Abstract
Natural biopolymers are an interesting resource for edible films production, as they are environmentally friendly packaging materials. The possibilities of the application of main animal proteins and natural polysaccharides are considered in the review, including the sources, structure, and limitations of usage. The main ways for overcoming the limitations caused by the physico-chemical properties of biopolymers are also discussed, including composites approaches, plasticizers, and the addition of crosslinking agents. Approaches for the production of biopolymer-based films and coatings are classified according to wet and dried processes and considered depending on biopolymer types. The methods for mechanical, physico-chemical, hydration, and uniformity estimation of edible films are reviewed.
1. Introduction
Inferior packaging or its absence causes significant food loss (about 20–25%) due to microbiological contamination and oxidative processes, which lead to a decrease in the quality of food products and makes them unsuitable for consumption [1].
The development and application of bioactive packaging systems is a relevant field of research. The application of such smart packaging systems is a tool for protecting food from spoilage and reducing the risk of growth of pathogenic microorganisms due to both the creation of a barrier and the action of active components at the border of the product with the packaging [2,3]. The currently used packaging materials are mainly produced from petrochemical products [4,5]. The global problem of environmental pollution makes alternative environmentally friendly and biodegradable polymers be in demand [6].
Every year, the problem of recycling polymer packaging materials becomes more acute [7] due to its accumulation in large quantities, which cause significant harm to the environment [8]. The incineration or pyrolysis of polymer waste, to some extent, solves the problem of their accumulation in landfills, but does not contribute to improving the overall environmental situation [9]. Recycling of polymer waste is more environmentally friendly, but in this case, significant labor and energy costs are required for sorting polymer materials and their subsequent processing [10,11]. It should be noted that the recycling of polymers is carried out a limited number of times, after which the problem of burial or incineration of these materials arises again [12].
Concerning environmental suitability, biopolymers are environmentally friendly packaging materials [13,14]. The main advantage of their use as bioplastics is the closed natural cycle, where the end of one cycle leads to the beginning of the next cycle [6].
Biopolymers can be divided into three main categories depending on the origin and method of production (Figure 1): directly extracted from biomass, synthesized bio-derived monomers, and produced by microorganisms [15]. Polysaccharides and proteins are the most promising biopolymers for the production of packaging materials [16,17]. Proteins are heteropolymers consisting of α-amino acids as monomeric units. The combinations of 20 amino acids to form a protein sequence allow for an almost unlimited number of various polymer chains with different physical and chemical properties. Proteins also contain a large number of functional groups that can be changed enzymatically, chemically, or physically for varying the properties of the films [16]. Polysaccharides are good candidates for replacing oil-based polymers due to their ability to form a film, affinity for paper-based materials, an appropriate barrier to gases and aromas, and good mechanical strength. Moreover, these biopolymers are biodegradable, non-toxic, and are used as a matrix for the inclusion of additives with specific functionality, such as active antimicrobial properties, for example [17].
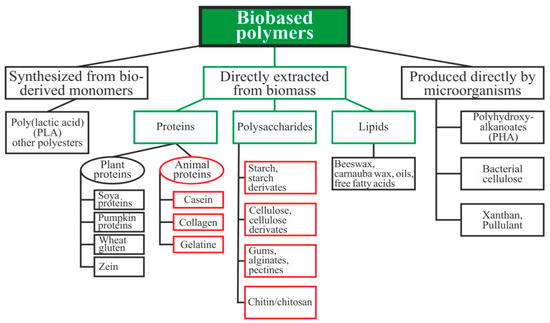
Figure 1.
Schematic classification of biopolymer types [15,18,19]. Reproduced with permission from Popović, S.Z. et al., Biopolymers for Food Design; published by Elsevier Inc., 2018.
The prefix “bio” indicates that biopolymers are biodegradable. The word “biodegradable” means that materials can be decomposed by bacteria, fungi, and yeast to the final products of biomass under anaerobic conditions—hydrocarbons and methane [20]. These types of polymers consist of monomers that are covalently connected, forming a chain of the molecule. They are also produced inside the cell as a result of complex metabolic processes. Biopolymers can be used for food packaging as a replacement for oil-based plastics made from petroleum due to their biodegradability, renewability, and wide distribution [21].
Environmental friendliness is a key ideology nowadays. The use of biopolymers from renewable resources could solve global plastic pollution. For many years, researchers have been trying to develop and design packaging materials based on natural biopolymers. However, animal proteins and natural polysaccharides are characterized by some undesirable properties caused by their chemical nature and structure. These disadvantages reduce their competitiveness with oil-based plastics but can be overcome. In this review, we summarize the description of such popular biopolymers as starch, cellulose, pectin, chitosan, alginate, casein, collagen and gelatin, considered for films and coatings production in general and for each biopolymer. The main characteristics of packaging materials based on animal proteins and natural polysaccharides, appropriateness for certain type of product based on properties of polymer, as well as ways to improve it are also described.
2. Biopolymers Used for Food Packaging
Biopolymers are widely implemented and can be used as coatings and films [4,22]. Coating involves the formation of a cover directly on the surface of food products, whereas films are structures that are used separately after formation [23].
Materials based on biopolymers must meet the basic requirements of health safety, mechanical and chemical resistance, and durability [24,25]. Therefore, food packaging should not only be biodegradable, but also functional. Compared to synthetic polymers derived from petroleum, biopolymers have a more complex chemical structure and side chain structure, which provides additional opportunities for the formation of packaging materials with specific characteristics for specific purposes [18,20].
Biopolymers directly extracted from biomass as polysaccharides and animal proteins, which are most often used for the preparation of packaging materials for food products, will be considered in this review [16,26,27].
2.1. Starch
Starch is one of the most readily available polysaccharides on the planet [28]. The plants from which it is obtained grow in almost all temperate climate zones. Corn, wheat, potatoes, and rice are the world leaders: 84%, 7%, 4%, and 1%, respectively [29]. This biopolymer is a mixture of amylose and amylopectin (Figure 2), the ratio of which varies depending on the type of starch.
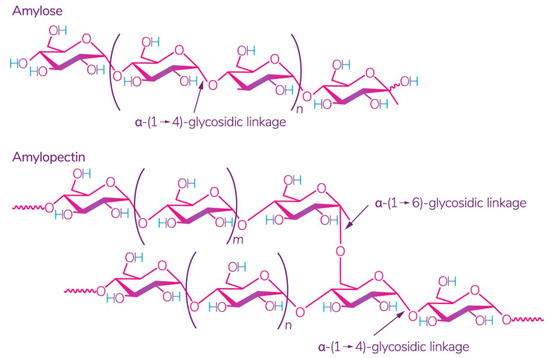
Figure 2.
Structure of amylose and amylopectin [30]. Reproduced from Kadokawa, Polymers; published by MDPI, Basel, Switzerland, 2012.
The ratio of amylose:amylopectin varies significantly not only between different plants, but also within a single plant species or plant organ [31]. The conditions and phase of growth also influence this ratio [32,33]. Several studies have shown that a change in this ratio implies a change in the physical and chemical characteristics of starch and its interaction with other molecules, which leads to different swelling capacity [34], solubility in water, microscopic properties [35], and stability and barrier/mechanical properties in starch films [36]. According to some studies, starch with a high content of amylose, for example, from peas, has the best mechanical and gas barrier properties [37]. It was also noted that lentil starch (30% amylose) has a strong tendency to gelatinize at a relatively low concentration compared to corn and potato starch [29].
Cereal (grain) starch is obtained by its physical separation from non-starch components. Various processes of wet grinding of grains have been developed for the production of starch. The main stages of these processes are soaking, grinding, and separation of grain components [38]. Potato starch is extracted from potato tubers in a process called bio-processing: the potatoes are ground, and the contents of the cells are released, including starch and protein [39].
Starch-based polymers have low moisture resistance, which limits their use in packaging. The usual form of natural starch is a crystalline molecular structure that is not flexible [40]. However, an interesting starch derivative is thermoplastic starch (TPS), which is more convenient for films production [41,42], which could be obtained with thermal and physical impacts in the presence of plasticizers [40]. Various physical and chemical reactions are involved in the heat treatment of starch-based polymers, such as water diffusion, granule expansion, gelatinization, melting, crystallization, and extrusion [43].
TPS with improving properties can be used in the field of food packaging since it is economical and available in large quantities. The production of flexible and solid packaging (biofilms, bags, laminated plastic, etc.) is the main sector of TPS application in the food industry [40]. Polymer films derived from starch are biodegradable and have good properties as oxygen barriers. However, the amount of plasticizer, humidity, and amylose content are the limiting factors that determine the mechanical properties of TPS. The type and amount of plasticizer used in the production of thermoplastic starch strongly affect the physical, chemical, and thermal properties of the film [44]. Various structural enhancers, such as microcrystalline cellulose, carboxymethylcellulose, carbon nanotubes, etc., are added to the starch-polymer matrix in order to improve its properties [45]. Various types of such reinforced starch are already successfully used in the packaging of bread, vegetables, and meat products stored in standard conditions [41,46,47].
2.2. Cellulose
Cellulose is the most common natural biopolymer and consists of β-(1–4)-D-glucopyranose monomers (Figure 3) [48]. It is biosynthesized by a number of living organisms, from lower to higher plants, marine animals, bacteria, and fungi [49]. It has been estimated that 1011–1012 tons are synthesized annually by photosynthesis in a fairly pure form, for example, in the seed hairs of the cotton plant, but mainly cellulose combines with lignin and other polysaccharides (hemicelluloses) in the cell wall of woody plants [50]. Although cellulose is primarily found in forests, where wood is the most important source, cellulose-containing materials include agricultural residues, aquatic plants, grasses, and other plant matter [51,52]. Commercial cellulose production is based on harvested sources, such as wood, or on natural sources with high biopolymer content, such as cotton [53]. In contrast with starch, cellulose is a linear polymer without winding and branching [48]. Numerous hydroxyl groups in cellulose form strong hydrogen bonds, which make the material non-fusible. Chemical modification of cellulose is required for the production of flexible materials, which often involves the replacement of hydroxyl groups with acetate or methyl groups (esterification), the purpose of which is to reduce the intensity of the hydrogen bonds [54]. The rate of esterification and type of replacers, as well as the length of the polymer chain, affect the further permeability, mechanical properties, and solubility. Methylcellulose (MC) and carboxymethylcellulose (CMC) are the most common cellulose esters and have good film-forming properties, which allow them to be used as packaging materials for food products [55,56,57,58].
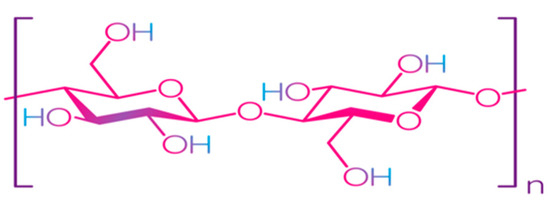
Figure 3.
Structure of cellulose [49]. Reproduced with permission from Trache, D. et al., International Journal of Biological Macromolecules; published by Elsevier Ltd., 2016.
Methylcellulose (MC) is formed when one or more hydroxyl groups (-OH) in the anhydroglucose are replaced by a methoxide group (-OCH3). The degree of substitution of MC ranges from 1.4 to 2.0, and it is soluble in cold water [59]. It is reported that MC forms unbreakable, flexible, transparent, tasteless, and non-toxic films that have good barrier properties for oxygen, but bad ones for water vapor [60]. MC has a relatively high tensile strength and elastic modulus, so it demonstrates a reinforcing effect [61] and exhibits improved mechanical properties in mixtures containing proteins, lipids, etc. [62].
Carboxymethylcellulose (CMC) is a cellulose derivative in which some hydroxyl groups of glucopyranose units in cellulose are replaced by carboxymethyl groups. CMC is formed by reaction with chloroacetic acid (ClCH2CO2H) and catalyzed by alkali [63]. The amount of carboxymethyl groups in the cellulose molecule improves the strength of CMC film due to strong intermolecular forces [64]. Initially, CMC was studied as a hydrogel polymer, but it was later discovered that its dry form could be considered a biodegradable alternative to petroleum-based food packaging materials [64]. CMC easily absorbs moisture, dissolves in cold water, and exhibits thermal gelatinization [65]. CMC-based films can be combined with MC, clay, chitosan, etc., which usually improves the mechanical properties of the film: the tensile strength and elastic modulus increase, while the strain at break of the films is reduced [65]. It was noted that such a plasticizer as glycerin significantly increases the flexibility of the film, but also reduces the tensile strength and elastic modulus [66].
Cellulose derivatives are more suitable for packaging that is in direct contact with food products [65]. Studies of the properties of various cellulose derivatives used for film formation are continuing with the aim of developing mixtures of cellulose derivatives with other biopolymers to improve the mechanical, barrier properties and increase the shelf life of packaged food products [46,67,68].
2.3. Pectin
Pectin is one of the main components of the plant cell wall, contributing to the integrity and rigidity of tissues, and is considered one of the most complex macromolecules in nature [69]. Although pectin is ubiquitous in the plant kingdom, pectin derived from apples, citrus fruits, sunflowers, and sugar beets is an undisputed commercial source for the processing industry due to their physical and chemical properties and the availability of biomass [70]. Pectin is a poly–α–1–4–galacturonic acid (Figure 4a) with different degrees of methylation of carboxylic acid residues and/or amidated polygalacturonic acids (Figure 4b) [71]. Carboxyl groups of galacturonic acid are esterificated with methanol, resulting in methoxylated carboxyl groups. On the other hand, amidated carboxyl groups are obtained when galacturonic acid is converted with ammonia to carboxylic acid amide.
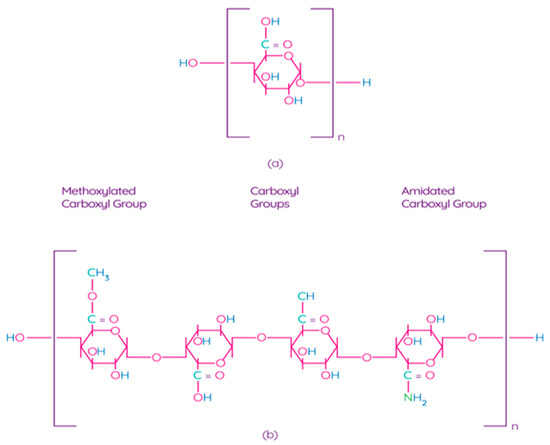
Figure 4.
Chemical structure of polygalacturonic acid (a) and representative chemical structure of pectin, showing typical repeating groups (b) [72]. Reproduced with permission from Espitia, P.J.P. et al., Food Hydrocolloids; published by Elsevier Ltd., 2013.
According to the degree of esterification by methanol (the ratio of esterified galacturonic acid groups to its total number), pectin can be classified as high-methoxylpectin (HMP, >50% of esterified carboxyl groups) pectin or low-methoxyl pectin (LMP, <50% of esterified carboxyl groups) [70]. The degree of esterification affects the gelling properties of pectins. For example, pectin with a low content of methoxyl groups forms a gel in the presence of multivalent ions, which bonds pairs of carboxyl groups of different pectin chains. Pectin with a high content of methoxyl groups forms a gel in acidic solutions with the addition of various sugars, such as sucrose or glucose [72,73]. LMP is used for encapsulation, food packaging film processing, and low-calorie gels in dietary foods [72]. Structurally, pectins are classified as rhamnogalacturonan-I (RG-I; “hairy” pectin), substituted galacturonans (RG-II or SG; “hairy” pectin), and homogalacturonans (HG; smooth pectin). The hairy region of pectins (RG-I and RG-II) has a high probiotic potential [74]. Thus, based on the macromolecular and microstructural characteristics of pectins, the scope of their application in food products is determined.
Pectin is an ingredient used in the food industry without any restrictions other than current good manufacturing practices, is generally recognized as safe (GRAS) by the U.S. Food and Drug Administration (FDA), and is used in food primarily as a gelling agent, stabilizing agent, or thickener in products such as jams, yogurt drinks, fruit milk drinks, and ice cream [75,76]. The ubiquitous presence, low cost, structural flexibility, and polymerization ability of pectin contribute to its use as a matrix for active food packaging materials [70]. Since bioactive packaging films made from pectin have very weak antimicrobial properties, their antimicrobial potential can be enhanced by integrating and combining them with various functional compounds, such as essential oils, phenolic compounds, nanomaterials, free fatty acids, and others [77]. The production of edible films from pectin can be carried out in various ways, such as casting, extrusion, spraying, and coating with a knife [78].
2.4. Chitosan
Chitosan is a linear polysaccharide consisting of randomly linked units of β-(1,4)-D-glucosamine and N-acetyl-D-glucosamine (Figure 5). Chitin is the second most common structural polysaccharide found in nature after cellulose and is usually deacetylated by an alkali for chitosan production [79,80]. Natively, chitin is presented in the form of ordered crystalline microfibrils that form structural components in the exoskeleton of arthropods or in the cell walls of fungi and yeast. So far, the main commercial sources of chitin are the shells of crabs and shrimps [81]. In industrial processing, chitin is extracted by acid treatment to dissolve calcium carbonate, and then by an alkaline solution to dissolve proteins. In addition, a discoloration stage is often added to remove the pigments and obtain a colorless pure chitin [82]. Chitin is usually isolated from the exoskeleton of crustaceans and, in particular, from shrimps and crabs, where α-chitin is produced [83]. Squid is another important source of chitin, in which it exists in the β-form, which has been found to be more amenable to deacetylation. Such chitin also shows higher solubility, reactivity, and affinity for solvents and swelling than α-chitin due to the much weaker intermolecular hydrogen bond attributed to the parallel arrangement of the main chains [83]. Some types of insects, fungi, bacteria, and algae can also be alternative sources of chitin/chitosan [84,85,86].
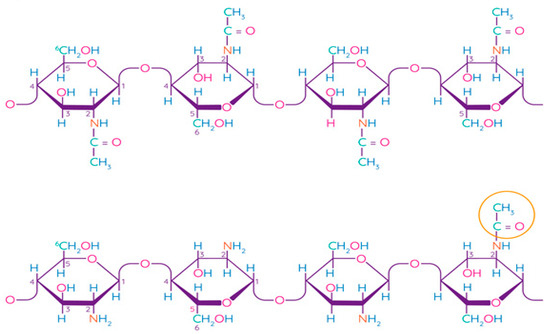
Figure 5.
Chemical structure of chitin and chitosan [87]. Reproduced with permission from Rasul, R.M. et al., Carbohydrate Polymers; published by Elsevier Ltd., 2020.
The term “chitosan” usually refers to a family of polymers produced after the deacetylation of chitin to varying degrees [81]. In fact, the acetylation degree, which reflects the balance between the two types of residues, distinguishes chitin from chitosan. When the acetylation degree is higher 60%, the product is called chitosan and becomes soluble in acidic aqueous solutions [88]. A depolymerization reaction also occurs during deacetylation, as evidenced by changes in the molecular weight of chitosan. Chitin can be converted into chitosan by enzyme treatment [89] or by a chemical process [90]. Chemical methods are widely used for commercial purposes of producing chitosan due to their low cost and suitability for mass production [81]. Chitosan has been found to be non-toxic, biodegradable, bio-functional, and demonstrates good film-forming qualities and antimicrobial properties [91].
However, films prepared only from chitosan do not meet the criteria for packaging materials. They are rigid and brittle; therefore, it is important to use plasticizers to improve their mechanical properties. Various plasticizers have an effect on the stability of plasticized films. It was found that polyethylene glycol (PEG) and glycerin are the best candidates as plasticizers of chitosan films [92]. It was found that other small-molecular organic materials also improve the mechanical properties of chitosan films, but their effectiveness decreases within a few weeks [92]. The addition of PEG and glycerol promotes the formation of relatively flexible and stable (at least for several months) chitosan films. In general, these substances prevent the formation of double bonds between adjacent polymer chains, which prevents recrystallization [93]. Plasticizers modify the mechanical properties of chitosan without changing its fundamental chemical structure [92].
In comparison with other biopolymers used for food packaging, chitosan has an advantage due to its ability to include such functional substances as minerals or vitamins and its antibacterial activity, which is important for maintaining the quality of products [94,95,96].
2.5. Alginate
Alginates are natural indigestible polysaccharides, usually produced and purified from various genera of brown algae (mainly Laminaria hyperborean, Macrocystis pyrifera, Ascophyllum nodosum, and to a lesser extent, Laminaria digitate, Laminaria japonica, Eclonia maxima, Lesonia negrescens, Sargassum sp.) [97]. Some bacteria, such as Azotobacter vinelandii or mucoid strains of Pseudomonas aeruginosa, also synthesize alginate-like polymers as exopolysaccharides [98,99]. Azotobacter vinelandii is a strict aerobe that can fix nitrogen and synthesize two polymers during vegetative growth: alginate and intracellular polyesters (polyhydroxybutyrate) [100]. A. vinelandii produce alginate during the encystment process as a mechanism for increasing resistance to drying in adverse environmental conditions to maintain cell hydration, being its structural element [101]. Moreover, it can act as a protective barrier against heavy metal toxicity, creating an ion exchange system with selectivity for Ca2+ [102]. P. aeruginosa (mucoid phenotype) produce alginate as the causative agent of cystic fibrosis, and it is associated with pathogenicity. Alginate biosynthesis in Pseudomonas spp. is induced under dehydration conditions and is probably a key component of stable biofilms in various media [103].
The molecular structure of alginates consists of unbranched linear binary copolymers of β-D-mannuronic acid (M) and α-L-guluronic acid (G) residues bound by 1–4 glycosidic bonds (Figure 6) [97]. The structure of alginate algae can be divided into three fractions (three blocks of uronic acid): these are the homopolymer regions of the M and G blocks and the alternating MG blocks containing both polyuronic acids [104]. Bacterial alginates have O-acetyl groups, while in the structure of alginate algae, they are absent [105]. In addition, bacterial alginates have a higher molecular weight compared to algae polymers [106]. The source of alginate affects the ratio of M and G residues, which has an impact on the physical and chemical properties of alginate, as well as on solution viscosity and thickness of the film [97].
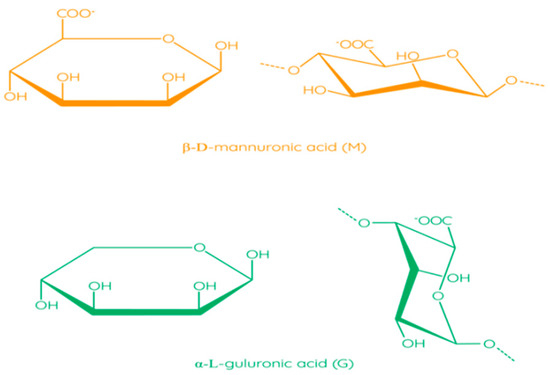
Figure 6.
Structural formulas of monomers in alginate [97]. Reproduced from Parreidt, T.S. et al., Foods; published by MDPI, Basel, Switzerland, 2018.
The solubility of various types of alginates in numerous solvents and solutions was indicated by Kimica Corporation [107]. The FDA classifies food grade sodium alginate as a GRAS (generally considered safe) in the Code of Federal Regulations (CFP) and list its use as an emulsifier, stabilizer, thickener, and gelling agent [108]. The European Commission (EC) has added alginic acid and its salts (E400–E404) to the list of permitted food additives [109].
Alginate is widely used in various industries, such as food, beverage, textile, printing, and pharmaceutical, as a thickener, stabilizer, emulsifier, chelating agent, encapsulator, suspending agent, or used for the formation of gels, films, and membranes [110]. About 30,000 metric tons of alginate derived from brown algae are produced annually [100]. Alginate is known for its biocompatible and biodegradable properties, as well as its low price. The ability of functional groups of G blocks to interact with polyvalent cations (for example, Ca2+, Al3+, and Fe3+) is an important characteristic of alginate [111]. Among divalent ions, calcium ions usually react with alginate to form a polymer with low solubility. In general, the length of the G-block characterizes the ability and selectivity of the alginate in the formation of these interactions, whereas the M and MG blocks have almost no selectivity. The M and G blocks bind via ions to form a three-dimensional structure («egg-box») [112]. This triggers the process of anionic exchange, in which the water-soluble alginate exchanges its counter ions for Ca2+. This ionic crosslinking leads to the formation of cold-setting and heat-resistant films. Alginate films are cross-linked to improve their water resistance, mechanical properties, and coherence [111].
2.6. Casein
Caseins are suitable hydrocolloids for the formation of edible films among proteins due to their high nutritional value, solubility in water, and ability to emulsify. Casein is the main protein extracted from milk (~80%) [113], consisting of four different protein fractions: αS1-, αS2-, β-, and κ-casein [114], which together form colloidal micelles in milk (Figure 7) stabilized by casein structures and calcium-phosphate bridges [115]. The unique properties of the four protein fractions affect the film-forming ability of casein [116].
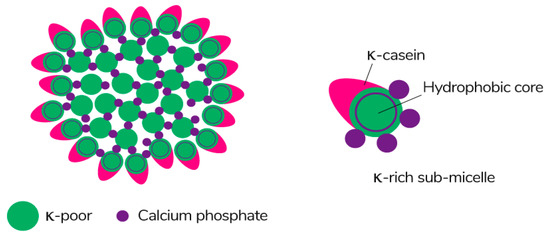
Figure 7.
The schematic of the submicelle model of the casein micelle [117]. Reproduced with permission from Horne, D.S., Current Opinion in Colloid & Interface Science; published by Elsevier Ltd., 2005.
The global production of caseins and caseinates is difficult to determine due to the lack of significant data. The largest casein producers are New Zealand (150,000 tons), Netherlands (85,000–10,000 tons) and Germany (25,000–40,000 tons). The global market for casein or caseinates used in the food industry ranges from 200,000 to 2,500,000 tons [118].
Casein is precipitated from skimmed milk by acidifying it to produce acidic casein, or the milk is treated with rennet to produce rennet casein. The precipitated casein clot is separated from the serum, washed, and dried [119]. Caseinates are water-soluble derivatives of acidic caseins formed by reaction with alkalis. Edible casein is a dairy byproduct that is used as an ingredient in many foods, including dairy products [120]. The development of food technologies and their applications has increased the production of casein and the demand for it. Its production differs from that of non-food casein (also called industrial casein) because food casein is produced under sanitary conditions [118]. In addition, food-grade chemicals are used for its production that undergoes sufficient heat treatment to ensure that casein is safe for human consumption [118]. Intensive research on production technologies over the years and their implementation into factories has significantly improved the approaches for food-grade casein production.
Depending on the coagulation method, different types of casein with certain characteristics are obtained; rennet and acid casein are the main available types [121]. Acidic casein is insoluble in water, has a pH of about 4.6, and is precipitated by acidifying milk with mineral acids or lactic acid [113]. Rennet casein is insoluble in water, has a pH of about 7.5, and is coagulated by the action of chymosin, which cleaves the k-casein tail and causes destabilization of the casein micelles [114]. Acidic casein can be solubilized by neutralization with alkali, such as sodium, potassium, or calcium hydroxide, to produce sodium, potassium, or calcium caseinates, respectively. This caseinate does not contain colloidal calcium phosphates, has a pH close to 7, and is highly soluble in water [122].
Casein films have good barrier properties for oxygen and other non-polar molecules due to the distribution of polar amino acids along the protein chain, which allows them to protect products that are prone to oxidation [122]. On the other hand, the interaction forces between the polar and non-polar amino acids in the casein structure form a cohesive film matrix that tends to shrink during drying and becomes brittle [123]. Food-grade plasticizers, such as glycerin or sorbitol, are added to the film-forming solution in order to solve this problem. Plasticizers increase the thermoplasticity of the protein film but reduce its strength [124].
Although casein films have potential for use as food packaging, some disadvantages need to be addressed before they can be widely used for commercial purposes. Casein-based films are very sensitive to moisture, they absorb and release water molecules, which greatly affect their mechanical and barrier properties, and moreover, they are mostly soluble in water, which limits their areas of application [125]. It is also worth noting that plasticized casein films cannot provide high mechanical strength or good elasticity compared with synthetic polymer materials. By crosslinking polymer chains, chemical and physical processing can be used for modification of the polymer matrix, which improves the functionality of the protein film [126]. Glutaraldehyde or the natural compound genipin, as well as transglutaminase, etc. are typical chemicals used as crosslinking agents [114,127,128]. In general, aldehydes are quite effective as binding agents of protein molecules, but their high toxicity is unacceptable for foods. Genipin and transglutaminase are known to be safe, but their high cost limits their further use in industry [114]. Thus, the search for new cheap crosslinking agents safe for use in the food industry is underway.
2.7. Collagen
Collagen is the most abundant protein in the extracellular matrix of vertebrates, accounting for approximately 30% of the total body protein mass. It is absent in plants and unicellular organism, where its role is played by polysaccharides and cellulose. Collagen is presented in the body walls and cuticle in invertebrates, while in mammals, it is found in the cornea, bones, blood vessels, cartilage, dentin of teeth, etc. [129].
Bovine hides are a by-product of meat production and mainly used for the production of leather, but the inner corium layer of the hide is rich in collagen [130]. This collagen has a higher denaturation temperature compared to collagen from marine sources [131]. Collagen can also be extracted from fish and pigs, but there are some limitations: the use of fish collagen is limited because its low hydroxyproline content [132] gives collagen a low denaturation temperature, while pork collagen is restricted due to religious considerations [133].
Collagen consists of three parallel chains of α-helices twisted in the form of a right triple helix [129] and constructed from frequently repeated fragments with a characteristic sequence -Gly-X-Y- (Figure 8). Glycine is every third amino acid residue, proline is often found in the X position, and the Y position can be occupied by both proline and 4-hydroxyproline. In addition, the collagen molecule contains residues of 3-hydroxyproline and 5-hydroxylysine. The right triple helix self-associates to form highly ordered cross-linked fibrils [129]. These fibrils form insoluble fibers that provide an extracellular matrix with high integrity and mechanical tensile strength due to its tightly wound triple helical structure. Collagen molecules are divided into 26 different types, which are grouped into 8 families, depending on its structure, chain bonding, and position in the organism. These families include fibril-forming, basement membrane, anchoring fibrils, microfibrillar, hexagonal network-forming, transmembrane, multiplexins and fibril-associated collagens with interrupted triple helix [134].
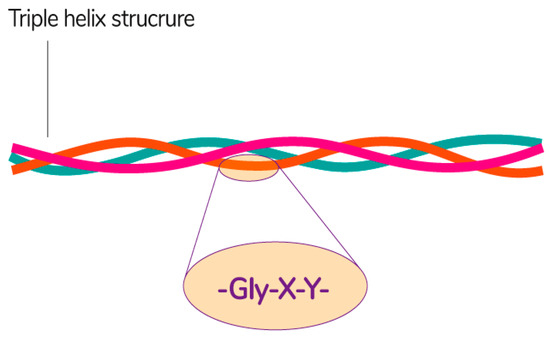
Figure 8.
The general structural features of collagen [135]. Reproduced from Sebald, A., proof-read/edited by Mitchell, D.A. Maxfacts; published by University of York, 2019.
Water-soluble collagen presents in a small percentage of total collagen. The solubility of collagen depends on the type of tissue and age. A neutral salt solution or dilute acetic acid are the most commonly used solvents for collagen extraction. Strong alkali or enzymes are used to extract the insoluble collagen to break down the additional cross-linked bonds [136]. Collagen can be used for the production of edible films in the meat industry, e.g., for sausages, salami, snacks [136], due to its good mechanical properties [137]. Fibrillar collagens can easily form stable films capable of contracting and stretching to adapt to the compression and expansion of meat raw materials during continuous processing [138,139]. Collagen films can become an embedded/edible part of meat products so they can provide safety benefits, control quality changes, and reduce the loss of shrinkage of meat and meat-based products during storage, thereby extending the shelf life and maintaining the visual appeal of products for a long time [140].
2.8. Gelatin
Gelatin is a naturally occurring water-soluble protein characterized by the absence of a noticeable odor and the random configuration of polypeptide chains in an aqueous solution. It is obtained by partial hydrolysis of collagen [141]; its structure is rigid rod-shaped molecules that are located in fibers connected by covalent bonds [142].
Pig skin was used as a raw material for the production of gelatin in the 1930s and continues to be the most important material for the large-scale industrial production of food gelatin [143]. For more expensive applications, such as pharmaceuticals, gelatin is usually obtained from cattle bones, which is considered a more complex and expensive extraction process [144]. However, in an effort to replace pork and bovine gelatin, fish gelatin production has increased over the past decade, accounting for more than 1.5% of total gelatin production. In recent years, by-products obtained in the fishing industry, such as heads, skin, bones, fins, muscle parts, scales, internal organs, and others, are considered as potential sources of gelatin and not recyclable waste [145]. The main disadvantages of fish gelatin are its rheological properties, since it is less stable than mammalian gelatin. Moreover, since the production of gelatin from fish and poultry is still limited, the resulting products are less competitive in price than products made from mammalian gelatin [146,147].
Gelatin is a heterogeneous polypeptide mixture of α-chains, β-chains, and γ-chains (Figure 9).
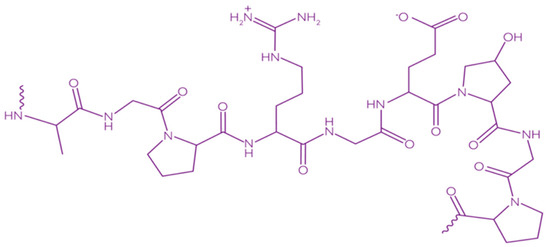
Figure 9.
Chemical structure of gelatin [148]. Reproduced with permission from Kariduraganavar, M.Y., et al., Natural and Synthetic Biomedical Polymers; published by Elsevier Inc., 2014.
Gelatin can be divided into two types depending on the processing method. Type A has an isoelectric point at pH ~ 8–9 and is obtained from acid-treated collagen. Type B has an isoelectric point at pH ~ 4–5 and is obtained from an alkali-treated collagen, during which asparagine and glutamine residues are converted to their respective acids, resulting in a higher viscosity [149]. Gelatin obtained from pig skin is commonly referred to as type A, and gelatin obtained from beef skin or cattle skins and bones is referred to as type B.
Gelatin has various functional properties that can be divided into two groups: properties related to the external surface, such as protective colloidal function, formation and stabilization of emulsion and foam, adhesion and cohesion, as well as the ability to form films and properties related to gelation, such as thickening, texturing, and water-binding ability [150,151,152]. Thus, a wide range of gelatin applications can be used in the food, packaging, pharmaceutical, cosmetic, and photographic industries, but the limited thermal stability and mechanical properties of gelatin, especially during processing, limit its implementation [139].
The film-forming properties of gelatin are widely used as an outer film to protect food products from drying out, exposure to light and/or oxygen during their shelf life [153]. Due to the high hygroscopicity of gelatin, it tends to swell or dissolve upon contact with the surface of foods with high moisture content [154].
Several scientific studies have been conducted to evaluate the overall effect of adding crosslinking and strengthening agents, plasticizers, or additives with antimicrobial or antioxidant properties to gelatin-based films to improve its functional properties and increase the shelf life of food products [155,156]. The improvement of these properties occurs when the intermolecular interactions of the protein chains decrease under the influence of molecular structures that modify their hydrophilic character or promote the formation of strong covalent bonds in the protein film [157].
Zhao et al. demonstrated the feasibility of using natural extracts as new natural crosslinking agents for the modification of gelatin (type B, from bovine bone) by forming hydrogen bonds between water and free hydroxyl groups of amino or polyphenolic groups, which significantly increased the strength of the gel compared to untreated gelatin [158]. Combining gelatin with other biopolymers, such as whey proteins, starch, chitosan, or pectin, can also be a good way to improve the mechanical properties and water resistance of gelatin-based films [159,160,161,162].
3. Approaches for the Production of Biopolymer-Based Films and Coatings
The film production is a physico-chemical process based on the intermolecular interaction of the biopolymer with the components of the solution in order to create a stable polymer matrix [163]. The process of film formation depends on the ability of the biopolymer to form a continuous crystalline or amorphous structure; therefore, at the initial stage, it is important to determine the type of polymer, taking into account its properties and the features of the mechanism of structure formation [164].
The film formation from polysaccharides is based on the breaking of polymer chains and the creation of new hydrophilic, hydrogen bonds and Van der Waals forces due to electrolytic and ion crosslinking [165]. Protein-based biopolymers, compared to other film-forming materials, have distinctive features: conformational denaturation of the protein (changes in secondary, tertiary, and quaternary structures), the presence of electrostatic charges, and amphiphilic nature (the presence of hydrophilic and hydrophobic molecules) [166]. The mechanism of formation of films from protein consists of its denaturation under the influence of acid/alkaline, mechanical, or enzymatic treatments, heat treatment, and various solvents, which leads to the formation of new molecular bonds between the peptide chains—ionic and hydrogen bonds, Van der Waals forces, hydrophobic interactions, covalent polar and nonpolar bonds-disulfide bridges, and cross-linking [116,167,168,169]. The molecular interaction of the protein-polysaccharide complex occurs on the basis of Van der Waals and electrostatic forces, hydrophobic and hydrogen bonds, the effects of excluded volume (the balance between steric repulsion of chain links and effective short-term attraction), or through chemical binding, for example, the Maillard reaction—the interaction of amino acids with sugars, including fructose, glucose, etc. [170].
The films and coatings are obtained by dissolving, dispersing, and emulsifying the biopolymer in a specific solvent. According to the safety and biodegradability requirements for food films and coatings, water, ethanol and their mixtures, and organic acids are used as solvents [166]. Magnetic or electric stirrers are used for acceleration of the dissolution of biopolymers [171,172], and a vacuum pump or ultrasound equipment are used to remove air bubbles from the solution [173].
3.1. Methods of Forming Edible Films
Edible food films are usually made in two ways: wet and dry processes, using the appropriate methods (Figure 10) [174].
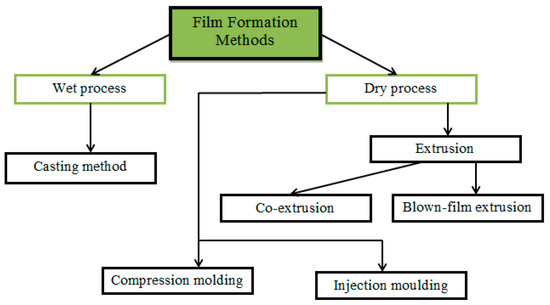
Figure 10.
Film formation methods of biopolymers.
In the wet process, the film-forming components are mixed with a solvent, followed by drying the solution to obtain a food film [175]. The dry process consists of the formation of a film by heat treatment of a film-forming composition with a low solvent content [176]. In both processes, the film composition may include a single biopolymer or a combination of different biopolymers [177]. The wet process is mainly represented by the casting method [178], while the dry process is more variable—extrusion, injection molding, compression molding, co-extrusion, blown-film extrusion, and others [179,180,181].
Also, the films can have a mono-, di-, or multi- layer structure [182]. The production of di- and multi- layer films is carried out using the “layer by layer” technology, alternating solutions of biopolymers [183]. This method of film production is less common in the food industry because it is more labor-intensive, despite the fact that the finished films have improved barrier and mechanical characteristics [184].
3.1.1. Casting Method
The most common method of forming edible films is the casting method (Figure 11) referring to the wet process [185]. This method is widely used in laboratory and pilot scales because it is quite simple and does not require special equipment. The film production process includes 3 main stages: (1) solubilization of the biopolymer in the solvent; (2) casting of the solution in the mold; (3) drying of the solution [186,187].
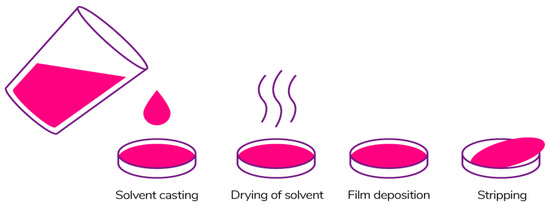
Figure 11.
Casting method [186]. Reproduced with permission from Suhag, R., et al., Food Research International; published by Elsevier Ltd., 2020.
Various materials with low adhesion are used as forms for drying film-forming solutions. Teflon [188], glass coating (can be coated with a polyester film) [189], Petri dishes [190], Plexiglass [191], and polycarbonate plates [192] are the most common. Films are dried in various ways: under ambient conditions, with hot air, infrared energy, or microwave energy, which influence the formation of physico-chemical and mechanical properties [193].
The continuous casting method allows the films to be formed through the continuous flow of the biopolymer solution to a conveyor belt and then dried. The advantage of this method is the ability to choose such modes as the speed of the conveyor and the casting cycles of the solution, thereby adjusting the film thickness, temperature, and duration of drying [194]. This method makes it possible to obtain multilayer films [182].
The casting method is one of the most common. Dai et al. [195] studied the properties of films based on native and modified starches from various plant sources. Bagheri et al. [196] examined the effect of drying temperature on films prepared from sodium alginate with glycerin. Films plasticized with glycerin were cast using chitosan of two different molecular weights [197] and cellulose sulfate [198]. Liu et al. [199] investigated casting parameters for a mixture of gelatin, glycerin, and transglutaminase.
3.1.2. Extrusion Method
Extrusion is a continuous technological process of obtaining a film material by pushing the molten film-forming composition through a forming hole of a certain shape (Figure 12) [200]. This process involves several operations simultaneously: melting, mixing, dosing, and forming [201].
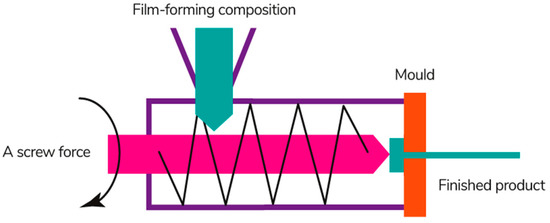
Figure 12.
Extrusion process [186]. Reproduced with permission from Suhag, R., et al., Food Research International; published by Elsevier Ltd., 2020.
The method is based on the thermoplastic properties of polymers during plasticization and heating above their glass transition temperature under conditions of low water content [202]. Therefore, the thermoplastic properties of film-forming materials must be well studied for the production of films in this way. It is necessary to take into account the influence of plasticizers that lower the glass transition temperature and any other additives on the thermoplasticity of film-forming materials [174].
The production of films by extrusion includes 3 main stages: (1) loading the film-forming composition in the form of a gel, powder, or concentrated solution into the feed hopper; (2) adding the composite components and mixing; (3) heating and applying to a conveyor belt or drum, followed by cooling on metal shafts [203]. The structure of biopolymer films becomes more homogeneous during the extrusion process, as well as their physicochemical, mechanical, and barrier properties are improved [204,205].
Processing parameters such as die temperature, die construction, speed, and number of screws directly affect the properties of the film [206]. The construction of the dies is chosen depending on the viscosity of the solution and the required film thickness: (1) sheet dies or slotted; (2) flat-film designed for blown-film dies most often annular, less viscous solutions are suitable for them; (3) pipe and tubing dies (annular); (4) profile extrusion dies (all possible geometric shapes—square, U and T-shaped); (5) dies for co-extrusion dies [207]. Single- or twin-screw extruders are used in the laboratory and industry to mix the ingredients evenly [208]. The optimal properties of the film also depend on the speed of the screw.
González-Seligra et al. [209] noted that, at a screw speed of 40 rpm, the starch/glycerol films were characterized by a large amount of non-gelated starch and an uneven distribution of glycerol. At a speed of 80 rpm, more homogeneous films were obtained, but with rapid retrogradation of starch (the transition of starch from a soluble state to an insoluble state, leading to an increase in the rigidity and brittleness of the film) [210]. At a speed of 120 rpm, thermoplastic films with good mechanical properties (tensile strength) and slower starch retrogradation were obtained. Calderón-Castro et al. [211] used pretreatment with a double-screw extruder with a speed of 120 rpm and a temperature of 100 °C with a cylindrical die and 3 heating zones to cast the extruded composition of physically modified starch. Compositions of pectin and starch with glycerol added in various combinations were extruded using a twin-screw extruder with a slit matrix to produce a thin film, which was dried in vacuum at room temperature before testing [212]. A twin-screw extruder with a cylindrical die was used to produce films from pectin and gelatin/sodium alginate [213]. A dry method was developed for the production of film sheets from rennet, acid casein, and sodium caseinate using a twin-screw extruder at a speed of 170 rpm and a temperature of 10–75 °C in various zones, and glycerin was added to the second zone; films from rennet casein, waxes, glycerin, and potassium sorbate were obtained by the same principle [122,214]. Collagen gels with plasma proteins, soy protein isolate, and gluten were processed using a single-screw extruder at 65 rpm, with saturated sodium chloride solutions at the exit of the annular die to remove the remaining moisture [215].
Blown-film extrusion is an alternative approach to achieve the industrial production of films, often using a single-screw extruder with an annular die and pressure shafts to pull the blown film [216]. This method is also used for the production of films from various biopolymers and their combinations: thermoplastic starch with plasticized chitosan processed in a twin-screw extruder to produce pellets with further blowing on a single-screw mechanism with a ring head [217]; film blown from granules of plasticized sodium caseinate [218]; granules of starch and gelatin reinforced with cellulose, pre-made by compression molding at 100 °C using a single screw for subsequent blow molding [219].
Co-extrusion is the process of simultaneous extrusion of two or more materials to form a multilayer film or coating [220]. This method is also used for applying an edible film/coating to food products, for example, a film of calcium alginate formed on the surface of minced meat when they simultaneously pass through a co-extrusion die [221]; alginate films with various polysaccharides (carrageenan, pectin, starches, cellulose, and gums) applied for sausage products packaging [222]; sodium alginate films with additives used for packaging sausages made by dry fermentation [223]; collagen films [224].
3.1.3. Compression Molding
Compression molding is one of the oldest and most convenient methods of material processing [225]. The main stage of this process consists of heating the material under high pressure in the mold until it solidifies as a result of the formation of new bonds of biopolymer chains [226].
The extrusion method is often used to prepare the material before the main thermoforming process. Krishna et al. [227] obtained films from fish gelatin with glycerin by double-screw extrusion with further compression molding on teflon plates. Ceballos et al. [228] treated manioc starch with glycerin obtained on a double-screw extruder with a temperature-controlled hydraulic press. The films could be produced in one stage. A mixture of fish gelatin solution with glycerin and citric acid was subjected to compression molding using the caver laboratory press [229], as well as the composition of corn starch with glycerin and citric acid, previously mixed in a two-roll mill, followed by pressing [230]. Between the press and the film, sheets of various materials are used, for example, polytetrafluoroethylene [228], steel [230], aluminum [225], etc.
3.1.4. Injection Molding
Injection molding is a widely used process for mass production of plastic products [231], but it can also be used for the production of edible films and consists of three main stages—filling, packing, and cooling [232]. Pre-injection pressure temperature, injection pressure, and molding temperature are the most important parameters [233].
Yu et al. [234] applied the method of twin-screw extrusion for the subsequent formation of granules from plasticized wheat starch with or without polyethylene oxide. At the second stage, injection molding was performed to produce films. Cho et al. [235] demonstrated the ability to process wheat gluten and glycerin pellets on a three-phase screw injection molding machine by the compression molding method. Pea isolate films with glycerol addition were made using the Injection Molding System [233].
3.2. Methods of Forming Coatings
Edible coatings of food products could be prepared in different approaches: dipping, spraying, brushing, fluidized bed, and panning methods (Figure 13) [236].
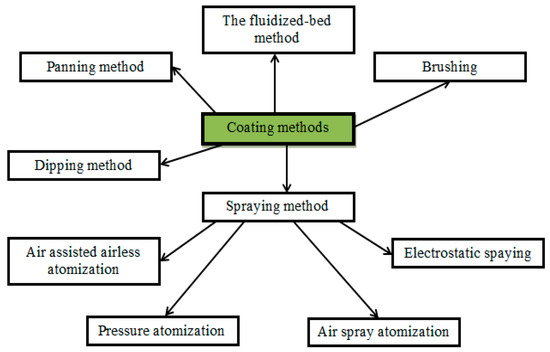
Figure 13.
Coating methods.
The use of a certain method depends on the type of coated product [237,238]. The feasibility of using coatings is explained by the following functional features: barrier properties to gases (O2, CO2, C2H4) and water, mechanical and optical properties, homogeneity, appearance, thickness, uniform distribution of the solution (wettability) on the surface, and adhesion (interaction between the surface of the product and the coating) [239].
The fluidized-bed and panning methods are difficult to perform and constitute in ways of spraying the film-forming solution: in the first case, it is produced on the surface of the fluidized bed and in the second case, it is sprayed on the processed product placed into a rotating pan [240,241]. Brushing is a method of applying the biopolymer solution with a brush [239]. Dipping and spraying are the most common methods due to simplicity and the low cost of the equipment [178]. The dipping method is most commonly used in the laboratory and is well suited for products with good surface adhesion [242], while spraying is convenient for implementation in industry [202].
3.2.1. Dipping Method
Dipping is a method of coating in which the product is immersed in a film-forming solution for a certain time, removed, and left to form a film on the surface of the product (Figure 14) [236]. If one dip is not enough, since part of the solution may not be completely distributed over the surface of the product, this procedure is repeated [243]. The properties of the coating depend on the density, viscosity, and surface tension of the solution [244].
Figure 14.
Dipping method [27]. Reproduced with permission from Mohamed, S.A.A., et al., Carbohydrate Polymer; published by Elsevier Ltd., 2020.
The disadvantage of the method is the possibility of forming an uneven and thick coating layer, which affects the appearance of the product, its shelf life, mechanical characteristics, and gas permeability [244]. Fruits are covered with a fairly thin layer, while vegetables and meat products have a thicker layer [245]. There are also a number of problems, such as possible dilution of the solution with pieces of product, resulting in the accumulation of product residues and the development of microbes in the immersion bath. This method is more suitable for shapeless products [246].
The dipping method is used for coating melon slices with alginate solution [247], sliced pears with chitosan and/or carboxymethylchitosan solutions mixed with sodium chloride [248], rainbow trout fillet with sodium alginate solution [249], fresh kiwifruit with sodium alginate solution mixed with calcium chloride as a cross-linking agent [250], and papaya with k-carrageenan solution plasticized with glycerin [251].
3.2.2. Spraying Method
The spraying method (Figure 15) is carried out using spray equipment, from which the solution is applied to the surface of the product under pressure using special nozzles. The quality of the coating depends on a set of these parameters [178]. This method is suitable for fluid solutions, since more viscous ones make it difficult to spray [97]. The spraying method allows the product to be evenly coated, the thickness of the coating to be controlled, the temperature of the solution to be controlled, and large surfaces of products to be covered, but can have large losses of the solution to the ambient surroundings [177].
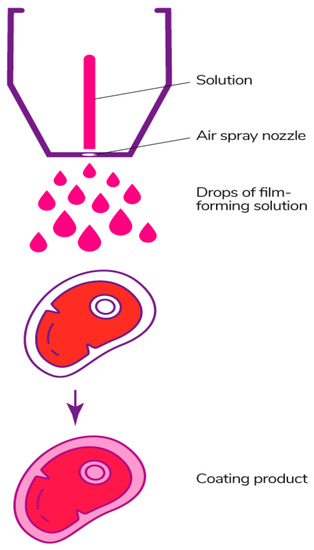
Figure 15.
Spraying method.
There are several main types of spraying. Air spray atomization is the method when the film-forming solution is sprayed under low pressure mixed with atmospheric air. Pressure atomization is carried out without air and under high pressure. Air assisted airless atomization is a combined type of atomization, which consists of feeding the solution under high pressure and flattening with air at the last stage of processing for a better distribution of the composition on the surface of the product [178,252]. The latter two methods can be used for more viscous solutions, but the equipment for them has a high cost [186].
Electrostatic spraying is a modern method, which is spread enough [253]. An electric charge is given to small particles of the film-forming solution, which is moved under the influence of an electric field [254]. The main difference from typical spraying methods is a uniform coating layer and an increase in the adhesion on the product surface, which reduces losses [253].
The spraying method is used for coating Mozzarella cheese by electrostatic spraying with alginate, chitosan, and soy protein isolate solutions [255], freshly cut lotus roots by pressure atomization with xanthan gum [256], strawberry by electrostatic and usual spraying with sodium alginate [257], and products by electrostatic spraying with chitosan solutions of different deacetylation degrees [258].
3.3. Edible Films Composition
The most important properties of edible films are density, thickness, transparency, degree of swelling, thermal stability, mechanical strength, barrier properties to oxygen, and water vapor and safety [259]. The possibility of adding ingredients to the composition of edible films for improving the physico-chemical, mechanical, optical, and microbiological parameters compared with their basic compositions is of great interest to researchers [72,245]. Auxiliary components include plasticizers, crosslinking agents, and other additives [260].
The addition of a plasticizer is an important step in the preparation of solutions from proteins and polysaccharides, which allow the formation of a brittle structure of edible films to be excluded [186]. Most plasticizers contain hydroxyl groups that form hydrogen bonds with biopolymers and increase the free space and flexibility of the polymer matrix [261]. Water, mono-, di-, and oligosaccharides (glucose, sucrose, and fructose) [262], polyatomic alcohols (glycerin and sorbitol) [263], and lipids and their derivatives (lecithin, oleic acid, and waxes) [264] are the main plasticizers.
Crosslinking agents are used to improve the hydration, mechanical, and barrier properties of films and coatings based on proteins and polysaccharides [265]. The process of crosslinking consists of the formation of a three-dimensional structure of the solution by binding the polymer chains with covalent or non-covalent bonds, which leads to an increase in the hydrophobicity of the biopolymer [4]. The choice of crosslinking agents depends on the chemical structure, the presence of active groups, and the molecular weight of the biopolymer, as well as its compatibility with the crosslinking agent [266]. Crosslinking agents and plasticizers must be safe and approved for use in the food industry [267].
3.3.1. Edible Starch Films
Starch is used to produce biodegradable films due to its ability to form a continuous matrix and low permeability to oxygen [268]. The production of edible films from starch is based on the process of gelatinization, i.e., the destruction of the native structure of the biopolymer. Gelatinization is accompanied by a retrogradation process, which includes a reduction of the amount of water-soluble substances in the dissolved starch [269]. The resulting mixture consists of solubilized amylose and partially residual amylopectin granules [270]. Amylose is more prone to retrogradation, the rate of which depends on the amount of starch in the film, the ratio of amylose:amylopectin, their structure, etc. [271]. It leads to undesirable changes in the mechanical and thermal characteristics of the film [272].
A sufficient amount of water (at least 65% of the biopolymer mass) and exposure to high temperatures ranging from 60 to 95 °C are important conditions for hydration of starch molecules for the film processing [273,274]. Water is a good plasticizer, but it is often not enough to obtain the necessary elasticity of the film [275]. The evaporation of water during the drying process can lead to increasing fragility of the starch films [276]. It is necessary to use a plasticizer, such as citric acid and glycerin [277,278], and/or modified starch to reduce its hydrophilic properties [175,279]. The characteristics of starch films depend on the source of the starch, the content and type of plasticizer, and the processing conditions [175]. The high content of amylose in starch results in an elevation of the strength properties of the obtained films since amylose can easily form crystallites and new bonds [269].
Nawab et al. [280] used starch plasticized with glycerin and/or sorbitol from mango seeds for coating of nuts. Mehyar and Han [281] studied mechanical and barrier properties of rice and pea starch films with a high content of amylose and plasticized with glycerin. The properties of cassava starch with glycerin, citric acid, and without it were investigated by Chiumarelli et al. [282] for preserving the quality of mango during the entire shelf life.
3.3.2. Edible Cellulose Films
Cellulose is insoluble in polar solvents, such as water, alcohols, etc. [283], due to its complex and dense linear structure, which includes a large number of intra- and intermolecular hydrogen bonds [284,285], as well as its ability to crystallize [286]. The solubility of cellulose is assumed to be related to its amphiphilic nature and hydrophobic interactions in the polymer structure [287,288].
A lot of studies has focused on finding new ways to dissolve cellulose using the following unusual solvents: N-methylmorpholine N-oxide (NMMO) [285], ionic liquids (a group of organic salts in the liquid state) [289], non-aqueous solvent LiCl/N,N-dimethylacetamide (LiCl/DMAc) [290], aqueous solution of NaOH [291], aqueous solution of alkalis/urea and aqueous solution of NaOH/thiourea [292], mixture of tetra-n-butylammonium fluoride trihydrate (TBAF×3H2O) and dimethyl sulfoxide (DMSO) [293], hydrates of inorganic molten salts, as an example ZnCl2×4H2O [294], and aqueous solutions of metal complexes consisting of transition metal ions and nitrogenous ligands, the most well-known of which are cuprammonium hydroxide (Cuam) and bis(ethylenediamine) copper (II) hydroxide (Cuen) [295].
The physical regeneration of a cellulose solution with coagulants (anti-solvents or non-solvents) is one of the methods of obtaining films and coatings [285]. Zhang et al. [296] used H2SO4, CH3COOH, H2SO4/Na2SO4, Na2SO4, etc., as coagulants for following cellulose regeneration in NaOH/urea. Geng et al. [297] studied a mixture of acetone with water for cellulose precipitation with following regeneration in NaOH/urea/water. The obtained films have good mechanical and barrier properties [298]. Cellophane and cuprofen are the main products prepared from regenerated cellulose and used in the food, medical, and other industries. However, they are losing popularity due to the high cost of production, as well as low barrier properties [299].
Films made of cellulose derivatives dissolve in polar solvents, including water, alcohols, etc., and, therefore, are of interest to researchers [252]. Cellulose derivatives have good mechanical and barrier properties to lipids and gases, including oxygen (O2) and carbon dioxide (CO2) [300]. The production of films from carboxymethylcellulose (CMC) and cellulose nanocrystals (CNC) is quite common. Cellulose nanocrystals or nanocellulose is a highly crystalline material with improved thermal, mechanical, and barrier properties, and is obtained by acid hydrolysis [301]. It is noted that the nanofiber of CMC has a negative charge and forms a film in the hydrogel state due to ion crosslinking [302]. Thus, Oun and Rhim [303] studied CMC films plasticized with glycerin with or without CNC from rice, barley, and wheat straw. Li et al. [304] used CMC with CNC from pea husks, and Singh et al. [305] used sodium CMC and hydroxyethylcellulose with citric acid as a crosslinking agent. A coating of blueberries from CMC were prepared with the addition of plasticizers—sorbitol and propylene glycol [306].
CNC in a matrix with another biopolymer form a percolated grid linked by hydrogen bonds [307,308]; therefore, cellulose is used as a reinforcing component [309], for example, in films of oxidized starch [310] or pea starch with CMC hydrogel [311,312], mango puree [313], and plasticized gelatin [314]. Moreover, CNC could be used for improving the barrier properties of chitosan films to water vapor [315,316].
3.3.3. Edible Pectin Films
Pectins are soluble in acid and water, most often used as a gelling agent [317]. Many studies consider homogalacturonan, rhamnogalacturonan I, and rhamnogalacturonan II the main monomers of pectin [318]. Pectins have a large number of negatively charged carboxyl groups in the chemical structure and exhibit the ability to interact with metal cations and can bind the active ingredients [319].
The molecular weight of the biopolymer, the degree of esterification, and acetyl esterification depend on the source and extraction conditions, and affect the gelation, texture, and stability of the pectin systems [320]. The degree of methoxylation of galacturonic acid residues is the main factor determining the functionality of pectin [321]. Gelation of low-methoxylated pectin (DM < 50) involves electrostatic interactions between cations and negatively charged regions of polymer chains [322]. This model of interaction is called “egg box”—ionic bonds through calcium bridges between carboxyl groups [323]. The stabilization of the system between two adjacent chains of “egg boxes” can occur by van der Waals forces, hydrogen bonds, and electrostatic interactions [321]. Amidated pectin is characterized by a low content of methoxyl groups. It requires less calcium ions to form a gel, is more stable to pH fluctuations compared to non-amidated pectin, and as a result, it is less sensitive to precipitation with a large amount of calcium, and its gels are thermally reversible [72]. Pectin with a high content of methoxyl groups (DM > 50) has a gel-forming ability in an acidic pH < 3.5 in the presence of cosolute with a high concentration, which promotes hydrophobic interactions between methoxyl groups by reducing the activity of water [321]. A low pH is necessary to reduce the dissociation of carboxyl groups, which can form hydrogen bonds with secondary alcohol groups [323].
It is known that pectin gels can be formed due to the formation of covalent cross-links [317], e.g., feruloylated pectins have ferulic acid in the side chains, which can be cross-linked using the oxidative enzymes laccase or peroxidase and form a covalently bound structure, since they have poor gelling properties [324]. The functional properties of such gels differ from those formed by other bonds: the rigidity of the gel increases and the elasticity decreases [321].
Pectin films have good mechanical and barrier properties to oxygen, high resistance to fats, but low stability to moisture [325]. Pectin films have a brittle structure, while the addition of a plasticizer does not always lead to high elasticity, so the use of crosslinking agents has a positive effect on the mechanical characteristics of such films [326,327]. Low pectin methoxyl from the cocoa peel was plasticized with glycerin/sorbitol [328], pectin film without additives was used for freshly cut pears [329], calcium lactate as a cross-linking agent was added to low pectin methoxyl for coating of melon [330], while high pectin methoxyl was plasticized with sorbitol for mango dipping [331].
3.3.4. Edible Chitosan Films
Chitosan has good film-forming properties and is characterized by an increased viscosity of the solution during hydration [332]. Antimicrobial activity is one of the features of chitosan due to the presence of active amino groups in the structure [333]. The biopolymer is insoluble in water and usual organic solvents [334]. Its solubility depends on the degree of deacetylation, the location of acetyl groups along the main chain, and the molecular weight. Chitosan is soluble in dilute acid solutions at a pH below 6.0–6.3 due to the presence of amino groups [335]. The mechanical characteristics of the film improve when increasing the molecular weight of the biopolymer [336]. The polycationic properties of chitosan allow the formation of films by breaking the fragments of the polymer chain and then converting them into a film matrix or gel, for example, by evaporation of the solvent, creating hydrophilic and hydrogen bonds and/or electrolytic and ionic crosslinking [337]. Chitosan can effectively improve the mechanical and barrier properties of the film due to the electrostatic attraction [338].
Chitosan-based films are transparent, flexible, and durable [339], have good resistance to fats, selective permeability to carbon dioxide (CO2) and oxygen (O2), but are very sensitive to moisture [340,341]. Chitosan is also used to enhance the emulsifying effect, reduce the acidity, and stabilize the color of the films [342]. Chitosan is not a thermoplastic material, as it decomposes to the melting point. Therefore, it cannot be extruded, and the films cannot be heat-sealed, but this problem is solved by using it in conjunction with thermoplastic polymers [343].
Chitosan was applied as the main biopolymer for creating edible films in many studies. Chitosan plasticized with glycerin was used for films production [335,344], a film-forming solution of chitosan in a 1% solution of acetic acid was used for dipping sardines [345] and its mixture with glycerin was used for packaging Mozzarella cheese [346] and rainbow trout together with high hydrostatic pressure technology [347], and a film based on chitosan dissolved in deionized water with the addition of glacial acetic acid was wrapped around the fillet of sea bass [348].
3.3.5. Edible Alginate Films
Alginate is a linear polysaccharide with moderate branching. Due to this, it is able to form films with high strength [349]. Sodium, potassium, and ammonium alginates are highly soluble in water, while alginic acid and calcium alginate are not [97]. This anionic polysaccharide with carboxyl groups in each monomer block can react with cations of polyvalent metals (calcium, magnesium, manganese, aluminum, iron, etc.), which makes it possible to obtain strong water-resistant films [350].
The composition, sequence, and ratio of alginate monomers M (β-d-mannuronic acid)/G (α-l-guluronic acid) directly affect the properties of alginate, alginate gels, and films [351]. A large number of G-monomers indicates the ability to form strong films, while the predominance of M-monomers often leads to more elastic films [352]. The unique property of alginates is based on the interaction with metal cations, especially with calcium ions [353]. The ions form bonds between the M and G blocks, resulting in a stable and ordered three-dimensional “egg box” model grid [354]. The formation of bonds occurs due to the length and selectivity of the G blocks, and the M and MG blocks are practically devoid of selectivity [355]. The crosslinking process is used to improve resistance to water, mechanical strength, cohesion and rigidity, and the ability to retain active components in a polymer matrix [244]. The process of interaction with ions is rapid; therefore, a fast-setting and thermally stable gel or film is formed, as a result of which the homogeneity of the resulting material can be disrupted, so it is important to control the rate of ion release [295].
Alginates form transparent, homogeneous, water-soluble films with high resistance to fats and low permeability to oxygen [353,356]. In a number of studies, alginates are used as the main component of biofilms; for example, the ratio of components was selected using the surface response methodology in films made of sodium alginate with glycerin crosslinked with calcium chloride and citric acid [357]; sodium alginate crosslinked with calcium chloride [354,358], and sodium alginate plasticized with glycerin [196]; alginate films plasticized with various types of compounds—PEG-8000, glycerin, fructose, or sorbitol [262].
3.3.6. Edible Casein Films
Caseins can bind ions and small molecules, have outstanding surface-active, stabilizing, and emulsifying properties, as well as good gel-forming and water-binding ability [359]. The preparation of edible films from aqueous solutions of caseinate occurs by its solubilization due to the disordered structure of the protein’s helix [360]. However, the effect of a buffer solution with pH 4.6 leads to the formation of water-insoluble substances, since the protein is in the isoelectric point [361,362].
Caseinates can form intermolecular hydrogen, hydrophobic and electrostatic bonds, which lead to increased interactions between the chains [363]. The structure of the biopolymer matrix is stabilized by hydrophobic, ionic, and hydrogen bonds [115], while binding with hydrophobic molecules occurs by hydrophobic interactions, Van der Waals forces, and hydrogen bonds [362].
Milk proteins form elastic transparent films without odor [113]. The casein films demonstrate resistance to denaturation and/or coagulation and remain stable in a wide range of temperatures, pH, and salt concentrations [364]. Casein-based films for food packaging have good mechanical properties due to the presence of calcium ions and salts of other metals, low oxygen permeability, poor elasticity, high sensitivity to moisture, as well as allergenicity [183,365]. Calcium caseinate films have better barrier properties but are more rigid, whereas sodium caseinate films have better optical properties and elongation under tension [363,366].
The application of caseins in the production of films is quite widespread, for example, films from casein cross-linked by transglutaminase [365] or tannic acid [114], calcium or sodium caseinates and β-casein isolate plasticized with glycerin [115,367,368], films based on casein treated with carbon dioxide [369], or cold plasma with dielectric barrier discharges for improving the functional properties of the films [370].
3.3.7. Edible Collagen Films
Type I collagen has a fibrous structure and is most often used for films processing [371]. The main amino acid composition of collagen includes glycine, proline, and hydroxyproline [372]. The amino acid composition and molecular weight affect the type and number of interactions involved in the stabilization of the protein matrix, which include disulfide covalent bonds, hydrogen bonds, electrostatic attraction forces, and hydrophobic interactions [373]. A hydrogen bond is formed between the glycine and the amide group in the adjacent chains, which is the main factor in the stabilization of the collagen triple helix [374]. Collagen is a hydrophilic protein, since it contains more acidic, basic, and hydroxylated amino acid residues than lipophilic ones [375].
Collagen has two main properties: gelling, which includes gelation, thickening, etc., and surface activity, including emulsification, adhesion and cohesion, film-forming ability, etc. [145]. The collagen film has good barrier properties to moisture, oxygen (O2), prevents the migration of dissolved substances, provides structural integrity and vapor permeability, but has disadvantages, including a rough surface and low thermal stability [376]. The low mechanical strength of the collagen films often leads to breaks during packing [375]. Crosslinking or aging treatments are two ways to solve these problems [377]. There are many additives that can improve the physical and chemical properties of films by chemical crosslinking—formaldehyde, carbodiimide, hexamethylene-1,6-diaminocarboxysulfonate, glutaraldehyde, heparin, transglutaminase, etc. [375], but most of them are cytotoxic [377]. Aging treatments is considered a method of physical crosslinking accompanied with changes in orientation of the collagen fibers, for its implementation, temperature, humidity, and time parameters can be used. The optimal conditions for improving the physical and chemical properties, such as mechanical parameters and reduced water absorption capacity, could be obtained by varying the aging parameters of the collagen films [375].
Collagen-based films have also been used in the food industry for a long time. For film processing, the extracted collagen is pre-treated with an acidic and/or alkaline solution [378], and the collagen fiber plasticized with glycerin [379] or lyophilized collagen are crosslinked with transglutaminase [380]. Many meat products and feed are packed in a collagen film [381].
3.3.8. Edible Gelatin Films
Gelatin is a hydrolyzed form of collagen [176]. The hydrolysis conditions (catalyst and its concentration, the process temperature and duration, etc.) determine the functional characteristics of the hydrolysate [153]. Collagen consisting of f-chains with a molecular weight of about 100 kDa can be dissolved in acid during hydrolysis without changing its original triple helix configuration. Non-covalent bonds are broken during this process [382]. The breaking of both hydrogen and covalent bonds occur during subsequent heat treatment; this leads to the destabilization of the triple helix as a result of the helix-helix transition [383] and the transformation into soluble gelatin [384]. The amino acid composition and molecular weight are the main factors affecting the physical and structural properties of gelatin, which form the mechanical and barrier properties, and the thermal stability of the obtained films and gels [384,385].
Gelatin demonstrates a film-forming ability and forms transparent, slightly colored films with high elongation [386], can form large grids due to its large molecular weight, and incorporate various components [314]. Gelatin-based materials have some serious application limitations due to their high sensitivity to moisture, solubility in water, poor mechanical properties (brittleness), and low thermal stability [387,388]. Hydrophobic plasticizers, such as citric acid esters, can be used for improving the barrier properties to water vapor [389]. Gelatin is dissolved in hot water and the solution is poured into a container and dried to obtain an edible film [390]. The edible films become denser, and the mechanical properties improve with an increase in the protein content, but the permeability to water vapor decreases [27].
Plasticized gelatin from farm animal and fish skin [391,392] or gelatin cross-linked with alginate dialdehyde are used for edible film processing [393]. Sorbitol [394] and triacetin [395] also could be used as plasticizers for films production from cattle and pork gelatin.
3.4. Ways of Edible Films Improving, Production and Application in Food Pachaging Based on Biopolymer Properties
Animal proteins and natural polysaccharides have both advantages and disadvantages caused by its chemical nature and structure. Taking into account these characteristic, undesirable properties could be improved, while positive ones could be considered for films and coatings production for each biopolymer and type of food product. The summarized information is presented in Table 1.

Table 1.
The main characteristics of packaging materials based on animal proteins and natural polysaccharides and ways of its improving, production and application.
Edible films based on one biopolymer are often characterized high sensitivity to moisture and and poor barrier to water wapor due to its hydrophilicity nature, as well as brittleness and insufficient mechanical properties. These disadvantages could be solved by different modifications, cross-linking agents and plasticizers addition, and especially by creating composite films and blending.
3.5. Edible Composite Films
The application of various combinations of biopolymers is of interest to researchers because it can eliminate the disadvantages of single-component films and achieve the desired barrier and physical-mechanical characteristics of edible films [163]. The properties of edible films and coatings can be changed based on the hydrophobic-hydrophilic characteristics of certain biopolymers [244]. Hydrophobic molecules have a positive effect on the barrier properties to moisture [456]. Oppositely, hydrophilic molecules contribute to the production of materials with higher strength characteristics and low gas permeability [457]. Table 2 shows some variations of edible films obtained by mixing different types of biopolymers and auxiliary ingredients to improve the properties of composite films.

Table 2.
Examples of composites edible films.
The knowledge of the nature, structure, and properties of biopolymers allows compositions to be combined for producing films and coatings with improved properties [23]. Composite materials are obtained in the form of a multilayer structure by alternating hydrophilic and hydrophobic layers, or in the form of single-layer films with a multicomponent composition [486].
3.6. Safety Requirements for Components of Edible Films
Materials in contact with food containing food additives, ingredients, and agents should not have a negative impact on the properties of the product and must meet current hygienic requirements [487]. Nowadays, there are no relevant legislative documents for biopolymer films and coatings, so manufacturers are guided by regulatory documents related to polymer materials in contact with food products [441].
In accordance with current manufacturing practices, edible packaging materials must have a permit from the Food and Drug Administration (FDA), after which they are usually qualified as Generally Recognized as Safe (GRAS) products [488]. GRAS materials can be used in edible films and coatings within the limits set by the FDA [489]. If the material does not qualify for GRAS, the manufacturer may apply for a safety confirmation of the component used or use it without approval, which applies to materials that received "prior approval" or were entered before 1958 in the FDA register [490]. The main risk for the use of components is the possible migration of substances from the packaging to the food [441].
Except for chitosan, polysaccharides, including cellulose and its derivatives (CMC, MC, HPMC), starches and its derivatives, pectins, and seaweed extracts (agar, alginates, carrageenan), are either approved food additives or GRAS substances [388]. The most commonly used proteins, such as corn zein, wheat gluten, soy protein and milk proteins, some types of collagen, and gelatin have GRAS status [491]. However, there are some concerns about possible allergenicity or intolerance of wheat or milk proteins for some consumers [492].
Edible food films and coatings are also biodegradable, which implies their complete decomposition by microorganisms during the composting process to products that do not harm the environment [492]. The problem with biodegradable films and coatings is that they must perform their functions safely and effectively for a certain period of time, and only then must they undergo a decomposition stage [490].
4. Methods for Estimating Edible Films Quality
Edible films are considered a special type of packaging material that can be eaten together with the product [237]. Such types of biopolymers as proteins, polysaccharides and lipids, as well as their various combinations, are used for edible films production [493]. The composition and structure of biopolymer films, as well as the introduction of various functional components, have a significant impact on the properties of the obtained material. In this regard, there is an obvious need for a comprehensive assessment of the properties of the developed film materials for their further targeted application. The main parameters include mechanical, barrier, optical, hydratative, and microstructural parameters [494]. A comprehensive study of the listed characteristics of the films is possible due to the use of modern research methods discussed in this section.
4.1. Basic Requirements for Testing the Condition of Films
Most researchers rely on the requirements of existing regulatory documents developed for synthetic polymer materials when studying the properties of biopolymer films. In this case, it is possible to adapt standardized methods depending on the equipment and the specific test conditions in a certain laboratory.
Special attention should be paid to maintaining the same conditions before and during the testing of films in order to achieve comparability and reliability of the obtained results. The ASTM D882–10 standard establishes the recommended sample preparation requirements for the following parameters: temperature 23 ± 2 °C and a relative humidity of 50 ± 10% for 40 h [495]. Previously, the films are placed in a desiccator containing a saturated solution of magnesium chloride MgCl2 [29], magnesium nitrate (Mg (NO3)2) [496], or sodium bromide (NaBr) [452], etc. Conditioning is necessary, since most edible materials tend to absorb excess moisture, which can lead to changes in the mechanical, physico-chemical, and hydration properties of the films [497].
An important parameter for testing is the precise determination of the thickness of the material, since some properties, such as mechanical or physico-chemical, depend significantly on this indicator. The film thickness is measured with a manual digital or mechanical micrometer with an accuracy of 0.001 mm in several repetitions, choosing arbitrary points in the center and around the perimeter [29]. The average value of the obtained results is taken as the film thickness.
4.2. Mechanical Properties
The mechanical strength of the edible film is one of the most important characteristics that allow the limit of the material’s resistance to physical destruction to be determined. The value of the mechanical strength indirectly indicates the degree of food product protection from physical and chemical impacts [498].
The determination of mechanical properties includes investigation of such indicators as tensile strength (TS), elongation at break (EB), elastic modulus (EM), and puncture force (PF). The tensile strength, elongation to break, and elastic modulus are the most common parameters used to evaluate mechanical properties [499].
The determination of TS and EB is carried out according to standards ASTM D882–10 and ISO 527–1:2019 [495,500]. Universal breaking machines or texture analyzers are usually used for testing edible films and providing a constant rate of force increase with a relative error of the breaking force of ±1.0 % and an absolute error of the elongation of ±1.0 mm [495].
The equipment must be fitted with tension clamps, which may be metal or in the form of sponges [401]. The use of cardboard gaskets to avoid slipping and cracking of the film in the clamps is allowed [501]. The ends of the gaskets must be at the level of the clamping planes, which limit the clamping length of the sample.
After conditioning, rectangular strips of a certain standard length and width are cut out from each film sample using a scissor [29], scalpel [502], blade [501], or guillotine [503]. According to the standard, the nominal width of the test samples is not less than 5.0 mm and not more than 25.4 mm [495]. The prepared samples must be free of visible damage, tears, and notches at the edges. The film is fixed in a strictly vertical position in the clamps with a set distance. The pre-force value is selected depending on the thickness (structure) of the films. The initial distance between the clamps and the required speed of the crosshead are set before testing. A breaking machine or texture analyzer records values using an appropriate software [504].
Tensile strength (TS, MPa) is calculated by dividing the maximum force on the initial cross section of the sample using the equation [505]:
where Fmax is the maximum force (H) and A is the sample cross-sectional square (m2).
Elongation at break (E, %) is calculated using the equation [506]:
where b is the sample length during deformation (mm) and a is the initial sample length (mm).
The calculation of the obtained data is carried out to the first decimal place, followed by rounding to an integer. The measurements are repeated at least 5 times, and the arithmetic mean value is calculated.
Elastic modulus (EM) is the characteristic of film rigidity. The equipment and the method of preparing films for testing are similar to the determination of tensile strength and elongation at break [507]. The elastic modulus is determined by the ratio of the normal force to the corresponding deformation within the limits of proportionality [508].
The EM (MPa) could be determined according to the angle of the curve tension-deformation using the equation [509]:
where εΤ and σΤ are the deformation and tension, respectively, and k is a constant coefficient.
Puncture force determines the resistance of the material to dynamic puncture and the spread of tear when making a puncture. The measurement of this indicator is carried out according to ASTM D6241–14 standard [510].
Studies of films are carried out on testing machines, for example, texture analyzers, equipped with special nozzles designed to measure the resistance to puncture. The film samples are pre-condensed before testing. Films of a certain shape and size, for example, 30 mm × 30 mm, are placed on the support ring of the texture analyzer. The round aluminum plate is fixed with screws to prevent the films from slipping off. Then, a spherical stainless-steel probe of a certain diameter is brought perpendicular to the surface of the film at a constant speed of 1 mm/s until it punctures the sample. The puncture force coefficient is obtained from the “force-deformation” curves recorded using the software [496]. Puncture deformation (PD, %) is calculated using the equation [511]:
where lo is the initial length of the film, equal to the radius of the annular space (mm) and D is the displacement of the probe at the rupture point (mm).
4.3. Physico-Chemical Properties
The study of physio-chemical characteristics is carried out in order to determine the barrier properties of edible films, which allow the level of permeability of biopolymer films in relation to water vapor and various gases, such as O2 and CO2, to be assessed. Permeability is one of the most important transport properties of films and coatings, and depends on both biopolymer nature and such properties, as roughness, porosity, arrangements of unidirectionally aligned fibers, area fractal dimension of pore and tortuosity fractal dimension. Factors such as the temperature or presence of electrolytes could also influence on permeability. Some theoretical models were developed to predict the transverse permeability depending on square, staggered, and hexagonal arrangements of fibers. Moreover, permeability can be expressed as a function of the unit cell aspect ratio, porosity, fiber size, net charge density, and viscosity and electrical conductivity of the electrolyte solution [512,513]. Nevertheless, permeability of films is usually evaluated by standard methods, described in ASTM E96 and ASTM D3985 [514,515].
4.3.1. Water Vapor Permeability
Water vapor permeability (WVP) is determined by the gravimetric method according to the ASTM E96 [514]. The method is based on the dependence of the change in the mass of the test sample on time. A weighing bottle with a certain depth and diameter with a screw or conventional lid is used for measuring WVP. The film sample is placed on top of the weighing bottle and fixed with a lid with a rubber O-ring [496].
There are two main ways of determining WVP. The most common method is the dry method, which is based on the transition of water vapor through the sample from moist air into a space with a desiccant (silica gel) where the humidity is close to zero [29].
According to the second way (wet method), water vapor from a cup of water, where the air humidity is close to 100%, passes through the sample into air having a lower humidity (about 30%). In this case, the samples are placed in ventilated chambers, providing a gradient of relative humidity between the two sides of the film at a level of 30–100% [471].
Water vapor permeability (WVP, g·m/m2·s·Pa) is calculated using the equation [29]:
where Δm/Δt is the mass of lost moisture per unit of time (g·s−1), A is the square of the film subject to moisture transfer (m2), e is the film thickness (m), and Δp is the pressure change of water vapor between the two sides of the film (Pa).
4.3.2. Gaseous Permeability
Gas permeability is an important property of biopolymer materials since each type of biopolymer has a different degree of gas permeability [516]. Oxygen and carbon dioxide have a great influence on the quality and safety of products during storage; therefore, the determination of gas permeability of films requires special attention [517].
The measurement of the gas permeability of the films is carried out according to the ASTM D3985 standard [515]. The test is performed on an appropriate equipment: a tester [518] or an oxygen permeability analyzer [519]. Films were cut into 4 cm diameter and covered with aluminum foil on both sides in order to avoid contact of the test gas with other areas of the film. An uncovered space in the form of a circle was left for the equipment cell [520]. During the test, the prepared film sample is placed in the cell of the analyzer or tester, where the film is isolated on both sides. Oxygen or carbon dioxide is supplied on one side, and a neutral gas consisting of 98% nitrogen and 2% hydrogen on the other [519].
Gas permeability against oxygen or carbon dioxide of films (OP or CP, cm3/m·Pa) is calculated using the equation [519]:
where OTR is the gas transmission rate (cm3/m2), A is the film thickness (m), and ∆p is the partial pressure difference between the film sides (Pa).
4.4. Hydration Properties
Different hydrocolloids are obtained from different natural sources of animal and plant origin, so the films based on them have different levels of solubility. Obviously, films prepared from proteins biopolymers (sodium caseinate, whey protein isolate, gelatin) and plant polysaccharides (CMC, sodium alginate, and potato starch) demonstrated different hydration properties [521,522]. The solubility of edible films is a key parameter for assessing their resistance to solvents, such as water, acid, and alkali [523].
4.4.1. Films Solubility
Most biopolymer films based on protein and polysaccharides without the introduction of crosslinking agents and/or tanning agents have a high solubility in water [523]. In many studies, the solubility of films is determined according to the method proposed by Gontard and Guilbert [523]. The film samples are ground into small rectangular pieces and dried at a temperature of 100 °C for 24 h to a constant mass. The samples are then placed in 100 mL of distilled water, and either hydrochloric acid (pH 4.0) or sodium hydroxide (pH 10.0), for 24 h. Next, the films are removed from the solutions and re-dried at 100 °C for 24 h, and the final mass is fixed.
The approach described by Gontard et al. [524] is used as an alternative method. The film samples are cut into square pieces (2 cm × 2 cm), and weighed and dried in a drying cabinet at a temperature of 105 °C for 12 h. Then, the dry films are re-weighed to determine the initial mass. The film samples are placed in 30 mL of solvent at a temperature of 25 °C and kept at room temperature for 24 h. The remaining parts of the undissolved film are removed and dried at 105 °C for 24 h and weighed to determine the final mass.
Films solubility (FS, %) is calculated using the equation [520]:
where Wᵢ is the initial dry film weight (g) and Wᵧ is the final dry film weight (g).
4.4.2. Swelling Index
The swelling index allows the level of moisture absorption (absorption capacity) of the edible film to be evaluated. This parameter has a high correlation with solubility [525] and the structure density and thickness of edible films [29], as well as with hydrophilicity of biopolymers used for films processing.
According to the method by Basiak et al. (2017), the film samples are cut into 2 cm × 2 cm pieces and weighed and immersed in distilled water (25 °C) for 2 min. Wet samples are blotted with filter paper to remove excess liquid and weighed. A similar Equation (7) is used to determine the swelling index [29].
4.4.3. Contact Angle Measurements
A contact angle measurement allows the level of film hydrophobicity to be determined and is based on the sessile-drop technique. The sessile-drop contact angle (θ) is formed due to the interfacial tension, and the vertex of the angle lies at the interface of three phases: γsv (solid-vapor), γlv (liquid-vapor), and γsl (solid-liquid) [499]. The schematic of a sessile-drop contact angle system is presented in Figure 16. The measurement of the sessile-drop contact angle is carried out by determining the tangent (angle) of the liquid drop to the solid surface at the base [526].
Figure 16.
Schematic of a sessile-drop contact angle system [526]. Reproduced with permission from Kwok, D.Y. and Neumann, A.W., Advances in Colloid and Interface Science; Elsevier Science B.V., 1999.
A standard liquid is used for the test, for example, glycerin. There are restrictions on the use of water as a standard liquid since food films have high hydrophilic properties, which lead to their rapid swelling [527]. A drop of 1.5–2.0 µL of the test liquid is applied to the horizontal surface of the film, which was in contact with the air at the time of drying [528]. The sessile-drop contact angle is measured on one or both sides of the drop using a digital microscope equipped with image analysis software [499]. The image of the edge angle is taken immediately, within 60 s after touching the liquid on the surface of the film, in order to avoid evaporation, dissolution, and swelling processes [528].
The obtained results allow the relationship between the surface tension energies of the three phases to be described, according to the Young equation [529]:
where γlv is the interfacial boundary tension: liquid-gas, γsv is the interfacial boundary tension: solid-gas, γsl is the interfacial boundary tension: solid-liquid, and Y is the Young’s edge angle.
4.5. Scanning Electron Microscopy
Scanning electron microscopy (SEM) is performed to determine the morphological features of the surface and cross-section of edible films. The study of the microstructure of films allows an expanded understanding of the influence of modifiers on the processes of structure formation to be obtained, with the identification of cracks, porosity, roughness, uniformity, and density of the material structure (Figure 17).
Figure 17.
Characteristics of the film structure determined by SEM.
There is a direct relationship between the structure and the mechanical, physico-chemical, and hydration properties of the films. SEM is recommended for composite films, for example, alginate/pectin [467], nanoemulsions [496], or when introducing functional additives, such as essential oils [530] or nanoparticles [531].
The microstructure of the films is studied using a scanning electron microscope. The films are pre-frozen in liquid nitrogen and randomly destroyed for the assessment of cross-section morphology. The films are treated with carbon, placed on an aluminum holder, and fixed to the support with double-sided tape. Gold or gold-palladium is applied to the surface of the samples for making electroconductive properties (visualization), and the samples are observed at an accelerating voltage of 5 to 15 kV and an operating distance of 10 mm [29].
5. Conclusions and Future Perspectives
Traditional plastics do not degrade and accumulate in large quantities, which cause significant harm to the environment. The development and application of bioactive packaging systems from environmentally friendly biopolymers is a relevant field of research.
Animal proteins and natural polysaccharides are the most common biopolymers for the production of edible films and coatings. Nevertheless, such biopolymers have a list of limitations for this application mainly due to its structure and properties, such us brittleness and unsufficient mechanical properties, as well as high hidrophilicity cased low water stability, high moisture sensitivity and water vapor permeability. These disadvantages could be overcome by blending and composite films production, implementation of crosslinking agents and plasticizers, as well as the application of appropriate ways of film production based on the biopolymer structure and type of food product. Novel edible films must meet the requirements of quality and safety.
We made an effort to summarize information about such popular biopolymers from renewable resources as starch, cellulose, pectin, chitosan, alginate, casein, collagen and gelatin, discuss the main advantages and disadvantages, approaches for properties improving, recommended film-forming solution, appropriate form and ways of production of films and coatings, as well as type of packaged food based on the compatibility and nature of a certain biopolymer. Moreover, approaches for films and coating production were also reviewed as in general so and for certain type of biopolymers. We also collected usual methods for assessment the properties of designed films. This information allows the reader to make the first insight into environmentally friendly packaging from nature of polimer till properties of packaging material.
Nevertheless, films based on one biopolymer could be not so competitive to traditional plastics. In this regard, blending and composite materials are particularly relevant, because combination of biopolymers and its modificated derivates could allow create packaging material with properties of plastics without microplastic addition. On the other hand, created new composites should be more deeply studied, because of containing two or more biopolymer chaines. In this regard, additional investigation approaches, such as scanning electron microscopy, could clear the interoperability of biopolymers. Scanning electron microscopy is also essential for investigation of films with introduced functional additives, which are widely used for improving the biological properties of packaging matherials.
Global ecologization dictates its own conditions to the manufacturer; consumer acceptance is also driven in biodegradability and eco-friendliness. Nowadays, due to the efforts of scientists worldwide, the creation of competitively packaging material based on biopolymers is a reality, but not a myth.
Author Contributions
Conceptualization, V.N. and E.K.; data curation, V.N. and E.K.; writing—original draft preparation, E.P. (Section 2), I.K. (Section 3) and N.R. (Section 4); writing—review and editing, E.K.; visualization, E.P., I.K., E.K. and N.R.; supervision, E.K. and A.S.; project administration, A.L. and A.S.; funding acquisition, A.L. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Ministry of Science and Higher Education of the Russian Federation for large scientific projects in priority areas of scientific and technological development (Grant No.075-15-2020-775).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Authors would like to thank Yana Uglitskikh for graphical and artwork https://www.behance.net/yanzaugb68e (accessed on 13 May 2021).
Conflicts of Interest
Authors declare no conflict of interest.
References
- Wohner, B.; Pauer, E.; Heinrich, V.; Tacker, M. Packaging-Related Food Losses and Waste: An Overview of Drivers and Issues. Sustainability 2019, 11, 264. [Google Scholar] [CrossRef]
- Arkoun, M.; Daigle, F.; Holley, R.A.; Heuzey, M.C.; Ajji, A. Chitosan-based nanofibers as bioactive meat packaging materials. Packag. Technol. Sci. 2018, 31, 185–195. [Google Scholar] [CrossRef]
- Moreira, D.; Gullón, B.; Gullón, P.; Gomes, A.; Tavaria, F. Bioactive packaging using antioxidant extracts for the prevention of microbial food-spoilage. Food Funct. 2016, 7, 3273–3282. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.-H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Datta, J.; Kopczyńska, P. From polymer waste to potential main industrial products: Actual state of recycling and recovering. Crit. Rev. Environ. Sci. Technol. 2016, 46, 905–946. [Google Scholar] [CrossRef]
- Faraca, G.; Astrup, T. Plastic waste from recycling centres: Characterisation and evaluation of plastic recyclability. Waste Manag. 2019, 95, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Mwanza, B.G.; Mbohwa, C. Drivers to Sustainable Plastic Solid Waste Recycling: A Review. Procedia Manuf. 2017, 8, 649–656. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Krepsztul, J.; Rydzkowski, T. Pyrolysis and incineration in polymer waste management system. J. Mech. Energy Eng. 2020, 3, 337–342. [Google Scholar] [CrossRef]
- Jakobek, L. Food packaging materials with polyphenols as active compounds. Meso 2019, 21, 469–474. [Google Scholar] [CrossRef]
- Stoica, M.; Marian Antohi, V.; Laura Zlati, M.; Stoica, D. The financial impact of replacing plastic packaging by biodegradable biopolymers—A smart solution for the food industry. J. Clean. Prod. 2020, 277, 124013. [Google Scholar] [CrossRef]
- Popović, S.Z.; Lazić, V.L.; Hromiš, N.M.; Šuput, D.Z.; Bulut, S.N. Chapter 8—Biopolymer Packaging Materials for Food Shelf-Life Prolongation. In Biopolymers for Food Design, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 10, pp. 223–277. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gavara, R.; Catalá, R.; Hernández-Muñoz, P. The Potential of Proteins for Producing Food Packaging Materials: A Review. Packag. Technol. Sci. 2016, 29, 203–224. [Google Scholar] [CrossRef]
- Nechita, P.; Roman (Iana-Roman), M. Review on Polysaccharides Used in Coatings for Food Packaging Papers. Coatings 2020, 10, 566. [Google Scholar] [CrossRef]
- Weber, C.J.; Haugaard, V.; Festersen, R.; Bertelsen, G. Production and applications of biobased packaging materials for the food industry. Food Addit. Contam. 2002, 19, 172–177. [Google Scholar] [CrossRef]
- Yadav, A.; Mangaraj, S.; Singh, R.; Das, K.; Kumar, N.; Arora, S. Biopolymers as packaging material in food and allied industry. Int. J. Chem. Stud. 2018, 6, 2411–2418. [Google Scholar]
- Adeyeye, O.A.; Sadiku, E.R.; Babu Reddy, A.; Ndamase, A.S.; Makgatho, G.; Sellamuthu, P.S.; Perumal, A.B.; Nambiar, R.B.; Fasiku, V.O.; Ibrahim, I.D.; et al. The Use of Biopolymers in Food Packaging. In Green Biopolymers and their Nanocomposites. Materials Horizons: From Nature to Nanomaterials, 1st ed.; Gnanasekaran, D., Ed.; Springer: Singapore, 2019; pp. 137–158. [Google Scholar] [CrossRef]
- Srivastava, P.; Bano, K.; Zaheer, M.R.; Kuddus, M. Biodegradable Smart Biopolymers for Food Packaging: Sustainable Approach Toward Green Environment. In Bio-Based Materials for Food Packaging, 1st ed.; Ahmed, S., Ed.; Springer: Singapore, 2018; pp. 197–216. [Google Scholar] [CrossRef]
- Xu, T.; Ma, C.; Aytac, Z.; Hu, X.; Ng, K.W.; White, J.C.; Demokritou, P. Enhancing Agrichemical Delivery and Seedling Development with Biodegradable, Tunable, Biopolymer-Based Nanofiber Seed Coatings. ACS Sustain. Chem. Eng. 2020, 8, 9537–9548. [Google Scholar] [CrossRef]
- Dhumal, C.V.; Sarkar, P. Composite edible films and coatings from food-grade biopolymers. J. Food Sci. Technol. 2018, 55, 4369–4383. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, B.; Oleyaei, S.A.; Almasi, H. Nanostructured Materials Utilized in Biopolymer-based Plastics for Food Packaging Applications. Crit. Rev. Food Sci. Nutr. 2015, 55, 1699–1723. [Google Scholar] [CrossRef] [PubMed]
- Wicochea-Rodríguez, J.D.; Chalier, P.; Ruiz, T.; Gastaldi, E. Active Food Packaging Based on Biopolymers and Aroma Compounds: How to Design and Control the Release. Front. Chem. 2019, 7, 398. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.-i. Preparation and Applications of Amylose Supramolecules by Means of Phosphorylase-Catalyzed Enzymatic Polymerization. Polymers 2012, 4, 116–133. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Barbosa, L.S.; Ramos, I.G.; Coelho, R.E.; Druzian, J.I. Characterization of amylose and amylopectin fractions separated from potato, banana, corn, and cassava starches. Int. J. Biol. Macromol. 2019, 132, 32–42. [Google Scholar] [CrossRef]
- Bertoft, E.; Blennow, A. Chapter 3—Structure of Potato Starch. In Advances in Potato Chemistry and Technology, 2nd ed.; Singh, J., Kaur, L., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 57–73. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, S.; Zhang, B.; Qiao, D.; Pu, H.; Liu, S.; Li, L. Structural features and thermal property of propionylated starches with different amylose/amylopectin ratio. Int. J. Biol. Macromol. 2017, 97, 123–130. [Google Scholar] [CrossRef]
- Keeratiburana, T.; Hansen, A.R.; Soontaranon, S.; Blennow, A.; Tongta, S. Porous high amylose rice starch modified by amyloglucosidase and maltogenic α-amylase. Carbohydr. Polym. 2020, 230, 115611. [Google Scholar] [CrossRef]
- Singh, J.; Colussi, R.; McCarthy, O.J.; Kaur, L. Chapter 8—Potato Starch and Its Modification. In Advances in Potato Chemistry and Technology, 2nd ed.; Singh, J., Kaur, L., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 195–247. [Google Scholar] [CrossRef]
- Li, H.; Prakash, S.; Nicholson, T.M.; Fitzgerald, M.A.; Gilbert, R.G. The importance of amylose and amylopectin fine structure for textural properties of cooked rice grains. Food Chem. 2016, 196, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Palviainen, P.; Heinämäki, J.; Myllärinen, P.; Lahtinen, R.; Yliruusi, J.; Forssell, P. Corn Starches as Film Formers in Aqueous-Based Film Coating. Pharm. Dev. Technol. 2001, 6, 353–361. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications-a review. Int. J. Food Sci. Technol. 2017, 52, 38–58. [Google Scholar] [CrossRef]
- Semeijn, C.; Buwalda, P.L. Chapter 9—Potato Starch. In Woodhead Publishing Series in Food Science, Technology and Nutrition, 2nd ed.; Sjöö, M., Nilsson, L., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 353–372. [Google Scholar] [CrossRef]
- Khan, B.; Bilal Khan Niazi, M.; Samin, G.; Jahan, Z. Thermoplastic Starch: A Possible Biodegradable Food Packaging Material-A Review. J. Food Process Eng. 2017, 40, e12447. [Google Scholar] [CrossRef]
- Gironès, J.; López, J.P.; Mutjé, P.; Carvalho, A.J.F.; Curvelo, A.A.S.; Vilaseca, F. Natural fiber-reinforced thermoplastic starch composites obtained by melt processing. Compos. Sci. Technol. 2012, 72, 858–863. [Google Scholar] [CrossRef]
- Díaz-Galindo, E.P.; Nesic, A.; Cabrera-Barjas, G.; Mardones, C.; von Baer, D.; Bautista-Baños, S.; Dublan Garcia, O. Physical-Chemical Evaluation of Active Food Packaging Material Based on Thermoplastic Starch Loaded with Grape cane Extract. Molecules 2020, 25, 1306. [Google Scholar] [CrossRef]
- Dorigato, A.; Perin, D.; Pegoretti, A. Effect of the Temperature and of the Drawing Conditions on the Fracture Behaviour of Thermoplastic Starch Films for Packaging Applications. J. Polym. Environ. 2020, 28, 3244–3255. [Google Scholar] [CrossRef]
- Schmitt, H.; Guidez, A.; Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P. Studies on the effect of storage time and plasticizers on the structural variations in thermoplastic starch. Carbohydr. Polym. 2015, 115, 364–372. [Google Scholar] [CrossRef]
- López, O.V.; Castillo, L.A.; García, M.A.; Villar, M.A.; Barbosa, S.E. Food packaging bags based on thermoplastic corn starch reinforced with talc nanoparticles. Food Hydrocoll. 2015, 43, 18–24. [Google Scholar] [CrossRef]
- Jabeen, N.; Majid, I.; Nayik, G.A.; Yildiz, F. Bioplastics and food packaging: A review. Cogent Food Agric. 2015, 1, 1117749. [Google Scholar] [CrossRef]
- Sengupta, T.; Han, J.H. Chapter 4—Surface Chemistry of Food, Packaging, and Biopolymer Materials. In Innovations in Food Packaging, 2nd ed.; Han, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 51–86. [Google Scholar] [CrossRef]
- Gardner, K.H.; Blackwell, J. The structure of native cellulose. Biopolymers 1974, 13, 1975–2001. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Hui Chuin, C.T.; Sabar, S.; Fazita, M.R.N.; Taiwo, O.F.A.; Hassan, T.M.; Haafiz, M.K.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kharkar, P.S.; Pethe, A.M. Biomass and waste materials as potential sources of nanocrystalline cellulose: Comparative review of preparation methods (2016–Till date). Carbohydr. Polym. 2019, 207, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Moser, C.; Lindström, M.E.; Henriksson, G.; Li, J. Cellulose Nanofibers from Softwood, Hardwood, and Tunicate: Preparation–Structure–Film Performance Interrelation. ACS Appl. Mater. Interfaces 2017, 9, 13508–13519. [Google Scholar] [CrossRef]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Production and Characteristics of Cellulose from Different Sources. In Cellulose Derivatives, 1st ed.; Springer Series on Polymer and Composite Materials; Heinze, T., El Seoud, O.A., Koschella, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–38. [Google Scholar] [CrossRef]
- Zhang, B.; Azuma, J.; Uyama, H. Preparation and characterization of a transparent amorphous cellulose film. RSC Adv. 2015, 5, 2900–2907. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012; 733p. [Google Scholar]
- Wu, Y.; Li, Q.; Zhang, X.; Li, Y.; Li, B.; Liu, S. Cellulose-based peptidopolysaccharides as cationic antimicrobial package films. Int. J. Biol. Macromol. 2019, 128, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Filipini, G.; Romani, V.P.; Guimarães Martins, V. Biodegradable and active-intelligent films based on methylcellulose and jambolão (Syzygium cumini) skins extract for food packaging. Food Hydrocoll. 2020, 109, 106139. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Rodríguez, F.; Guarda, A.; Galotto, M.J. Antioxidant films based on cross-linked methyl cellulose and native Chilean berry for food packaging applications. Carbohydr. Polym. 2016, 136, 1052–1060. [Google Scholar] [CrossRef]
- Yoo, S.; Krochta, J.M. Starch-methylcellulose-whey protein film properties. Int. J. Food Sci. Technol. 2012, 47, 255–261. [Google Scholar] [CrossRef]
- Debeaufort, F. Lipid hydrophobicity and physical state effects on the properties of bilayer edible films. J. Membr. Sci. 2000, 180, 47–55. [Google Scholar] [CrossRef]
- Heinze, T.; Pfeiffer, K. Studies on the synthesis and characterization of carboxymethylcellulose. Die Angew. Makromol. Chem. 1999, 266, 37–45. [Google Scholar] [CrossRef]
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Biodegradation of PVP–CMC hydrogel film: A useful food packaging material. Carbohydr. Polym. 2012, 89, 346–353. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Natural polymer based cling films for food packaging. Int. J. Pharm. Pharm. Sci. 2015, 7, 10–18. [Google Scholar]
- Rachtanapun, P.; Rattanapanone, N. Synthesis and characterization of carboxymethyl cellulose powder and films from Mimosa pigra. J. Appl. Polym. Sci. 2011, 122, 3218–3226. [Google Scholar] [CrossRef]
- He, X.; Lu, W.; Sun, C.; Khalesi, H.; Mata, A.; Andaleeb, R.; Fang, Y. Cellulose and cellulose derivatives: Different colloidal states and food-related applications. Carbohydr. Polym. 2021, 255, 117334. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Jolie, R.P.; Duvetter, T.; Van Loey, A.M.; Hendrickx, M.E. Pectin methylesterase and its proteinaceous inhibitor: A review. Carbohydr. Res. 2010, 345, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tomar, M.; Saurabh, V.; Mahajan, T.; Punia, S.; Contreras, M. del M.; Rudra, S.G.; Kaur, C.; Kennedy, J.F. Emerging trends in pectin extraction and its anti-microbial functionalization using natural bioactives for application in food packaging. Trends Food Sci. Technol. 2020, 105, 223–237. [Google Scholar] [CrossRef]
- Espitia, P.; Avena-Bustillos, R.J.; Du, W.-X.; Teófilo, R.F.; Soares, N.F.F.; McHugh, T.H. Optimal antimicrobial formulation and physical–mechanical properties of edible films based on açaí and pectin for food preservation. Food Packag. Shelf Life 2014, 2, 38–49. [Google Scholar] [CrossRef]
- Espitia, P.; Du, W.-X.; Avena-Bustillos, R. de J.; Soares, N. de F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Mishra, R.K.; Banthia, A.K.; Majeed, A.B.A. Pectin based formulations for biomedical applications: A review. Asian J. Pharm. Clin. Res. 2012, 5, 1–7. [Google Scholar]
- Gómez, B.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J.L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Ilharco, L.M.; Pagliaro, M. Pectin production and global market. Agro Food Ind. Hi Tech 2016, 27, 17–20. [Google Scholar]
- Raei, M.; Shahidi, F.; Farhoodi, M.; Jafari, S.M.; Rafe, A. Application of whey protein-pectin nano-complex carriers for loading of lactoferrin. Int. J. Biol. Macromol. 2017, 105, 281–291. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Rosa, G.B.; Ferreira, A.L.A.; Rosa, C.G.d.; Beling, P.C.; Xavier, L.O.; Hansen, C.M.; Ferrareze, J.P.; Nunes, M.R.; Barreto, P.L.M.; et al. Bioactive food packaging based on starch, citric pectin and functionalized with Acca sellowiana waste by-product: Characterization and application in the postharvest conservation of apple. Int. J. Biol. Macromol. 2020, 147, 295–303. [Google Scholar] [CrossRef]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M. Natural Pectin Polysaccharides as Edible Coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Sánchez-Machado, D.I.; López-Cervantes, J.; Soto Valdez, H. Chitosan/Hydrophilic Plasticizer-Based Films: Preparation, Physicochemical and Antimicrobial Properties. J. Polym. Environ. 2014, 22, 41–51. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, L.; Soetemans, L.; D’Hondt, E.; Elst, K. Chapter 1 Sources of Chitin and Chitosan and their Isolation. In Chitin and Chitosan: Properties and Applications, 1st ed.; van den Broek, L.A.M., Boeriu, C.G., Eds.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2019; pp. 1–34. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Cakmak, Y.S.; Baran, T.; Asan-Ozusaglam, M.; Mentes, A.; Tozak, K.O. New chitin, chitosan, and O-carboxymethyl chitosan sources from resting eggs of Daphnia longispina (Crustacea); with physicochemical characterization, and antimicrobial and antioxidant activities. Biotechnol. Bioprocess Eng. 2014, 19, 58–69. [Google Scholar] [CrossRef]
- Majtán, J.; Bíliková, K.; Markovič, O.; Gróf, J.; Kogan, G.; Šimúth, J. Isolation and characterization of chitin from bumblebee (Bombus terrestris). Int. J. Biol. Macromol. 2007, 40, 237–241. [Google Scholar] [CrossRef]
- Yen, M.-T.; Mau, J.-L. Physico-chemical characterization of fungal chitosan from shiitake stipes. LWT Food Sci. Technol. 2007, 40, 472–479. [Google Scholar] [CrossRef]
- Rasul, R.M.; Muniandy, M.T.; Zakaria, Z.; Shah, K.; Chee, C.F.; Dabbagh, A.; Rahman, N.A.; Wong, T.W. A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydr. Polym. 2020, 250, 116800. [Google Scholar] [CrossRef] [PubMed]
- Yeul, V.S.; Rayalu, S.S. Unprecedented Chitin and Chitosan: A Chemical Overview. J. Polym. Environ. 2013, 21, 606–614. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181. [Google Scholar] [CrossRef]
- Domján, A.; Bajdik, J.; Pintye-Hódi, K. Understanding of the Plasticizing Effects of Glycerol and PEG 400 on Chitosan Films Using Solid-State NMR Spectroscopy. Macromolecules 2009, 42, 4667–4673. [Google Scholar] [CrossRef]
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J. Food Eng. 2013, 116, 588–597. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Chiou, B.-S.; Williams, T.; Wood, D.; Du, W.-X.; Sedej, I.; Ban, Z.; Rodov, V.; Poverenov, E.; Vinokur, Y.; et al. Vitamin D-fortified chitosan films from mushroom waste. Carbohydr. Polym. 2017, 167, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kalaycıoğlu, Z.; Torlak, E.; Akın-Evingür, G.; Özen, İ.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, S.Y.; Park, H.J. Effect of halloysite nanoclay on the physical, mechanical, and antioxidant properties of chitosan films incorporated with clove essential oil. Food Hydrocoll. 2018, 84, 58–67. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Emmerichs, N.; Wingender, J.; Flemming, H.-C.; Mayer, C. Interaction between alginates and manganese cations: Identification of preferred cation binding sites. Int. J. Biol. Macromol. 2004, 34, 73–79. [Google Scholar] [CrossRef]
- Evans, L.R.; Linker, A. Production and Characterization of the Slime Polysaccharide of Pseudomonas aeruginosa. J. Bacteriol. 1973, 116, 915–924. [Google Scholar] [CrossRef]
- Hay, I.D.; Rehman, Z.U.; Moradali, M.F.; Wang, Y.; Rehm, B.H.A. Microbial alginate production, modification and its applications. Microb. Biotechnol. 2013, 6, 637–650. [Google Scholar] [CrossRef]
- Urtuvia, V.; Maturana, N.; Acevedo, F.; Peña, C.; Díaz-Barrera, A. Bacterial alginate production: An overview of its biosynthesis and potential industrial production. World J. Microbiol. Biotechnol. 2017, 33, 198. [Google Scholar] [CrossRef]
- Flores, C.; Díaz-Barrera, A.; Martínez, F.; Galindo, E.; Peña, C. Role of oxygen in the polymerization and de-polymerization of alginate produced by Azotobacter vinelandii. J. Chem. Technol. Biotechnol. 2015, 90, 356–365. [Google Scholar] [CrossRef]
- Hay, I.D.; Wang, Y.; Moradali, M.F.; Rehman, Z.U.; Rehm, B.H.A. Genetics and regulation of bacterial alginate production. Environ. Microbiol. 2014, 16, 2997–3011. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I. Chapter 29 Alginates. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 807–828. [Google Scholar]
- Davidson, I.W.; Sutherland, I.W.; Lawson, C.J. Localization of O-Acetyl Groups of Bacterial Alginate. J. Gen. Microbiol. 1977, 98, 603–606. [Google Scholar] [CrossRef]
- Clementi, F. Alginate Production by Azotobacter Vinelandii. Crit. Rev. Biotechnol. 1997, 17, 327–361. [Google Scholar] [CrossRef] [PubMed]
- Kimica Corporation. How to Use Alginates. Available online: https://kimica-algin.com/alginate/usage/ (accessed on 11 May 2020).
- U.S. Food & Drug Administration. Code for Federal Regulations Title 21 Part 184—Direct Food Substances Affirmed as Generally Recognized as Safe. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1724 (accessed on 11 May 2020).
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of alginic acid and its sodium, potassium, ammonium and calcium salts (E 400–E 404) as food additives. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Yoon, K.-J.; Ko, S.-W. Preparation and properties of alginate superabsorbent filament fibers crosslinked with glutaraldehyde. J. Appl. Polym. Sci. 2000, 78, 1797–1804. [Google Scholar] [CrossRef]
- Makaremi, M.; Yousefi, H.; Cavallaro, G.; Lazzara, G.; Goh, C.B.S.; Lee, S.M.; Solouk, A.; Pasbakhsh, P. Safely Dissolvable and Healable Active Packaging Films Based on Alginate and Pectin. Polymers 2019, 11, 1594. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Alvarez Igarzabal, C.I. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Bonnaillie, L.M.; Zhang, H.; Akkurt, S.; Yam, K.L.; Tomasula, P.M. Casein Films: The Effects of Formulation, Environmental Conditions and the Addition of Citric Pectin on the Structure and Mechanical Properties. Polymers 2014, 6, 2018–2036. [Google Scholar] [CrossRef]
- Chiralt, A.; González-Martínez, C.; Vargas, M.; Atarés, L. 18 - Edible films and coatings from proteins. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 477–500. [Google Scholar] [CrossRef]
- Horne, D.S. Casein micelle structure: Models and muddles. Curr. Opin. Colloid Interface Sci. 2006, 11, 148–153. [Google Scholar] [CrossRef]
- Sarode, A.R.; Sawale, P.D.; Khedkar, C.D.; Kalyankar, S.D.; Pawshe, R.D. Casein and Caseinate: Methods of Manufacture. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 676–682. [Google Scholar] [CrossRef]
- Lawrence, N.D.; Kentish, S.E.; O’Connor, A.J.; Barber, A.R.; Stevens, G.W. Microfiltration of skim milk using polymeric membranes for casein concentrate manufacture. Sep. Purif. Technol. 2008, 60, 237–244. [Google Scholar] [CrossRef]
- Alekseev, G.V.; Khripov, A.A. Method of Rapid Remote Control of Casein Concentration in Dairy Products in Unopened Packages. J. Food Process Eng. 2015, 38, 11–18. [Google Scholar] [CrossRef]
- Carr, A.; Golding, M. Functional Milk Proteins Production and Utilization: Casein-Based Ingredients. In Advanced Dairy Chemistry, 4th ed.; McSweeney, P., O’Mahony, J., Eds.; Springer: New York, NY, USA, 2016; Volume 1B, pp. 35–66. [Google Scholar] [CrossRef]
- Chevalier, E.; Assezat, G.; Prochazka, F.; Oulahal, N. Development and characterization of a novel edible extruded sheet based on different casein sources and influence of the glycerol concentration. Food Hydrocoll. 2018, 75, 182–191. [Google Scholar] [CrossRef]
- Xu, J.-L.; Gowen, A.A. Investigation of plasticizer aggregation problem in casein based biopolymer using chemical imaging. Talanta 2019, 193, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Ucpinar Durmaz, B.; Aytac, A. Poly (vinyl alcohol) and casein films: The effects of glycerol amount on the properties of films. Res. Eng. Struct. Mater. 2019, 5, 155–165. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocoll. 2007, 21, 575–584. [Google Scholar] [CrossRef]
- Lin, H.-C.; Wang, B.-J.; Weng, Y.-M. Development and characterization of sodium caseinate edible films cross-linked with genipin. LWT 2020, 118, 108813. [Google Scholar] [CrossRef]
- Sabbah, M.; Giosafatto, C.V.L.; Esposito, M.; Di Pierro, P.; Mariniello, L.; Porta, R. Chapter 21—Transglutaminase Cross-Linked Edible Films and Coatings for Food Applications. In Enzymes in Food Biotechnology, 1st ed.; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 369–388. [Google Scholar] [CrossRef]
- Silvipriya, K.; Kumar, K.; Bhat, A.; Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 123–127. [Google Scholar] [CrossRef]
- Bhagwat, P.K.; Dandge, P.B. Collagen and collagenolytic proteases: A review. Biocatal. Agric. Biotechnol. 2018, 15, 43–55. [Google Scholar] [CrossRef]
- Song, E.; Yeon Kim, S.; Chun, T.; Byun, H.-J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Goyal, D.; Goyal, A.; Brittberg, M. Consideration of religious sentiments while selecting a biological product for knee arthroscopy. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1577–1586. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Sebald, A. Collagen. 2019. Available online: https://maxfacts.uk/treatment/other/medication/miscellaneous/collagen (accessed on 25 February 2021).
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macromol. 2020, 160, 340–351. [Google Scholar] [CrossRef]
- Kozlowska, J.; Sionkowska, A.; Skopinska-Wisniewska, J.; Piechowicz, K. Northern pike (Esox lucius) collagen: Extraction, characterization and potential application. Int. J. Biol. Macromol. 2015, 81, 220–227. [Google Scholar] [CrossRef]
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Kerry, J.P.; Hopkins, D.L. Meat packaging solutions to current industry challenges: A review. Meat Sci. 2018, 144, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Development of active gelatin films by means of valorisation of food processing waste: A review. Food Hydrocoll. 2017, 68, 192–198. [Google Scholar] [CrossRef]
- Kaewruang, P.; Benjakul, S.; Prodpran, T. Molecular and functional properties of gelatin from the skin of unicorn leatherjacket as affected by extracting temperatures. Food Chem. 2013, 138, 1431–1437. [Google Scholar] [CrossRef]
- Mariod, A.A.; Fadol, H.A. Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Mhd Sarbon, N.; Badii, F.; Howell, N.K. Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocoll. 2013, 30, 143–151. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Karayannakidis, P.D.; Zotos, A. Fish Processing By-Products as a Potential Source of Gelatin: A Review. J. Aquat. Food Prod. Technol. 2016, 25, 65–92. [Google Scholar] [CrossRef]
- Siburian, W.Z.; Rochima, E.; Andriani, Y.; Praseptiangga, D. Fish gelatin (definition, manufacture, analysis of quality characteristics, and application): A review. Int. J. Fish. Aquat. Stud. 2020, 8, 90–95. [Google Scholar]
- Kariduraganavar, M.Y.; Kittur, A.A.; Kamble, R.R. Chapter 1—Polymer Synthesis and Processing. In Natural and Synthetic Biomedical Polymers, 1st ed.; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–31. [Google Scholar] [CrossRef]
- Lee, B.; Lum, N.; Seow, L.; Lim, P.; Tan, L. Synthesis and Characterization of Types A and B Gelatin Methacryloyl for Bioink Applications. Materials 2016, 9, 797. [Google Scholar] [CrossRef]
- Aksun Tümerkan, E.T.; Cansu, Ü.; Boran, G.; Mac Regenstein, J.; Özoğul, F. Physiochemical and functional properties of gelatin obtained from tuna, frog and chicken skins. Food Chem. 2019, 287, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.V.; Shamasundar, B.A. Texture Profile Analysis and Functional Properties of Gelatin from the Skin of Three Species of Fresh Water Fish. Int. J. Food Prop. 2015, 18, 572–584. [Google Scholar] [CrossRef]
- Lassoued, I.; Jridi, M.; Nasri, R.; Dammak, A.; Hajji, M.; Nasri, M.; Barkia, A. Characteristics and functional properties of gelatin from thornback ray skin obtained by pepsin-aided process in comparison with commercial halal bovine gelatin. Food Hydrocoll. 2014, 41, 309–318. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin–chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Scopel, B.S.; Ribeiro, M.E.; Dettmer, A.; Baldasso, C. Cornstarch-Gelatin Films: Commercial Gelatin Versus Chromed Leather Waste Gelatin and Evaluation of Drying Conditions. J. Polym. Environ. 2018, 26, 1998–2006. [Google Scholar] [CrossRef]
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; del Carmen Garrigós, M.; Jiménez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci 2016, 133. [Google Scholar] [CrossRef]
- Ortiz-Zarama, M.A.; Jiménez-Aparicio, A.R.; Solorza-Feria, J. Obtainment and partial characterization of biodegradable gelatin films with tannic acid, bentonite and glycerol. J. Sci. Food Agric. 2016, 96, 3424–3431. [Google Scholar] [CrossRef]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. The effect of plasticizers on the functional properties of biodegradable gelatin-based film: A review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Yang, W.; Xue, C.; Wang, Y.; Dong, J.; Xue, Y. Modification of Gelatine with Galla chinensis Extract, a Natural Crosslinker. Int. J. Food Prop. 2016, 19, 731–744. [Google Scholar] [CrossRef]
- Alemán, A.; González, F.; Arancibia, M.Y.; López-Caballero, M.E.; Montero, P.; Gómez-Guillén, M.C. Comparative study between film and coating packaging based on shrimp concentrate obtained from marine industrial waste for fish sausage preservation. Food Control 2016, 70, 325–332. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Chambin, O.; Karbowiak, T.; Debeaufort, F. Release behavior of quercetin from chitosan-fish gelatin edible films influenced by electron beam irradiation. Food Control 2016, 66, 315–319. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Taylor, M.M.; Lee, J.; Bumanlag, L.P.; Latona, R.J.; Brown, E.M. Biopolymers produced from gelatin and whey protein concentrate using polyphenols. J. Am. Leather Chem. Assoc. 2014, 109, 82–88. [Google Scholar]
- Paul, S.K. Edible Films and Coatings for Fruits and Vegetables. In Encyclopedia of Renewable and Sustainable Materials, 1st ed.; Choudhury, I.A., Hashmi, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, pp. 363–376. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Kouhi, M.; Prabhakaran, M.P.; Ramakrishna, S. Edible polymers: An insight into its application in food, biomedicine and cosmetics. Trends Food Sci. Technol. 2020, 103, 248–263. [Google Scholar] [CrossRef]
- Han, J.H. Chapter 9—Edible Films and Coatings: A Review. In Innovations in Food Packaging, 2nd ed.; Han, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 213–255. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Physical and mechanical properties of compression molded and solution casting soybean protein concentrate based films. Food Hydrocoll. 2014, 38, 193–204. [Google Scholar] [CrossRef]
- Hammann, F.; Schmid, M. Determination and Quantification of Molecular Interactions in Protein Films: A Review. Materials 2014, 7, 7975–7996. [Google Scholar] [CrossRef]
- Park, H.J.; Byun, Y.J.; Kim, Y.T.; Whiteside, W.S.; Bae, H.J. Chapter 10—Processes and Applications for Edible Coating and Film Materials from Agropolymers In Innovations in Food Packaging, 2nd ed.; Han, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 257–275. [Google Scholar] [CrossRef]
- Semenova, M.G.; Moiseenko, D.V.; Grigorovich, N.V.; Anokhina, M.S.; Antipova, A.S.; Belyakova, L.E.; Polikarpov, Y.N.; Tsapkina, E.N. Chapter 6—Protein–Polysaccharide Interactions and Digestion of the Complex Particles. In Food Structures, Digestion and Health, 1st ed.; Boland, M., Golding, M., Singh, H., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 169–192. [Google Scholar] [CrossRef]
- Ochoa-Yepes, O.; Di Giogio, L.; Goyanes, S.; Mauri, A.; Famá, L. Influence of process (extrusion/thermo-compression, casting) and lentil protein content on physicochemical properties of starch films. Carbohydr. Polym. 2019, 208, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Hou, H.; Guo, P.; Dong, H. Effects of extrusion and glycerol content on properties of oxidized and acetylated corn starch-based films. Carbohydr. Polym. 2012, 87, 707–712. [Google Scholar] [CrossRef]
- Jiang, W.J.; Tsai, M.L.; Liu, T. Chitin nanofiber as a promising candidate for improved salty taste. LWT Food Sci. Technol. 2017, 75, 65–71. [Google Scholar] [CrossRef]
- Guilbert, S.; Cuq, B.; Gontard, N. Recent innovations in edible and/or biodegradable packaging materials. Food Addit. Contam. 1997, 14, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and Biodegradable Starch Films: A Review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Umaraw, P.; Verma, A.K. Comprehensive review on application of edible film on meat and meat products: An eco-friendly approach. Crit. Rev. Food Sci. Nutr. 2017, 57, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 2467–2480. [Google Scholar] [CrossRef]
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing Spray Systems for Application of Edible Coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Anukiruthika, T.; Sethupathy, P.; Wilson, A.; Kashampur, K.; Moses, J.A.; Anandharamakrishnan, C. Multilayer packaging: Advances in preparation techniques and emerging food applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1156–1186. [Google Scholar] [CrossRef] [PubMed]
- Jeya, J.J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Britto Joseph, G.; Mageshwaran, G.; Durairaj, R.B. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Regubalan, B.; Pandit, P.; Maiti, S.; Nadathur, G.T.; Mallick, A. Potential bio-based edible films, foams, and hydrogels for food packaging. In Bio-Based Materials for Food Packaging, 1st ed.; Ahmed, S., Ed.; Springer: Singapore, 2018; pp. 105–123. [Google Scholar] [CrossRef]
- Zhang, W.; Shu, C.; Chen, Q.; Cao, J.; Jiang, W. The multi-layer film system improved the release and retention properties of cinnamon essential oil and its application as coating in inhibition to penicillium expansion of apple fruit. Food Chem. 2019, 299, 125109. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Poverenov, E. Improving food products’ quality and storability by using Layer by Layer edible coatings. Trends Food Sci. Technol. 2018, 75, 81–92. [Google Scholar] [CrossRef]
- Debeaufort, F.; Voilley, A. Effect of surfactants and drying rate on barrier properties of emulsified edible films. Int. J. Food Sci. Technol. 2007, 30, 183–190. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Joya, J.A.; De Leon-Zapata, M.A.; Alvarez-Perez, O.B.; Torres-León, C.; Nieto-Oropeza, D.E.; Ventura-Sobrevilla, J.M.; Aguilar, M.A.; Ruelas-Chacón, X.; Rojas, R.; Ramos-Aguiñaga, M.E.; et al. Chapter 1—Basic and Applied Concepts of Edible Packaging for Foods. In Handbook of Food Bioengineering, Food Packaging and Preservation, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 9, pp. 1–61. [Google Scholar] [CrossRef]
- Sharma, L.; Singh, C. Sesame protein based edible films: Development and characterization. Food Hydrocoll. 2016, 61, 139–147. [Google Scholar] [CrossRef]
- De Moura, M.R.; Avena-Bustillos, R.J.; Mchugh, T.H.; Krochta, J.M.; Mattoso, L.H.C. Properties of Novel Hydroxypropyl Methylcellulose Films Containing Chitosan Nanoparticles. J. Food Sci. 2008, 73, 31–37. [Google Scholar] [CrossRef]
- Chen, X.; Cui, F.; Zi, H.; Zhou, Y.; Liu, H.; Xiao, J. Development and characterization of a hydroxypropyl starch/zein bilayer edible film. Int. J. Biol. Macromol. 2019, 141, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, W.; Wang, L. Developing a green and edible film from Cassia gum: The effects of glycerol and sorbitol. J. Clean. Prod. 2018, 175, 276–282. [Google Scholar] [CrossRef]
- Fishman, M.L.; Coffin, D.R.; Onwulata, C.I.; Konstance, R.P. Extrusion of pectin and glycerol with various combinations of orange albedo and starch. Carbohydr. Polym. 2004, 57, 401–413. [Google Scholar] [CrossRef]
- Dangaran, K.; Tomasula, P.M.; Qi, P. Structure and Function of Protein-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications, 1st ed.; Huber, K., Embuscado, M., Eds.; Springer: New York, NY, USA, 2009; pp. 25–56. [Google Scholar] [CrossRef]
- Cai, J.; Xiao, J.; Chen, X.; Liu, H. Essential oil loaded edible films prepared by continuous casting method: Effects of casting cycle and loading position on the release properties. Food Packag. Shelf Life 2020, 26, 100555. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, J.; Cheng, F. Effects of starches from different botanical sources and modification methods on physicochemical properties of starch-based edible films. Int. J. Biol. Macromol. 2019, 132, 897–905. [Google Scholar] [CrossRef]
- Bagheri, F.; Radi, M.; Amiri, S. Drying conditions highly influence the characteristics of glycerol-plasticized alginate films. Food Hydrocoll. 2019, 90, 162–171. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Ziani, K.; Pedroza-Islas, R.; Maté, J.I. Effect of drying conditions on the mechanical and barrier properties of films based on Chitosan. Dry. Technol. 2010, 28, 1350–1358. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, B.; Zhao, J.; Chen, H. Development and characterization of food packaging film fromcellulose sulfate. Food Hydrocoll. 2014, 35, 476–483. [Google Scholar] [CrossRef]
- Liu, F.; Majeed, H.; Antoniou, J.; Li, Y.; Ma, Y.; Yokoyama, W.; Ma, J.; Zhong, F. Tailoring physical properties of transglutaminase-modified gelatin films by varying drying temperature. Food Hydrocoll. 2016, 58, 20–28. [Google Scholar] [CrossRef]
- Hernandez-Izquierdo, V.M.; Reid, D.S.; McHugh, T.H.; De, J. Berrios, J.; Krochta, J.M. Thermal Transitions and Extrusion of Glycerol-Plasticized Whey Protein Mixtures. J. Food Sci. 2008, 73, E169–E175. [Google Scholar] [CrossRef] [PubMed]
- Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Properties and characteristics of dual-modified rice starch based biodegradable films. Int. J. Biol. Macromol. 2014, 67, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Skurtys, O.; Acevedo, C.; Pedreschi, F.; Enronoe, J.; Osorio, F.; Aguilera, J.M. Food Hydrocolloid Edible Films and Coatings; Nova Science Publishers Inc: New York, NY, USA, 2010; 66p. [Google Scholar]
- Liu, L.; Kerry, J.F.; Kerry, J.P. Selection of optimum extrusion technology parameters in the manufacture of edible/ biodegradable packaging films derived from food-based polymers. J. Food Agric. Environ. 2005, 3, 51–58. [Google Scholar] [CrossRef]
- Fitch-Vargas, P.R.; Aguilar-Palazuelos, E.; de Jesús Zazueta-Morales, J.; Vega-García, M.O.; Valdez-Morales, J.E.; Martínez-Bustos, F.; Jacobo-Valenzuela, N. Physicochemical and Microstructural Characterization of Corn Starch Edible Films Obtained by a Combination of Extrusion Technology and Casting Technique. J. Food Sci. 2016, 81, E2224–E2232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gu, W.C.; Cheng, Z.Y.; Li, Y.; Gu, W.J. Development of edible packaging materials. Adv. Mater. Res. 2014, 904, 189–191. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Yamashita, F.; Grossmann, M.V.E. Extrusion parameters related to starch/chitosan active films properties. Int. J. Food Sci. Technol. 2011, 46, 702–710. [Google Scholar] [CrossRef]
- Kostic, M.M.; Reifschneider, L.G. Design of Extrusion Dies. In Encyclopedia of Chemical Processing, 1st ed.; Lee, S., Ed.; Taylor & Francis Group: Abingdon-on-Thames, UK, 2006; pp. 633–649. [Google Scholar] [CrossRef]
- Hernandez-Izquierdo, V.M.; Krochta, J.M. Thermoplastic processing of proteins for film formation—A review. J. Food Sci. 2008, 73, R30–R39. [Google Scholar] [CrossRef]
- González-Seligra, P.; Guz, L.; Ochoa-Yepes, O.; Goyanes, S.; Famá, L. Influence of extrusion process conditions on starch film morphology. LWT 2017, 84, 520–528. [Google Scholar] [CrossRef]
- Gilfillan, W.N.; Moghaddam, L.; Bartley, J.; Doherty, W.O.S. Thermal extrusion of starch film with alcohol. J. Food Eng. 2015, 170, 92–99. [Google Scholar] [CrossRef]
- Calderón-Castro, A.; Vega-García, M.O.; de Jesús Zazueta-Morales, J.; Fitch-Vargas, P.R.; Carrillo-López, A.; Gutiérrez-Dorado, R.; Limón-Valenzuela, V.; Aguilar-Palazuelos, E. Effect of extrusion process on the functional properties of high amylose corn starch edible films and its application in mango (Mangifera indica L.) cv. Tommy Atkins. J. Food Sci. Technol. 2018, 55, 905–914. [Google Scholar] [CrossRef]
- Fishman, M.L.; Coffin, D.R.; Konstance, R.P.; Onwulata, C.I. Extrusion of pectin/starch blends plasticized with glycerol. Carbohydr. Polym. 2000, 41, 317–325. [Google Scholar] [CrossRef]
- Liu, L.; Kerry, J.F.; Kerry, J.P. Application and assessment of extruded edible casings manufactured from pectin and gelatin/sodium alginate blends for use with breakfast pork sausage. Meat Sci. 2007, 75, 196–202. [Google Scholar] [CrossRef]
- Chevalier, E.; Chaabani, A.; Assezat, G.; Prochazka, F.; Oulahal, N. Casein/wax blend extrusion for production of edible films as carriers of potassium sorbate—A comparative study of waxes and potassium sorbate effect. Food Packag. Shelf Life 2018, 16, 41–50. [Google Scholar] [CrossRef]
- Oechsle, A.M.; Häupler, M.; Weigel, F.; Gibis, M.; Kohlus, R.; Weiss, J. Modulation of extruded collagen films by the addition of co-gelling proteins. J. Food Eng. 2016, 171, 164–173. [Google Scholar] [CrossRef]
- Belyamani, I.; Prochazka, F.; Assezat, G. Production and characterization of sodium caseinate edible films made by blown-film extrusion. J. Food Eng. 2014, 121, 39–47. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Development of thermoplastic starch blown film by incorporating plasticized chitosan. Carbohydr. Polym. 2015, 115, 575–581. [Google Scholar] [CrossRef]
- Colak, B.Y.; Peynichou, P.; Galland, S.; Oulahal, N.; Assezat, G.; Prochazka, F.; Degraeve, P. Active biodegradable sodium caseinate films manufactured by blown-film extrusion: Effect of thermo-mechanical processing parameters and formulation on lysozyme stability. Ind. Crops Prod. 2015, 72, 142–151. [Google Scholar] [CrossRef]
- Rodríguez-Castellanos, W.; Martínez-Bustos, F.; Rodrigue, D.; Trujillo-Barragán, M. Extrusion blow molding of a starch-gelatin polymer matrix reinforced with cellulose. Eur. Polym. J. 2015, 73, 335–343. [Google Scholar] [CrossRef]
- Dierickx, L.; Saerens, L.; Almeida, A.; De Beer, T.; Remon, J.P.; Vervaet, C. Co-extrusion as manufacturing technique for fixed-dose combination mini-matrices. Eur. J. Pharm. Biopharm. 2012, 81, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Hilbig, J.; Hartlieb, K.; Herrmann, K.; Weiss, J.; Gibis, M. Influence of calcium on white efflorescence formation on dry fermented sausages with co-extruded alginate casings. Food Res. Int. 2020, 131, 109012. [Google Scholar] [CrossRef]
- Harper, B.A.; Barbut, S.; Smith, A.; Marcone, M.F. Mechanical and Microstructural Properties of “Wet” Alginate and Composite Films Containing Various Carbohydrates. J. Food Sci. 2015, 80, E84–E92. [Google Scholar] [CrossRef] [PubMed]
- Marcos, B.; Gou, P.; Arnau, J.; Guàrdia, M.D.; Comaposada, J. Co-extruded alginate as an alternative to collagen casings in the production of dry-fermented sausages: Impact of coating composition. Meat Sci. 2020, 169, 108184. [Google Scholar] [CrossRef]
- Barbut, S.; Ioi, M.; Marcone, M. Co-extrusion of collagen casings. Effects of preparation, brining, and heating on strength, rheology and microstructure. Ital. J. Food Sci. 2020, 32, 91–106. [Google Scholar] [CrossRef]
- Guerrero, P.; Stefani, P.M.; Ruseckaite, R.A.; De La Caba, K. Functional properties of films based on soy protein isolate and gelatin processed by compression molding. J. Food Eng. 2011, 105, 65–72. [Google Scholar] [CrossRef]
- Tatara, R.A. 14 - Compression Molding. In Plastics Design Library, Applied Plastics Engineering Handbook, 2nd ed.; Kutz, M., Ed.; William Andrew Publishing: New York, NY, USA, 2017; pp. 291–320. [Google Scholar] [CrossRef]
- Krishna, M.; Nindo, C.I.; Min, S.C. Development of fish gelatin edible films using extrusion and compression molding. J. Food Eng. 2012, 108, 337–344. [Google Scholar] [CrossRef]
- Ceballos, R.L.; Ochoa-Yepes, O.; Goyanes, S.; Bernal, C.; Famá, L. Effect of yerba mate extract on the performance of starch films obtained by extrusion and compression molding as active and smart packaging. Carbohydr. Polym. 2020, 244, 116495. [Google Scholar] [CrossRef]
- Uranga, J.; Etxabide, A.; Guerrero, P.; de la Caba, K. Development of active fish gelatin films with anthocyanins by compression molding. Food Hydrocoll. 2018, 84, 313–320. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Jiménez, A.; Talens, P.; Chiralt, A. Properties of starch-hydroxypropyl methylcellulose based films obtained by compression molding. Carbohydr. Polym. 2014, 109, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, L.; Li, Q. Optimization of injection molding process parameters using combination of artificial neural network and genetic algorithm method. J. Mater. Process. Technol. 2007, 183, 412–418. [Google Scholar] [CrossRef]
- Nussinovitch, A. CHAPTER 10—Biopolymer Films and Composite Coatings. In Modern Biopolymer Science, 1st ed.; Kasapis, S., Norton, I.T., Ubbink, J.B., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 295–326. [Google Scholar] [CrossRef]
- Perez, V.; Felix, M.; Romero, A.; Guerrero, A. Characterization of pea protein-based bioplastics processed by injection moulding. Food Bioprod. Process. 2016, 97, 100–108. [Google Scholar] [CrossRef]
- Yu, F.; Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P. Plasticized-starch/poly(ethylene oxide) blends prepared by extrusion. Carbohydr. Polym. 2013, 91, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Gällstedt, M.; Johansson, E.; Hedenqvist, M.S. Injection-molded nanocomposites and materials based on wheat gluten. Int. J. Biol. Macromol. 2011, 48, 146–152. [Google Scholar] [CrossRef]
- Raghav, P.K.; Agarwal, N.; Saini, M. Edible coating of fruits and vegetables: A review. Int. J. Sci. Res. Mod. Educ. 2016, 1, 188–204. [Google Scholar]
- Pavlath, A.E.; Orts, W. Edible Films and Coatings: Why, What, and How? In Edible Films and Coatings for Food Applications, 1st ed.; Huber, K., Embuscado, M., Eds.; Springer: New York, NY, USA, 2009; pp. 1–23. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Ferreira, D.C.; Louzada, L.B.; Dos Santos, F.; Corrêa, A.C.; Moreira, F.K.V.; Mattoso, L.H. Chapter 10—Starch-Based Edible Films and Coatings: An Eco-friendly Alternative for Food Packaging. In Starches for Food Application, 1st ed.; Clerici, M.T.P.S., Schmiele, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 359–420. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Geschwindner, G.; Drouven, H. 18 - Manufacturing processes: Chocolate panning and inclusions. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Science and Technology of Enrobed and Filled Chocolate, Confectionery and Bakery Products, 1st ed.; Talbot, G., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 397–413. [Google Scholar] [CrossRef]
- Solís-Morales, D.; Sáenz-Hernández, C.M.; Ortega-Rivas, E. Attrition reduction and quality improvement of coated puffed wheat by fluidised bed technology. J. Food Eng. 2009, 93, 236–241. [Google Scholar] [CrossRef]
- Wongphan, P.; Harnkarnsujarit, N. Characterization of starch, agar and maltodextrin blends for controlled dissolution of edible films. Int. J. Biol. Macromol. 2020, 156, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The effect of edible coatings on the nutritional quality of ‘Bravo de Esmolfe’ fresh-cut apple through shelf-life. LWT Food Sci. Technol. 2017, 75, 210–219. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and Incorporation of Functional Ingredients in Edible Films and Coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Atieno, L.; Owino, W.; Ateka, E.M.; Ambuko, J. Influence of Coating Application Methods on the Postharvest Quality of Cassava. Int. J. Food Sci. 2019, 2019, 2148914. [Google Scholar] [CrossRef] [PubMed]
- Parreidt, T.S.; Schmid, M.; Müller, K. Effect of Dipping and Vacuum Impregnation Coating Techniques with Alginate Based Coating on Physical Quality Parameters of Cantaloupe Melon. J. Food Sci. 2018, 83, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Luo, Y.; Luo, Y.; Wang, Q. Combined effects of sodium chlorite dip treatment and chitosan coatings on the quality of fresh-cut d’Anjou pears. Postharvest Biol. Technol. 2011, 62, 319–326. [Google Scholar] [CrossRef]
- Hamzeh, A.; Rezaei, M. The Effects of Sodium Alginate on Quality of Rainbow Trout (Oncorhynchus mykiss) Fillets Stored at 4 ± 2 °C. J. Aquat. Food Prod. Technol. 2012, 21, 14–21. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Combined effect of active coating and MAP to prolong the shelf life of minimally processed kiwifruit (Actinidia deliciosa cv. Hayward). Food Res. Int. 2011, 44, 1224–1230. [Google Scholar] [CrossRef]
- Hamzah, H.M.; Osman, A.; Tan, C.P.; Mohamad Ghazali, F. Carrageenan as an alternative coating for papaya (Carica papaya L. cv. Eksotika). Postharvest Biol. Technol. 2013, 75, 142–146. [Google Scholar] [CrossRef]
- Kumar, N.; Neeraj. Polysaccharide-based component and their relevance in edible film/coating: A review. Nutr. Food Sci. 2019, 49, 793–823. [Google Scholar] [CrossRef]
- Barringer, S.A.; Sumonsiri, N. Electrostatic Coating Technologies for Food Processing. Annu. Rev. Food Sci. Technol. 2015, 6, 157–169. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhuang, C.; Gu, W.; Zhao, Y. Effect of molecular weight on the properties of chitosan films prepared using electrostatic spraying technique. Carbohydr. Polym. 2019, 212, 197–205. [Google Scholar] [CrossRef]
- Zhong, Y.; Cavender, G.; Zhao, Y. Investigation of different coating application methods on the performance of edible coatings on Mozzarella cheese. LWT Food Sci. Technol. 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Lara, G.; Yakoubi, S.; Villacorta, C.M.; Uemura, K.; Kobayashi, I.; Takahashi, C.; Nakajima, M.; Neves, M.A. Spray technology applications of xanthan gum-based edible coatings for fresh-cut lotus root (Nelumbo nucifera). Food Res. Int. 2020, 137, 109723. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.X.; Avena-Bustillos, R.J.; Berrios, J.D.J.; Sambo, P.; McHugh, T.H. Electrostatic and Conventional Spraying of Alginate-Based Edible Coating with Natural Antimicrobials for Preserving Fresh Strawberry Quality. Food Bioprocess Technol. 2017, 10, 165–174. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhong, Y.; Zhao, Y. Effect of deacetylation degree on properties of Chitosan films using electrostatic spraying technique. Food Control 2019, 97, 25–31. [Google Scholar] [CrossRef]
- Khanzadi, M.; Jafari, S.M.; Mirzaei, H.; Chegini, F.K.; Maghsoudlou, Y.; Dehnad, D. Physical and mechanical properties in biodegradable films of whey protein concentrate-pullulan by application of beeswax. Carbohydr. Polym. 2015, 118, 24–29. [Google Scholar] [CrossRef]
- Ferreira Saraiva, L.E.; Naponucena, L.d.O.M.; de Silva Santos, V.; Silva, R.P.D.; de Souza, C.O.; Souza, I.E.G.L.; de Oliveira Mamede, M.E.; Druzian, J.I. Development and application of edible film of active potato starch to extend mini panettone shelf life. LWT Food Sci. Technol. 2016, 73, 311–319. [Google Scholar] [CrossRef]
- Da Rocha, M.; de Souza, M.M.; Prentice, C. Chapter 9—Biodegradable Films: An Alternative Food Packaging. In Handbook of Food Bioengineering, Food Packaging and Preservation, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 9, pp. 307–342. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Alginate-calcium films: Water vapor permeability and mechanical properties as affected by plasticizer and relative humidity. LWT Food Sci. Technol. 2008, 41, 359–366. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Heikkinen, S.; Soovre, A.; Peura, M.; Serimaa, R.; Talja, R.A.; Helén, H.; Hyvönen, L.; Tenkanen, M. Films from oat spelt arabinoxylan plasticized with glycerol and sorbitol. J. Appl. Polym. Sci. 2009, 114, 457–466. [Google Scholar] [CrossRef]
- Ansorena, M.R.; Pereda, M.; Marcovich, N.E. Edible films. In Polymers for Food Applications, 1st ed.; Gutiérrez, T., Ed.; Springer: Cham, Switzerland, 2018; pp. 5–24. [Google Scholar] [CrossRef]
- Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and pectin composite films crosslinked with Ca2+ ions: Effect of the plasticizer concentration. Carbohydr. Polym. 2009, 77, 736–742. [Google Scholar] [CrossRef]
- Tillet, G.; Boutevin, B.; Ameduri, B. Chemical reactions of polymer crosslinking and post-crosslinking at room and medium temperature. Prog. Polym. Sci. 2011. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Dole, P.; Joly, C.; Espuche, E.; Alric, I.; Gontard, N. Gas transport properties of starch based films. Carbohydr. Polym. 2004, 58, 335–343. [Google Scholar] [CrossRef]
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-based films and food coatings: An overview. Starch 2016, 68, 1026–1037. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Sivakumar, V.; Thirugnanasambandham, K.; Kandasamy, S. Modeling and analysis of film composition on mechanical properties of maize starch based edible films. Int. J. Biol. Macromol. 2013, 62, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Flores, S.; Famá, L.; Rojas, A.M.; Goyanes, S.; Gerschenson, L. Physical properties of tapioca-starch edible films: Influence of filmmaking and potassium sorbate. Food Res. Int. 2007, 40, 257–265. [Google Scholar] [CrossRef]
- García, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.; Goyanes, S. Effect of glycerol on the morphology of nanocomposites made from thermoplastic starch and starch nanocrystals. Carbohydr. Polym. 2011, 84, 203–210. [Google Scholar] [CrossRef]
- Avila-De La Rosa, G.; Carrillo-Navas, H.; Echeverría, J.C.; Bello-Pérez, L.A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Mechanisms of elastic turbulence in gelatinized starch dispersions. Chaos Solitons Fractals 2015, 77, 29–38. [Google Scholar] [CrossRef]
- Carrillo-Navas, H.; Avila-De La Rosa, G.; Gómez-Luría, D.; Meraz, M.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Impact of ghosts on the viscoelastic response of gelatinized corn starch dispersions subjected to small strain deformations. Carbohydr. Polym. 2014, 110, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xia, R.; Zheng, L.; Yuan, T.; Sun, R. Plasticized hemicelluloses/chitosan-based edible films reinforced by cellulose nanofiber with enhanced mechanical properties. Carbohydr. Polym. 2019, 224, 115164. [Google Scholar] [CrossRef] [PubMed]
- Lumdubwong, N. Applications of Starch-Based Films in Food Packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Olsson, E.; Hedenqvist, M.S.; Johansson, C.; Järnström, L. Influence of citric acid and curing on moisture sorption, diffusion and permeability of starch films. Carbohydr. Polym. 2013, 94, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Menzel, C.; Johansson, C.; Andersson, R.; Koch, K.; Järnström, L. The effect of pH on hydrolysis, cross-linking and barrier properties of starch barriers containing citric acid. Carbohydr. Polym. 2013, 98, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Menzel, C. Improvement of starch films for food packaging through a three-principle approach: Antioxidants, cross-linking and reinforcement. Carbohydr. Polym. 2020, 250, 116828. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Haq, M.A.; Lutfi, Z.; Hasnain, A. Effect of mango kernel starch coatings on the shelf life of almond (Prunus dulcis) kernels. J. Food Process. Preserv. 2018, 42, e13449. [Google Scholar] [CrossRef]
- Mehyar, G.F.; Han, J.H. Physical and Mechanical Properties of High-amylose Rice and Pea Starch Films as Affected by Relative Humidity and Plasticizer. J. Food Sci. 2006, 69, E449–E454. [Google Scholar] [CrossRef]
- Chiumarelli, M.; Pereira, L.M.; Ferrari, C.C.; Sarantópoulos, C.I.G.L.; Hubinger, M.D. Cassava Starch Coating and Citric Acid to Preserve Quality Parameters of Fresh-Cut “Tommy Atkins” Mango. J. Food Sci. 2010, 75, E297–E304. [Google Scholar] [CrossRef] [PubMed]
- Medronho, B.; Lindman, B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface Sci. 2015, 222, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Medronho, B.; Lindman, B. Competing forces during cellulose dissolution: From solvents to mechanisms. Curr. Opin. Colloid Interface Sci. 2014, 19, 32–40. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Cruz-Romero, M.C.; Cruz-Romero, M.; Kerry, J.P.; Kerry, J. Crop-based biodegradable packaging and its environmental implications. Nutr. Nat. Resour. 2008, 3, 1–25. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing cellulose (in)solubility: Reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 2012, 19, 581–587. [Google Scholar] [CrossRef]
- Abe, M.; Fukaya, Y.; Ohno, H. Fast and facile dissolution of cellulose with tetrabutylphosphonium hydroxide containing 40 wt% water. Chem. Commun. 2012, 48, 1808–1810. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Heinze, T. Organic esters of cellulose: New perspectives for old polymers. Adv. Polym. Sci. 2005, 186, 103–149. [Google Scholar] [CrossRef]
- Egal, M.; Budtova, T.; Navard, P. Structure of aqueous solutions of microcrystalline cellulose/sodium hydroxide below 0 °C and the limit of cellulose dissolution. Biomacromolecules 2007, 8, 2282–2287. [Google Scholar] [CrossRef]
- Lue, A.; Zhang, L. Advances in aqueous cellulose solvents. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification, 1st ed.; Liebert, T., Heinze, T., Edgar, K.J., Eds.; American Chemical Society: Washington, DC, USA, 2010; Volume 1033, pp. 67–89. [Google Scholar] [CrossRef]
- Heinze, T.; Köhler, S. Dimethyl sulfoxide and ammonium fluorides-novel cellulose solvents. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification, 1st ed.; Liebert, T., Heinze, T., Edgar, K.J., Eds.; American Chemical Society: Washington, DC, USA, 2010; Volume 1033, pp. 103–118. [Google Scholar] [CrossRef]
- Yang, Y.J.; Shin, J.M.; Kang, T.H.; Kimura, S.; Wada, M.; Kim, U.J. Cellulose dissolution in aqueous lithium bromide solutions. Cellulose 2014, 21, 1175–1181. [Google Scholar] [CrossRef]
- Ferreira, A.; Alves, V.; Coelhoso, I. Polysaccharide-Based Membranes in Food Packaging Applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mao, Y.; Zhou, J.; Cai, J. Effects of Coagulation Conditions on the Properties of Regenerated Cellulose Films Prepared in NaOH/Urea Aqueous Solution. Ind. Eng. Chem. Res. 2005, 44, 522–529. [Google Scholar] [CrossRef]
- Geng, H.; Yuan, Z.; Fan, Q.; Dai, X.; Zhao, Y.; Wang, Z.; Qin, M. Characterisation of cellulose films regenerated from acetone/water coagulants. Carbohydr. Polym. 2014, 102, 438–444. [Google Scholar] [CrossRef]
- Qi, H.; Chang, C.; Zhang, L. Properties and applications of biodegradable transparent and photoluminescent cellulose films prepared via a green process. Green Chem. 2009, 11, 177–184. [Google Scholar] [CrossRef]
- Bedane, A.H.; Eić, M.; Farmahini-Farahani, M.; Xiao, H. Water vapor transport properties of regenerated cellulose and nanofibrillated cellulose films. J. Membr. Sci. 2015, 493, 46–57. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Benito-González, I.; Jaén-Cano, C.M.; López-Rubio, A.; Martínez-Abad, A.; Martínez-Sanz, M. Valorisation of vine shoots for the development of cellulose-based biocomposite films with improved performance and bioactivity. Int. J. Biol. Macromol. 2020, 165, 1540–1551. [Google Scholar] [CrossRef]
- Kwak, H.; Shin, S.; Kim, J.; Kim, J.; Lee, D.; Lee, H.; Lee, E.J.; Hyun, J. Protective coating of strawberries with cellulose nanofibers. Carbohydr. Polym. 2021, 258, 117688. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.W. Isolation of cellulose nanocrystals from grain straws and their use for the preparation of carboxymethyl cellulose-based nanocomposite films. Carbohydr. Polym. 2016, 150, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, H.; He, Y.; Fei, X.; Peng, L. Preparation and characterization of carboxymethyl cellulose-based composite films reinforced by cellulose nanocrystals derived from pea hull waste for food packaging applications. Int. J. Biol. Macromol. 2020, 164, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Magalhães, S.; Alves, L.; Antunes, F.; Miguel, M.; Lindman, B.; Medronho, B. Cellulose-based edible films for probiotic entrapment. Food Hydrocoll. 2019, 88, 68–74. [Google Scholar] [CrossRef]
- Osorio, F.A.; Molina, P.; Matiacevich, S.; Enrione, J.; Skurtys, O. Characteristics of hydroxy propyl methyl cellulose (HPMC) based edible film developed for blueberry coatings. Procedia Food Sci. 2011, 1, 287–293. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Avena-Bustillos, R.J.; Filho, G.C.; Munford, M.L.; Wood, D.; McHugh, T.H. Nanocellulose reinforced chitosan composite films as affected by nanofiller loading and plasticizer content. J. Food Sci. 2010, 75, N1–N7. [Google Scholar] [CrossRef] [PubMed]
- Savadekar, N.R.; Karande, V.S.; Vigneshwaran, N.; Bharimalla, A.K.; Mhaske, S.T. Preparation of nano cellulose fibers and its application in kappa-carrageenan based film. Int. J. Biol. Macromol. 2012, 51, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Benito-González, I.; López-Rubio, A.; Martínez-Sanz, M. High-performance starch biocomposites with celullose from waste biomass: Film properties and retrogradation behaviour. Carbohydr. Polym. 2019, 216, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Xia, Y.; Yang, G.; Chen, J.; Ji, D. Improved film formability of oxidized starch-based blends through controlled modification with cellulose nanocrystals. Ind. Crops Prod. 2019, 140, 111665. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical properties of starch-CMC-nanoclay biodegradable films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem. Cent. J. 2011, 5, 6. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Wood, D.; Williams, T.G.; Avena-Bustillos, R.J.; McHugh, T.H. Nanocomposite edible films from mango puree reinforced with cellulose nanofibers. J. Food Sci. 2009, 74, N31–N35. [Google Scholar] [CrossRef]
- Andrade-Pizarro, R.D.; Skurtys, O.; Osorio-Lira, F. Effect of cellulose nanofibers concentration on mechanical, optical, and barrier properties of gelatin-based edible films. DYNA 2015, 82, 219–226. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Miranda, K.W.E.; Ribeiro, H.L.; Rosa, M.F.; Nascimento, D.M. Nanoreinforced alginate-acerola puree coatings on acerola fruits. J. Food Eng. 2012, 113, 505–510. [Google Scholar] [CrossRef]
- Wu, T.; Farnood, R.; O’Kelly, K.; Chen, B. Mechanical behavior of transparent nanofibrillar cellulose-chitosan nanocomposite films in dry and wet conditions. J. Mech. Behav. Biomed. Mater. 2014, 32, 279–286. [Google Scholar] [CrossRef]
- Gutierrez-Pacheco, M.M.; Ortega-Ramirez, L.A.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Chapter 50—Combinational Approaches for Antimicrobial Packaging: Pectin and Cinnamon Leaf Oil. In Antimicrobial Food Packaging, 1st ed.; Barros-Velázquez, J., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 609–617. [Google Scholar] [CrossRef]
- Coenen, G.J.; Bakx, E.J.; Verhoef, R.P.; Schols, H.A.; Voragen, A.G.J. Identification of the connecting linkage between homo- or xylogalacturonan and rhamnogalacturonan type I. Carbohydr. Polym. 2007, 70, 224–235. [Google Scholar] [CrossRef]
- Eça, K.S.; Machado, M.T.C.; Hubinger, M.D.; Menegalli, F.C. Development of Active Films From Pectin and Fruit Extracts: Light Protection, Antioxidant Capacity, and Compounds Stability. J. Food Sci. 2015, 80, C2389–C2396. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Fraeye, I.; Duvetter, T.; Doungla, E.; Van Loey, A.; Hendrickx, M. Fine-tuning the properties of pectin-calcium gels by control of pectin fine structure, gel composition and environmental conditions. Trends Food Sci. Technol. 2010, 21, 219–228. [Google Scholar] [CrossRef]
- Videcoq, P.; Garnier, C.; Robert, P.; Bonnin, E. Influence of calcium on pectin methylesterase behaviour in the presence of medium methylated pectins. Carbohydr. Polym. 2011, 86, 1657–1664. [Google Scholar] [CrossRef]
- Thibault, J.-F.; Ralet, M.-C. Physico-Chemical Properties of Pectins in the Cell Walls and After Extraction. In Advances in Pectin and Pectinase Research, 1st ed.; Springer: Dordrecht, The Netherlands, 2003; pp. 91–105. [Google Scholar] [CrossRef]
- Kuuva, T.; Lantto, R.; Reinikainen, T.; Buchert, J.; Autio, K. Rheological properties of laccase-induced sugar beet pectin gels. Food Hydrocoll. 2003, 17, 679–684. [Google Scholar] [CrossRef]
- Galus, S.; Turska, A.; Lenart, A. Sorption and Wetting Properties of Pectin Edible Films. Czech J. Food Sci. 2012, 30, 446–455. [Google Scholar] [CrossRef]
- Giancone, T.; Torrieri, E.; Pierro, P. Di, Cavella, S.; Giosafatto, C.V.L.; Masi, P. Effect of Surface Density on the Engineering Properties of High Methoxyl Pectin-Based Edible Films. Food Bioprocess Technol. 2011, 4, 1228–1236. [Google Scholar] [CrossRef]
- Sucheta; Chaturvedi, K.; Sharma, N.; Yadav, S.K. Composite edible coatings from commercial pectin, corn flour and beetroot powder minimize post-harvest decay, reduces ripening and improves sensory liking of tomatoes. Int. J. Biol. Macromol. 2019, 133, 284–293. [Google Scholar] [CrossRef]
- Darni, Y.; Utami, H.; Septiana, R.; Fitriana, R.A. Comparative studies of the edible film based on low pectin methoxyl with glycerol and sorbitol plasticizers. J. Bahan Alam Terbarukan 2017, 6, 158–167. [Google Scholar] [CrossRef][Green Version]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol. Technol. 2008, 50, 87–94. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Sarantópoulos, C.I.G.L.; Carmello-Guerreiro, S.M.; Hubinger, M.D. Effect of Osmotic Dehydration and Pectin Edible Coatings on Quality and Shelf Life of Fresh-Cut Melon. Food Bioprocess Technol. 2013, 6, 80–91. [Google Scholar] [CrossRef]
- Moalemyan, M.; Ramaswamy, H.S.; Maftoonazad, N. Pectin-based edible coating for shelf-life extension of ataulfo mango. J. Food Process Eng. 2012, 35, 572–600. [Google Scholar] [CrossRef]
- Sathivel, S.; Liu, Q.; Huang, J.; Prinyawiwatkul, W. The influence of chitosan glazing on the quality of skinless pink salmon (Oncorhynchus gorbuscha) fillets during frozen storage. J. Food Eng. 2007, 83, 366–373. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Thi Dao, U.T.; Thi Bui, Q.P.; Bach, G.L.; Ha Thuc, C.N.; Ha Thuc, H. Enhanced antimicrobial activities and physiochemical properties of edible film based on chitosan incorporated with Sonneratia caseolaris (L.) Engl. leaf extract. Prog. Org. Coat. 2020, 140, 105487. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. The versatile biopolymer chitosan: Potential sources, evaluation of extraction methods and applications. Crit. Rev. Microbiol. 2014, 40, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Leceta, I.; Guerrero, P.; De La Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.T.; Kim, J.T.; Jung, S.T.; Cho, G.S.; Park, H.J. Properties of chitosan-based biopolymer films with various degrees of deacetylation and molecular weights. J. Appl. Polym. Sci. 2003, 89, 3476–3484. [Google Scholar] [CrossRef]
- Fernandez-Saiz, P.; Lagaron, J.M.; Ocio, M.J. Optimization of the biocide properties of chitosan for its application in the design of active films of interest in the food area. Food Hydrocoll. 2009, 23, 913–921. [Google Scholar] [CrossRef]
- Gao, H.X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.C. A functional polysaccharide film forming by pectin, chitosan, and tea polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Effects of Interactions between the Constituents of Chitosan-Edible Films on Their Physical Properties. Food Bioprocess Technol. 2012, 5, 3181–3192. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Nayik, G.A.; Majid, I.; Kumar, V. Characterization of Indian Honeys View project Sprouted onion View project Developments in Edible films and Coatings for the extension of Shelf Life of Fresh Fruits. Am. J. Nutr. Food Sci. 2015, 2, 16–20. [Google Scholar] [CrossRef]
- Duran, A.; Kahve, H.I. The use of chitosan as a coating material. Acad. J. Sci. 2016, 05, 167–172. [Google Scholar]
- Van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Pavinatto, A.; de Almeida Mattos, A.V.; Malpass, A.C.G.; Okura, M.H.; Balogh, D.T.; Sanfelice, R.C. Coating with chitosan-based edible films for mechanical/biological protection of strawberries. Int. J. Biol. Macromol. 2020, 151, 1004–1011. [Google Scholar] [CrossRef]
- Mohan, C.O.; Ravishankar, C.N.; Lalitha, K.V.; Srinivasa Gopal, T.K. Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocoll. 2012, 26, 167–174. [Google Scholar] [CrossRef]
- Duan, J.; Park, S.I.; Daeschel, M.A.; Zhao, Y. Antimicrobial chitosan-lysozyme (CL) films and coatings for enhancing microbial safety of mozzarella cheese. J. Food Sci. 2007, 72, M355–M362. [Google Scholar] [CrossRef]
- Günlü, A.; Sipahioǧlu, S.; Alpas, H. The effect of chitosan-based edible film and high hydrostatic pressure process on the microbiological and chemical quality of rainbow trout (Oncorhynchus mykiss Walbaum) fillets during cold storage (4±1 °C). High Press. Res. 2014, 34, 110–121. [Google Scholar] [CrossRef]
- Günlü, A.; Koyun, E. Effects of Vacuum Packaging and Wrapping with Chitosan-Based Edible Film on the Extension of the Shelf Life of Sea Bass (Dicentrarchus labrax) Fillets in Cold Storage (4 °C). Food Bioprocess Technol. 2013, 6, 1713–1719. [Google Scholar] [CrossRef]
- Vu, C.H.T.; Won, K. Novel water-resistant UV-activated oxygen indicator for intelligent food packaging. Food Chem. 2013, 140, 52–56. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, H.J.; Rhim, J.W. Effect of sulfur nanoparticles on properties of alginate-based films for active food packaging applications. Food Hydrocoll. 2021, 110, 106155. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Blanco-Pascual, N.; Montero, M.P.; Gómez-Guillén, M.C. Antioxidant film development from unrefined extracts of brown seaweeds Laminaria digitata and Ascophyllum nodosum. Food Hydrocoll. 2014, 37, 100–110. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, B.; Qin, Y.; Li, F.; Yang, S.; Lu, P.; Wang, L.; Fan, J. Preparation and characterization of antifungal coating films composed of sodium alginate and cyclolipopeptides produced by Bacillus subtilis. Int. J. Biol. Macromol. 2020, 143, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical properties of alginate-based films: Effect of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef]
- Singh, P.; Baisthakur, P.; Yemul, O.S. Synthesis, characterization and application of crosslinked alginate as green packaging material. Heliyon 2020, 6, e03026. [Google Scholar] [CrossRef] [PubMed]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef]
- Rangel-Marrón, M.; Mani-López, E.; Palou, E.; López-Malo, A. Effects of alginate-glycerol-citric acid concentrations on selected physical, mechanical, and barrier properties of papaya puree-based edible films and coatings, as evaluated by response surface methodology. LWT 2019, 101, 83–91. [Google Scholar] [CrossRef]
- Koushki, M.R.; Azizi, M.H.; Azizkhani, M.; Koohy-Kamaly, P. Effect of Different Formulations on Mechanical and Physical Properties of Calcium Alginate Edible Films. J. Food Qual. Hazards Control 2015, 2, 45–50. [Google Scholar]
- Elzoghby, A.O.; Abo El-Fotoh, W.S.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release 2011, 153, 206–216. [Google Scholar] [CrossRef]
- Jeya, J.; Chandrasekaran, M.; Durairaj, R.; Mageshwaran, G.; Joseph, G.B. A Brief Review on Edible Food Packaging Materials. J. Glob. Eng. Probl. Solut. 2017, 1, 9–19. [Google Scholar]
- Khwaldia, K.; Ferez, C.; Banon, S.; Desobry, S.; Hardy, J. Milk proteins for edible films and coatings. Crit. Rev. Food Sci. Nutr. 2004, 44, 239–251. [Google Scholar] [CrossRef]
- Shendurse, A.; Gopikrishna, G.; Profile, S.; Pandya, A.J. Milk protein based edible films and coatings-preparation, properties and food applications. J. Nutr. Heal. Food Eng. 2018, 8, 219–226. [Google Scholar] [CrossRef]
- Tkaczewska, J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings—A review. Trends Food Sci. Technol. 2020, 106, 298–311. [Google Scholar] [CrossRef]
- Singh, P.; Singh, T.P.; Gandhi, N. Prevention of lipid oxidation in muscle foods by milk proteins and peptides: A review. Food Rev. Int. 2018, 34, 226–247. [Google Scholar] [CrossRef]
- Chambi, H.; Grosso, C. Edible films produced with gelatin and casein cross-linked with transglutaminase. Food Res. Int. 2006, 39, 458–466. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Influence of calcium on tensile, optical and water vapor permeability properties of sodium caseinate edible films. J. Food Eng. 2010, 96, 356–364. [Google Scholar] [CrossRef]
- Mauer, L.J.; Smith, D.E.; Labuza, T.P. Water vapor permeability, mechanical, and structural properties of edible β-casein films. Int. Dairy J. 2000, 10, 353–358. [Google Scholar] [CrossRef]
- Schou, M.; Longares, A.; Montesinos-Herrero, C.; Monahan, F.J.; O’Riordan, D.; O’Sullivan, M. Properties of edible sodium caseinate films and their application as food wrapping. LWT Food Sci. Technol. 2005, 38, 605–610. [Google Scholar] [CrossRef]
- Tomasula, P.M.; Parris, N.; Yee, W.; Coffin, D. Properties of Films Made from CO2-Precipitated Casein. J. Agric. Food Chem. 1998, 46, 4470–4474. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; O’Neill, L.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Physicochemical characterization of plasma-treated sodium caseinate film. Food Res. Int. 2014, 66, 438–444. [Google Scholar] [CrossRef]
- Ma, Y.; Teng, A.; Zhao, K.; Zhang, K.; Zhao, H.; Duan, S.; Li, S.; Guo, Y.; Wang, W. A top-down approach to improve collagen film’s performance: The comparisons of macro, micro and nano sized fibers. Food Chem. 2020, 309, 125624. [Google Scholar] [CrossRef]
- Yamauchi, K.; Takeuchi, N.; Kurimoto, A.; Tanabe, T. Films of collagen crosslinked by S-S bonds: Preparation and characterization. Biomaterials 2001, 22, 855–863. [Google Scholar] [CrossRef]
- Mauri, A.N.; Añón, M.C. Mechanical and Physical Properties of Soy Protein Films with pH-Modified Microstructures. Food Sci. Technol. Int. 2008, 14, 119–125. [Google Scholar] [CrossRef]
- Matmaroh, K.; Benjakul, S.; Prodpran, T.; Encarnacion, A.B.; Kishimura, H. Characteristics of acid soluble collagen and pepsin soluble collagen from scale of spotted golden goatfish (Parupeneus heptacanthus). Food Chem. 2011, 129, 1179–1186. [Google Scholar] [CrossRef]
- Shi, D.; Liu, F.; Yu, Z.; Chang, B.; Goff, H.D.; Zhong, F. Effect of aging treatment on the physicochemical properties of collagen films. Food Hydrocoll. 2019, 87, 436–447. [Google Scholar] [CrossRef]
- Hashim, P.; Mohd Ridzwan, M.S.; Bakar, J.; Mat Hashim, D. Collagen in food and beverage industries. Int. Food Res. J. 2015, 22, 1–8. [Google Scholar]
- Oechsle, A.M.; Wittmann, X.; Gibis, M.; Kohlus, R.; Weiss, J. Collagen entanglement influenced by the addition of acids. Eur. Polym. J. 2014, 58, 144–156. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Gómez-Guillén, M.C. A state-of-the-art review on the elaboration of fish gelatin as bioactive packaging: Special emphasis on nanotechnology-based approaches. Trends Food Sci. Technol. 2018, 79, 125–135. [Google Scholar] [CrossRef]
- Wolf, K.L.; Sobral, P.J.A.; Telis, V.R.N. Physicochemical characterization of collagen fibers and collagen powder for self-composite film production. Food Hydrocoll. 2009, 23, 1886–1894. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, W.; Li, Y.; Gao, G.; Zhang, K.; Zhou, J.; Wu, Z. Cross-linking and film-forming properties of transglutaminase-modified collagen fibers tailored by denaturation temperature. Food Chem. 2019, 271, 527–535. [Google Scholar] [CrossRef]
- Gennadios, A.; Hanna, M.A.; Kurth, L.B. Application of edible coatings on meats, poultry and seafoods: A review. LWT Food Sci. Technol. 1997, 30, 337–350. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Structure and rheology of gelatin gels: Recent progress. Polymer 1992, 33, 2622–2627. [Google Scholar] [CrossRef]
- Djabourov, M.; Lechaire, J.P.; Gaill, F. Structure and rheology of gelatin and collagen gels. Biorheology 1993, 30, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Deiber, J.A.; Ottone, M.L.; Piaggio, M.V.; Peirotti, M.B. Characterization of cross-linked polyampholytic gelatin hydrogels through the rubber elasticity and thermodynamic swelling theories. Polymer 2009, 50, 6065–6075. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Díaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Sobral, P.J.A.; Thomazine, M.; Habitante, A.M.Q.B.; Giménez, B.; Gómez-Guillén, M.C.; Montero, P. Development of edible films based on differently processed Atlantic halibut (Hippoglossus hippoglossus) skin gelatin. Food Hydrocoll. 2008, 22, 1117–1123. [Google Scholar] [CrossRef]
- Mu, C.; Guo, J.; Li, X.; Lin, W.; Li, D. Preparation and properties of dialdehyde carboxymethyl cellulose crosslinked gelatin edible films. Food Hydrocoll. 2012, 27, 22–29. [Google Scholar] [CrossRef]
- Ustunol, Z. Edible Films and Coatings for Meat and Poultry. In Edible Films and Coatings for Food Applications, 1st ed.; Huber, K., Embuscado, M., Eds.; Springer: New York, NY, USA, 2009; pp. 245–268. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Carvalho, R.A.; Grosso, C.R.F. Effect of hydrophobic plasticizers on functional properties of gelatin-based films. Food Res. Int. 2009, 42, 1113–1121. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; Nashy, E.S.H.A.; Othman, A.M. Novel natural composite films as packaging materials with enhanced properties. Int. J. Biol. Macromol. 2019, 136, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Min, S.C. Trout Skin Gelatin-Based Edible Film Development. J. Food Sci. 2012, 77, E240–E246. [Google Scholar] [CrossRef]
- Sompie, M.; Tinangon, R.M.; Surtijono, S.E.; Said, M.I. Effect of long-time immersion in edible film solution from local chicken claw on the physical and chemical properties of chicken meat. IOP Conf. Ser. Earth Environ. Sci. 2020, 492, 012056. [Google Scholar] [CrossRef]
- Park, J.; Nam, J.; Yun, H.; Jin, H.J.; Kwak, H.W. Aquatic polymer-based edible films of fish gelatin crosslinked with alginate dialdehyde having enhanced physicochemical properties. Carbohydr. Polym. 2020, 254, 117317. [Google Scholar] [CrossRef]
- Sobral, P.J.A.; Menegalli, F.C.; Hubinger, M.D.; Roques, M.A. Mechanical, water vapor barrier and thermal properties of gelatin based edible films. Food Hydrocoll. 2001, 15, 423–432. [Google Scholar] [CrossRef]
- Bertan, L.C.; Tanada-Palmu, P.S.; Siani, A.C.; Grosso, C.R.F. Effect of fatty acids and “Brazilian elemi” on composite films based on gelatin. Food Hydrocoll. 2005, 19, 73–82. [Google Scholar] [CrossRef]
- Ribba, L.; Garcia, N.L.; D’Accorso, N.; Goyanes, S. Chapter 3Disadvantages of Starch-Based Materials, Feasible Alternatives in Order to Overcome These Limitations. In Starch-Based Materials in Food Packaging, 1st ed.; Villar, M.A., Barbosa, S.E., García, M.A., Castillo, L.A., López, O.V., Eds.; Academic Press: London, UK, 2017; pp. 37–76. [Google Scholar] [CrossRef]
- Molavi, H.; Behfar, S.; Shariati, M.A.; Kaviani, M.; Atarod, S. A review on biodegradable starch based film. J. Microbiol. Biotech. Food Sci. 2015, 4, 456–461. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Arab-Tehrany, E.; Cháfer, M.; González-Martínez, C.; Chiralt, A. Active Edible and Biodegradable Starch Films. In Polysaccharides, 1st ed.; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Cham, Switzerland, 2014; pp. 1–15. [Google Scholar] [CrossRef]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Compr Rev Food Sci Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef]
- Perez Sira, E.; Dufour, D. Native and Modified Starches as Matrix for Edible Films and Covers. Nutr. Food Sci. Int. J. 2017, 3, 555615. [Google Scholar] [CrossRef]
- Farahnaky, A.; Saberi, B.; Majzoobi, M. Effect of glycerol on physical and mechanical properties of wheat starch edible films. J. Texture Stud. 2013, 44, 176–186. [Google Scholar] [CrossRef]
- Ugalde, M.L.; de Cezaro, A.M.; Vedovatto, F.; Paroul, N.; Steffens, J.; Valduga, E.; Backes, G.T.; Franceschi, E.; Cansian, R.L. Active starch biopolymeric packaging film for sausages embedded with essential oil of Syzygium aromaticum. J. Food Sci. Technol. 2017, 54, 2171–2175. [Google Scholar] [CrossRef]
- Sapper, M.; Chiralt, A. Starch-Based Coatings for Preservation of Fruits and Vegetables. Coatings 2018, 8, 152. [Google Scholar] [CrossRef]
- Khumkomgool, A.; Saneluksana, T.; Harnkarnsujarit, N. Active meat packaging from thermoplastic cassava starch containing sappan and cinnamon herbal extracts via LLDPE blown-film extrusion. Food Packag. Shelf Life 2020, 26, 100557. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Kandeepan, G. Selection of biopolymers to develop a biodegradable and edible film for packaging of luncheon chicken meat slices. Asian J. Dairy Food Res. 2017, 36, 67–71. [Google Scholar] [CrossRef][Green Version]
- Huber, K.; Embuscado, M. Edible Films and Coatings for Food Applications, 1st ed.; Springer: New York, NY, USA, 2009; 411p. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Averous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-Based Bio- and Nanocomposites: A Review. Int. J. Polym. Sci. 2011, 2011, 837875. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P.M. Edible Polymers: Challenges and Opportunities. J. Polym. 2014, 2014, 427259. [Google Scholar] [CrossRef]
- Fotie, G.; Limbo, S.; Piergiovanni, L. Manufacturing of Food Packaging Based on Nanocellulose: Current Advances and Challenges. Nanomaterials 2020, 10, 1726. [Google Scholar] [CrossRef]
- Tajeddin, B. Chapter 21. Cellulose-Based Polymers for Packaging Applications. In Lignocellulosic Polymer Composites, 1st ed.; Thakur, V.K., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2014; pp. 477–498. [Google Scholar] [CrossRef]
- Silva, F.A.G.S.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose Bio-Based Composites for Food Packaging. Nanomaterials 2020, 10, 2041. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Katiyar, V. Cellulose-Based Hydrogel Films for Food Packaging. In Cellulose-Based Superabsorbent Hydrogels. Polymers and Polymeric Composites: A Reference Series, 1st ed.; Mondal, M., Ed.; Springer: Cham, Switzerland, 2019; pp. 1061–1084. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Yang, B.; Weng, Y. Hemicellulose-Based Film: Potential Green Films for Food Packaging. Polymers 2020, 12, 1775. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Ukkola, J.; Liimatainen, H. Effect of plasticizers on the mechanical and thermomechanical properties of cellulose-based biocomposite films. Ind. Crops Prod. 2018, 122, 513–521. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Khan, A.; Niazi, M.B.K.; Naqvi, S.R.; Farooq, W. Influence of Plasticizers on Mechanical and Thermal Properties of Methyl Cellulose-Based Edible Films. J. Polym. Environ. 2018, 26, 291–300. [Google Scholar] [CrossRef]
- Indrarti, L.; Indriyati; Syampurwadi, A.; Pujiastuti, S. Physical and Mechanical Properties of Modified Bacterial Cellulose Composite Films. AIP Conf. Proc. 2016, 1711, 050007. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent Trends in the Use of Pectin from Agro-Waste Residues as a Natural-Based Biopolymer for Food Packaging Applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Pinheiro, A.C.; Cerqueira, M.A.; Rocha, C.M.R.; Avides, M.C.; Quintas, M.A.C.; Vicente, A.A. Physico-chemical characterization of chitosan-based edible films incorporating bioactive compounds of different molecular weight. J. Food Eng. 2011, 106, 111–118. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan. In Sustainable Agriculture Reviews 35. Sustainable Agriculture Reviews., 1st ed.; Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2019; Volume 35, pp. 49–123. [Google Scholar] [CrossRef]
- Cissé, M.; Montet, D.; Loiseau, G.; Ducamp-Collin, M.-N. Influence of the Concentrations of Chitosan and Glycerol on Edible Film Properties Showed by Response Surface Methodology. J. Polym. Environ. 2012, 20, 830–837. [Google Scholar] [CrossRef]
- Singh, T.P.; Chatli, M.K.; Sahoo, J. Development of chitosan based edible films: Process optimization using response surface methodology. J. Food Sci. Technol. 2015, 52, 2530–2543. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Abdullah, N.A.S.; Mohamad, Z.; Khan, Z.I.; Jusoh, M.; Zakaria, Z.Y.; Ngadi, N. Alginate Based Sustainable Films and Composites for Packaging: A Review. Chem. Eng. Trans. 2021, 83, 271–276. [Google Scholar] [CrossRef]
- Marismandani, A.D.P.; Husni, A. Development and Characterization of Biobased Alginate/Glycerol/Virgin Coconut Oil as Biodegradable Packaging. E3S Web Conf. 2020, 147, 03016. [Google Scholar] [CrossRef]
- Nehchiri, N.; Amiri, S.; Radi, M. Improving the water barrier properties of alginate packaging films by submicron coating with drying linseed oil. Packag. Technol. Sci. 2021, 34, 283–295. [Google Scholar] [CrossRef]
- Gheorghita, R.; Gutt, G.; Amariei, S. The Use of Edible Films Based on Sodium Alginate in Meat Product Packaging: An Eco-Friendly Alternative to Conventional Plastic Materials. Coatings 2020, 10, 166. [Google Scholar] [CrossRef]
- Nataraj, D.; Reddy, N. Chemical modifications of alginate and its derivatives. Int. J. Chem. Res. 2020, 4, 1–17. [Google Scholar] [CrossRef]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Bourtoom, T. Edible protein films: Properties enhancement. Int. Food Res. J. 2009, 16, 1–9. [Google Scholar]
- Wagh, Y.R.; Pushpadass, H.A.; Emerald, F.M.; Nath, B.S. Preparation and characterization of milk protein films and their application for packaging of Cheddar cheese. J. Food Sci. Technol. 2014, 51, 3767–3775. [Google Scholar] [CrossRef]
- Khwaldia, K.; Banon, S.; Perez, C.; Desobry, S. Properties of Sodium Caseinate Film-Forming Dispersions and Films. J. Dairy Sci. 2004, 87, 2011–2016. [Google Scholar] [CrossRef]
- Abdalrazeq, M.; Giosafatto, C.V.L.; Esposito, M.; Fenderico, M.; Di Pierro, P.; Porta, R. Glycerol-Plasticized Films Obtained from Whey Proteins Denatured at Alkaline pH. Coatings 2019, 9, 322. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peltzer, M.A.; del Carmen Garrigós, M.; Jiménez, A. Structure and mechanical properties of sodium and calcium caseinate edible active films with carvacrol. J. Food Eng. 2013, 114, 486–494. [Google Scholar] [CrossRef]
- Maruddin, F.; Malaka, R.; Baba, S.; Amqam, H.; Taufik, M.; Sabil, S. Brightness, elongation and thickness of edible film with caseinate sodium using a type of plasticizer. IOP Conf. Ser. Earth Environ. Sci. 2020, 492, 012043. [Google Scholar] [CrossRef]
- Saez-Orviz, S.; Laca, A.; Rendueles, M.; Díaz, M. Approaches for casein film uses in food stuff packaging. Afinidad –Barcelona 2017, 74, 26–29. [Google Scholar]
- Coltelli, M.B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A.; et al. State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: An extensive review. Coatings 2016, 6, 1. [Google Scholar] [CrossRef]
- Said, M.I.; Erwanto, Y.; Abustam, E. Properties of Edible Film Produced using Combination of Collagen Extracts of Bligon Goatskin with Glycerol. Am. J. Anim. Vet. Sci. 2016, 11, 151–159. [Google Scholar] [CrossRef]
- Alizadeh, A.; Behfar, S. Properties of collagen based edible films in food packaging: A review. Ann. Biol. Res. 2013, 4, 253–256. [Google Scholar]
- Mihalca, V.; Kerezsi, A.D.; Weber, A.; Gruber-Traub, C.; Schmucker, J.; Vodnar, D.C.; Dulf, F.V.; Socaci, S.A.; Fărcaș, A.; Mureșan, C.I.; et al. Protein-Based Films and Coatings for Food Industry Applications. Polymers 2021, 13, 769. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, F.; Yu, Z.; Ma, Y.; Goff, H.D.; Zhong, F. Improvement in physicochemical properties of collagen casings by glutaraldehyde cross-linking and drying temperature regulating. Food Chem. 2020, 318, 126404. [Google Scholar] [CrossRef]
- Ikeuchi, Y. 24 - Recent advances in the application of high pressure technology to processed meat products. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Processed Meats, 1st ed.; Kerry, J.P., Kerry, J.F., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 590–616. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Wang, W.; Han, Y.; Liu, A. Improved mechanical and thermal properties of gelatin films using a nano inorganic filler. J. Food Process Eng. 2017, 40, e12469. [Google Scholar] [CrossRef]
- Wihodo, M.; Moraru, C.I. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. J. Food Eng. 2013, 114, 292–302. [Google Scholar] [CrossRef]
- Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Improvement of barrier properties of fish gelatin films promoted by gelatin glycation with lactose at high temperatures. LWT Food Sci. Technol. 2015, 63, 315–321. [Google Scholar] [CrossRef]
- Thomazine, M.; Carvalho, R.A.; Sobral, P.J.A. Physical Properties of Gelatin Films Plasticized by Blends of Glycerol and Sorbitol. J. Food Sci. 2005, 70, E172–E176. [Google Scholar] [CrossRef]
- Arpi, N.; Fahrizal; Martunis; Hardianti, E. Preparation and characterization of biodegradable film based on skin and bone fish gelatin. IOP Conf. Ser. Earth Environ. Sci. 2018, 207, 012050. [Google Scholar] [CrossRef]
- Al-Hassan, A.A.; Norziah, M.H. Starch-gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Thi, M.T.L.; Hiroki, M.; Emiko, O.; Kazufumi, O. Effect of preparing conditions on properties of gelatin film from horse mackerel scale. KnE Life Sci. 2015, 32–34. [Google Scholar] [CrossRef]
- Jorge, M.F.C.; Alexandre, E.M.C.; Flaker, C.H.C.; Bittante, A.M.Q.B.; Sobral, P.J.d.A. Biodegradable Films Based on Gelatin and Montmorillonite Produced by Spreading. Int. J. Polym. Sci. 2015, 2015, 806791. [Google Scholar] [CrossRef]
- Azmi, N.S.; Basha, R.K.; Arifin, N.N.T.; Othman, S.H.; Mohammed, M.A.P. Functional Properties of Tilapia’s Fish Scale Gelatin Film: Effects of Different Type of Plasticizers. Sains Malays. 2020, 49, 2221–2229. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: Possibilities and limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Li, Y.; Zhu, L.; Fang, Z.; Shi, Q. Physicochemical, mechanical and structural properties of composite edible films based on whey protein isolate/psyllium seed gum. Int. J. Biol. Macromol. 2020, 153, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vergara, L.D.; Cifuentes, M.T.; Franco, A.P.; Pérez-Cervera, C.E.; Andrade-Pizarro, R.D. Development and characterization of edible films based on native cassava starch, beeswax, and propolis. NFS J. 2020, 21, 39–49. [Google Scholar] [CrossRef]
- Suwanprateep, S.; Kumsapaya, C.; Sayan, P. Structure and Thermal Properties of Rice Starch-based Film Blended with Mesocarp Cellulose Fiber. Mater. Today 2019, 17, 2039–2047. [Google Scholar] [CrossRef]
- Dias, A.B.; Müller, C.M.O.; Larotonda, F.D.S.; Laurindo, J.B. Mechanical and barrier properties of composite films based on rice flour and cellulose fibers. LWT Food Sci. Technol. 2011, 44, 535–542. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohydr. Polym. 2008, 72, 369–375. [Google Scholar] [CrossRef]
- Tosati, J.V.; Messias, V.C.; Carvalho, P.I.N.; Rodrigues Pollonio, M.A.; Meireles, M.A.A.; Monteiro, A.R. Antimicrobial Effect of Edible Coating Blend Based on Turmeric Starch Residue and Gelatin Applied onto Fresh Frankfurter Sausage. Food Bioprocess Technol. 2017, 10, 2165–2175. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Kolehmainen, A.; Liimatainen, H.; Niinimäki, J.; Hormi, O.E.O. Biocomposite cellulose-alginate films: Promising packaging materials. Food Chem. 2014, 151, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Yang, J.; Liu, P.; Xu, M.; Zhang, X.; Zhang, L. Fabrication, properties and bioapplications of cellulose/collagen hydrolysate composite films. Carbohydr. Polym. 2013, 92, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Luo, X.; Li, W.; Song, R.; Li, J.; Li, Y.; Li, B.; Liu, S. Green and biodegradable composite films with novel antimicrobial performance based on cellulose. Food Chem. 2016, 197, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Brito, T.B.; Carrajola, J.F.; Gonçalves, E.C.B.A.; Martelli-Tosi, M.; Ferreira, M.S.L. Fruit and vegetable residues flours with different granulometry range as raw material for pectin-enriched biodegradable film preparation. Food Res. Int. 2019, 121, 412–421. [Google Scholar] [CrossRef]
- Nešić, A.; Onjia, A.; Davidović, S.; Dimitrijević, S.; Errico, M.E.; Santagata, G.; Malinconico, M. Design of pectin-sodium alginate based films for potential healthcare application: Study of chemico-physical interactions between the components of films and assessment of their antimicrobial activity. Carbohydr. Polym. 2017, 157, 981–990. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Di Pierro, P.; Gunning, P.; Mackie, A.; Porta, R.; Mariniello, L. Characterization of Citrus pectin edible films containing transglutaminase-modified phaseolin. Carbohydr. Polym. 2014, 106, 200–208. [Google Scholar] [CrossRef]
- Ahmad, M.; Nirmal, N.P.; Danish, M.; Chuprom, J.; Jafarzedeh, S. Characterisation of composite films fabricated from collagen/chitosan and collagen/soy protein isolate for food packaging applications. RSC Adv. 2016, 6, 82191–82204. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Nkenmogne Kamdem, I.E.; Saidou, C.; Ngassoum, M.B.; Ndjouenkeu, R. Synergistic interactions in dilute aqueous solutions between alginate and tropical vegetal hydrocolloids. Heliyon 2020, 6, e04348. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; Rodrigues, J.M.; Valadares, A.C.F.; de Almeida, A.B.; de Lima, T.M.; Takeuchi, K.P.; Alves, C.C.F.; de Figueiredo Sousa, H.A.; da Silva, E.R.; Dyszy, F.H.; et al. Active food packaging: Alginate films with cottonseed protein hydrolysates. Food Hydrocoll. 2019, 92, 267–275. [Google Scholar] [CrossRef]
- Gomaa, M.; Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M. Use of seaweed and filamentous fungus derived polysaccharides in the development of alginate-chitosan edible films containing fucoidan: Study of moisture sorption, polyphenol release and antioxidant properties. Food Hydrocoll. 2018, 82, 239–247. [Google Scholar] [CrossRef]
- Chick, J.; Hernandez, R.J. Physical, Thermal, and Barrier Characterization of Casein-Wax-Based Edible Films. J. Food Sci. 2002, 67, 1073–1079. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Wang, R.; Chai, Y. Preparation and characterization of homogeneous and enhanced casein protein-based composite films via incorporating cellulose microgel. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Eghbal, N.; Degraeve, P.; Oulahal, N.; Yarmand, M.S.; Mousavi, M.E.; Gharsallaoui, A. Low methoxyl pectin/sodium caseinate interactions and composite film formation at neutral pH. Food Hydrocoll. 2017, 69, 132–140. [Google Scholar] [CrossRef]
- Ben Slimane, E.; Sadok, S. Collagen from Cartilaginous Fish By-Products for a Potential Application in Bioactive Film Composite. Mar. Drugs 2018, 16, 211. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, M.; Li, G. Preparation and characterization of collagen/hydroxypropyl methylcellulose (HPMC) blend film. Carbohydr. Polym. 2015, 119, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Lima, Á.M.; Cerqueira, M.A.; Souza, B.W.S.; Santos, E.C.M.; Teixeira, J.A.; Moreira, R.A.; Vicente, A.A. New edible coatings composed of galactomannans and collagen blends to improve the postharvest quality of fruits—Influence on fruits gas transfer rate. J. Food Eng. 2010, 97, 101–109. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J.A. Gelatin-chitosan edible film activated with Boldo extract for improving microbiological and antioxidant stability of sliced Prato cheese. Int. J. Food Sci. Technol. 2019, 54, 1617–1624. [Google Scholar] [CrossRef]
- Jridi, M.; Hajji, S.; Ben Ayed, H.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, structural, antioxidant and antimicrobial properties of gelatin-chitosan composite edible films. Int. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Wang, L.; Auty, M.A.E.; Kerry, J.P. Physical assessment of composite biodegradable films manufactured using whey protein isolate, gelatin and sodium alginate. J. Food Eng. 2010, 96, 199–207. [Google Scholar] [CrossRef]
- Tabari, M. Investigation of Carboxymethyl Cellulose (CMC) on Mechanical Properties of Cold Water Fish Gelatin Biodegradable Edible Films. Foods 2017, 6, 41. [Google Scholar] [CrossRef]
- Kurek, M.; Galus, S.; Debeaufort, F. Surface, mechanical and barrier properties of bio-based composite films based on chitosan and whey protein. Food Packag. Shelf Life 2014, 1, 56–67. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan- and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Garrigós, M.C.; Jiménez, A. Natural additives and agricultural wastes in biopolymer formulations for food packaging. Front. Chem. 2014, 2, 6. [Google Scholar] [CrossRef]
- Nor, S.M.; Ding, P. Trends and advances in edible biopolymer coating for tropical fruit: A review. Food Res. Int. 2020, 134, 109208. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zheng, Q. Ecomaterials based on food proteins and polysaccharides. Polym. Rev. 2014, 54, 514–571. [Google Scholar] [CrossRef]
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Zondervan-van den Beuken, E.; Tobback, P. Active and intelligent food packaging: Legal aspects and safety concerns. Trends Food Sci. Technol. 2008, 19, S103–S112. [Google Scholar] [CrossRef]
- Salgado, P.R.; Ortiz, C.M.; Musso, Y.S.; Di Giorgio, L.; Mauri, A.N. Edible films and coatings containing bioactives. Curr. Opin. Food Sci. 2015, 5, 86–92. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Guilbert, S. Edible films and coatings as active layers. In Active Food Packaging, 1st ed.; Rooney, M.L., Ed.; Springer: Boston, MA, USA, 1995; pp. 111–142. [Google Scholar] [CrossRef]
- Suput, D.; Lazic, V.; Popovic, S.; Hromis, N. Edible films and coatings: Sources, properties and application. Food Feed Res. 2015, 42, 11–22. [Google Scholar] [CrossRef]
- ASTM D882-10 Standard Test Method for Tensile Properties of Thin Plastic Sheeting. 2010. Available online: https://doi.org/10.1520/D0882-10 (accessed on 24 December 2020).
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Han, J.H.; Aristippos, G. 15 - Edible films and coatings: A review. In Innovations in Food Packaging, 1st ed.; Han, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 239–262. [Google Scholar] [CrossRef]
- Lacroix, M. Mechanical and Permeability Properties of Edible Films and Coatings for Food and Pharmaceutical Applications. In Edible Films and Coatings for Food Applications, 1st ed.; Huber, K., Embuscado, M., Eds.; Springer: New York, NY, USA, 2009; pp. 347–366. [Google Scholar] [CrossRef]
- Avramescu, S.M.; Butean, C.; Popa, C.V.; Ortan, A.; Moraru, I.; Temocico, G. Edible and Functionalized Films/Coatings—Performances and Perspectives. Coatings 2020, 10, 687. [Google Scholar] [CrossRef]
- ISO 527-1:2019(en), Plastics—Determination of Tensile Properties—Part 1: General Principles. 2019. Available online: https://www.iso.org/ru/standard/75824.html (accessed on 24 December 2020).
- McHugh, T.H.; Krochta, J.M. Sorbitol-vs Glycerol-Plasticized Whey Protein Edible Films: Integrated Oxygen Permeability and Tensile Property Evaluation. J. Agric. Food Chem. 1994, 42, 841–845. [Google Scholar] [CrossRef]
- Nandane, A.S.; Jain, R. Study of mechanical properties of soy protein based edible film as affected by its composition and process parameters by using RSM. J. Food Sci. Technol. 2015, 52, 3645–3650. [Google Scholar] [CrossRef]
- Pena-Serna, C.; Lopes Filho, J.F. Biodegradable zein-based blend films: Structural, mechanical and barrier properties. Food Technol. Biotechnol. 2015, 53, 348–353. [Google Scholar] [CrossRef]
- Seung, Y.C.; Rhee, C. Mechanical properties and water vapor permeability of edible films made from fractionated soy proteins with ultrafiltration. LWT Food Sci. Technol. 2004, 37, 833–839. [Google Scholar] [CrossRef]
- Arham, R.; Mulyati, M.T.; Metusalach, M.; Salengke, S. Physical and mechanical properties of agar based edible film with glycerol plasticizer. Int. Food Res. J. 2016, 23, 1669–1675. [Google Scholar] [CrossRef]
- Choi, W.S.; Han, J.H. Physical and mechanical properties of pea-protein-based edible films. J. Food Sci. 2001, 66, 319–322. [Google Scholar] [CrossRef]
- Razmjoo, F.; Sadeghi, E.; Rouhi, M.; Mohammadi, R.; Noroozi, R.; Safajoo, S. Polyvinyl alcohol—Zedo gum edible film: Physical, mechanical and thermal properties. J. Appl. Polym. Sci. 2021, 138, 49875. [Google Scholar] [CrossRef]
- Talja, R.A.; Helén, H.; Roos, Y.H.; Jouppila, K. Effect of type and content of binary polyol mixtures on physical and mechanical properties of starch-based edible films. Carbohydr. Polym. 2008, 71, 269–276. [Google Scholar] [CrossRef]
- Chillo, S.; Flores, S.; Mastromatteo, M.; Conte, A.; Gerschenson, L.; Del Nobile, M.A. Influence of glycerol and chitosan on tapioca starch-based edible film properties. J. Food Eng. 2008, 88, 159–168. [Google Scholar] [CrossRef]
- ASTM D6241 - 14 Standard Test Method for Static Puncture Strength of Geotextiles and Geotextile-Related Products Using a 50-mm Probe. 2014. Available online: https://doi.org/10.1520/D6241-14 (accessed on 25 March 2021).
- Nur Hazirah, M.A.S.P.; Isa, M.I.N.; Sarbon, N.M. Effect of xanthan gum on the physical and mechanical properties of gelatin-carboxymethyl cellulose film blends. Food Packag. Shelf Life 2016, 9, 55–63. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, Y.; Wang, Y.; Jiang, G.; Liang, M.; Chen, X.; Long, G. A Fractal Model for Kozeny-Carman Constant and Dimensionless Permeability of Fibrous Porous Media with Roughened Surfaces. Fractals 2019, 27, 1950116. [Google Scholar] [CrossRef]
- Liang, M.; Liu, Y.; Xiao, B.; Yang, S.; Wang, Z.; Han, H. An analytical model for the transverse permeability of gas diffusion layer with electrical double layer effects in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2018, 43, 17880–17888. [Google Scholar] [CrossRef]
- ASTM E96 - 00 Standard Test Methods for Water Vapor Transmission of Materials. 2000. Available online: https://doi.org/10.1520/E0096-00 (accessed on 25 December 2020).
- ASTM D3985 - 17 Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. 2017. Available online: https://doi.org/10.1520/D3985-17 (accessed on 25 December 2020).
- Sanchez-Tamayo, M.I.; Pasos, C.V.; Ochoa-Martinez, C.I. Methods for gas permeability measurement in edible films for fruits and vegetables: A review. Food Sci. Technol. 2020, in press. [Google Scholar] [CrossRef]
- Lee, K.T. Quality and safety aspects of meat products as affected by various physical manipulations of packaging materials. Meat Sci. 2010, 86, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Butler, B.L.; Vergano, P.J.; Testin, R.F.; Bunn, J.M.; Wiles, J.L. Mechanical and barrier properties of edible chitosan films as affected by composition and storage. J. Food Sci. 1996, 61, 953–956. [Google Scholar] [CrossRef]
- Wongphan, P.; Harnkarnsujarit, N. Edible packaging from hydroxypropyl thermoplastic cassava starch, agar and maltodextrin blends produced by cast extrusion. Int. J. Food Sci. Technol. 2020, 56, 62–772. [Google Scholar] [CrossRef]
- Sadeghi-Varkani, A.; Emam-Djomeh, Z.; Askari, G. Physicochemical and microstructural properties of a novel edible film synthesized from Balangu seed mucilage. Int. J. Biol. Macromol. 2018, 108, 1110–1119. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use of beef, pork and fish gelatin sources in the manufacture of films and assessment of their composition and mechanical properties. Food Hydrocoll. 2012, 29, 144–151. [Google Scholar] [CrossRef]
- Wang, L.Z.; Liu, L.; Holmes, J.; Kerry, J.F.; Kerry, J.P. Assessment of film-forming potential and properties of protein and polysaccharide-based biopolymer films. Int. J. Food Sci. Technol. 2007, 42, 1128–1138. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S. Bio-packaging: Technology and properties of edible and/or biodegradable material of agricultural origin. In Food Packaging and Preservation, 1st ed.; Mathlouthi, M., Ed.; Springer: Boston, MA, USA, 1994; pp. 159–181. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.-L. Edible Wheat Gluten Films: Influence of the Main Process Variables on Film Properties using Response Surface Methodology. J. Food Sci. 1992, 57, 190–195. [Google Scholar] [CrossRef]
- Da Rosa Zavareze, E.; Storck, C.R.; de Castro, L.A.S.; Schirmer, M.A.; Dias, A.R.G. Effect of heat-moisture treatment on rice starch of varying amylose content. Food Chem. 2010, 121, 358–365. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Walczak, M.; Michalska-Sionkowska, M.; Kaczmarek, B.; Sionkowska, A. Surface and antibacterial properties of thin films based on collagen and thymol. Mater. Today Commun. 2020, 22, 100949. [Google Scholar] [CrossRef]
- Gutiérrez, T.J. Surface and nutraceutical properties of edible films made from starchy sources with and without added blackberry pulp. Carbohydr. Polym. 2017, 165, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Kwok, D.Y.; Neumann, A.W. Contact angle interpretation in terms of solid surface tension. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 161, 31–48. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-based composite edible films containing Origanum vulgare L. essential oil. Ind. Crops Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- De Moura, M.R.; Aouada, F.A.; Avena-Bustillos, R.J.; McHugh, T.H.; Krochta, J.M.; Mattoso, L.H.C. Improved barrier and mechanical properties of novel hydroxypropyl methylcellulose edible films with chitosan/tripolyphosphate nanoparticles. J. Food Eng. 2009, 92, 448–453. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).