Latest Advances on the Synthesis of Linear ABC-Type Triblock Terpolymers and Star-Shaped Polymers by RAFT Polymerization
Abstract
1. Introduction
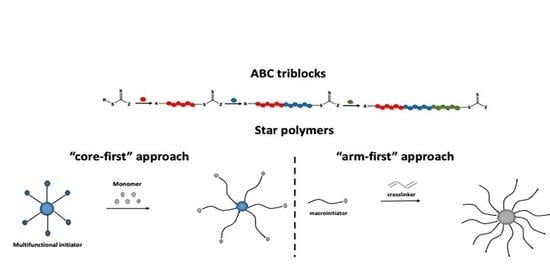
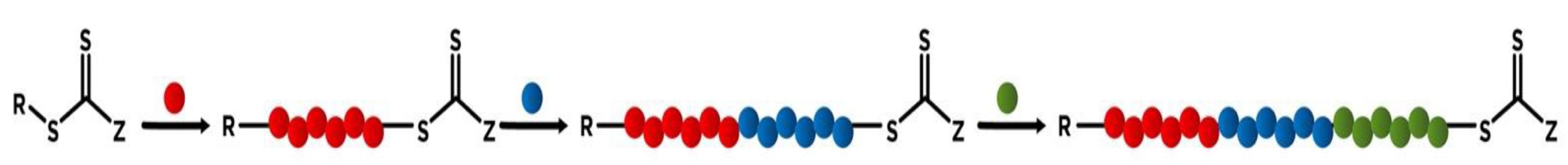
2. ABC-Type Linear Triblock Terpolymers
2.1. General Synthetic Strategies for ABC Triblock Copolymers by RAFT
2.2. Stimuli-Responsive ABC Triblock Copolymers
2.3. Triblock Terpolymers Self-Assembly Properties in Solvent Exchange Protocols
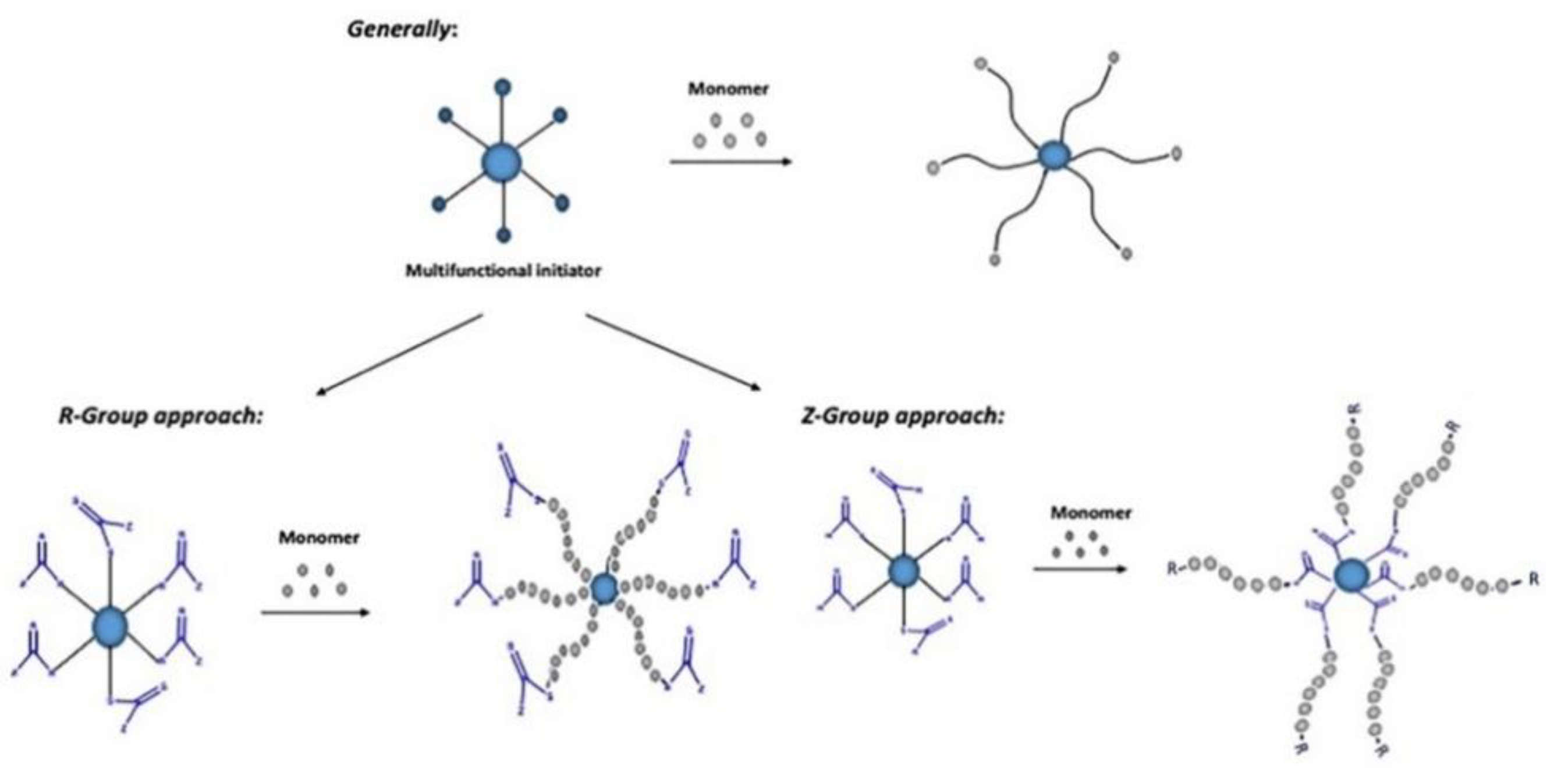
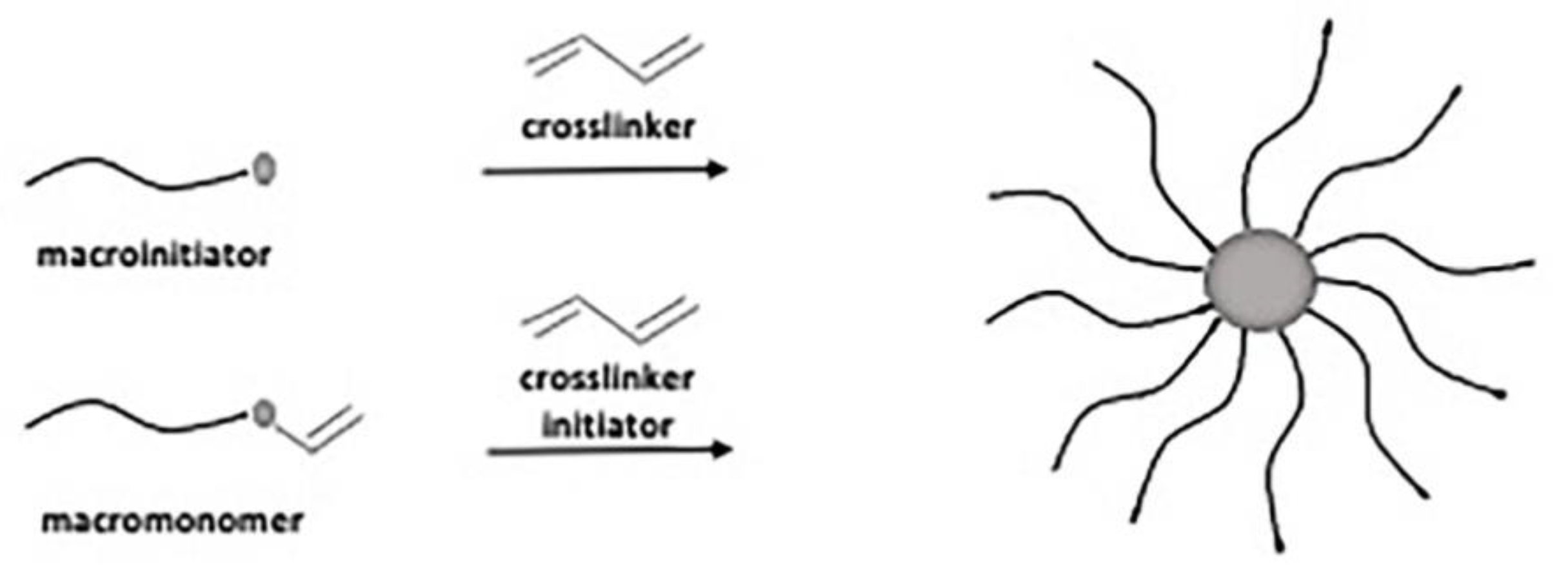
3. Star-Shaped Polymers
3.1. “Core-First” Approach
3.2. “Arm-First” Approach
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moad, G.; Rizzardo, E.; Thang, S.H. Toward living radical polymerization. Acc. Chem. Res. 2008, 41, 1133–1142. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process—A Second Update. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process—A First Update. Aust. J. Chem. 2006, 59, 669–692. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Radical addition–fragmentation chemistry in polymer synthesis. Polymer 2008, 49, 1079–1131. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization: A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Keddie, D.J. A guide to the synthesis of block copolymers using reversible-addition fragmentation chain transfer (RAFT) polymerization. Chem. Soc. Rev. 2014, 43, 496–505. [Google Scholar] [CrossRef]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef]
- Alfurhood, J.A.; Bachler, P.R.; Sumerlin, B.S. Hyperbranched polymers via RAFT self-condensing vinyl polymerization. Polym. Chem. 2016, 7, 3361–3369. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Moad, G. RAFT polymerization to form stimuli-responsive polymers. Polym. Chem. 2017, 8, 177–219. [Google Scholar] [CrossRef]
- Smith, A.E.; Xu, X.; McCormick, C.L. Stimuli-responsive amphiphilic (co)polymers via RAFT polymerization. Prog. Polym. Sci. 2010, 35, 45–93. [Google Scholar] [CrossRef]
- McCormick, C.L.; Lowe, A.B. Aqueous RAFT Polymerization: Recent Developments in Synthesis of Functional Water-Soluble (Co)polymers with Controlled Structures. Acc. Chem. Res. 2004, 37, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.B.; McCormick, C.L. Reversible addition–fragmentation chain transfer (RAFT) radical polymerization and the synthesis of water-soluble (co)polymers under homogeneous conditions in organic and aqueous media. Prog. Polym. Sci. 2007, 32, 283–351. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Luo, J.; Zeng, M.; Peng, B.; Tang, Y.; Zhang, L.; Wang, P.; He, L.; Huang, D.; Wang, L.; Wang, X.; et al. Electrostatic-Driven Dynamic Jamming of 2D Nanoparticles at Interfaces for Controlled Molecular Diffusion. Angew. Chem. Int. Ed. Engl. 2018, 57, 11752–11757. [Google Scholar] [CrossRef]
- Tumnantong, D.; Rempel, G.L.; Prasassarakich, P. Polyisoprene-Silica Nanoparticles Synthesized via RAFT Emulsifier-Free Emulsion Polymerization Using Water-Soluble Initiators. Polymers 2017, 9, 637. [Google Scholar] [CrossRef]
- Zeng, M.; Shah, S.A.; Huang, D.; Parviz, D.; Yu, Y.H.; Wang, X.; Green, M.J.; Cheng, Z. Aqueous Exfoliation of Graphite into Graphene Assisted by Sulfonyl Graphene Quantum Dots for Photonic Crystal Applications. ACS Appl. Mater. Interfaces 2017, 9, 30797–30804. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, W.; Liu, Q.; Chen, T.; Zhou, X.; Yang, C.; Zhang, K.; Xie, Z. Atom-economical, room-temperature, and high-efficiency synthesis of polyamides via a three-component polymerization involving benzoxazines, odorless isocyanides, and water. Polym. Chem. 2018, 9, 5566–5571. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Liu, Y.; Sun, J.Z.; Qin, A.; Tang, B.Z. Facile Polymerization of Water and Triple-Bond Based Monomers toward Functional Polyamides. Macromolecules 2017, 50, 8554–8561. [Google Scholar] [CrossRef]
- Keddie, D.J.; Moad, G.; Rizzardo, E.; Thang, S.H. RAFT Agent Design and Synthesis. Macromolecules 2012, 45, 5321–5342. [Google Scholar] [CrossRef]
- Destarac, M.; Charmot, D.; Franck, X.; Zard, S.Z. Dithiocarbamates as universal reversible addition-fragmentation chain transfer agents. Macromol. Rapid Commun. 2000, 21, 1035–1039. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Pispas, S.; Avgeropoulos, A. Linear and non-linear triblock terpolymers. Synthesis, self-assembly in selective solvents and in bulk. Prog. Polym. Sci. 2005, 30, 725–782. [Google Scholar] [CrossRef]
- Moad, G.; Mayadunne, R.T.A.; Rizzardo, E.; Skidmore, M.; Thang, S.H. Synthesis of novel architectures by radical polymerization with reversible addition fragmentation chain transfer (RAFT polymerization). Macromol. Symp. 2003, 192, 1–12. [Google Scholar] [CrossRef]
- Konishcheva, E.; Daubian, D.; Gaitzsch, J.; Meier, W. Synthesis of Linear ABC Triblock Copolymers and Their Self-Assembly in Solution. Helv. Chim. Acta 2018, 101. [Google Scholar] [CrossRef]
- Wyman, I.W.; Liu, G. Micellar structures of linear triblock terpolymers: Three blocks but many possibilities. Polymer 2013, 54, 1950–1978. [Google Scholar] [CrossRef]
- Kříž, J.; Masař, B.; Pleštil, J.; Tuzar, Z.; Pospíšil, H.; Doskočilová, D. Three-Layer Micelles of an ABC Block Copolymer: NMR, SANS, and LS Study of a Poly(2−ethylhexyl acrylate)-block-poly(methyl methacrylate)-block-poly(acrylic acid) Copolymer in D2O. Macromolecules 1998, 31, 41–51. [Google Scholar] [CrossRef]
- Hu, J.; Njikang, G.; Liu, G. Twisted ABC Triblock Copolymer Cylinders with Segregated A and C Coronal Chains. Macromolecules 2008, 41, 7993–7999. [Google Scholar] [CrossRef]
- Velez-Herrera, P.; Doyama, K.; Abe, H.; Ishida, H. Synthesis and Characterization of Highly Fluorinated Polymer with the Benzoxazine Moiety in the Main Chain. Macromolecules 2008, 41, 9704–9714. [Google Scholar] [CrossRef]
- Moughton, A.O.; Hillmyer, M.A.; Lodge, T.P. Multicompartment Block Polymer Micelles. Macromolecules 2012, 45, 2–19. [Google Scholar] [CrossRef]
- Erhardt, R.; Böker, A.; Zettl, H.; Kaya, H.; Pyckhout-Hintzen, W.; Krausch, G.; Abetz, V.; Müller, A.H.E. Janus Micelles. Macromolecules 2001, 34, 1069–1075. [Google Scholar] [CrossRef]
- Gregory, A.; Stenzel, M.H. Complex polymer architectures via RAFT polymerization: From fundamental process to extending the scope using click chemistry and nature’s building blocks. Prog. Polym. Sci. 2012, 37, 38–105. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H.; Driva, P.; Sakellariou, G.; Chatzichristidi, M. 6.03—Polymers with Star-Related Structures: Synthesis, Properties, and Applications. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 29–111. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pispas, S.; Pitsikalis, M.; Iatrou, H.; Vlahos, C. Asymmetric star polymers: Synthesis and properties. In Branched Polymers I.; Roovers, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 71–127. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Mays, J. Macromolecular architectures by living and controlled/living polymerizations. Prog. Polym. Sci. 2006, 31, 1068–1132. [Google Scholar] [CrossRef]
- Hu, J.; Qiao, R.; Whittaker, M.R.; Quinn, J.F.; Davis, T.P. Synthesis of star polymers by RAFT polymerization as versatile nanoparticles for biomedical applications. Aust. J. Chem. 2017, 70, 1161–1170. [Google Scholar] [CrossRef]
- Blencowe, A.; Tan, J.F.; Goh, T.K.; Qiao, G.G. Core cross-linked star polymers via controlled radical polymerisation. Polymer 2009, 50, 5–32. [Google Scholar] [CrossRef]
- Gao, H.; Matyjaszewski, K. Arm-first method as a simple and general method for synthesis of miktoarm star copolymers. J. Am. Chem. Soc. 2007, 129, 11828–11834. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, M.R.; Monteiro, M.J. Synthesis and aggregation behavior of four-arm star amphiphilic block copolymers in water. Langmuir 2006, 22, 9746–9752. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, L.; Collins, J.; Chen, G.; Wallis, R.; Mitchell, D.A.; Haddleton, D.M.; Becer, C.R. Dendritic cell lectin-targeting sentinel-like unimolecular glycoconjugates to release an anti-HIV drug. J. Am. Chem. Soc. 2014, 136, 4325–4332. [Google Scholar] [CrossRef]
- Barner, L.; Davis, T.P.; Stenzel, M.H.; Barner-Kowollik, C. Complex macromolecular architectures by reversible addition fragmentation chain transfer chemistry: Theory and practice. Macromol. Rapid Commun. 2007, 28, 539–559. [Google Scholar] [CrossRef]
- Wu, W.; Wang, W.; Li, J. Star polymers: Advances in biomedical applications. Prog. Polym. Sci. 2015, 46, 55–85. [Google Scholar] [CrossRef]
- Altintas, O.; Artar, M.; ter Huurne, G.; Voets, I.K.; Palmans, A.R.A.; Barner-Kowollik, C.; Meijer, E.W. Design and Synthesis of Triblock Copolymers for Creating Complex Secondary Structures by Orthogonal Self-Assembly. Macromolecules 2015, 48, 8921–8932. [Google Scholar] [CrossRef]
- Germack, D.S.; Wooley, K.L. RAFT-Based Synthesis and Characterization of ABC versus ACB Triblock Copolymers Containingtert-Butyl Acrylate, Isoprene, and Styrene Blocks. Macromol. Chem. Phys. 2007, 208, 2481–2491. [Google Scholar] [CrossRef]
- Ghamkhari, A. Antimicrobial activity evaluation of a novel triblock cationic copolymer (PHEMA-b-PNIPAM-b-PVEAH). J. Hum. Health Halal Metr. 2020, 1, 35–41. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Y.; Li, X.; He, J.; Tan, J.; Zhang, L. Enzyme catalysis-induced RAFT polymerization in water for the preparation of epoxy-functionalized triblock copolymer vesicles. Polym. Chem. 2018, 9, 4908–4916. [Google Scholar] [CrossRef]
- Zhang, K.; Fahs, G.B.; Aiba, M.; Moore, R.B.; Long, T.E. Nucleobase-functionalized ABC triblock copolymers: Self-assembly of supramolecular architectures. Chem. Commun. 2014, 50, 9145–9148. [Google Scholar] [CrossRef] [PubMed]
- Messerschmidt, M.; Komber, H.; Häußler, L.; Hanzelmann, C.; Stamm, M.; Raether, B.; da Costa e Silva, O.; Uhlmann, P. Amphiphilic ABC Triblock Copolymers Tailored via RAFT Polymerization as Textile Surface Modifiers with Dual-Action Properties. Macromolecules 2013, 46, 2616–2627. [Google Scholar] [CrossRef]
- Chen, S.; Alcouffe, P.; Rousseau, A.; Gérard, J.-F.; Lortie, F.; Zhu, J.; Bernard, J. Design of Semicrystalline Elastomeric Glassy Triblock Copolymers from Oligoamide-Based RAFT Agents. Macromolecules 2020, 54, 94–104. [Google Scholar] [CrossRef]
- Mable, C.J.; Thompson, K.L.; Derry, M.J.; Mykhaylyk, O.O.; Binks, B.P.; Armes, S.P. ABC Triblock Copolymer Worms: Synthesis, Characterization, and Evaluation as Pickering Emulsifiers for Millimeter-Sized Droplets. Macromolecules 2016, 49, 7897–7907. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Banerjee, S.L.; Ghosh, S.K.; Singha, N.K. Smart Polyacrylate Emulsion Based on a New ABC-Type Triblock Copolymer via RAFT-Mediated Surfactant-Free Miniemulsion Polymerization: Its Multifunctional Properties. ACS Appl. Mater. Interfaces 2019, 11, 44722–44734. [Google Scholar] [CrossRef]
- Pal, S.; Kather, M.; Banerjee, S.L.; Saha, P.; Pich, A.; Singha, N.K. Dual Stimuli-Responsive Self-Assembly Behavior of a Tailor-Made ABC-Type Amphiphilic Tri-Block Copolymer. J. Polym. Sci. 2020, 58, 843–851. [Google Scholar] [CrossRef]
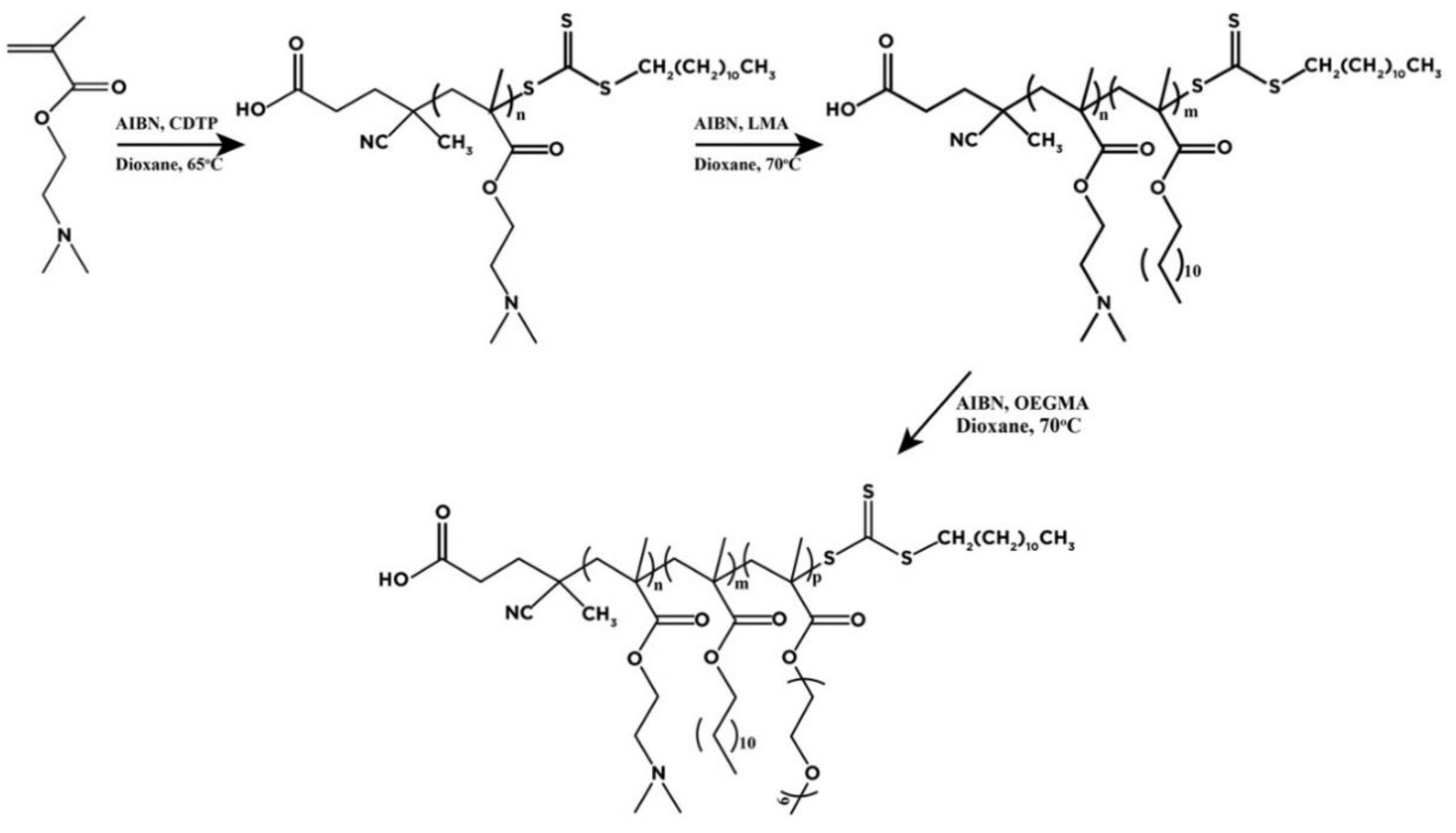
- Skandalis, A.; Pispas, S. PDMAEMA-b-PLMA-b-POEGMA triblock terpolymers via RAFT polymerization and their self-assembly in aqueous solutions. Polym. Chem. 2017, 8, 4538–4547. [Google Scholar] [CrossRef]
- Skandalis, A.; Uchman, M.; Štěpánek, M.; Kereїche, S.; Pispas, S. Complexation of DNA with QPDMAEMA-b-PLMA-b-POEGMA Cationic Triblock Terpolymer Micelles. Macromolecules 2020, 53, 5747–5755. [Google Scholar] [CrossRef]
- Skandalis, A.; Murmiliuk, A.; Stepanek, M.; Pispas, S. Physicochemical Evaluation of Insulin Complexes with QPDMAEMA-b-PLMA-b-POEGMA Cationic Amphiphlic Triblock Terpolymer Micelles. Polymers 2020, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, A.; Pispas, S. pH- and thermo-responsive solution behavior of amphiphilic, linear triblock terpolymers. Polymer 2018, 157, 9–18. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Meristoudi, A.; Pispas, S.; Keiderling, U. Thermal response of self-organization in an amphiphilic triblock polyelectrolyte and the influence of the globular protein lysozyme. Eur. Polym. J. 2018, 99, 49–57. [Google Scholar] [CrossRef]
- Giaouzi, D.; Pispas, S. Effects of Chemical Modifications on the Thermoresponsive Behavior of a PDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymer. Polymers 2020, 12, 1382. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tian, L.; Mao, H.; Ning, W.; Shang, P.; Wu, J.; Shi, X. Micellization and sol-gel transition of novel thermo- and pH-responsive ABC triblock copolymer synthesized by RAFT. J. Polym. Res. 2018, 25, 1–12. [Google Scholar] [CrossRef]
- Mahmoodzadeh, F.; Abbasian, M.; Jaymand, M.; Amirshaghaghi, A. A novel dual stimuli-responsive thiol-end-capped ABC triblock copolymer: Synthesis via reversible addition-fragmentation chain transfer technique, and investigation of its self-assembly behavior. Polym. Int. 2017, 66, 1651–1661. [Google Scholar] [CrossRef]
- Guragain, S.; Perez-Mercader, J. Light-mediated one-pot synthesis of an ABC triblock copolymer in aqueous solution via RAFT and the effect of pH on copolymer self-assembly. Polym. Chem. 2018, 9, 4000–4006. [Google Scholar] [CrossRef]
- Ye, Z.; Li, Y.; An, Z.; Wu, P. Exploration of Doubly Thermal Phase Transition Process of PDEGA-b-PDMA-b-PVCL in Water. Langmuir 2016, 32, 6691–6700. [Google Scholar] [CrossRef]
- Mäkinen, L.; Varadharajan, D.; Tenhu, H.; Hietala, S. Triple Hydrophilic UCST–LCST Block Copolymers. Macromolecules 2016, 49, 986–993. [Google Scholar] [CrossRef]
- Davaran, S.; Ghamkhari, A.; Alizadeh, E.; Massoumi, B.; Jaymand, M. Novel dual stimuli-responsive ABC triblock copolymer: RAFT synthesis, “schizophrenic” micellization, and its performance as an anticancer drug delivery nanosystem. J. Colloid Interface Sci. 2017, 488, 282–293. [Google Scholar] [CrossRef]
- Huang, Y.; Yong, P.; Chen, Y.; Gao, Y.; Xu, W.; Lv, Y.; Yang, L.; Reis, R.L.; Pirraco, R.P.; Chen, J. Micellization and gelatinization in aqueous media of pH- and thermo-responsive amphiphilic ABC (PMMA82-b-PDMAEMA150-b-PNIPAM65) triblock copolymer synthesized by consecutive RAFT polymerization. RSC Adv. 2017, 7, 28711–28722. [Google Scholar] [CrossRef]
- Banerjee, R.; Dhara, D. Functional group-dependent self-assembled nanostructures from thermo-responsive triblock copolymers. Langmuir 2014, 30, 4137–4146. [Google Scholar] [CrossRef] [PubMed]
- Koonar, I.; Zhou, C.; Hillmyer, M.A.; Lodge, T.P.; Siegel, R.A. ABC triblock terpolymers exhibiting both temperature- and pH-sensitive micellar aggregation and gelation in aqueous solution. Langmuir 2012, 28, 17785–17794. [Google Scholar] [CrossRef]
- Guang, N.-e.; Liu, S.-x.; Li, X.; Tian, L.; Mao, H.-g. Micellization and gelation of the double thermoresponsive ABC-type triblock copolymer synthesized by RAFT. Chin. J. Polym. Sci. 2016, 34, 965–980. [Google Scholar] [CrossRef]
- Li, Q.; Gao, C.; Li, S.; Huo, F.; Zhang, W. Doubly thermo-responsive ABC triblock copolymer nanoparticles prepared through dispersion RAFT polymerization. Polym. Chem. 2014, 5, 2961–2972. [Google Scholar] [CrossRef]
- Huo, F.; Li, S.; Li, Q.; Qu, Y.; Zhang, W. In-Situ Synthesis of Multicompartment Nanoparticles of Linear BAC Triblock Terpolymer by Seeded RAFT Polymerization. Macromolecules 2014, 47, 2340–2349. [Google Scholar] [CrossRef]
- Qu, Y.; Huo, F.; Li, Q.; He, X.; Li, S.; Zhang, W. In situ synthesis of thermo-responsive ABC triblock terpolymer nano-objects by seeded RAFT polymerization. Polym. Chem. 2014, 5, 5569–5577. [Google Scholar] [CrossRef]
- Li, Z.; Ma, J.; Lee, N.S.; Wooley, K.L. Dynamic cylindrical assembly of triblock copolymers by a hierarchical process of covalent and supramolecular interactions. J. Am. Chem. Soc. 2011, 133, 1228–1231. [Google Scholar] [CrossRef]
- Muslim, A.; Malik, D.; Hojiahmat, M. RAFT polymerization of linear ABC triblock copolymer PtBA-b-PS-b-P2VP and regulation of its hierarchical self-assembly structure in solution. Chem. Pap. 2015, 69, 1512–1518. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, R.; Hlavatovičová, E.; Rodzeń, K.; Strachota, A.; Kereïche, S.; Matějíček, P.; Cabrera-González, J.; Núñez, R.; Uchman, M. Synthesis and self-assembly of a carborane-containing ABC triblock terpolymer: Morphology control on a dual-stimuli responsive system. Polym. Chem. 2019, 10, 2774–2780. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Davis, T.P.; Stenzel, M.H. Synthesis of star polymers using RAFT polymerization: What is possible? Aust. J. Chem. 2006, 59, 719–727. [Google Scholar] [CrossRef]
- Mayadunne, R.T.; Jeffery, J.; Moad, G.; Rizzardo, E. Living free radical polymerization with reversible addition− fragmentation chain transfer (raft polymerization): Approaches to star polymers. Macromolecules 2003, 36, 1505–1513. [Google Scholar] [CrossRef]
- Altintas, O.; Abbasi, M.; Riazi, K.; Goldmann, A.S.; Dingenouts, N.; Wilhelm, M.; Barner-Kowollik, C. Stability of star-shaped RAFT polystyrenes under mechanical and thermal stress. Polym. Chem. 2014, 5, 5009–5019. [Google Scholar] [CrossRef]
- Allison-Logan, S.; Karimi, F.; Sun, Y.; McKenzie, T.G.; Nothling, M.D.; Bryant, G.; Qiao, G.G. Highly living stars via core-first photo-RAFT polymerization: Exploitation for ultra-high molecular weight star synthesis. ACS Macro Lett. 2019, 8, 1291–1295. [Google Scholar] [CrossRef]
- Chaffey-Millar, H.; Stenzel, M.H.; Davis, T.P.; Coote, M.L.; Barner-Kowollik, C. Design criteria for star polymer formation processes via living free radical polymerization. Macromolecules 2006, 39, 6406–6419. [Google Scholar] [CrossRef]
- Cao, M.; Han, G.; Duan, W.; Zhang, W. Synthesis of multi-arm star thermo-responsive polymers and topology effects on phase transition. Polym. Chem. 2018, 9, 2625–2633. [Google Scholar] [CrossRef]
- Bian, Q.; Xiao, Y.; Lang, M. R-RAFT approach for the polymerization of N-isopropylacrylamide with a star poly (ε-caprolactone) core. J. Polym. Sci. Part. A Polym. Chem. 2012, 50, 571–580. [Google Scholar] [CrossRef]
- Herfurth, C.; de Molina, P.M.; Wieland, C.; Rogers, S.; Gradzielski, M.; Laschewsky, A. One-step RAFT synthesis of well-defined amphiphilic star polymers and their self-assembly in aqueous solution. Polym. Chem. 2012, 3, 1606–1617. [Google Scholar] [CrossRef]
- Liu, J.; Tao, L.; Xu, J.; Jia, Z.; Boyer, C.; Davis, T.P. RAFT controlled synthesis of six-armed biodegradable star polymeric architectures via a ‘core-first’methodology. Polymer 2009, 50, 4455–4463. [Google Scholar] [CrossRef]
- Liao, X.; Walden, G.; Falcon, N.D.; Donell, S.; Raxworthy, M.J.; Wormstone, M.; Riley, G.P.; Saeed, A. A direct comparison of linear and star-shaped poly (dimethylaminoethyl acrylate) polymers for polyplexation with DNA and cytotoxicity in cultured cell lines. Eur. Polym. J. 2017, 87, 458–467. [Google Scholar] [CrossRef]
- Skandalis, A.; Pispas, S. Synthesis of (AB) n-, AnBn-, and AxBy-type amphiphilic and double-hydrophilic star copolymers by RAFT polymerization. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1771–1783. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Liu, Q.; Li, S.; Perrier, S.; Zhao, Y. Facile synthesis of hyperbranched and star-shaped polymers by RAFT polymerization based on a polymerizable trithiocarbonate. Macromolecules 2011, 44, 2034–2049. [Google Scholar] [CrossRef]
- Qiu, Q.; Liu, G.; An, Z. Efficient and versatile synthesis of star polymers in water and their use as emulsifiers. Chem. Commun. 2011, 47, 12685–12687. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Syrett, J.; Whittaker, M.; Haddleton, D.; Davis, T.P.; Boyer, C. Optimizing the generation of narrow polydispersity ‘arm-first’star polymers made using RAFT polymerization. Polym. Chem. 2011, 2, 1671–1677. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y. Allyl functionalized telechelic linear polymer and star polymer via RAFT polymerization. Polymer 2006, 47, 5259–5266. [Google Scholar] [CrossRef]
- Audouin, F.; Knoop, R.J.; Huang, J.; Heise, A. Star polymers by cross-linking of linear poly (benzyl-L-glutamate) macromonomers via free-radical and RAFT polymerization. A simple route toward peptide-stabilized nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4602–4610. [Google Scholar] [CrossRef]
- Boyer, C.; Teo, J.; Phillips, P.; Erlich, R.B.; Sagnella, S.; Sharbeen, G.; Dwarte, T.; Duong, H.T.; Goldstein, D.; Davis, T.P. Effective delivery of siRNA into cancer cells and tumors using well-defined biodegradable cationic star polymers. Mol. Pharm. 2013, 10, 2435–2444. [Google Scholar] [CrossRef]
- Teo, J.; McCarroll, J.A.; Boyer, C.; Youkhana, J.; Sagnella, S.M.; Duong, H.T.; Liu, J.; Sharbeen, G.; Goldstein, D.; Davis, T.P. A rationally optimized nanoparticle system for the delivery of RNA interference therapeutics into pancreatic tumors in vivo. Biomacromolecules 2016, 17, 2337–2351. [Google Scholar] [CrossRef]
- Wei, X.; Moad, G.; Muir, B.W.; Rizzardo, E.; Rosselgong, J.; Yang, W.; Thang, S.H. An Arm-First Approach to Cleavable Mikto-Arm Star Polymers by Raft Polymerization. Macromol. Rapid Commun. 2014, 35, 840–845. [Google Scholar] [CrossRef]
- Chen, C.; Guo, X.; Du, J.; Choi, B.; Tang, H.; Feng, A.; Thang, S.H. Synthesis of multifunctional miktoarm star polymers via an RGD peptide-based RAFT agent. Polym. Chem. 2019, 10, 228–234. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Sun, Z.; Hao, B.; Lee, T.-C.; Feng, A.; Zhang, L.; Thang, S.H. Synthesis of star-shaped polyzwitterions with adjustable UCST and fast responsiveness by a facile RAFT polymerization. Polym. Chem. 2020, 11, 3162–3168. [Google Scholar] [CrossRef]
- Sharker, K.K.; Takeshima, S.; Toyama, Y.; Ida, S.; Kanaoka, S.; Yusa, S.-I. pH-and thermo-responsive behavior of PNIPAM star containing terminal carboxy groups in aqueous solutions. Polymer 2020, 203, 122735. [Google Scholar] [CrossRef]
- Bray, C.; Peltier, R.; Kim, H.; Mastrangelo, A.; Perrier, S. Anionic multiblock core cross-linked star copolymers via RAFT polymerization. Polym. Chem. 2017, 8, 5513–5524. [Google Scholar] [CrossRef]
- Vlassi, E.; Papagiannopoulos, A.; Pispas, S. Amphiphilic AxBy mikto-arm star copolymers with PLMA and POEGMA arms: Self-assembly and drug encapsulation. J. Polym. Sci. 2021, 59, 775–786. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skandalis, A.; Sentoukas, T.; Giaouzi, D.; Kafetzi, M.; Pispas, S. Latest Advances on the Synthesis of Linear ABC-Type Triblock Terpolymers and Star-Shaped Polymers by RAFT Polymerization. Polymers 2021, 13, 1698. https://doi.org/10.3390/polym13111698
Skandalis A, Sentoukas T, Giaouzi D, Kafetzi M, Pispas S. Latest Advances on the Synthesis of Linear ABC-Type Triblock Terpolymers and Star-Shaped Polymers by RAFT Polymerization. Polymers. 2021; 13(11):1698. https://doi.org/10.3390/polym13111698
Chicago/Turabian StyleSkandalis, Athanasios, Theodore Sentoukas, Despoina Giaouzi, Martha Kafetzi, and Stergios Pispas. 2021. "Latest Advances on the Synthesis of Linear ABC-Type Triblock Terpolymers and Star-Shaped Polymers by RAFT Polymerization" Polymers 13, no. 11: 1698. https://doi.org/10.3390/polym13111698
APA StyleSkandalis, A., Sentoukas, T., Giaouzi, D., Kafetzi, M., & Pispas, S. (2021). Latest Advances on the Synthesis of Linear ABC-Type Triblock Terpolymers and Star-Shaped Polymers by RAFT Polymerization. Polymers, 13(11), 1698. https://doi.org/10.3390/polym13111698