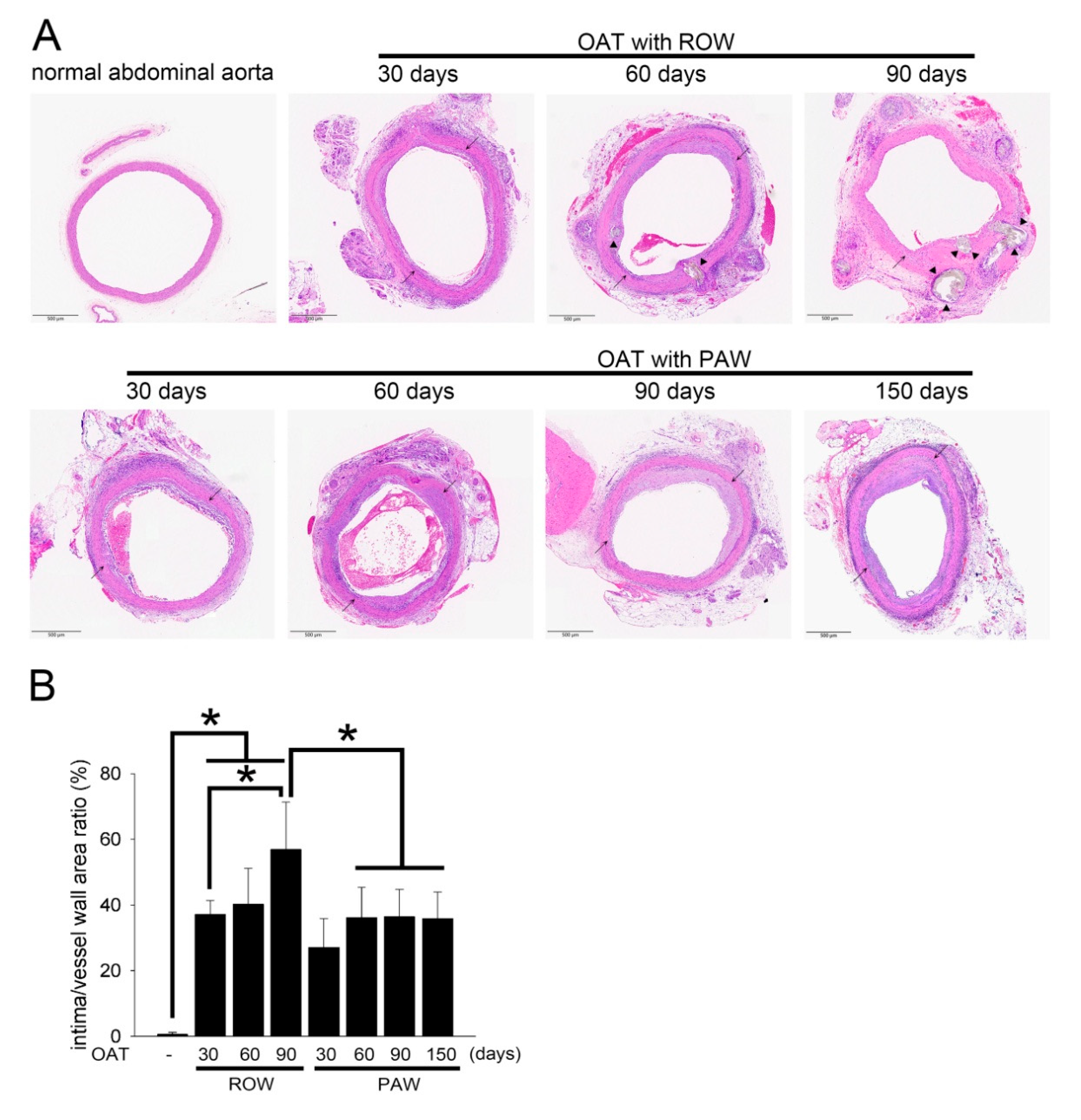
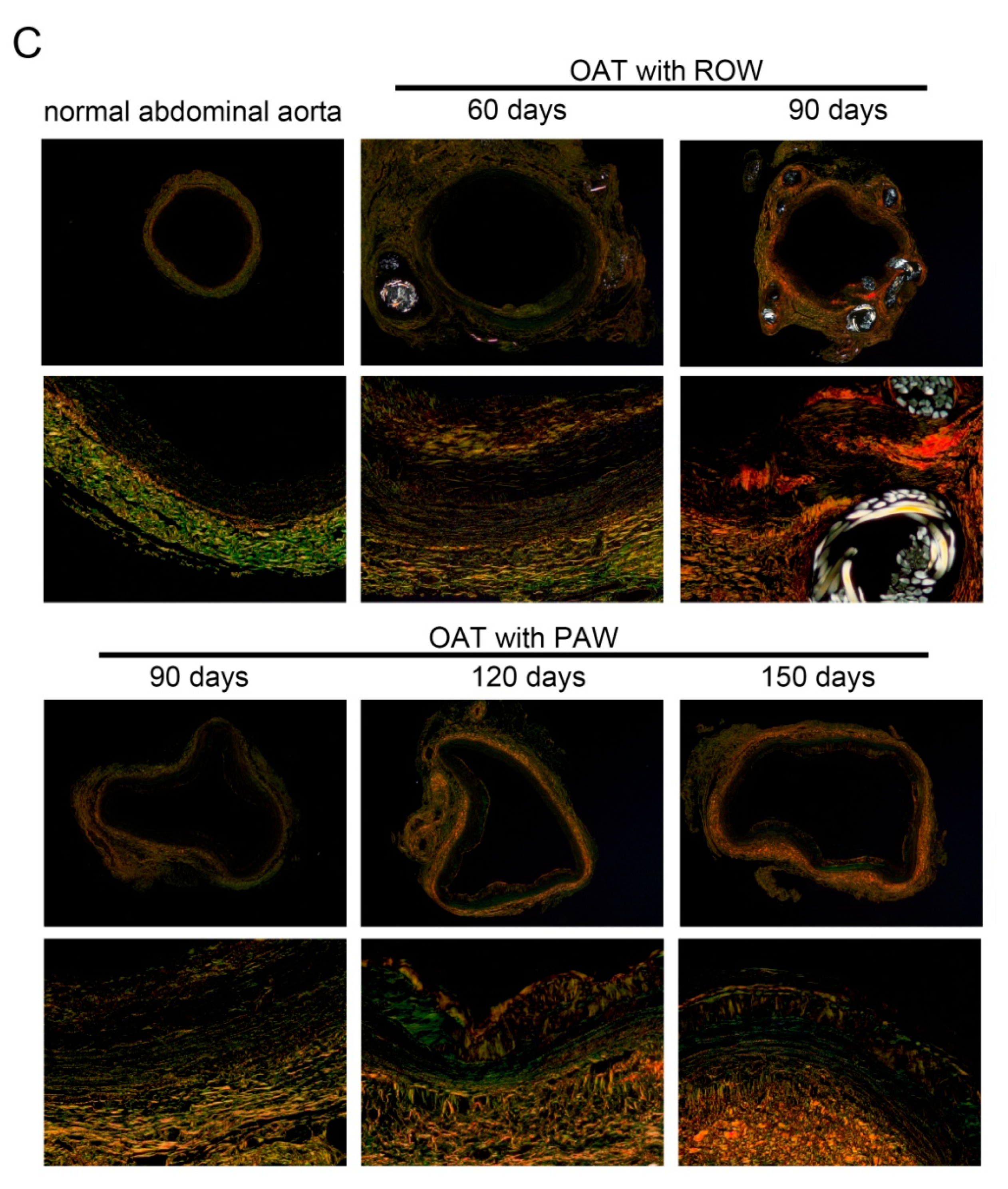
3.2. PAW Decreases Vasculopathy and Collagen Accumulation in OAT ACI/NKyo Rats
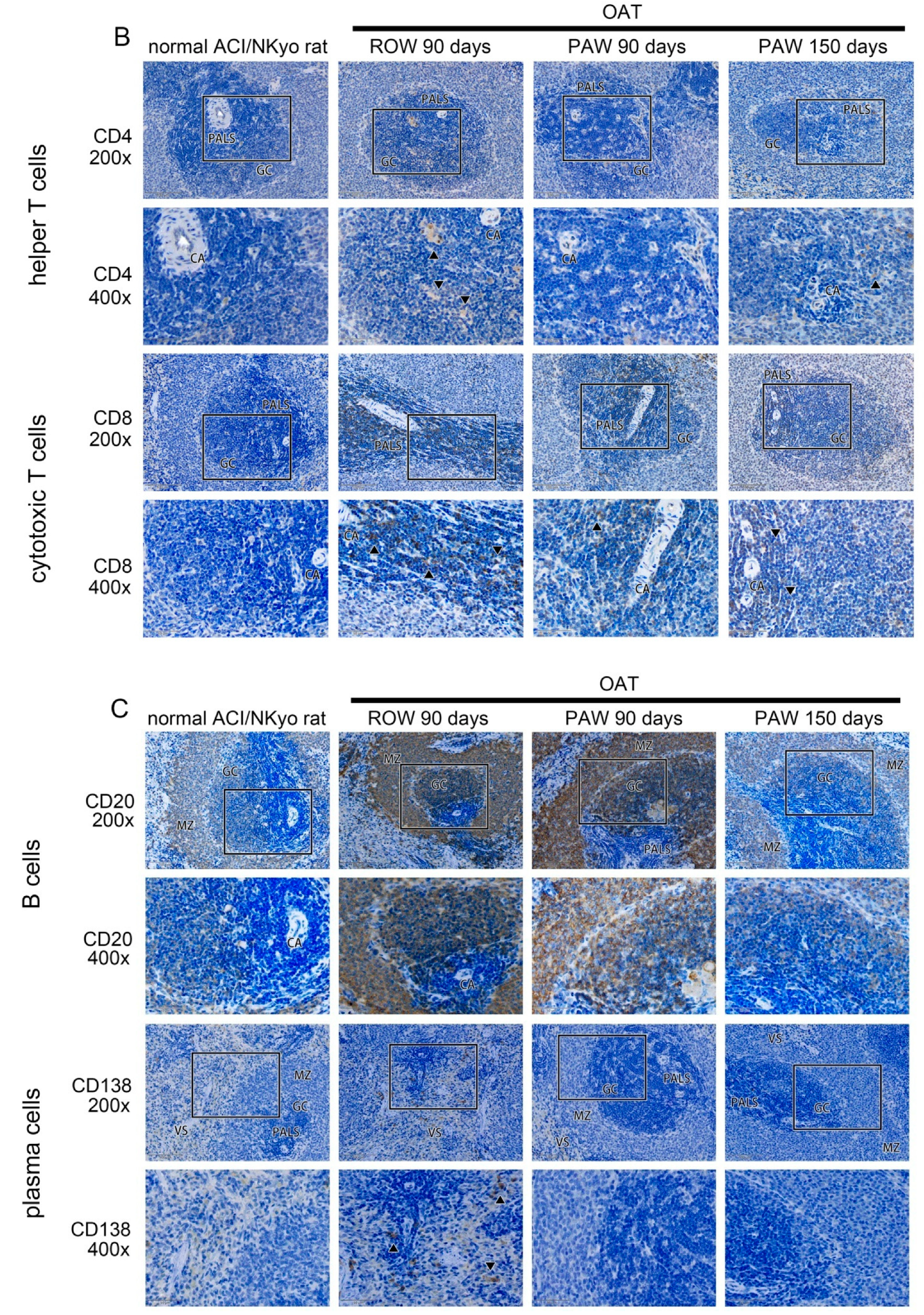
3.3. Reduced Proliferation and Accumulation of Immune Cells Was Observed in the Vessel Wall of PAW Administered OAT ACI/NKyo Rats
3.4. Administration of PAW Results in Lower Plasma Concentration of Inflammatory Factors and Cytokines in OAT ACI/NKyo Rats
3.5. Administration of PAW Results in Reduced Cell-Mediated and Humoral Immune Responses inOAT ACI/NKyo Rats
3.6. Administration of PAW Promotes Increased Mobilization of Circulating EPCs and Differentiation of Early EPCs Than Administration of ROW in ACI/NKyo Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.C.; Hwang, B.J.; Mai, F.D.; Liu, Y.C.; Lin, C.M.; Kuo, H.S.; Chou, D.S.; Lee, M.J.; Yang, K.H.; Yu, C.C.; et al. Active and stable liquid water innovatively prepared using resonantly illuminated gold nanoparticles. ACS Nano 2014, 8, 2704–2713. [Google Scholar] [CrossRef]
- Chen, H.C.; Lin, H.C.; Chen, H.H.; Mai, F.D.; Liu, Y.C.; Lin, C.M.; Chang, C.C.; Tsai, H.Y.; Yang, C.P. Innovative strategy with potential to increase hemodialysis efficiency and safety. Sci. Rep. 2014, 4, 4425. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Chen, H.C.; Wang, C.C.; Tsai, P.W.; Ho, C.W.; Liu, Y.C. Effective Energy Transfer via Plasmon-Activated High-Energy Water Promotes Its Fundamental Activities of Solubility, Ionic Conductivity, and Extraction at Room Temperature. Sci. Rep. 2015, 5, 18152. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Wang, C.K.; Chen, H.C.; Fang, S.U.; Ho, C.W.; Tai, C.J.; Yang, C.P.; Liu, Y.C. Innovatively Therapeutic Strategy on Lung Cancer by Daily Drinking Antioxidative Plasmon-Induced Activated Water. Sci. Rep. 2018, 8, 6316. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lin, K.J.; Hong, C.T.; Wu, D.; Chang, H.M.; Liu, C.H.; Hsiao, I.T.; Yang, C.P.; Liu, Y.C.; Hu, C.J. Plasmon-Activated Water Reduces Amyloid Burden and Improves Memory in Animals with Alzheimer’s Disease. Sci. Rep. 2019, 9, 13252. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.H.; Edwards, L.B.; Kucheryavaya, A.Y.; Dipchand, A.I.; Benden, C.; Christie, J.D.; Dobbels, F.; Kirk, R.; Rahmel, A.O.; Yusen, R.D.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report--2013; focus theme: Age. J. Heart Lung Transplant. 2013, 32, 951–964. [Google Scholar] [CrossRef]
- Costello, J.P.; Mohanakumar, T.; Nath, D.S. Mechanisms of chronic cardiac allograft rejection. Tex. Heart Inst. J. 2013, 40, 395–399. [Google Scholar]
- Nath, D.S.; Basha, H.I.; Mohanakumar, T. Antihuman leukocyte antigen antibody-induced autoimmunity: Role in chronic rejection. Curr. Opin. Organ. Transplant. 2010, 15, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, I.; Deutsch, M.A.; Kauke, T.; Beiras-Fernandez, A.; Schmoeckel, M.; Vicol, C.; Sodian, R.; Reichart, B.; Spannagl, M.; Ueberfuhr, P. Donor-specific HLA alloantibodies: Long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Exp. Clin. Transplant. 2008, 6, 229–235. [Google Scholar] [PubMed]
- Weiss, M.J.; Madsen, J.C.; Rosengard, B.R.; Allan, J.S. Mechanisms of chronic rejection in cardiothoracic transplantation. Front. Biosci. 2008, 13, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Daly, K.P.; Seifert, M.E.; Chandraker, A.; Zurakowski, D.; Nohria, A.; Givertz, M.M.; Karumanchi, S.A.; Briscoe, D.M. VEGF-C, VEGF-A and related angiogenesis factors as biomarkers of allograft vasculopathy in cardiac transplant recipients. J. Heart Lung Transplant. 2013, 32, 120–128. [Google Scholar] [CrossRef]
- Benatti, R.D.; Taylor, D.O. Evolving concepts and treatment strategies for cardiac allograft vasculopathy. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 278. [Google Scholar] [CrossRef]
- Zhang, Q.; Cecka, J.M.; Gjertson, D.W.; Ge, P.; Rose, M.L.; Patel, J.K.; Ardehali, A.; Kobashigawa, J.A.; Fishbein, M.C.; Reed, E.F. HLA and MICA: Targets of antibody-mediated rejection in heart transplantation. Transplantation 2011, 91, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Jane-wit, D.; Qin, L.; Tellides, G. Interacting mechanisms in the pathogenesis of cardiac allograft vasculopathy. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1609–1614. [Google Scholar] [CrossRef]
- Hwang, B.J.; Chen, H.C.; Mai, F.D.; Tsai, H.Y.; Yang, C.P.; Rick, J.; Liu, Y.C. Innovative Strategy on Hydrogen Evolution Reaction Utilizing Activated Liquid Water. Sci. Rep. 2015, 5, 16263. [Google Scholar] [CrossRef]
- Bedi, D.S.; Riella, L.V.; Tullius, S.G.; Chandraker, A. Animal models of chronic allograft injury: Contributions and limitations to understanding the mechanism of long-term graft dysfunction. Transplantation 2010, 90, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Poston, R.S.; Billingham, M.; Hoyt, E.G.; Pollard, J.; Shorthouse, R.; Morris, R.E.; Robbins, R.C. Rapamycin reverses chronic graft vascular disease in a novel cardiac allograft model. Circulation 1999, 100, 67–74. [Google Scholar] [CrossRef]
- Kato, G.J.; Hebbel, R.P.; Steinberg, M.H.; Gladwin, M.T. Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. Am. J. Hematol. 2009, 84, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.T.; Mirza, A.B.; Zahid, R.; Haleem, A.; Al Hussaini, H.; Al Sulaiman, M.; Mousa, D. Antibody-mediated rejection: Importance of lactate dehydrogenase and neutrophilia in early diagnosis. Saudi J. Kidney Dis. Transpl. 2011, 22, 525–530. [Google Scholar]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Zou, H.; Yang, Y.; Gao, M.; Zhang, B.; Ming, B.; Sun, Y.; Chen, H.; Tang, X.; Chen, Z.; Xiong, P.; et al. HMGB1 is involved in chronic rejection of cardiac allograft via promoting inflammatory-like mDCs. Am. J. Transplant. 2014, 14, 1765–1777. [Google Scholar] [CrossRef]
- Fadini, G.P.; Sartore, S.; Albiero, M.; Baesso, I.; Murphy, E.; Menegolo, M.; Grego, F.; Vigili de Kreutzenberg, S.; Tiengo, A.; Agostini, C.; et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Pintavorn, P.; Ballermann, B.J. TGF-β and the endothelium during immune injury. Kidney Int. 1997, 51, 1401–1412. [Google Scholar] [CrossRef]
- Weis, M.; Wildhirt, S.M.; Schulze, C.; Pehlivanli, S.; Fraunberger, P.; Meiser, B.M.; von Scheidt, W. Modulation of coronary vasomotor tone by cytokines in cardiac transplant recipients. Transplantation 1999, 68, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Rua, R.; McGavern, D.B. Elucidation of monocyte/macrophage dynamics and function by intravital imaging. J. Leukoc. Biol. 2015, 98, 319–332. [Google Scholar] [CrossRef]
- Del Papa, N.; Pignataro, F. The Role of Endothelial Progenitors in the Repair of Vascular Damage in Systemic Sclerosis. Front. Immunol. 2018, 9, 1383. [Google Scholar] [CrossRef] [PubMed]
- Tilling, L.; Chowienczyk, P.; Clapp, B. Progenitors in motion: Mechanisms of mobilization of endothelial progenitor cells. Br. J. Clin. Pharmacol. 2009, 68, 484–492. [Google Scholar] [CrossRef]
- Oh, S.A.; Li, M.O. TGF-β: Guardian of T cell function. J. Immunol. 2013, 191, 3973–3979. [Google Scholar] [CrossRef]
- Knight, R.J.; Liu, H.; Fishman, E.; Reis, E.D. Cold ischemic injury, aortic allograft vasculopathy, and pro-inflammatory cytokine expression. J. Surg. Res. 2003, 113, 201–207. [Google Scholar] [CrossRef]
- Slaughter, S.L.; Ellis, P.R.; Jackson, E.C.; Butterworth, P.J. The effect of guar galactomannan and water availability during hydrothermal processing on the hydrolysis of starch catalysed by pancreatic alpha-amylase. Biochim. Biophys. Acta 2002, 1571, 55–63. [Google Scholar] [CrossRef]
- de Meis, L.; Suzano, V.A. Role of water activity on the rates of acetyl phosphate and ATP hydrolysis. FEBS Lett. 1988, 232, 73–77. [Google Scholar] [CrossRef]
- Rivard, U.; Thomas, V.; Bruhacs, A.; Siwick, B.; Iftimie, R. Donor-Bridge-Acceptor Proton Transfer in Aqueous Solution. J. Phys. Chem. Lett. 2014, 5, 3200–3205. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, M.; Gargiulo, S.; Iborra, S.; Arends, I.W.; Hollmann, F.; Corma, A. Photobiocatalytic chemistry of oxidoreductases using water as the electron donor. Nat. Commun. 2014, 5, 3145. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.; Spijker, P.; Voitchovsky, K. Water-induced correlation between single ions imaged at the solid-liquid interface. Nat. Commun. 2014, 5, 4400. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Shi, L.; Zhu, Y.; Mideksa, M.F.; Hou, K.; Zhao, W.; Wang, D.; Zhao, M.; Zhang, X.; et al. Boosting Hot Electrons in Hetero-superstructures for Plasmon-Enhanced Catalysis. J. Am. Chem. Soc. 2017, 139, 17964–17972. [Google Scholar] [CrossRef]
- Mukherjee, S.; Libisch, F.; Large, N.; Neumann, O.; Brown, L.V.; Cheng, J.; Lassiter, J.B.; Carter, E.A.; Nordlander, P.; Halas, N.J. Hot electrons do the impossible: Plasmon-induced dissociation of H2 on Au. Nano Lett. 2013, 13, 240–247. [Google Scholar] [CrossRef]
- Huang, Y.F.; Zhang, M.; Zhao, L.B.; Feng, J.M.; Wu, D.Y.; Ren, B.; Tian, Z.Q. Activation of oxygen on gold and silver nanoparticles assisted by surface plasmon resonances. Angew. Chem. Int. Ed. Engl. 2014, 53, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Li, Y.; Li, M.Y.; Zhang, H.; Zhang, J. Boosting electrocatalytic hydrogen evolution by plasmon-driven hot-electron excitation. Nanoscale 2018, 10, 2236–2241. [Google Scholar] [CrossRef]
- Yang, C.P.; Liu, Y.C. Therapeutics for Inflammatory-Related Diseases Based on Plasmon-Activated Water: A Review. Int. J. Mol. Sci. 2018, 19, 1589. [Google Scholar] [CrossRef]
- Chen, H.C.; Cheng, C.Y.; Lin, H.C.; Chen, H.H.; Chen, C.H.; Yang, C.P.; Yang, K.H.; Lin, C.M.; Lin, T.Y.; Shih, C.M.; et al. Multifunctions of Excited Gold Nanoparticles Decorated Artificial Kidney with Efficient Hemodialysis and Therapeutic Potential. ACS Appl. Mater. Interfaces 2016, 8, 19691–19700. [Google Scholar] [CrossRef]
- Gonzalez-Ortegon, E.; Vay, L.L.; Walton, M.E.M.; Gimenez, L. Maternal Trophic Status and Offpsring Phenotype in a Marine Invertebrate. Sci. Rep. 2018, 8, 9618. [Google Scholar] [CrossRef]
- Huibers, M.; De Jonge, N.; Van Kuik, J.; Koning, E.S.; Van Wichen, D.; Dullens, H.; Schipper, M.; De Weger, R. Intimal fibrosis in human cardiac allograft vasculopathy. Transpl. Immunol. 2011, 25, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Cruz, R.P.; Granville, D.J.; McManus, B.M. Allograft vasculopathy versus atherosclerosis. Circ. Res. 2006, 99, 801–815. [Google Scholar] [CrossRef]
- Chen, P.Y.; Qin, L.; Barnes, C.; Charisse, K.; Yi, T.; Zhang, X.; Ali, R.; Medina, P.P.; Yu, J.; Slack, F.J.; et al. FGF regulates TGF-β signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. 2012, 2, 1684–1696. [Google Scholar] [CrossRef]
- Kovacic, J.C.; Mercader, N.; Torres, M.; Boehm, M.; Fuster, V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: From cardiovascular development to disease. Circulation 2012, 125, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- von Gise, A.; Pu, W.T. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ. Res. 2012, 110, 1628–1645. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Horsley, J.; Rhind-Tutt, S.; Charman, S.; Phillpotts, C.J.; Wallwork, J.; Goddard, M.J. Neointimal smooth muscle cells in human cardiac allograft coronary artery vasculopathy are of donor origin. J. Heart Lung Transplant. 2004, 23, 427–435. [Google Scholar] [CrossRef]
- Borthwick, L.A.; Parker, S.M.; Brougham, K.A.; Johnson, G.E.; Gorowiec, M.R.; Ward, C.; Lordan, J.L.; Corris, P.A.; Kirby, J.A.; Fisher, A.J. Epithelial to mesenchymal transition (EMT) and airway remodelling after human lung transplantation. Thorax 2009, 64, 770–777. [Google Scholar] [CrossRef]
- Hillebrands, J.L.; Onuta, G.; Rozing, J. Role of progenitor cells in transplant arteriosclerosis. Trends Cardiovasc. Med. 2005, 15, 1–8. [Google Scholar] [CrossRef]
- Hillebrands, J.L.; Klatter, F.A.; Rozing, J. Origin of vascular smooth muscle cells and the role of circulating stem cells in transplant arteriosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 380–387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- D’Alessandro, D.A.; Kajstura, J.; Hosoda, T.; Gatti, A.; Bello, R.; Mosna, F.; Bardelli, S.; Zheng, H.; D’Amario, D.; Padin-Iruegas, M.E.; et al. Progenitor cells from the explanted heart generate immunocompatible myocardium within the transplanted donor heart. Circ. Res. 2009, 105, 1128–1140. [Google Scholar] [CrossRef]
- Sathya, C.J.; Sheshgiri, R.; Prodger, J.; Tumiati, L.; Delgado, D.; Ross, H.J.; Rao, V. Correlation between circulating endothelial progenitor cell function and allograft rejection in heart transplant patients. Transpl. Int. 2010, 23, 641–648. [Google Scholar] [CrossRef]
- Simper, D.; Wang, S.; Deb, A.; Holmes, D.; McGregor, C.; Frantz, R.; Kushwaha, S.S.; Caplice, N.M. Endothelial progenitor cells are decreased in blood of cardiac allograft patients with vasculopathy and endothelial cells of noncardiac origin are enriched in transplant atherosclerosis. Circulation 2003, 108, 143–149. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Piera-Velazquez, S.; Jimenez, S.A. Molecular mechanisms of endothelial to mesenchymal cell transition (EndoMT) in experimentally induced fibrotic diseases. Fibrogenesis Tissue Repair 2012, 5, S7. [Google Scholar] [CrossRef]
- Evrard, S.M.; Lecce, L.; Michelis, K.C.; Nomura-Kitabayashi, A.; Pandey, G.; Purushothaman, K.R.; d’Escamard, V.; Li, J.R.; Hadri, L.; Fujitani, K.; et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat. Commun. 2016, 7, 11853. [Google Scholar] [CrossRef]
- Tellides, G.; Pober, J.S. Interferon-γ axis in graft arteriosclerosis. Circ. Res. 2007, 100, 622–632. [Google Scholar] [CrossRef]
- Bolinger, B.; Engeler, D.; Krebs, P.; Miller, S.; Firner, S.; Hoffmann, M.; Palmer, D.C.; Restifo, N.P.; Tian, Y.; Clavien, P.A.; et al. IFN-γ-receptor signaling ameliorates transplant vasculopathy through attenuation of CD8+ T-cell-mediated injury of vascular endothelial cells. Eur. J. Immunol. 2010, 40, 733–743. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- van Loosdregt, J.; van Oosterhout, M.F.; Bruggink, A.H.; van Wichen, D.F.; van Kuik, J.; de Koning, E.; Baan, C.C.; de Jonge, N.; Gmelig-Meyling, F.H.; de Weger, R.A. The chemokine and chemokine receptor profile of infiltrating cells in the wall of arteries with cardiac allograft vasculopathy is indicative of a memory T-helper 1 response. Circulation 2006, 114, 1599–1607. [Google Scholar] [CrossRef]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef]
- Messmer, D.; Yang, H.; Telusma, G.; Knoll, F.; Li, J.; Messmer, B.; Tracey, K.J.; Chiorazzi, N. High mobility group box protein 1: An endogenous signal for dendritic cell maturation and Th1 polarization. J. Immunol. 2004, 173, 307–313. [Google Scholar] [CrossRef]
- Rendon-Mitchell, B.; Ochani, M.; Li, J.; Han, J.; Wang, H.; Yang, H.; Susarla, S.; Czura, C.; Mitchell, R.A.; Chen, G.; et al. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J. Immunol. 2003, 170, 3890–3897. [Google Scholar] [CrossRef]
- Bonaldi, T.; Talamo, F.; Scaffidi, P.; Ferrera, D.; Porto, A.; Bachi, A.; Rubartelli, A.; Agresti, A.; Bianchi, M.E. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003, 22, 5551–5560. [Google Scholar] [CrossRef]
- Park, J.S.; Svetkauskaite, D.; He, Q.; Kim, J.Y.; Strassheim, D.; Ishizaka, A.; Abraham, E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004, 279, 7370–7377. [Google Scholar] [CrossRef]
- Treutiger, C.J.; Mullins, G.E.; Johansson, A.S.; Rouhiainen, A.; Rauvala, H.M.; Erlandsson-Harris, H.; Andersson, U.; Yang, H.; Tracey, K.J.; Andersson, J.; et al. High mobility group 1 B-box mediates activation of human endothelium. J. Intern. Med. 2003, 254, 375–385. [Google Scholar] [CrossRef]
- Fiuza, C.; Bustin, M.; Talwar, S.; Tropea, M.; Gerstenberger, E.; Shelhamer, J.H.; Suffredini, A.F. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 2003, 101, 2652–2660. [Google Scholar] [CrossRef]
- Lin, F.Y.; Shih, C.M.; Huang, C.Y.; Tsai, Y.T.; Loh, S.H.; Li, C.Y.; Lin, C.Y.; Lin, Y.W.; Tsai, C.S. Dipeptidyl Peptidase-4 Inhibitor Decreases Allograft Vasculopathy Via Regulating the Functions of Endothelial Progenitor Cells in Normoglycemic Rats. Cardiovasc. Drugs Ther. 2020. [Google Scholar] [CrossRef]



| Control ACI/NKyo (Non-OAT) | OAT + ROW (PVG/Seac to ACI/NKyo) | OAT + PAW (PVG/Seac to ACI/NKyo) | ||||||
|---|---|---|---|---|---|---|---|---|
| baseline | 90 days | baseline | 90 days | p | baseline | 90 days | p | |
| Body weight (g) | 240.3 ± 18.6 | 350.1 ± 16.7 | 220.9 ± 12.8 | 332.5 ± 15.6 | 0.123 | 234.6 ± 10.2 | 365.7 ± 13.4 | 0.071 |
| BUN (mg/dL) | 26.5 ± 5.6 | 30.0 ± 5.9 | 26.8 ± 9.0 | 29.5 ± 6.1 | 0.898 | 25.4 ± 7.1 | 29.8 ± 6.8 | 0.956 |
| Creatinine(mg/dL) | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.8 ± 0.5 | 0.8 ± 0.3 | 0.552 | 0.9 ± 0.3 | 0.9 ± 0.2 | 0.091 |
| ALT (IU/L) | 19.9 ± 6.7 | 22.8 ± 9.1 | 21.3 ± 6.5 | 23.5 ± 9.1 | 0.906 | 25.4 ± 3.8 | 20.6 ± 1.9 | 0.461 |
| AST (IU/L) | 30.5 ± 6.1 | 32.5 ± 8.1 | 30.5 ± 3.6 | 30.6 ± 8.1 | 0.720 | 29.8 ± 9.9 | 30.5 ± 9.1 | 0.685 |
| Control ACI/NKyo (Non-OAT) | OAT + ROW (PVG/Seac to ACI/NKyo) | OAT + PAW (PVG/Seac to ACI/NKyo) | ||||
|---|---|---|---|---|---|---|
| baseline | 90 days | baseline | 90 days | baseline | 90 days | |
| LDH (IU/L) | 900.5 ± 99.5 | 895.9 ± 125.6 | 854.6 ± 152.5 | 1888.78 ± 323.0 a,b | 802.3 ± 152.0 | 968.8 ± 116.4 c |
| CRP (mg/dL) | 40.2 ± 13.5 | 39.6 ± 9.1 | 39.4 ± 9.8 | 163.5 ± 22.5 a,b | 26.5 ± 8.1 | 72.1 ± 5.4 a,b,c |
| HMGB1 (ng/mL) | 2.7 ± 1.4 | 2.8 ± 1.3 | 3.4 ± 1.5 | 98.7 ± 19.7 a,b | 2.5 ± 1.4 | 55.4 ± 13.1 a,b,c |
| SDF-1α (pg/mL) | 200.3 ± 22.6 | 196.5 ± 52.1 | 220.1 ± 41.5 | 432.6 ± 100.4 a,b | 241.3 ± 54.1 | 1211.4 ± 130.2 a,b,c |
| TGF-β1 (ng/mL) | 66.3 ± 21.2 | 78.9 ± 25.4 | 72.4 ± 19.7 | 378.1 ± 55.9 a,b | 72.1 ± 22.0 | 129.7 ± 54.1 a,b,c |
| INF-γ (pg/mL) | 2.5 ± 0.8 | 3.1 ± 0.9 | 1.5 ± 0.7 | 18.7 ± 5.3 a,b | 1.9 ± 0.8 | 3.5 ± 1.2 c |
| IL-2 (pg/mL) | 45.7 ± 13.5 | 56.8 ± 18.9 | 53.7 ± 21.5 | 899.4 ± 102.4 a,b | 45.2 ± 21.0 | 352.4 ± 95.7 a,b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-S.; Lin, F.-Y.; Liu, Y.-C.; Lin, Y.-W.; Tsai, Y.-T.; Huang, C.-Y.; Lin, S.-J.; Li, C.-Y.; Lin, C.-Y.; Tseng, H.-T.; et al. Transferring Plasmon Effect on a Biological System: Expression of Biological Polymers in Chronic Rejection and Inflammatory Rat Model. Polymers 2021, 13, 1827. https://doi.org/10.3390/polym13111827
Tsai C-S, Lin F-Y, Liu Y-C, Lin Y-W, Tsai Y-T, Huang C-Y, Lin S-J, Li C-Y, Lin C-Y, Tseng H-T, et al. Transferring Plasmon Effect on a Biological System: Expression of Biological Polymers in Chronic Rejection and Inflammatory Rat Model. Polymers. 2021; 13(11):1827. https://doi.org/10.3390/polym13111827
Chicago/Turabian StyleTsai, Chien-Sung, Feng-Yen Lin, Yu-Chuan Liu, Yi-Wen Lin, Yi-Ting Tsai, Chun-Yao Huang, Shing-Jong Lin, Chi-Yuan Li, Cheng-Yen Lin, Horng-Ta Tseng, and et al. 2021. "Transferring Plasmon Effect on a Biological System: Expression of Biological Polymers in Chronic Rejection and Inflammatory Rat Model" Polymers 13, no. 11: 1827. https://doi.org/10.3390/polym13111827
APA StyleTsai, C.-S., Lin, F.-Y., Liu, Y.-C., Lin, Y.-W., Tsai, Y.-T., Huang, C.-Y., Lin, S.-J., Li, C.-Y., Lin, C.-Y., Tseng, H.-T., & Shih, C.-M. (2021). Transferring Plasmon Effect on a Biological System: Expression of Biological Polymers in Chronic Rejection and Inflammatory Rat Model. Polymers, 13(11), 1827. https://doi.org/10.3390/polym13111827