Surface Modification of Poly(l-lactic acid) through Stereocomplexation with Enantiomeric Poly(d-lactic acid) and Its Copolymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
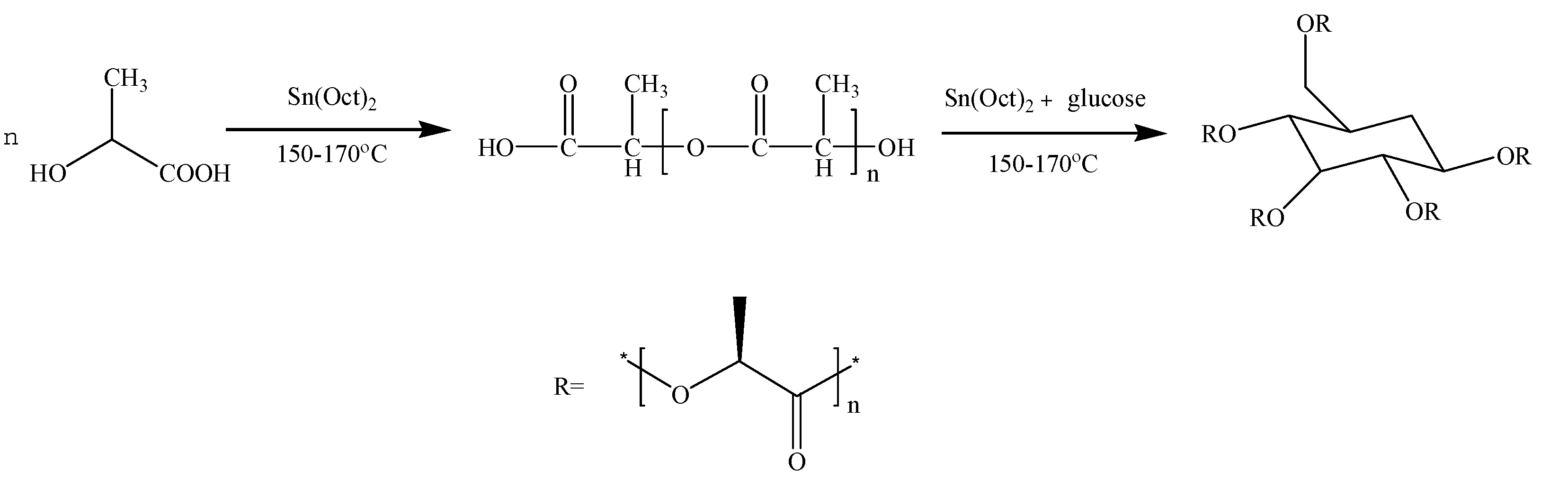
2.2.1. Synthesis of Poly(d-lactic acid) (PDLA)
2.2.2. Preparation of Poly(d-lactic acid-co-glucose) Copolymer (PDLAG)
2.2.3. Preparation and Modification of PLLA Film
2.3. Characterization Methods
3. Results and Discussion
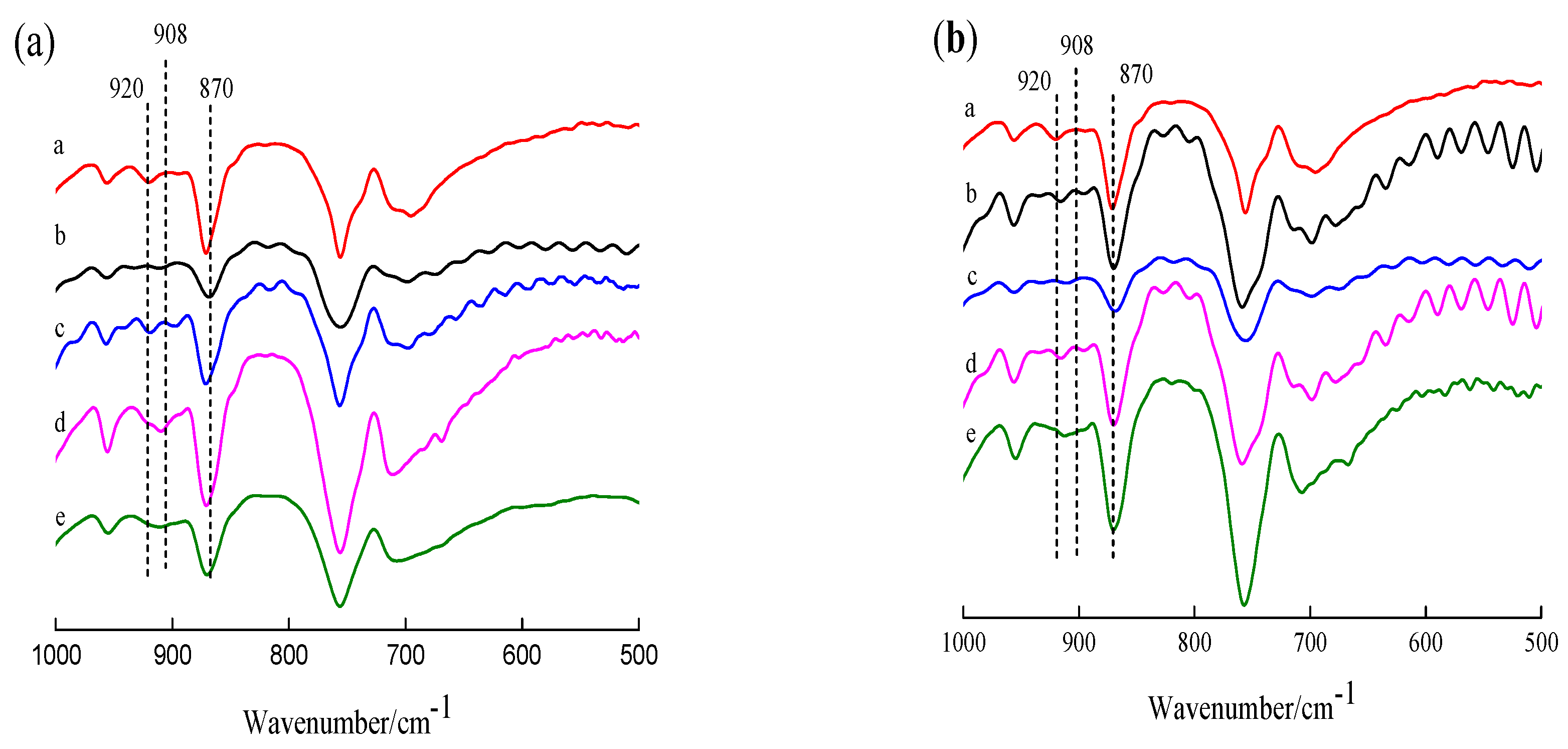
3.1. FT-IR Analysis of the Crystal Structure of Modified PLLA Films
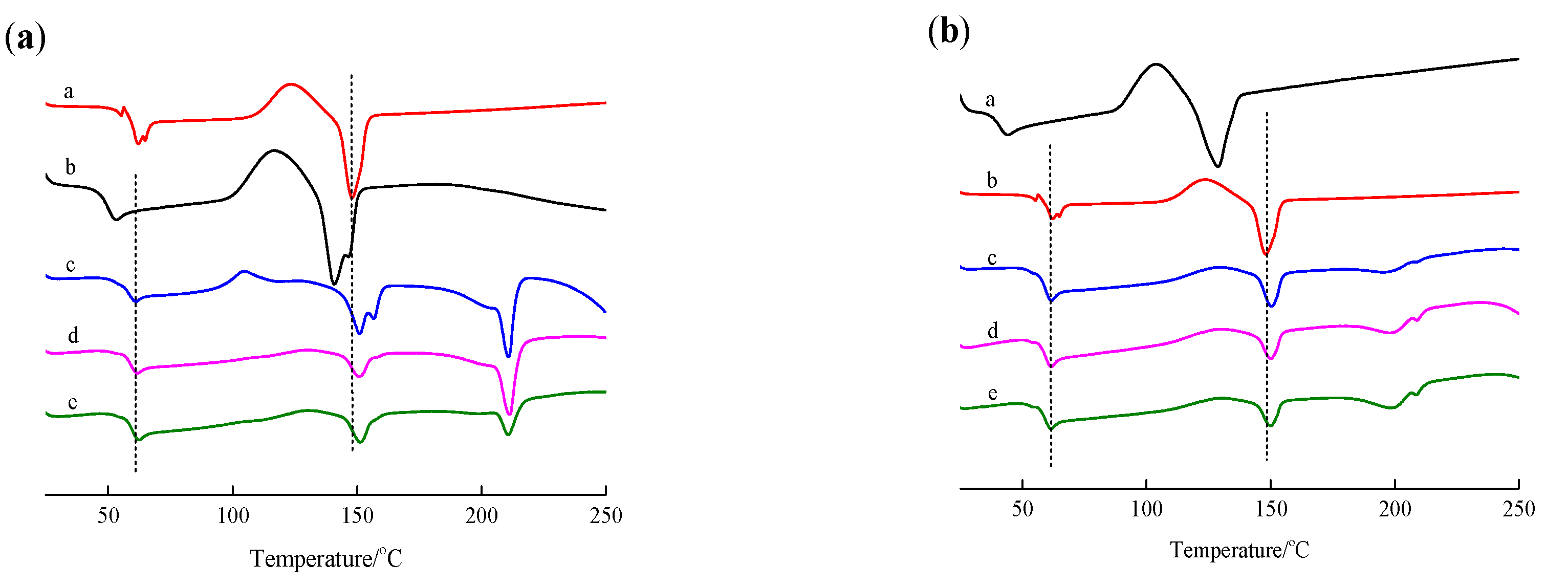
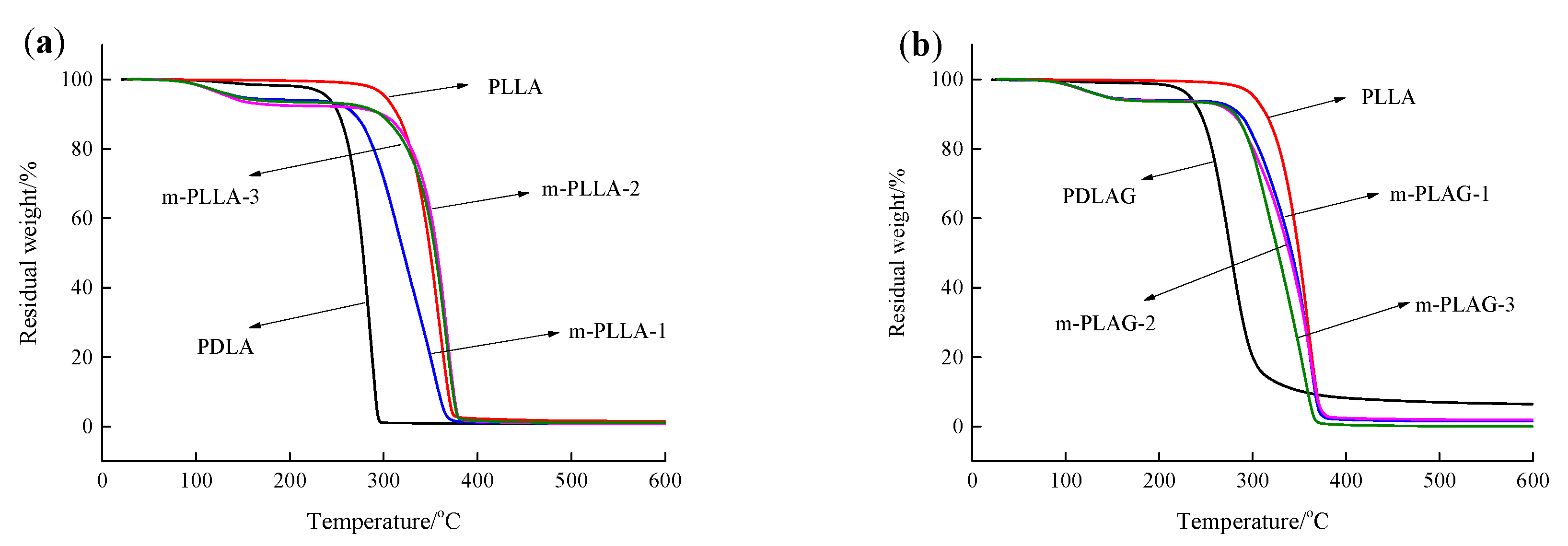
3.2. Analysis of Thermal Performance of Modified PLLA Film
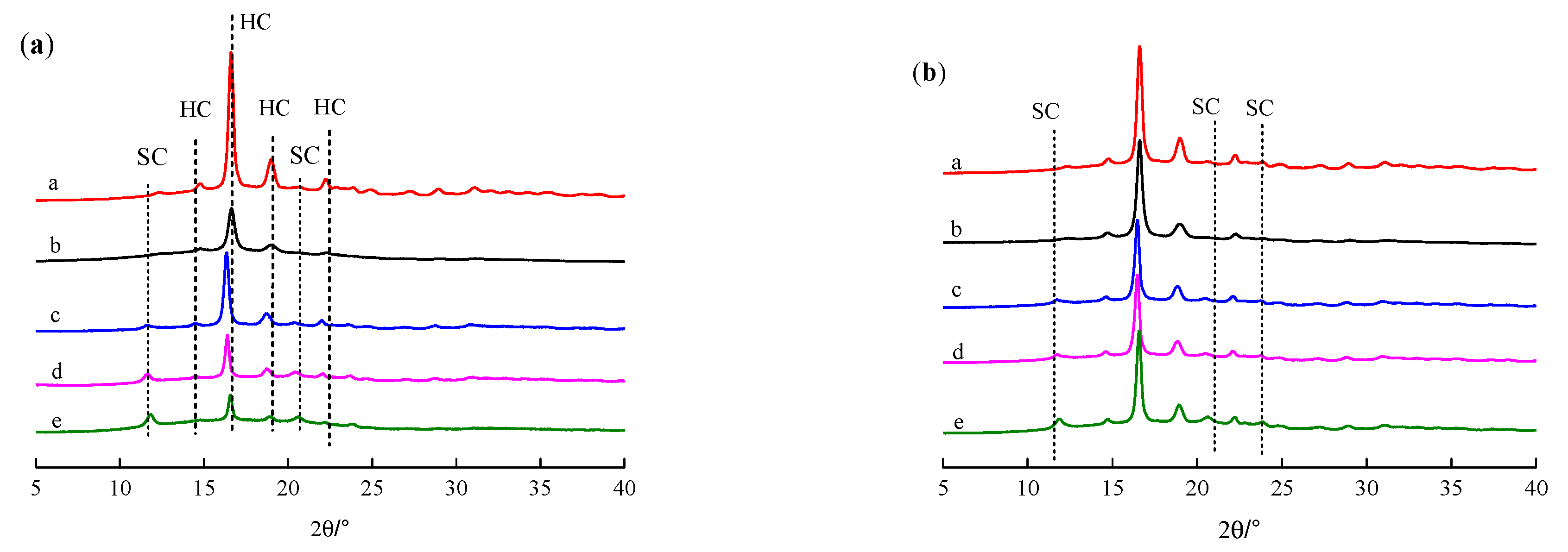
3.3. XRD Analysis of Modified PLLA Film
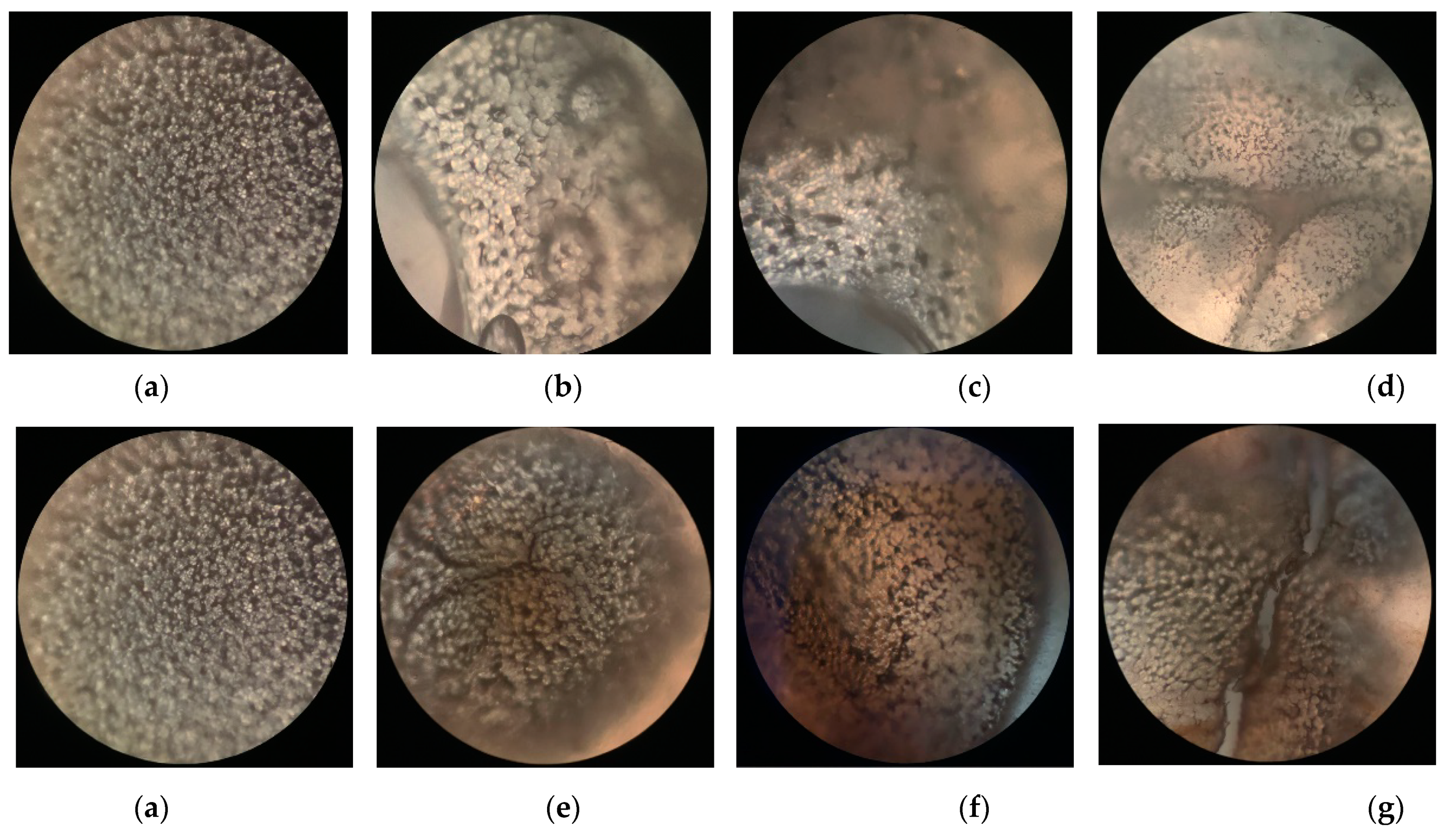
3.4. POM Analysis of the Crystal Morphology of Modified PLLA Films
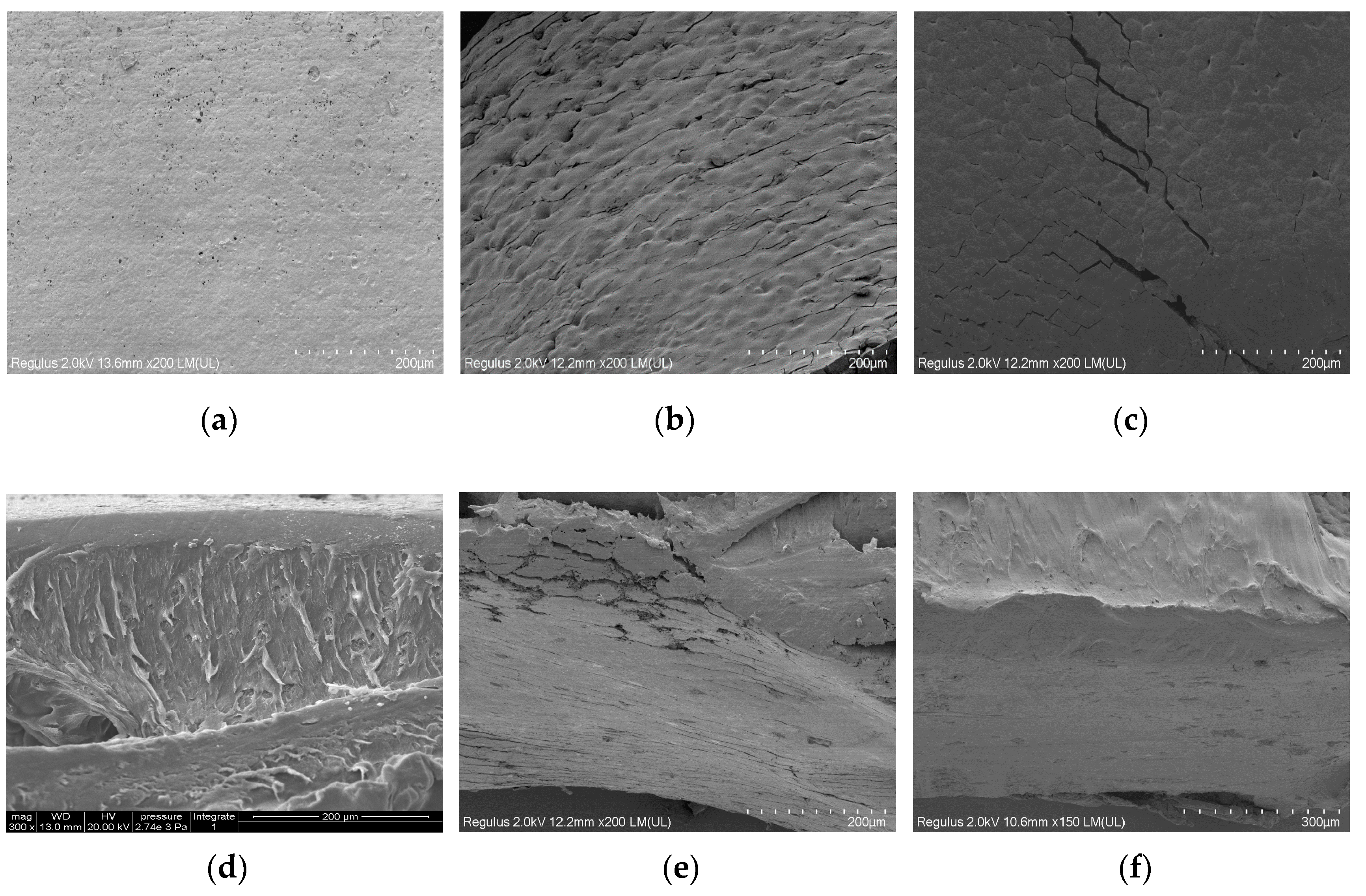
3.5. SEM Analysis of the Morphology of Modified PLLA Films
3.6. Hydrophilic Analysis of of Surface-Modified PLLA Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, P.; Zhu, D.-H.; Feng, K.; Liu, F.-J.; Lou, W.-Y.; Li, N.; Zong, M.-H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef]
- Sudamrao Getme, A.; Patel, B. A Review: Bio-fiber’s as reinforcement in composites of polylactic acid (PLA). Mater. Today: Proc. 2020, 26, 2116–2122. [Google Scholar] [CrossRef]
- Gupta, A.; Katiyar, V. Cellulose Functionalized High Molecular Weight Stereocomplex Polylactic Acid Biocomposite Films with Improved Gas Barrier, Thermomechanical Properties. ACS Sustain. Chem. Eng. 2017, 5, 6835–6844. [Google Scholar] [CrossRef]
- Gartner, H.; Li, Y.; Almenar, E. Improved wettability and adhesion of polylactic acid/chitosan coating for bio-based multilayer film development. Appl. Surf. Sci. 2015, 332, 488–493. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Villegas, C.; Garrido, L.; Roa, K.; Torres, A.; Galotto, M.J.; Rojas, A.; Romero, J. Modifying an Active Compound’s Release Kinetic Using a Supercritical Impregnation Process to Incorporate an Active Agent into PLA Electrospun Mats. Polymers 2018, 10, 479. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Velásquez, E.; Garrido, L.; Galotto, M.J.; López de Dicastillo, C. Design of active electrospun mats with single and core-shell structures to achieve different curcumin release kinetics. J. Food Eng. 2020, 273, 109900. [Google Scholar] [CrossRef]
- Behnoodfar, D.; Dadbin, S.; Frounchi, M. PLA Microspheres-Embedded PVA Hydrogels Prepared by Gamma-Irradiation and Freeze-Thaw Methods as Drug Release Carriers. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 28–33. [Google Scholar] [CrossRef]
- Xie, P.; Wang, J.; Li, J.; Cheng, Q.; Zhou, K.; Ren, J. Miktoarm star-shaped poly(lactic acid) copolymer: Synthesis and stereocomplex crystallization behavior. J. Polym. Sci. Part A: Polym. Chem. 2019, 57, 814–826. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Q.; Chen, W.; Lin, H.; Liu, Y.; Liang, H.; Zhu, F. Tacrolimus-loaded methoxy poly(ethylene glycol)-block-poly(D,L)-lactic–co-glycolic acid micelles self-assembled in aqueous solution for treating cornea immune rejection after allogenic penetrating keratoplasty in rats. Eur. J. Pharm. Sci. 2019, 133, 104–114. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Izzati Ayob, N.A.; Blanford, C.F.; Mohammad Rawi, N.F.; Szekely, G. Nonwoven Membrane Supports from Renewable Resources: Bamboo Fiber Reinforced Poly(Lactic Acid) Composites. ACS Sustain. Chem. Eng. 2019, 7, 11885–11893. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, Z.; Xu, X.; Liu, X.; Liu, L.; Huang, G.; Liu, L.; Wang, H.; Song, P. Grafting Lignin with Bioderived Polyacrylates for Low-Cost, Ductile, and Fully Biobased Poly(lactic acid) Composites. ACS Sustain. Chem. Eng. 2020, 8, 2267–2276. [Google Scholar] [CrossRef]
- Yang, Z.; Fu, H.J.; Zhang, R. Modification of layered α-zirconium phosphate and its application in poly (lactic acid)/α-zirconium phosphate composites. J. For. Eng. 2019, 4, 100–106. [Google Scholar]
- Janorkar, A.V.; Metters, A.T.; Hirt, D.E. Modification of Poly(lactic acid) Films: Enhanced Wettability from Surface-Confined Photografting and Increased Degradation Rate Due to an Artifact of the Photografting Process. Macromolecules 2004, 37, 9151–9159. [Google Scholar] [CrossRef]
- Yokohara, T.; Yamaguchi, M. Structure and properties for biomass-based polyester blends of PLA and PBS. Eur. Polym. J. 2008, 44, 677–685. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, K.; Wang, S.; Yuan, S.; Chen, W.; Konishi, T.; Miyoshi, T. Stoichiometry and Packing Structure of Poly(lactic acid) Stereocomplex as Revealed by Solid-State NMR and 13C Isotope Labeling. ACS Macro Lett. 2018, 7, 667–671. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Yang, G.; Ming, R.; Yu, M.; Zhang, H.; Shao, H. Evaluation of thermal resistance and mechanical properties of injected molded stereocomplex of poly(l-lactic acid) and poly(d-lactic acid) with various molecular weights. Adv. Polym. Technol. 2018, 37, 1674–1681. [Google Scholar] [CrossRef]
- Schwaiger, D.; Lohstroh, W.; Müller-Buschbaum, P. Investigation of Molecular Dynamics of a PTB7:PCBM Polymer Blend with Quasi-Elastic Neutron Scattering. ACSAppl. Polym. Mater. 2020, 2, 3797–3804. [Google Scholar] [CrossRef]
- Ignacz, G.; Fei, F.; Szekely, G. Ion-Stabilized Membranes for Demanding Environments Fabricated from Polybenzimidazole and Its Blends with Polymers of Intrinsic Microporosity. ACS Appl. Nano Mater. 2018, 1, 6349–6356. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Ogueri, K.S.; Allcock, H.R.; Laurencin, C.T. A Regenerative Polymer Blend Composed of Glycylglycine Ethyl Ester-Substituted Polyphosphazene and Poly(lactic-co-glycolic acid). ACS Appl. Polym. Mater. 2020, 2, 1169–1179. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Ajiro, H.; Takahama, S.; Mizukami, M.; Kan, K.; Akashi, M.; Kurihara, K. Force Estimation on the Contact of Poly(l,l-lactide) and Poly(d,d-lactide) Surfaces Regarding Stereocomplex Formation. Langmuir 2016, 32, 9501–9506. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Kato, K.; Iwata, H. Adsorption of Enantiomeric Poly(lactide)s on Surface-Grafted Poly(l-lactide). Langmuir 2004, 20, 6748–6753. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699–6708. [Google Scholar] [CrossRef]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Zhong, L.; Gong, X. Phase separation-induced superhydrophobic polylactic acid films. Soft Matter 2019, 15, 9500–9506. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Wang, W.; Fan, D.; He, P. Effects of femtosecond laser micromachining on the surface and substrate properties of poly-lactic acid (PLA). Appl. Surf. Sci. 2021, 538, 148117. [Google Scholar] [CrossRef]
- Pal, A.K.; Katiyar, V. Nanoamphiphilic chitosan dispersed poly(lactic acid) bionanocomposite films with improved thermal, mechanical, and gas barrier properties. Biomacromolecules 2016, 17, 2603–2618. [Google Scholar] [CrossRef]
- Miletić, A.; Ristić, I.; Coltelli, M.-B.; Pilić, B. Modification of PLA-Based Films by Grafting or Coating. J. Funct. Biomater. 2020, 11, 30. [Google Scholar] [CrossRef]
- Aulin, C.; Karabulut, E.; Tran, A.; Wågberg, L.; Lindström, T. Correction to Transparent Nanocellulosic Multilayer Thin Films on Polylactic Acid with Tunable Gas Barrier Properties. ACS Appl. Mater. Interfaces 2013, 5, 10395–10396. [Google Scholar] [CrossRef]
- Guo, B.; Finne-Wistrand, A.; Albertsson, A.-C. Electroactive Hydrophilic Polylactide Surface by Covalent Modification with Tetraaniline. Macromolecules 2012, 45, 652–659. [Google Scholar] [CrossRef]
- Cao, D.; Ming, W.; Qi, L.; Zhao, Y.; Gao, Q. Preparation and Properties of Poly(Lactic Acid) Stereocomplex Containing Glucose Groups. Chem. Ind. For. Prod. 2018, 38, 17–22. [Google Scholar]
- Qi, L.; Zhu, Q.; Cao, D.; Liu, T.; Zhu, K.; Chang, K.; Gao, Q. Preparation and Properties of Stereocomplex of Poly(lactic acid) and Its Amphiphilic Copolymers Containing Glucose Groups. Polymers 2020, 12, 760. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Pal, A.K.; Woo, E.M.; Katiyar, V. Effects of amphiphilic chitosan on stereocomplexation and properties of poly(lactic acid) nano-biocomposite. Sci. Rep. 2018, 8, 4351. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, C. Synthesis and stereocomplex crystallization of poly(lactide)-graphene oxide nanocomposites. ACS Macro Lett. 2012, 1, 709–713. [Google Scholar] [CrossRef]
- Meaurio, E.; López-Rodríguez, N.; Sarasua, J.R. Infrared Spectrum of Poly(l-lactide): Application to Crystallinity Studies. Macromolecules 2006, 39, 9291–9301. [Google Scholar] [CrossRef]
- Qi, F.; Tang, M.; Chen, X.; Chen, M.; Guo, G.; Zhang, Z. Morphological structure, thermal and mechanical properties of tough poly(lactic acid) upon stereocomplexes. Eur. Polym. J. 2015, 71, 314–324. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: Their formation, properties, and application. Polym. Int. 2006, 55, 626–642. [Google Scholar] [CrossRef]
- Bai, H.; Liu, H.; Bai, D.; Zhang, Q.; Wang, K.; Deng, H.; Chen, F.; Fu, Q. Enhancing the melt stability of polylactide stereocomplexes using a solid-state cross-linking strategy during a melt-blending process. Polym. Chem. 2014, 5, 5985–5993. [Google Scholar] [CrossRef]
- Jia, S.; Yu, D.; Wang, Z.; Zhang, X.; Chen, L.; Fu, L. Morphologies, crystallization, and mechanical properties of PLA-based nanocomposites: Synergistic effects of PEG/HNTs. J. Appl. Polym. Sci. 2019, 136, 47385–47395. [Google Scholar] [CrossRef]
- Toncheva, A.; Mincheva, R.; Kancheva, M.; Manolova, N.; Rashkov, I.; Dubois, P.; Markova, N. Antibacterial PLA/PEG electrospun fibers: Comparative study between grafting and blending PEG. Eur. Polym. J. 2016, 75, 223–233. [Google Scholar] [CrossRef]
- Tsuji, H.; Sato, S.; Masaki, N.; Arakawa, Y.; Kuzuya, A.; Ohya, Y. Synthesis, stereocomplex crystallization and homo-crystallization of enantiomeric poly(lactic acid-co-alanine)s with ester and amide linkages. Polym. Chem. 2018, 9, 565–575. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, P.; Shi, X.; Zhang, G.; Wang, C. Preparation of open-porous stereocomplex PLA/PBAT scaffolds and correlation between their morphology, mechanical behavior, and cell compatibility. RSC Adv. 2018, 8, 12933–12943. [Google Scholar] [CrossRef]
- Shibata, M.; Katoh, M.; Takase, H.; Shibita, A. Stereocomplex formation in stereoblock copolymer networks composed of 4-armed star-shaped lactide oligomers and a 2-armed ε-caprolactone oligomer. Polym. Chem. 2015, 6, 4123–4132. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Li, Z.; Muiruri, J.K.; Thitsartarn, W.; Zhang, X.; Tan, B.H.; He, C. Biodegradable silica rubber core-shell nanoparticles and their stereocomplex for efficient PLA toughening. Compos. Sci. Technol. 2018, 159, 11–17. [Google Scholar] [CrossRef]
- Liang, Y.-Y.; Xu, J.-Z.; Li, Y.; Zhong, G.-J.; Wang, R.; Li, Z.-M. Promoting Interfacial Transcrystallization in Polylactide/Ramie Fiber Composites by Utilizing Stereocomplex Crystals. ACS Sustain. Chem. Eng. 2017, 5, 7128–7136. [Google Scholar] [CrossRef]
- Pan, P.; Bao, J.; Han, L.; Xie, Q.; Shan, G.; Bao, Y. Stereocomplexation of high-molecular-weight enantiomeric poly(lactic acid)s enhanced by miscible polymer blending with hydrogen bond interactions. Polymer 2016, 98, 80–87. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Yang, J.; Chen, S.; Ding, Y.; Wang, Z. Significantly accelerated spherulitic growth rates for semicrystalline polymers through the layer-by-layer film method. J. Phys. Chem. C 2013, 117, 5882–5893. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Jiang, F.; Bai, J.; Wang, Z. Effect of miscibility on spherulitic growth rate for double-layer polymer films. Soft Matter 2013, 9, 5771–5778. [Google Scholar] [CrossRef]
- Makowski, T.; Svyntkivska, M.; Piorkowska, E.; Kregiel, D. Multifunctional polylactide nonwovens with 3D network of multiwall carbon nanotubes. Appl. Surf. Sci. 2020, 527, 146898–146905. [Google Scholar] [CrossRef]


| Sample | Modification Time in PDLA Solution (min) | Sample | Modification Time in PDLAG Solution (min) |
|---|---|---|---|
| m-PLLA-1 | 0.5 | m-PLAG-1 | 0.5 |
| m-PLLA-2 | 1.0 | m-PLAG-2 | 1.0 |
| m-PLLA-3 | 3.0 | m-PLAG-3 | 3.0 |
| Sample | Modification Time/min | Tm, HC/°C | Tm, SC/°C | Tg/°C | fc,HC/% | fc,SC% | Tb/°C | Tmax/°C | Wr/% |
|---|---|---|---|---|---|---|---|---|---|
| PLLA | 0 | 147.9 | / | 61.9 | 21.8 | / | 301.7 | 349.6 | 2.0 |
| PDLA | 0 | 140.6 | / | 52.8 | 18.1 | / | 239.7 | 278.1 | 0.9 |
| PDLAG | 0 | 128.8 | / | 44.2 | 17.6 | / | 233.1 | 276.8 | 6.5 |
| m-PLLA-1 | 0.5 | 150.8 | 210.8 | 61.6 | 8.2 | 8.2 | 249.2 | 321.2 | 1.1 |
| m-PLLA-2 | 1 | 151.0 | 211.2 | 62.4 | 3.4 | 5.7 | 278.1 | 357.1 | 1.4 |
| m-PLLA-3 | 3 | 151.6 | 211.4 | 63.2 | 3.1 | 2.1 | 256.3 | 355.0 | 1.7 |
| m-PLAG-1 | 0.5 | 150.6 | 208.4 | 61.2 | 4.9 | 0.4 | 253.7 | 342.1 | 2.0 |
| m-PLAG-2 | 1 | 151.0 | 208.6 | 61.4 | 4.7 | 0.7 | 252.1 | 339.2 | 2.0 |
| m-PLAG-3 | 3 | 150.2 | 208.8 | 61.8 | 4.1 | 1.2 | 249.3 | 327.4 | 0.6 |
| Sample | Number | Sum | Mean | Variance | / | / |
|---|---|---|---|---|---|---|
| m-PLLA-1 | 3 | 632.4 | 210.8 | 0.01 | / | / |
| m-PLAG-1 | 3 | 625.2 | 208.4 | 0.09 | / | / |
| Source of difference | SS | df | MS | F | p-value | F crit |
| Between groups | 8.64 | 1 | 8.64 | 172.8 | 0.000193 | 7.708647 |
| Within groups | 0.2 | 4 | 0.05 | / | / | / |
| sum | 8.84 | 5 | / | / | / | / |
| Sample | Modification Time/min | Water Contact Angle/° |
|---|---|---|
| PLLA | 0.0 | 84.1 |
| m-PLLA-1 | 0.5 | 76.5 |
| m-PLLA-2 | 1.0 | 72.5 |
| m-PLLA-3 | 3.0 | 68.5 |
| m-PLAG-1 | 0.5 | 69.5 |
| m-PLAG-2 | 1.0 | 63.2 |
| m-PLAG-3 | 3.0 | 60.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Chang, K.; Qi, L.; Li, X.; Gao, W.; Gao, Q. Surface Modification of Poly(l-lactic acid) through Stereocomplexation with Enantiomeric Poly(d-lactic acid) and Its Copolymer. Polymers 2021, 13, 1757. https://doi.org/10.3390/polym13111757
Zhu Q, Chang K, Qi L, Li X, Gao W, Gao Q. Surface Modification of Poly(l-lactic acid) through Stereocomplexation with Enantiomeric Poly(d-lactic acid) and Its Copolymer. Polymers. 2021; 13(11):1757. https://doi.org/10.3390/polym13111757
Chicago/Turabian StyleZhu, Qianjin, Kaixin Chang, Liyan Qi, Xinyi Li, Woming Gao, and Qinwei Gao. 2021. "Surface Modification of Poly(l-lactic acid) through Stereocomplexation with Enantiomeric Poly(d-lactic acid) and Its Copolymer" Polymers 13, no. 11: 1757. https://doi.org/10.3390/polym13111757
APA StyleZhu, Q., Chang, K., Qi, L., Li, X., Gao, W., & Gao, Q. (2021). Surface Modification of Poly(l-lactic acid) through Stereocomplexation with Enantiomeric Poly(d-lactic acid) and Its Copolymer. Polymers, 13(11), 1757. https://doi.org/10.3390/polym13111757







