Feasibility of Using Carvacrol/Starch Edible Coatings to Improve the Quality of Paipa Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
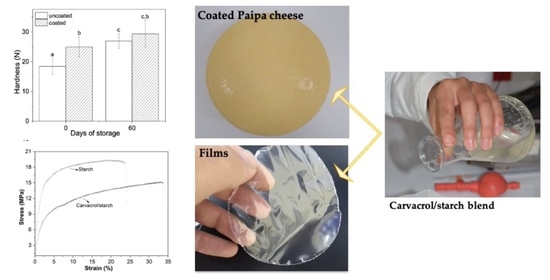
2.2. Preparation of Coatings and Films
2.3. Application of Coatings
2.4. Cheese Characterization
2.4.1. Proximal Analysis
2.4.2. Color Attributes
2.4.3. PH and Water Activity
2.4.4. Texture Profile Analysis
2.4.5. Microbiological Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Film Characterization
3.2. Effect of Edible Coatings on Paipa Cheeses
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castellanos-Rozo, J.; Pérez Pulido, R.; Grande, M.J.; Lucas, R.; Gálvez, A. Analysis of the Bacterial Diversity of Paipa Cheese (A Traditional Raw Cow’s Milk Cheese from Colombia) by High-Throughput Sequencing. Microorganisms 2020, 8, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luz, C.; Torrijos, R.; Quiles, J.; Mañes, J.; Meca, G. Shelf Life Extension of Mozzarella Cheese Contaminated with Penicillium spp. Using the Antifungal Compound ε-Polylysine. Food Sci. Technol. Int. 2019, 25, 295–302. [Google Scholar] [CrossRef]
- Embuena, A.I.C.; Nácher, M.C.; Boix, A.C.; Pons, M.P.M.; Llopis, M.B.; Martínez, M.C.B.; Martínez, C.G. Quality of Goat′s Milk Cheese as Affected by Coating with Edible Chitosan-Essential Oil Films. Int. J. Dairy Technol. 2017, 70, 68–76. [Google Scholar] [CrossRef]
- Youssef, A.M.; Assem, F.; Abdel-Aziz, M.E.; Elaaser, M.M.; Ibrahim, O.; Mahmoud, M.; El-Salam, M.H.A. Development of Bionanocomposite Materials and its Use in Coating of Ras Cheese. Food Chem. 2019, 270, 467–475. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; El-Sayed, H.; Ibrahim, O.; Youssef, A.M. Rational Design of Chitosan/Guar Gum/Zinc Oxide Bionanocomposites Based on Roselle Calyx Extract for Ras Cheese Coating. Carbohydr. Polym. 2020, 239, 116234. [Google Scholar] [CrossRef]
- Costa, M.J.; Maciel, L.C.; Teixeira, J.; Vicente, A.A.; Cerqueira, M.A. Use of Edible Films and Coatings in Cheese Preservation: Opportunities and Challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Bagheripoor, N.; Khoshgozaran-Abras, S.; Sohrabvandi, S.; Khorshidian, N.; Mortazavian, A.M.; Mollakhalili, N.; Jazaeri, S. Application of Active Edible Coatings to Improve the Shelf-Life of Cheese. Food Sci. Technol. Res. 2018, 24, 949–962. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Estevez-Areco, S.; Goyanes, S.; López-Córdoba, A. Characterization of Starches Isolated from Colombian Native Potatoes and Their Application as Novel Edible Coatings for Wild Andean Blueberries (Vaccinium Meridionale Swartz). Polymers 2019, 11, 1937. [Google Scholar] [CrossRef] [Green Version]
- Berti, S.; Resa, C.P.O.; Basanta, F.; Gerschenson, L.N.; Jagus, R.J. Edible Coatings on Gouda Cheese as a Barrier against External Contamination during Ripening. Food Biosci. 2019, 31, 100447. [Google Scholar] [CrossRef]
- González-Forte, L.D.S.; Amalvy, J.I.; Bertola, N. Corn Starch-Based Coating Enriched with Natamycin as an Active Compound to Control Mold Contamination on Semi-Hard Cheese during Ripening. Heliyon 2019, 5, e01957. [Google Scholar] [CrossRef] [Green Version]
- Berti, S.; Flores, S.; Jagus, R.J. Improvement of the Microbiological Quality of Argentinian Port Salut Cheese by Applying Starch-Based Films and Coatings Reinforced with Rice Bran and Containing Natural Antimicrobials. J. Food Process. Preserv. 2020, 44, 14827. [Google Scholar] [CrossRef]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Evaluation of Antifungal Activity of Antimicrobial Agents on Cheddar Cheese. Packag. Technol. Sci. 2012, 27, 49–58. [Google Scholar] [CrossRef]
- Doporto, M.C.; Dini, C.; Mugridge, A.; Viña, S.; García, M.A. Physicochemical, Thermal and Sorption Properties of Nutritionally Differentiated Flours and Starches. J. Food Eng. 2012, 113, 569–576. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Estevez-Areco, S.; Goyanes, S. Potato Starch-Based Biocomposites with Enhanced Thermal, Mechanical and Barrier Properties Comprising Water-Resistant Electrospun Poly (Vinyl Alcohol) Fibers and Yerba Mate Extract. Carbohydr. Polym. 2019, 215, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Medina-Jaramillo, C.; Quintero-Pimiento, C.; Díaz-Díaz, D.; Goyanes, S.; López-Córdoba, A. Improvement of Andean Blueberries Postharvest Preservation Using Carvacrol/Alginate-Edible Coatings. Polymers 2020, 12, 2352. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC International: Rockville, MD, USA, 2005; ISBN 0935584773. [Google Scholar]
- International Standard Organization. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. Available online: https://www.iso.org/standard/53728.html (accessed on 27 July 2021).
- International Standard Organization. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95. Available online: https://www.iso.org/standard/38276.html (accessed on 27 July 2021).
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Effects of Controlled Storage on Thermal, Mechanical and Barrier Properties of Plasticized Films from Different Starch Sources. J. Food Eng. 2006, 75, 453–460. [Google Scholar] [CrossRef]
- Bastos, M.D.S.R.; Laurentino, L.D.S.; Canuto, K.M.; Mendes, L.G.; Martins, C.M.; Silva, S.M.F.; Furtado, R.; Kim, S.; Biswas, A.; Cheng, H. Physical and Mechanical Testing of Essential Oil-Embedded Cellulose Ester Films. Polym. Test. 2016, 49, 156–161. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The Antibacterial Properties of Phenolic Isomers, Carvacrol and Thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible Cassava Starch Films Carrying Rosemary Antioxidant Extracts for Potential Use as Active Food Packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, Antibacterial and Antioxidant Properties of Chitosan Films Incorporated with Thyme Oil for Potential Wound Healing Applications. J. Mater. Sci. Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef] [Green Version]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Physico-Mechanical Properties of Starch-Based Films Containing Naturally Derived Antimicrobial Agents. Packag. Technol. Sci. 2013, 27, 149–159. [Google Scholar] [CrossRef]
- Ramos, M.; Beltrán, A.; Peltzer, M.A.; Valente, A.J.; Garrigós, M.D.C. Release and Antioxidant Activity of Carvacrol and Thymol from Polypropylene Active Packaging Films. LWT 2014, 58, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical Properties, Antioxidant and Antimicrobial Activity of Chitosan Films Containing Carvacrol and Pomegranate Peel Extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neira, L.M.; Martucci, J.F.; Stejskal, N.; Ruseckaite, R.A. Time-Dependent Evolution of Properties of Fish Gelatin Edible Films Enriched with Carvacrol During Storage. Food Hydrocoll. 2019, 94, 304–310. [Google Scholar] [CrossRef]
- Mastelić, J.; Jerkovic, I.; Blažević, I.; Poljak-Blaži, M.; Borović, S.; Ivančić-Baće, I.; Smrecki, V.; Žarković, N.; Brčić-Kostic, K.; Vikić-Topić, D.; et al. Comparative Study on the Antioxidant and Biological Activities of Carvacrol, Thymol, and Eugenol Derivatives. J. Agric. Food Chem. 2008, 56, 3989–3996. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry and Multibeneficial Bioactivities of Carvacrol (4-Isopropyl-2-Methylphenol), a Component of Essential Oils Produced by Aromatic Plants and Spices. J. Agric. Food Chem. 2014, 62, 7652–7670. [Google Scholar] [CrossRef]
- Quintanilla, P.; Beltrán, M.; Molina, A.; Escriche, I.; Molina, M. Characteristics of Ripened Tronchón Cheese from Raw Goat Milk Containing Legally Admissible Amounts of Antibiotics. J. Dairy Sci. 2019, 102, 2941–2953. [Google Scholar] [CrossRef] [Green Version]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of Water Activity (aw) on Microbial Stability as a Hurdle in Food Preservation. Water Act. Foods 2020, 323–355. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.; Souza, B.W.S.; Avides, M.D.C.; Vicente, A. Shelf Life Extension of Ricotta Cheese Using Coatings of Galactomannans from Nonconventional Sources Incorporating Nisin against Listeria Monocytogenes. J. Agric. Food Chem. 2010, 58, 1884–1891. [Google Scholar] [CrossRef] [Green Version]
- Henriques, M.; Santos, G.; Rodrigues, A.; Gomes, D.; Pereira, C.; Gil, M. Replacement of Conventional Cheese Coatings by Natural whey Protein Edible Coatings with Antimicrobial Activity. J. Hyg. Eng. Des. 2013, 3, 34–47. [Google Scholar]
- Zhong, Y.; Cavender, G.; Zhao, Y. Investigation of Different Coating Application Methods on the Performance of Edible Coatings on Mozzarella Cheese. LWT 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Pieretti, G.G.; Pinheiro, M.P.; Scapim, M.R.D.S.; Mikcha, J.M.G.; Madrona, G.S. Effect of an Edible Alginate Coating with Essential Oil to Improve the Quality of a Fresh Cheese. Acta Sci. Technol. 2019, 41, 36402. [Google Scholar] [CrossRef]
- Bianchi, A.; Mallmann, S.; Gazoni, I.; Cavalheiro, D.; Rigo, E. Effect of Acid Casein Freezing on the Industrial Production of Processed Cheese. Int. Dairy J. 2021, 118, 105043. [Google Scholar] [CrossRef]
- Everard, C.; O’Callaghan, D.; Howard, T.; O’Donnell, C.; Sheehan, E.; Delahunty, C. Relationships between Sensory and Rheological Measurements of Texture in Maturing Commercial Cheddar Cheese over a Range of Moisture and ph at the Point of Manufacture. J. Texture Stud. 2006, 37, 361–382. [Google Scholar] [CrossRef]
- Serna, C.P.; Penna, A.L.B.; Filho, J.F.L. Zein-based blend coatings: Impact on the Quality of a Model Cheese of Short Ripening Period. J. Food Eng. 2016, 171, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Kuorwel, K.K.; Cran, M.; Sonneveld, K.; Miltz, J.; Bigger, S. Migration of Antimicrobial Agents from Starch-Based Films into a Food Simulant. LWT 2013, 50, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Velasco, C.E.; Pérez-Pérez, J.C.; Varillas-Torres, J.M.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Munguía-Pérez, R.; Cid-Pérez, T.S.; Avila-Sosa, R. Starch Edible Films/Coatings Added with Carvacrol and Thymol: In Vitro and In Vivo Evaluation against Colletotrichum Gloeosporioides. Foods 2021, 10, 175. [Google Scholar] [CrossRef]
- Artigas, M.A.; Acevedo-Fani, A.; Martín-Belloso, O. Improving the Shelf Life of Low-Fat Cut Cheese using Nanoemulsion-Based Edible Coatings Containing Oregano Essential Oil and Mandarin Fiber. Food Control. 2017, 76, 1–12. [Google Scholar] [CrossRef] [Green Version]
- De Souza, A.G.; dos Santos, N.M.A.; Torin, R.F.D.S.; Rosa, D.D.S. Synergic Antimicrobial Properties of Carvacrol Essential Oil and Montmorillonite in Biodegradable Starch Films. Int. J. Biol. Macromol. 2020, 164, 1737–1747. [Google Scholar] [CrossRef]
- Di Pierro, P.; Sorrentino, A.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Chitosan/whey Protein Film as Active Coating to Extend Ricotta Cheese Shelf-Life. LWT 2011, 44, 2324–2327. [Google Scholar] [CrossRef]


| Parameters | Starch | Carvacrol/Starch |
|---|---|---|
| Moisture content (%) | 19.2 ± 1.5 a | 20.6 ± 0.9 a |
| Water vapor permeability (×10−10 g s−1 m−1 Pa−1) | 4.9 ± 0.2 a | 4.9 ± 0.8 a |
| Transparency | 11.4 ± 0.3 a | 10.9 ± 0.9 a |
| Water solubility (%) | 23.7 ± 1.2 a | 19.7 ± 1.1 b |
| Swelling (%) | 51.9 ± 6.4 a | 52.2 ± 4.1 a |
| Tensile strength (MPa) | 17.7 ± 0.5 a | 15.3 ± 0.4 b |
| Strain at break (%) | 23.7 ± 3.1 a | 33.4 ± 2.0 b |
| Young’s modulus (MPa) | 6.5 ± 0.1 a | 2.9 ± 0.6 b |
| DPPH-scavenging activity (inhibition %) | - | 25.8 ± 1.7 |
| Parameters | Sample | Day 0 | Day 60 |
|---|---|---|---|
| L* | Uncoated | 66.7 ± 1.1 a | 68.3 ± 0.6 b |
| Coated | 67.9 ± 1.5 a | 67.9 ± 1.5 a | |
| a* | Uncoated | 3.7 ± 0.3 a | 7.7 ± 0.1 b |
| Coated | 3.9 ± 0.2 a | 6.7 ± 0.2 b | |
| b* | Uncoated | 26.4 ± 1.0 a | 26.0 ± 1.4 a |
| Coated | 26.0 ± 1.0 a | 25.1 ± 0.8 a | |
| Hue angle | Uncoated | 81.9 ± 0.8 a | 73.4 ± 0.8 b |
| Coated | 81.5 ± 0.6 a | 75.1 ± 0.7 b | |
| ΔE | Uncoated | 1.3 | 1.5 |
| Coated |
| Sample | Days of Storage | Moisture Content (%) | Water Activity (aw) | PH |
|---|---|---|---|---|
| Uncoated | 0 | 32.0 ± 3.0 a | 0.95 ± 0.01 a | 5.12 ± 0.01 a |
| 60 | 30.3 ± 1.6 a | 0.96 ± 0.02 a | 5.31 ± 0.01 b | |
| Coated | 0 | 31.7 ± 1.8 a | 0.95 ± 0.01 a | 5.45 ± 0.02 c |
| 60 | 31.0 ± 1.2 a | 0.95 ± 0.03 a | 5.45 ± 0.01 c |
| Days of Storage | Total Mesophilic Aerobic Bacteria (log CFU × g−1) | Yeast and Molds (log CFU × g−1) | ||
|---|---|---|---|---|
| Uncoated | Coated | Uncoated | Coated | |
| 0 | 3.0 ± 0.3 a | 3.0 ± 0.2 a | 3.3 ± 0.3 a | 3.0 ± 0.2 a |
| 60 | 4.7 ± 0.1 b | 4.8 ± 0.3 b | 3.8 ± 0.2 b | 3.9 ± 0.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Córdoba, A. Feasibility of Using Carvacrol/Starch Edible Coatings to Improve the Quality of Paipa Cheese. Polymers 2021, 13, 2516. https://doi.org/10.3390/polym13152516
López-Córdoba A. Feasibility of Using Carvacrol/Starch Edible Coatings to Improve the Quality of Paipa Cheese. Polymers. 2021; 13(15):2516. https://doi.org/10.3390/polym13152516
Chicago/Turabian StyleLópez-Córdoba, Alex. 2021. "Feasibility of Using Carvacrol/Starch Edible Coatings to Improve the Quality of Paipa Cheese" Polymers 13, no. 15: 2516. https://doi.org/10.3390/polym13152516
APA StyleLópez-Córdoba, A. (2021). Feasibility of Using Carvacrol/Starch Edible Coatings to Improve the Quality of Paipa Cheese. Polymers, 13(15), 2516. https://doi.org/10.3390/polym13152516