Polysaccharide Derived from Nelumbo nucifera Lotus Plumule Shows Potential Prebiotic Activity and Ameliorates Insulin Resistance in HepG2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Purification of N. nucifera Polysaccharides
2.2. Characterization of NNP-2
2.2.1. Determination of the Purity and Composition of NNP-2
2.2.2. Determination of the Average Molecular Weight of NNP-2
2.2.3. Monosaccharide Composition
2.2.4. Fourier Transform Infrared (FT-IR) Analysis
2.3. In Vitro Prebiotic Activity of NNP
2.4. α-Glucosidase Inhibitory Assay
2.5. HepG2 Cell Culture and Cell Viability Assays
2.6. Anti-Insulin Resistance Activity
2.7. Reverse Transcription-Quantitative PCR (RT-qPCR)
2.8. Statistical Analysis
3. Results and Discussion
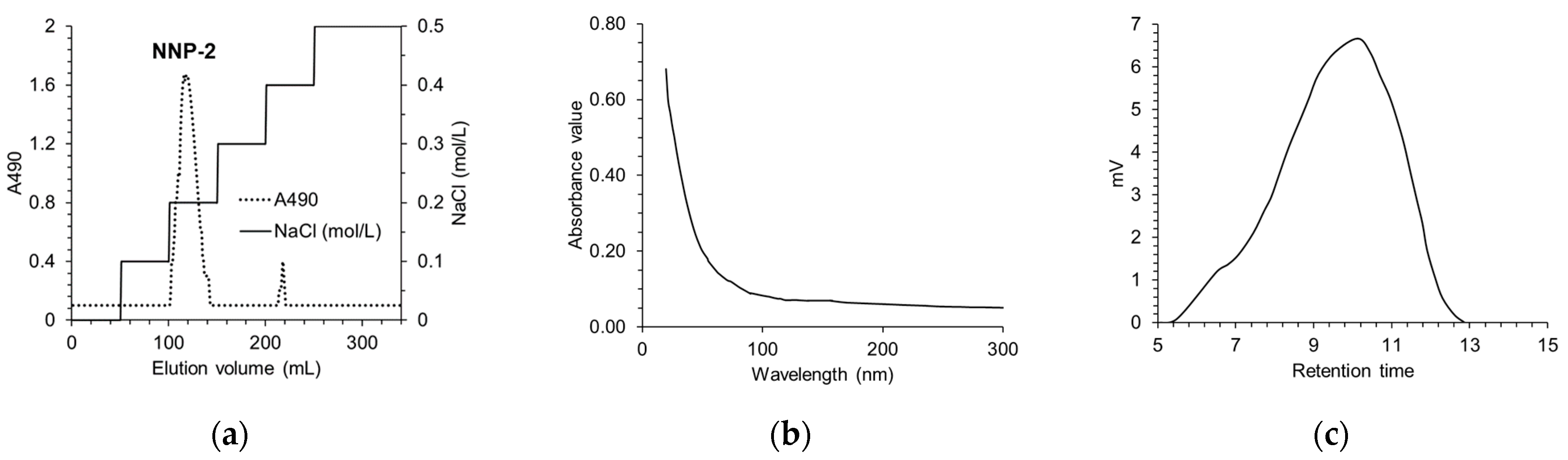
3.1. Determination of Purity and Characterization of NNP
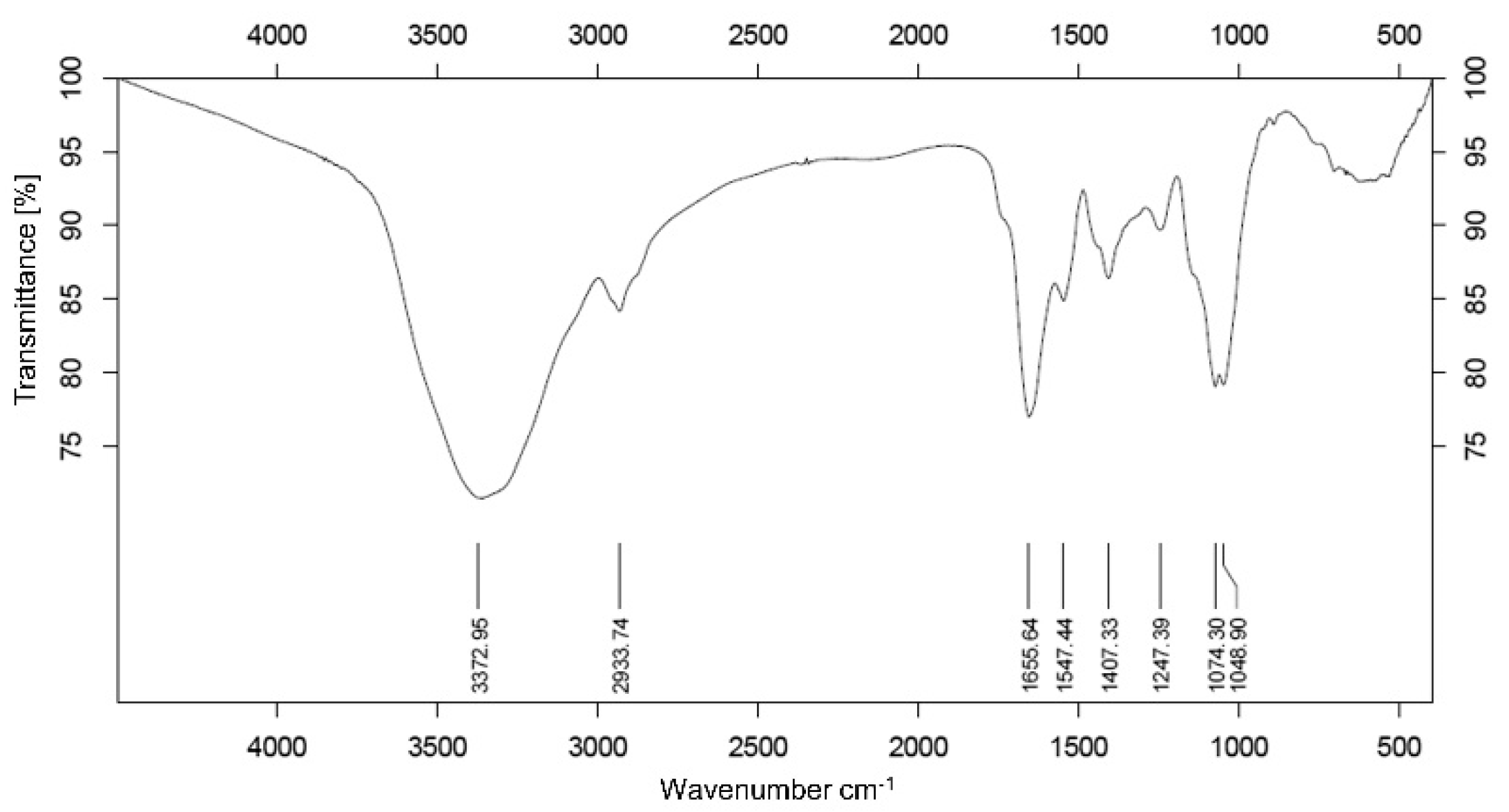
3.2. Analysis of Infrared Spectroscopy Spectrum
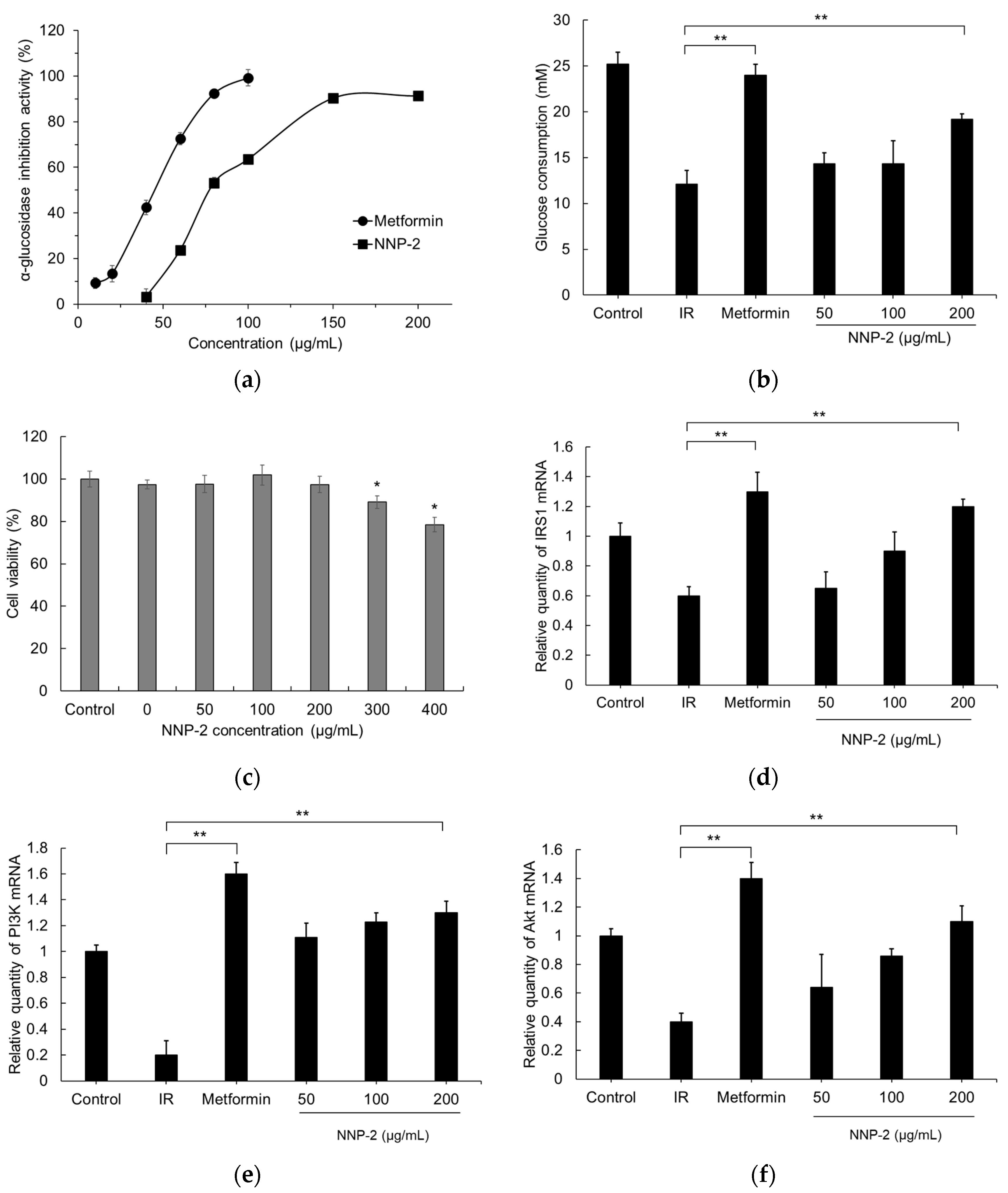
3.3. Prebiotic Properties of NNP-2
3.4. Inhibition of α-Glucosidase by NNP-2
3.5. Effects of NNP-2 on Glucose Uptake in Insulin-Resistant HepG2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmet, P.Z.; Magliano, D.J.; Herman, W.H.; Shaw, J.E. Diabetes: A 21st century challenge. Lancet Diabetes Endocrinol. 2014, 2, 56–64. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, F.; Li, W.; Cao, Y.; Wang, C.; Wang, Y.; Liang, D.; Zhang, R.; Zhang, S.; Wang, H. Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol. Cell. Biochem. 2011, 353, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Paolisso, P.; Sacra, C.; Santamaria, M.; de Lucia, C.; Ruocco, A.; Mauro, C.; Paolisso, G.; Rizzo, M.R.; Barbieri, M. Cardiac resynchronization therapy with a defibrillator (CRTd) in failing heart patients with type 2 diabetes mellitus and treated by glucagon-like peptide 1 receptor agonists (GLP-1 RA) therapy vs. conventional hypoglycemic drugs: Arrhythmic burden, hospitalizations for heart failure, and CRTd responders rate. Cardiovasc. Diabetol. 2018, 17, 1–16. [Google Scholar]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Tho, N.T.M.; An, T.N.M.; Tri, M.D.; Sreekanth, T.V.M.; Lee, J.-S.; Nagajyothi, P.C.; Lee, K.D. Green synthesis of silver nanoparticles using Nelumbo nucifera seed extract and its antibacterial activity. Acta Chim. Slov. 2013, 60, 673–678. [Google Scholar]
- Liu, W.; Yi, D.-D.; Guo, J.-L.; Xiang, Z.-X.; Deng, L.-F.; He, L. Nuciferine, extracted from Nelumbo nucifera Gaertn, inhibits tumor-promoting effect of nicotine involving Wnt/β-catenin signaling in non-small cell lung cancer. J. Ethnopharmacol. 2015, 165, 83–93. [Google Scholar] [CrossRef]
- Liao, C.-H.; Lin, J.-Y. Purified active lotus plumule (Nelumbo nucifera Gaertn) polysaccharides exert anti-inflammatory activity through decreasing Toll-like receptor-2 and-4 expressions using mouse primary splenocytes. J. Ethnopharmacol. 2013, 147, 164–173. [Google Scholar] [CrossRef]
- Sohn, D.-H.; Kim, Y.-C.; Oh, S.-H.; Park, E.-J.; Li, X.; Lee, B.-H. Hepatoprotective and free radical scavenging effects of Nelumbo nucifera. Phytomedicine 2003, 10, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Khatua, T.N.; Venkatesh, P.; Saha, B.; Mukherjee, P.K. Immunomodulatory potential of rhizome and seed extracts of Nelumbo nucifera Gaertn. J. Ethnopharmacol. 2010, 128, 490–494. [Google Scholar] [CrossRef]
- Poornima, P.; Weng, C.F.; Padma, V.V. Neferine, an alkaloid from lotus seed embryo, inhibits human lung cancer cell growth by MAPK activation and cell cycle arrest. BioFactors 2014, 40, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y.-P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L.M.; Morris-Natschke, S.L. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure–activity correlations with related alkaloids. Biorg. Med. Chem. 2005, 13, 443–448. [Google Scholar] [CrossRef]
- Liao, C.-H.; Guo, S.-J.; Lin, J.-Y. Characterisation of the chemical composition and in vitro anti-inflammation assessment of a novel lotus (Nelumbo nucifera Gaertn) plumule polysaccharide. Food Chem. 2011, 125, 930–935. [Google Scholar] [CrossRef]
- Liao, C.-H.; Lin, J.-Y. Lotus (Nelumbo nucifera Gaertn) plumule polysaccharide protects the spleen and liver from spontaneous inflammation in non-obese diabetic mice by modulating pro-/anti-inflammatory cytokine gene expression. Food Chem. 2011, 129, 245–252. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.; Wu, X.; Cai, W.; Lin, Q.; Zhu, D.; Liu, H. The effect of natural soluble polysaccharides on the type 2 diabetes through modulating gut microbiota: A review. Curr. Med. Chem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-H.; Teng, J.-F.; Hsu, T.-H.; Lin, F.-Y.; Yang, P.-W.; Lo, H.-C. 28-day oral safety evaluation of extracellular polysaccharopeptides produced in submerged culture from the turkey tail medicinal mushroom Trametes versicolor (L.: Fr.) Pilát LH-1 in mice. Int. J. Med. Mushrooms 2011, 13, 417–429. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Wu, T.; Xu, M.; Cai, H.; Zhang, Z. Effects of D-pinitol on insulin resistance through the PI3K/Akt signaling pathway in type 2 diabetes mellitus rats. J. Agric. Food Chem. 2015, 63, 6019–6026. [Google Scholar] [CrossRef]
- He, Z.; Opland, D.M.; Way, K.J.; Ueki, K.; Bodyak, N.; Kang, P.M.; Izumo, S.; Kulkarni, R.N.; Wang, B.; Liao, R. Regulation of vascular endothelial growth factor expression and vascularization in the myocardium by insulin receptor and PI3K/Akt pathways in insulin resistance and ischemia. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Kim, D.-S.; Kim, D.-Y.; Shin, H.-S. Exploiting fruit waste grape pomace for silver nanoparticles synthesis, assessing their antioxidant, antidiabetic potential and antibacterial activity against human pathogens: A novel approach. Nanomaterials 2020, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Kim, D.-S.; Saratale, G.D. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif. Cells Nanomed. Biotechnol. 2018, 46, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.M.; Pomeranz, Y. Effect of borate on colorimetric determinations of carbohydrates by the phenol-sulfuric acid method. Anal. Biochem. 1968, 24, 128–131. [Google Scholar]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dodgson, K.; Price, R. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Le, B.; Pham, T.N.A.; Yang, S.H. Prebiotic potential and anti-inflammatory activity of soluble polysaccharides obtained from soybean residue. Foods 2020, 9, 1808. [Google Scholar] [CrossRef]
- Sorourian, R.; Khajehrahimi, A.E.; Tadayoni, M.; Azizi, M.H.; Hojjati, M. Ultrasound-assisted extraction of polysaccharides from Typha domingensis: Structural characterization and functional properties. Int. J. Biol. Macromol. 2020, 160, 758–768. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, F.; Ni, H.; Mo, Z.; Wan, J.-B.; Hua, D.; Yan, C. Structural characterization, α-glucosidase inhibitory and DPPH scavenging activities of polysaccharides from guava. Carbohydr. Polym. 2016, 144, 106–114. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, H.; Yang, W.; Yang, S.; Chen, C. Chemical constituents from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Chem. Nat. Compd. 2011, 47, 316–318. [Google Scholar] [CrossRef]
- Hinterstoisser, B.; Salmén, L. Two-dimensional step-scan FTIR: A tool to unravel the OH-valency-range of the spectrum of Cellulose, I. Cellulose 1999, 6, 251–263. [Google Scholar] [CrossRef]
- Mihály, J.; Deák, R.; Szigyártó, I.C.; Bóta, A.; Beke-Somfai, T.; Varga, Z. Characterization of extracellular vesicles by IR spectroscopy: Fast and simple classification based on amide and CH stretching vibrations. Biochim. Biophys. Acta Biomembr. 2017, 1859, 459–466. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Matějka, P.; Machovič, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Schindler, B.; Legentil, L.; Allouche, A.-R.; Ferrières, V.; Compagnon, I. Spectroscopic diagnostic for the ring-size of carbohydrates in the gas phase: Furanose and pyranose forms of GalNAc. Phys. Chem. Chem. Phys. 2019, 21, 12460–12467. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Santamarina, A.; Miranda, J.M.; Mondragon, A.d.C.; Lamas, A.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Potential use of marine seaweeds as prebiotics: A review. Molecules 2020, 25, 1004. [Google Scholar] [CrossRef] [PubMed]
- Manning, T.S.; Gibson, G.R. Prebiotics. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 287–298. [Google Scholar] [CrossRef]
- Gibson, G.R. Dietary modulation of the human gut microflora using prebiotics. Br. J. Nutr. 1998, 80, S209–S212. [Google Scholar] [CrossRef]
- Jackson, S.A.; Schoeni, J.L.; Vegge, C.; Pane, M.; Stahl, B.; Bradley, M.; Goldman, V.S.; Burguière, P.; Atwater, J.B.; Sanders, M.E. Improving end-user trust in the quality of commercial probiotic products. Font. Microbiol. 2019, 10, 739. [Google Scholar] [CrossRef]
- Lin, S.-H.; Chou, L.-M.; Chien, Y.-W.; Chang, J.-S.; Lin, C.-I. Prebiotic effects of xylooligosaccharides on the improvement of microbiota balance in human subjects. Gastroenterol. Res. Pract. 2016, 2016, 5789232. [Google Scholar] [CrossRef]
- Iliev, I.; Vasileva, T.; Bivolarski, V.; Momchilova, A.; Ivanova, I. Metabolic profiling of xylooligosaccharides by Lactobacilli. Polymers 2020, 12, 2387. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharm. Rev. 2011, 5, 19. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Zhang, J.Y.; Chen, L.J.; Liu, X.C.; Liu, Y.; Wang, W.X.; Zhang, Y.M. Comparative evaluation of polysaccharides isolated from Astragalus, oyster mushroom, and yacon as inhibitors of α-glucosidase. Chin. J. Nat. Med. 2014, 12, 290–293. [Google Scholar] [CrossRef]
- Dou, Z.; Chen, C.; Fu, X. The effect of ultrasound irradiation on the physicochemical properties and α-glucosidase inhibitory effect of blackberry fruit polysaccharide. Food Hydrocoll. 2019, 96, 568–576. [Google Scholar] [CrossRef]
- Lv, Q.-Q.; Cao, J.-J.; Liu, R.; Chen, H.-Q. Structural characterization, α-amylase and α-glucosidase inhibitory activities of polysaccharides from wheat bran. Food Chem. 2021, 341, 128218. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-H.; Cao, J.-J.; Zhang, B.; Chen, H.-Q. Structural characterization, physicochemical properties and α-glucosidase inhibitory activity of polysaccharide from the fruits of wax apple. Carbohydr. Polym. 2019, 211, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Lizák, B.; Szarka, A.; Kim, Y.; Choi, K.-s.; Németh, C.E.; Marcolongo, P.; Benedetti, A.; Bánhegyi, G.; Margittai, É. Glucose transport and transporters in the endomembranes. Int. J. Mol. Sci. 2019, 20, 5898. [Google Scholar] [CrossRef]
- Stewart, M.L.; Zimmer, J.P. Postprandial glucose and insulin response to a high-fiber muffin top containing resistant starch type 4 in healthy adults: A double-blind, randomized, controlled trial. Nutrition 2018, 53, 59–63. [Google Scholar] [CrossRef]
- Bessell, E.; Fuller, N.R.; Markovic, T.P.; Lau, N.S.; Burk, J.; Hendy, C.; Picone, T.; Li, A.; Caterson, I.D. Effects of α-cyclodextrin on cholesterol control and hydrolyzed ginseng extract on glycemic control in people with prediabetes: A randomized clinical trial. JAMA Netw. Open 2020, 3, e2023491. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Lin, C.-Y.; Hsu, C.-L.; Fan, K.-H.; Huang, S.-F.; Liao, C.-T.; Lee, L.-Y.; Ng, S.-K.; Yen, T.-C.; Chang, J.T.-C. Incorporation of Astragalus polysaccharides injection during concurrent chemoradiotherapy in advanced pharyngeal or laryngeal squamous cell carcinoma: Preliminary experience of a phase II double-blind, randomized trial. J. Cancer Res. Clin. Oncol. 2020, 146, 33–41. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483. [Google Scholar] [CrossRef]
- Garabadu, D.; Krishnamurthy, S. Metformin attenuates hepatic insulin resistance in type-2 diabetic rats through PI3K/Akt/GLUT-4 signalling independent to bicuculline-sensitive GABAA receptor stimulation. Pharm. Biol. 2017, 55, 722–728. [Google Scholar] [CrossRef]
- Sesti, G.; Federici, M.; Hribal, M.L.; Lauro, D.; Sbraccia, P.; Lauro, R. Defects of the insulin receptor substrate (IRS) system in human metabolic disorders. FASEB J. 2001, 15, 2099–2111. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.-B.; Li, Z.-R.; Wu, J.; Ou, Z.-R.; Zhu, Q.; Sun, B.; Lin, L.; Zhao, M. Physicochemical properties of polysaccharide fractions from Sargassum fusiforme and their hypoglycemic and hypolipidemic activities in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 147, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, Z.-N.; Mehmood, S.; Wang, Y.; Pan, W.-J.; Zhang, W.-N.; Lu, Y.-M.; Chen, Y. Polysaccharide FMP-1 from Morchella esculenta attenuates cellular oxidative damage in human alveolar epithelial A549 cells through PI3K/AKT/Nrf2/HO-1 pathway. Int. J. Biol. Macromol. 2018, 120, 865–875. [Google Scholar] [CrossRef]
- Zhong, Q.-W.; Zhou, T.-S.; Qiu, W.-H.; Wang, Y.-K.; Xu, Q.-L.; Ke, S.-Z.; Wang, S.-J.; Jin, W.-H.; Chen, J.-W.; Zhang, H.-W. Characterization and hypoglycemic effects of sulfated polysaccharides derived from brown seaweed Undaria pinnatifida. Food Chem. 2021, 341, 128148. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward (5′ → 3′) | Reverse (3′ → 5′) |
|---|---|---|
| IRS1 | GCATCGAGAAGAACAATGTG | TCCACACTCCTCGTTGTCAG |
| PI3K | GGTGAGGATTCAGTTGGACA | CGAGGATCCAAACCAGCTTC |
| Akt | AGAAGCAGGAGGACCATTTG | GCTACTCGTTCATGGTCACG |
| β-actin | TGCGAGAGGCATCCTCACCT | TACATGGCTGAGGTTTGAAG |
| Monosaccharide Compositions (mol%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Galactose | Mannose | Fucose | Xylose | Glucuronic Acid | Fructose | Glucose | Arabinose |
| NNP-2 | - | 0.03 | 0.2 | 3.25 | - | 1 | 1 | - |
| Colony Forming Units (log CFU/mL) 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Basal Medium | Glucose | NNP-2 Concentration (%) | Inulin Concentration (%) | |||||
| Lactobacillus acidophilus | 0 | 0.76 ± 0.08 a | 0.28 ± 0.06 d | 0.68 ± 0.17 ab | 0.73 ± 0.09 a | 0.28 ± 0.05 d | 0.53 ± 0.08 c | 0.64 ± 0.02 b |
| Bifidobacterium adolescentis | 0 | 0.84 ± 0.03 a | 0.24 ± 0.01 e | 0.44 ± 0.09 d | 0.54 ± 0.03 c | 0.21 ± 0.06 e | 0.55 ± 0.03 c | 0.69 ± 0.03 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, B.; Anh, P.-T.-N.; Yang, S.-H. Polysaccharide Derived from Nelumbo nucifera Lotus Plumule Shows Potential Prebiotic Activity and Ameliorates Insulin Resistance in HepG2 Cells. Polymers 2021, 13, 1780. https://doi.org/10.3390/polym13111780
Le B, Anh P-T-N, Yang S-H. Polysaccharide Derived from Nelumbo nucifera Lotus Plumule Shows Potential Prebiotic Activity and Ameliorates Insulin Resistance in HepG2 Cells. Polymers. 2021; 13(11):1780. https://doi.org/10.3390/polym13111780
Chicago/Turabian StyleLe, Bao, Pham-Thi-Ngoc Anh, and Seung-Hwan Yang. 2021. "Polysaccharide Derived from Nelumbo nucifera Lotus Plumule Shows Potential Prebiotic Activity and Ameliorates Insulin Resistance in HepG2 Cells" Polymers 13, no. 11: 1780. https://doi.org/10.3390/polym13111780
APA StyleLe, B., Anh, P.-T.-N., & Yang, S.-H. (2021). Polysaccharide Derived from Nelumbo nucifera Lotus Plumule Shows Potential Prebiotic Activity and Ameliorates Insulin Resistance in HepG2 Cells. Polymers, 13(11), 1780. https://doi.org/10.3390/polym13111780








