Biosynthesized Poly(3-Hydroxybutyrate) on Coated Pineapple Leaf Fiber Papers for Biodegradable Packaging Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Bacterial Strain and Media
2.3. Production and Purification of Poly (3-Hydroxybutyrate)
2.4. Characterization of Poly (3-Hydroxybutyrate)
2.5. Preparation of Pineapple Leaf Fiber Paper (PLFP)
2.6. Coating Procedure of P(3-HB) on Pineapple Leaf Fiber Paper
2.7. Physical Characterization of P(3-HB) Non-Coated and Coated Pineapple Fiber Paper
2.7.1. Thickness Basis Weight and Percentage of Increasing in Weight
2.7.2. Color Properties
2.8. Scanning Electron Microscopy (SEM) of P(3-HB) Non-Coated and Coated Pineapple Fiber Paper
2.9. Fourier Transform Infrared Spectroscopy (FTIR) of P(3-HB) Non-Coated and Coated Pineapple Fiber Paper
2.10. Mechanical Characterization of P(3-HB) Non-Coated and Coated Pineapple Fiber Paper
2.10.1. Tensile Properties (Tensile Index and Elongation at Break)
2.10.2. Folding Endurance
2.10.3. Tear Index
2.10.4. Burst Index
2.11. Soil Burial Biodegradability Test
2.12. Water Drop Penetration Test
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Biosynthesized P(3-HB)
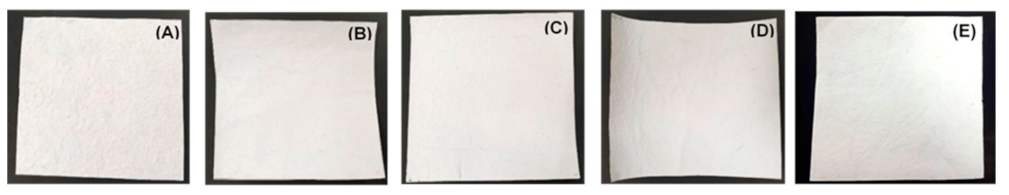
3.2. Physical and Color Properties of P(3-HB) Non-Coated and Coted Pineapple Leaf Fiber Papers (PLFP)
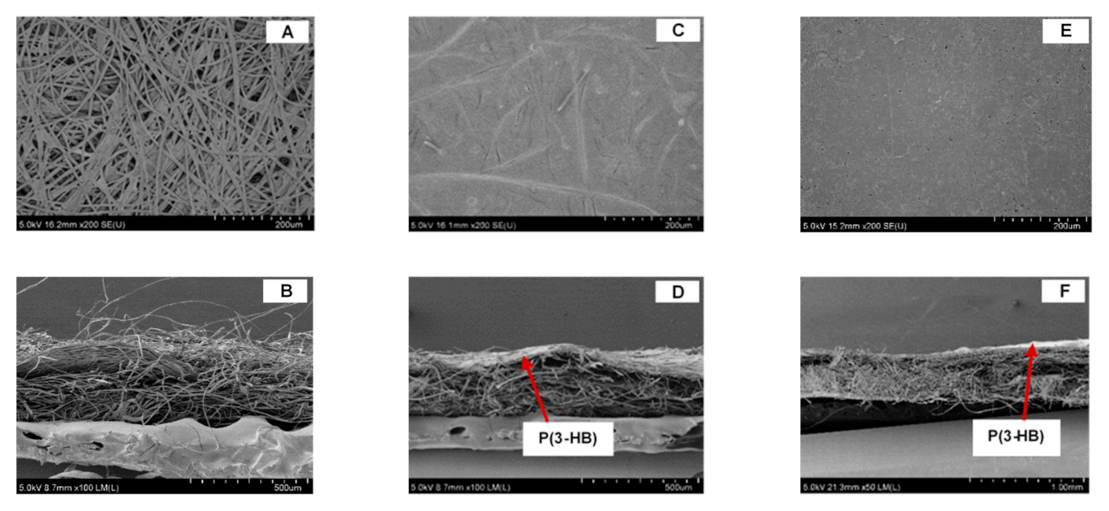
3.3. Scanning Electron Microscopy (SEM)
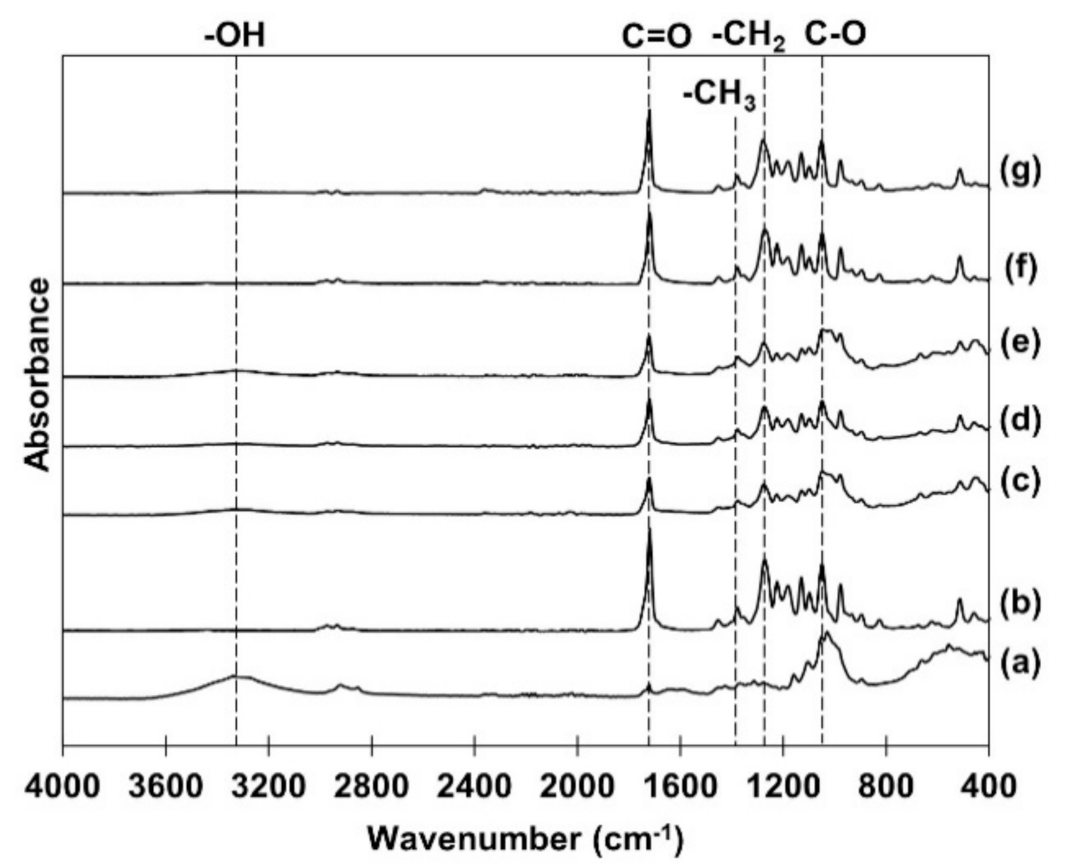
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
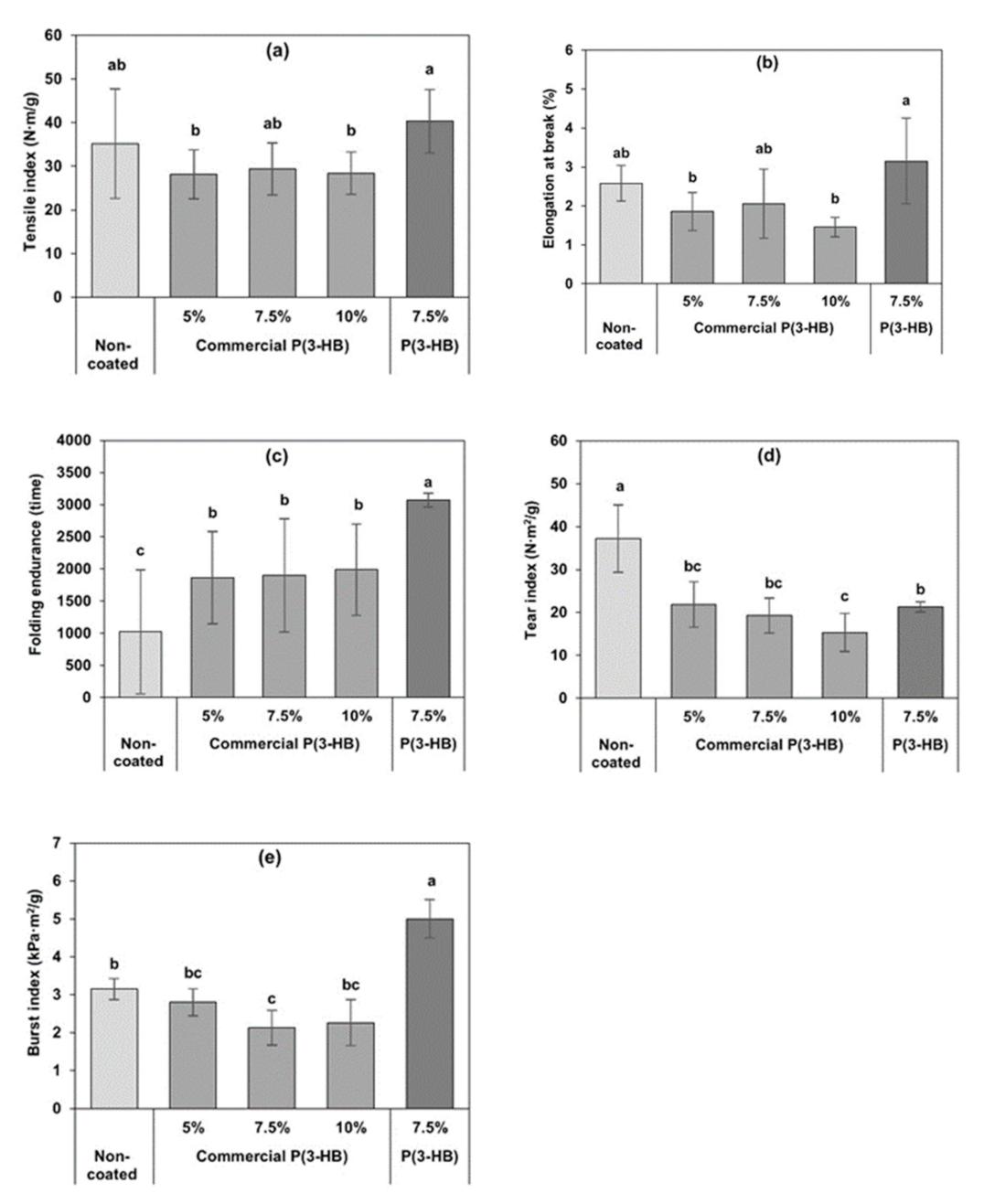
3.5. Mechanical Properties
3.6. Water Drop Penetration
3.7. Soil Burial Biodegradability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laftah, W.A.; Wan Abdul Rahman, W.A. Pulping process and the potential of using non-wood pineapple leaves fiber for pulp and paper production: A review. J. Nat. Fibers 2015, 13, 85–102. [Google Scholar] [CrossRef]
- Todkar, S.S.; Patil, S.A. Review on mechanical properties evaluation of pineapple leaf fibre (PALF) reinforced polymer composites. Compos. B Eng. 2019, 174, 106927. [Google Scholar] [CrossRef]
- Chollakup, R.; Tantatherdtam, R.; Ujjin, S.; Sriroth, K. Pineapple leaf fiber reinforced thermoplastic composites: Effects of fiber length and fiber content on their characteristics. J. Appl. Polym. Sci. 2011, 119, 1952–1960. [Google Scholar] [CrossRef]
- Smitthipong, W.; Tantatherdtam, R.; Chollakup, R. Effect of pineapple leaf fiber-reinforced thermoplastic starch/poly(lactic acid) green composite. J. Thermoplast. Compos. Mater. 2013, 28, 717–729. [Google Scholar] [CrossRef]
- Suwanruji, P.; Tuechart, T.; Smitthipong, W.; Chollakup, R. Modification of pineapple leaf fiber surfaces with silane and isocyanate for reinforcing thermoplastic. J. Thermoplast. Compos. Mater. 2016, 30, 1344–1360. [Google Scholar] [CrossRef]
- Chollakup, R.; Askanian, H.; Delor-Jestin, F. Initial properties and ageing behaviour of pineapple leaf and palm fibre as reinforcement for polypropylene. J. Thermoplast. Compos. Mater. 2016, 30, 174–195. [Google Scholar] [CrossRef]
- Sibaly, S.; Jeetah, P. Production of paper from pineapple leaves. J. Environ. Chem. Eng. 2017, 5, 5978–5986. [Google Scholar] [CrossRef]
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible films from carrageenan/orange essential oil/trehalose—structure, optical properties, and antimicrobial activity. Polymers 2021, 13, 332. [Google Scholar] [CrossRef]
- Khwaldia, K.; Arab-Tehrany, E.; Desobry, S. Biopolymer coatings on paper packaging materials. Compr. Rev. Food Sci. Food Saf. 2010, 9, 82–91. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Samyn, P. Bio-based coatings for paper applications. Coatings 2015, 5, 887–930. [Google Scholar] [CrossRef]
- Pena, C.; Castillo, T.; Garcia, A.; Millan, M.; Segura, D. Biotechnological strategies to improve production of microbial poly-(3-hydroxybutyrate): A review of recent research work. Microb. Biotechnol. 2014, 7, 278–293. [Google Scholar] [CrossRef]
- Kalia, V.C.; Singh Patel, S.K.; Shanmugam, R.; Lee, J.K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef] [PubMed]
- Ragaert, P.; Buntinx, M.; Maes, C.; Vanheusden, C.; Peeters, R.; Wang, S.; D’Hooge, D.R.; Cardon, L. Polyhydroxyalkanoates for Food Packaging Applications. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Obeso, C.G.; Sousa, M.P.; Song, W.; Rodriguez-Pérez, M.A.; Bhushan, B.; Mano, J.F. Modification of paper using polyhydroxybutyrate to obtain biomimetic superhydrophobic substrates. Colloids Surf. Physicochem. Eng. Aspects 2013, 416, 51–55. [Google Scholar] [CrossRef]
- Cyras, V.P.; Soledad, C.M.; Analía, V. Biocomposites based on renewable resource: Acetylated and non acetylated cellulose cardboard coated with polyhydroxybutyrate. Polymer 2009, 50, 6274–6280. [Google Scholar] [CrossRef]
- Cyras, V.P.; Commisso, M.S.; Mauri, A.N.; Vázquez, A. Biodegradable double-layer films based on biological resources: Polyhydroxybutyrate and cellulose. J. Appl. Polym. Sci. 2007, 106, 749–756. [Google Scholar] [CrossRef]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci. Rep. 2015, 5, 17884. [Google Scholar] [CrossRef]
- Kuusipalo, J. PHB/V in extrusion coating of paper and paperboard: Part I: Study of functional properties. J. Polym. Environ. 2000, 8, 39–47. [Google Scholar] [CrossRef]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P. Bilayer biocomposites based on coated cellulose paperboard with films of polyhydroxybutyrate/cellulose nanocrystals. Cellulose 2018, 25, 2419–2434. [Google Scholar] [CrossRef]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P. Properties and processing relationship of polyhydroxybutyrate and cellulose biocomposites. Procedia Mater. Sci. 2015, 8, 807–813. [Google Scholar] [CrossRef]
- Seoane, I.T.; Luzi, F.; Puglia, D.; Cyras, V.P.; Manfredi, L.B. Enhancement of paperboard performance as packaging material by layering with plasticized polyhydroxybutyrate/nanocellulose coatings. J. Appl. Polym. Sci. 2018, 135, 46872. [Google Scholar] [CrossRef]
- Trakunjae, C.; Boondaeng, A.; Apiwatanapiwat, W.; Kosugi, A.; Arai, T.; Sudesh, K.; Vaithanomsat, P. Enhanced polyhydroxybutyrate (PHB) production by newly isolated rare actinomycetes Rhodococcus sp. strain BSRT1-1 using response surface methodology. Sci. Rep. 2021, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Altaee, N.; Fahdil, A.; Yousif, E.; Sudesh, K. Recovery and subsequent characterization of polyhydroxybutyrate from Rhodococcus equi cells grown on crude palm kernel oil. J. Taibah Univ. Sci. 2016, 10, 543–550. [Google Scholar] [CrossRef]
- Chollakup, R.; Kongtud, W.; Sukatta, U.; Piriyasatits, K.; Premchookiat, M.; Jarerat, A. Development of rice straw paper coated with pomelo peel extract for bio-based and antibacterial packaging. Key Eng. Mater. 2020, 847, 141–146. [Google Scholar] [CrossRef]
- TAPPI. Standards Brightness of Pulp, Paper, and Paperboard (Directional Reflectance at 457 nm), Test Method. 2009. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T452.aspx (accessed on 19 January 2021).
- TAPPI. Tensile Properties of Paper and Paperboard (Using Constant Rate of Elongation Apparatus), Test Method TAPPI/ANSI 2009. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T494.aspx (accessed on 19 January 2021).
- TAPPI. Folding Endurance of Paper (Schopper Type Tester), Test Method 2007. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T423.aspx (accessed on 19 January 2021).
- TAPPI. Internal Tearing Resistance of Paper (Elmendorf-Type Method), Test Method. 2009. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T414.aspx (accessed on 19 January 2021).
- TAPPI. Bursting Strength of Paper, Test Method 2009. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T414.aspx (accessed on 19 January 2021).
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P.; Torre, L.; Fortunati, E.; Puglia, D. Effect of cellulose nanocrystals and bacterial cellulose on disintegrability in composting conditions of plasticized PHB nanocomposites. Polymers 2017, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- TAPPI. Water Absorption of Corrugating Medium: Water Drop Absoption Test, Test Method TAPPI/ANSI 2009. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T835.aspx (accessed on 19 January 2021).
- Penloglou, G.; Kretza, E.; Chatzidoukas, C.; Parouti, S.; Kiparissides, C. On the control of molecular weight distribution of polyhydroxybutyrate in Azohydromonas lata cultures. Biochem. Eng. J. 2012, 62, 39–47. [Google Scholar] [CrossRef]
- Nunes, R.W.; Martin, J.R.; Johnson, J.F. Influence of molecular weight and molecular weight distribution on mechanical properties of polymers. Polym. Eng. Sci. 1982, 22, 205–228. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Fiorese, M.n.L.; Freitas, F.; Pais, J.; Ramos, A.M.; de Aragão, G.u.M.F.; Reis, M.A.M. Recovery of polyhydroxybutyrate (PHB) from Cupriavidus necator biomass by solvent extraction with 1,2-propylene carbonate. Eng. Life Sci. 2009, 9, 454–461. [Google Scholar] [CrossRef]
- Sathiyanarayanan, G.; Saibaba, G.; Seghal Kiran, G.; Selvin, J. A statistical approach for optimization of polyhydroxybutyrate production by marine Bacillus subtilis MSBN17. Int. J. Biol. Macromol. 2013, 59, 170–177. [Google Scholar] [CrossRef]
- Al-Battashi, H.; Annamalai, N.; Al-Kindi, S.; Nair, A.S.; Al-Bahry, S.; Verma, J.P.; Sivakumar, N. Production of bioplastic (poly-3-hydroxybutyrate) using waste paper as a feedstock: Optimization of enzymatic hydrolysis and fermentation employing Burkholderia sacchari. J. Clean. Prod. 2019, 214, 236–247. [Google Scholar] [CrossRef]
- Oliveira, F.C.; Dias, M.L.; Castilho, L.R.; Freire, D.M. Characterization of poly(3-hydroxybutyrate) produced by Cupriavidus necator in solid-state fermentation. Bioresour. Technol. 2007, 98, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Chaijamrus, S.; Udpuay, N. Production and Characterization of Polyhydroxybutyrate from Molasses and Corn Steep Liquor Produced by Bacillus megaterium ATCC 6748. Available online: https://cigrjournal.org/index.php/Ejounral/article/view/1216 (accessed on 19 January 2021).
- Kulkarni, S.O.; Kanekar, P.P.; Jog, J.P.; Sarnaik, S.S.; Nilegaonkar, S.S. Production of copolymer, poly (hydroxybutyrate-co-hydroxyvalerate) by Halomonas campisalis MCM B-1027 using agro-wastes. Int. J. Biol. Macromol. 2015, 72, 784–789. [Google Scholar] [CrossRef]
- Khwaldia, K. Physical and mechanical properties of hydroxypropyl methylcellulose–coated paper as affected by coating weight and coating composition. BioResources 2013, 8, 3438–3452. [Google Scholar] [CrossRef]
- Sothornvit, R. Effect of hydroxypropyl methylcellulose and lipid on mechanical properties and water vapor permeability of coated paper. Food. Res. Inter. 2009, 42, 307–311. [Google Scholar] [CrossRef]
- Chauhan, V.S.; Bhardwaj, N.K.; Chakrabarti, S.K. Effect of particle size of magnesium silicate filler on physical properties of paper. Can. J. Chem. Eng. 2013, 91, 855–861. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under composting conditions of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef]
- Siakeng, R.; Jawaid, M.; Asim, M.; Siengchin, S. Accelerated weathering and soil burial effect on biodegradability, colour and textureof coir/pineapple leaf fibres/PLA biocomposites. Polymers 2020, 12, 458. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the use of PLA-PHB blends for sustainable food packaging applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef] [PubMed]



| Polymer/Carbon Source | Bacterial Strains | Mw (g/mol) | Mn (g/mol) | PDI (Mw/Mn) | References |
|---|---|---|---|---|---|
| P(3-HB) (fructose) | R. pyridinivorans BSRT1-1 | 6.07 × 105 | 2.96 × 105 | 2.0 | This study |
| Commercial P(3-HB) | - | 5.05 × 105 | - | - | This study |
| PHB (crude palm kernel oil) | R. equi | 6.42 × 105 | 3.73 × 105 | 1.72 | [23] |
| PHB (soy) | Cupriavidis necator | 7.90 × 105 | 3.49 × 105 | 2.26 | [38] |
| PHB (molasses and corn steep liquor) | Bacillus megaterium ATCC 6748 | 3.90 × 106 | 2.65 × 106 | 1.47 | [39] |
| PHB (pulp industry waste) | Bacillus subtilis MSBN17 | 6.40 × 105 | 3.80 × 105 | 1.68 | [36] |
| P(3HB-co-3HV) (bagasse extract) | Halomonas campisalis | 1.39 × 106 | 8.39 × 105 | 1.66 | [40] |
| Properties | Non-Coated PLFP | Commercial P(3-HB) | Biosynthesized P(3-HB) | ||
|---|---|---|---|---|---|
| 5% | 7.5% | 10% | 7.5% | ||
| Weight (g) | 1.55 ± 0.19 b | 1.79 ± 0.26 b | 2.35 ± 0.50 a | 2.62 ± 0.47 a | 2.50 ± 0.06 a |
| Basic weight (g/m2) | 99.64 ± 12.73 b | 114.35 ± 16.56 b | 150.09 ± 31.88 a | 171.30 ± 30.02 a | 159.82 ± 3.73 a |
| The percentage increase in weight (%) | - | 60.81 ± 16.04 b | 64.69 ± 16.17 b | 98.78 ± 12.13 a | 59.62 ± 7.64 b |
| Thickness (mm) | 0.27 ± 0.05 b | 0.29 ± 0.05 b | 0.29 ± 0.06 b | 0.27 ± 0.03 b | 0.59 ± 0.01 a |
| Color Parameters | Non-Coated PLFP | Commercial P(3-HB) | Biosynthesized P(3-HB) | ||
|---|---|---|---|---|---|
| 5% | 7.5% | 10% | 7.5% | ||
| L* | 87.92 ± 0.89 b | 91.22 ± 0.35 a | 91.23 ± 0.33 a | 91.23 ± 0.39 a | 87.63 ± 0.22 b |
| a* | 1.23 ± 0.04 a | 0.86 ± 0.15 c | 1.00 ± 0.12 b | 0.86 ± 0.06 c | 1.01 ± 0.02 b |
| b* | 9.21 ± 0.09 a | 6.91 ± 0.28 b | 7.17 ± 0.47 b | 7.11 ± 0.39 b | 8.16 ± 0.02 b |
| ∆E* | - | 4.03 ± 0.07 b | 6.14 ± 0.17 a | 6.32 ± 0.36 a | 1.03 ± 0.25 c |
| Brightness (%) | 27.39 ± 0.60 c | 41.98 ± 13.40 b | 44.06 ± 2.38 b | 45.13 ± 1.59 b | 62.57 ± 0.60 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaithanomsat, P.; Kongsin, K.; Trakunjae, C.; Boonyarit, J.; Jarerat, A.; Sudesh, K.; Chollakup, R. Biosynthesized Poly(3-Hydroxybutyrate) on Coated Pineapple Leaf Fiber Papers for Biodegradable Packaging Application. Polymers 2021, 13, 1733. https://doi.org/10.3390/polym13111733
Vaithanomsat P, Kongsin K, Trakunjae C, Boonyarit J, Jarerat A, Sudesh K, Chollakup R. Biosynthesized Poly(3-Hydroxybutyrate) on Coated Pineapple Leaf Fiber Papers for Biodegradable Packaging Application. Polymers. 2021; 13(11):1733. https://doi.org/10.3390/polym13111733
Chicago/Turabian StyleVaithanomsat, Pilanee, Kunat Kongsin, Chanaporn Trakunjae, Jirachaya Boonyarit, Amnat Jarerat, Kumar Sudesh, and Rungsima Chollakup. 2021. "Biosynthesized Poly(3-Hydroxybutyrate) on Coated Pineapple Leaf Fiber Papers for Biodegradable Packaging Application" Polymers 13, no. 11: 1733. https://doi.org/10.3390/polym13111733
APA StyleVaithanomsat, P., Kongsin, K., Trakunjae, C., Boonyarit, J., Jarerat, A., Sudesh, K., & Chollakup, R. (2021). Biosynthesized Poly(3-Hydroxybutyrate) on Coated Pineapple Leaf Fiber Papers for Biodegradable Packaging Application. Polymers, 13(11), 1733. https://doi.org/10.3390/polym13111733







