Biomimetic Mineralization of Tannic Acid-Supplemented HEMA/SBMA Nanocomposite Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydrogel Materials
2.3. Characterization
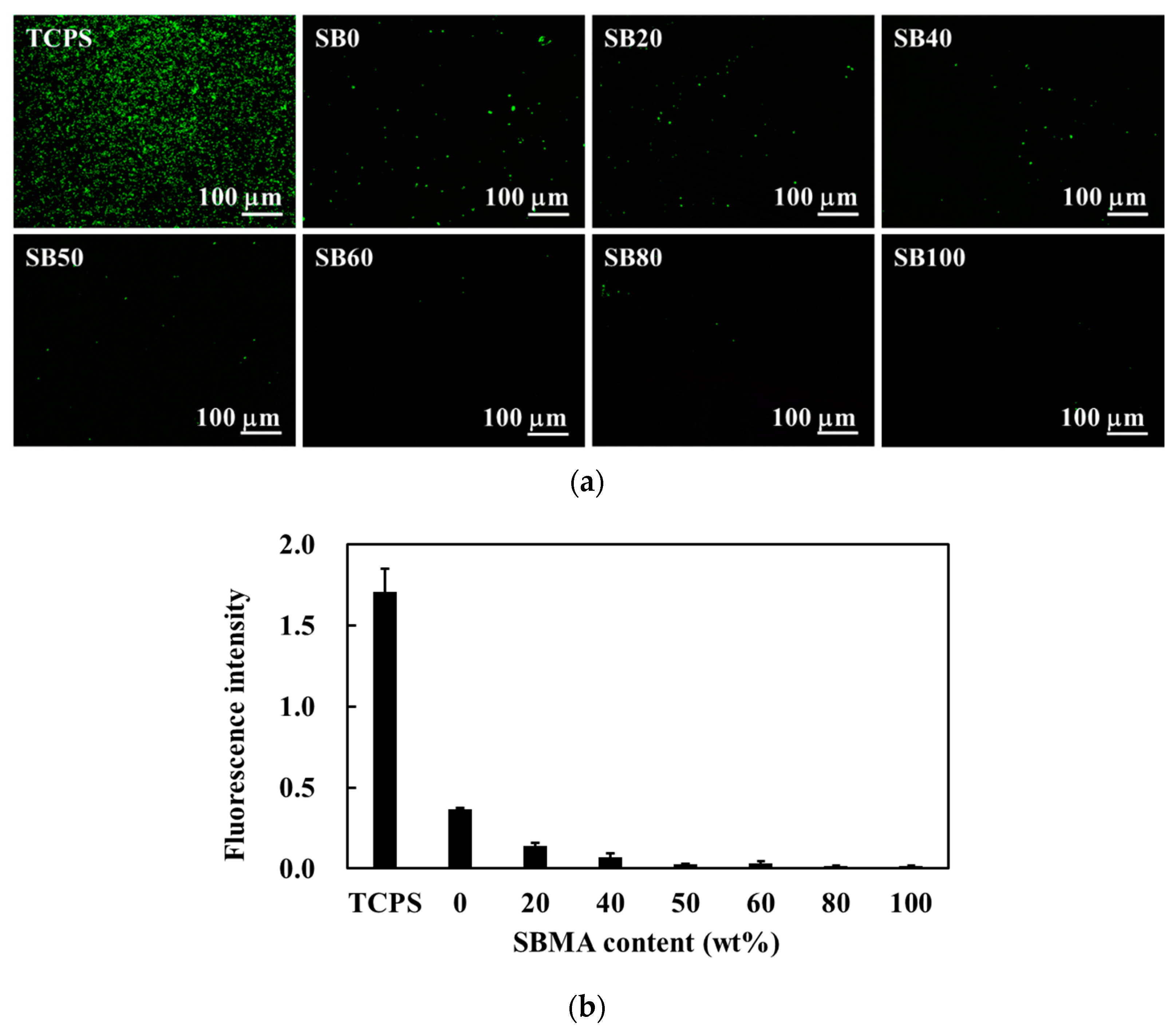
2.4. Evaluation of Bacterial Attachment
2.5. Mineralization
2.6. Statistical Analysis
3. Results
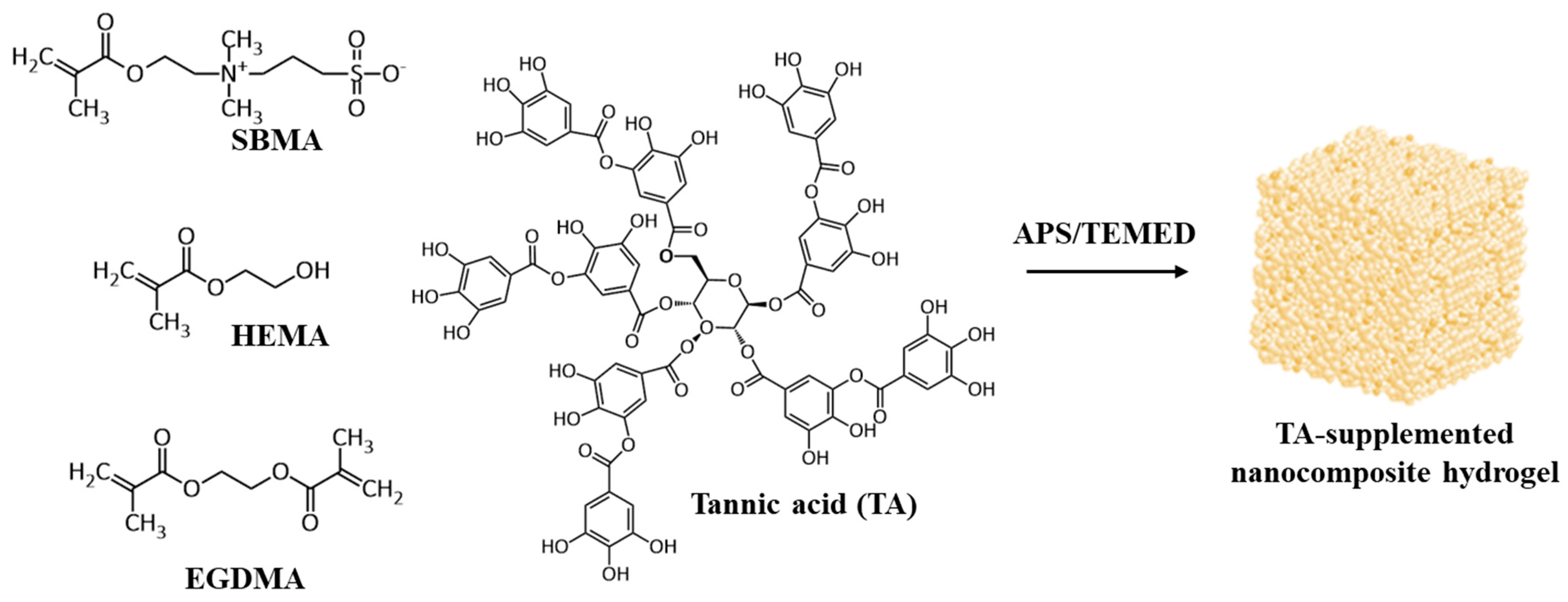
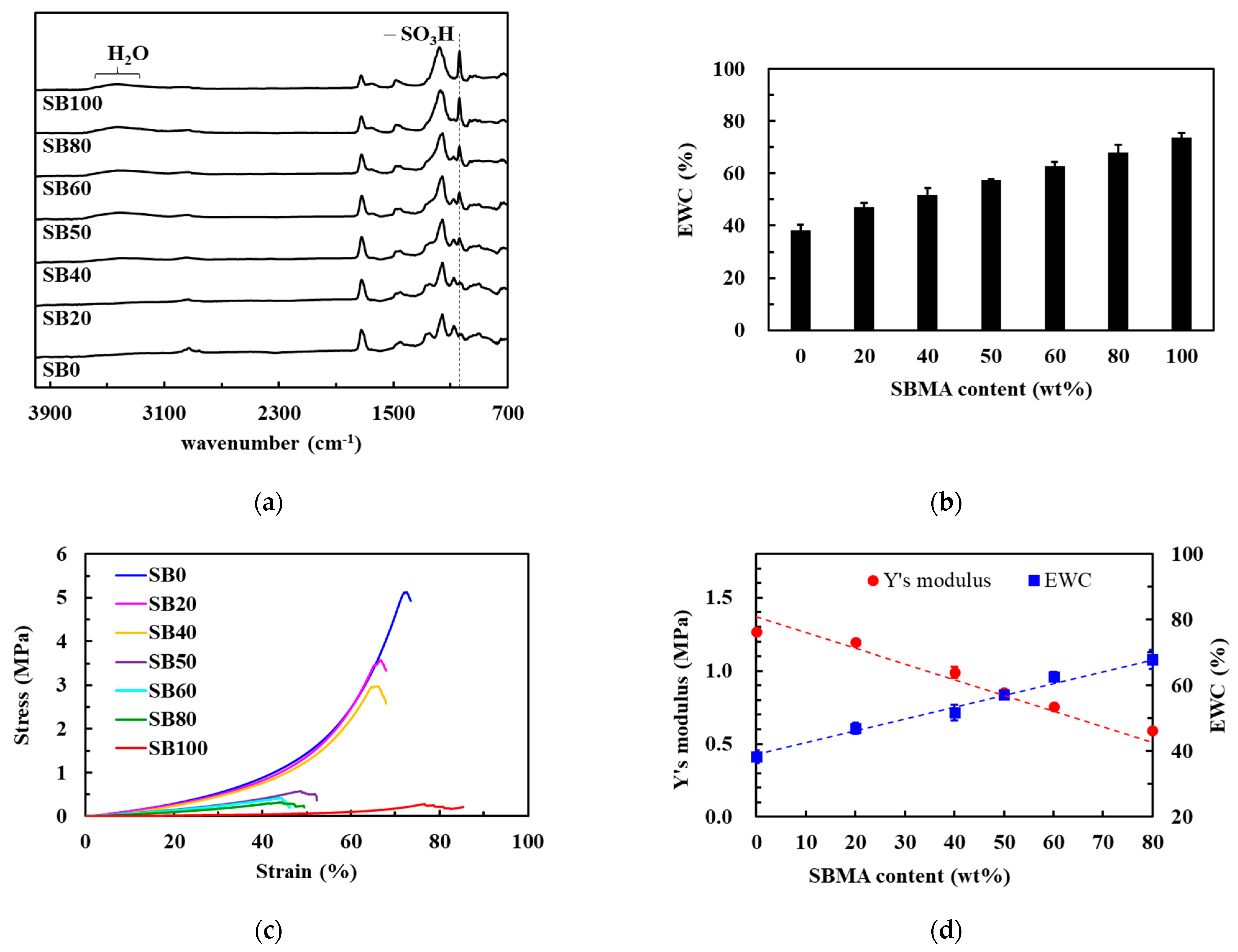
3.1. Preparation of Hybrid HEMA/SBMA Hydrogels
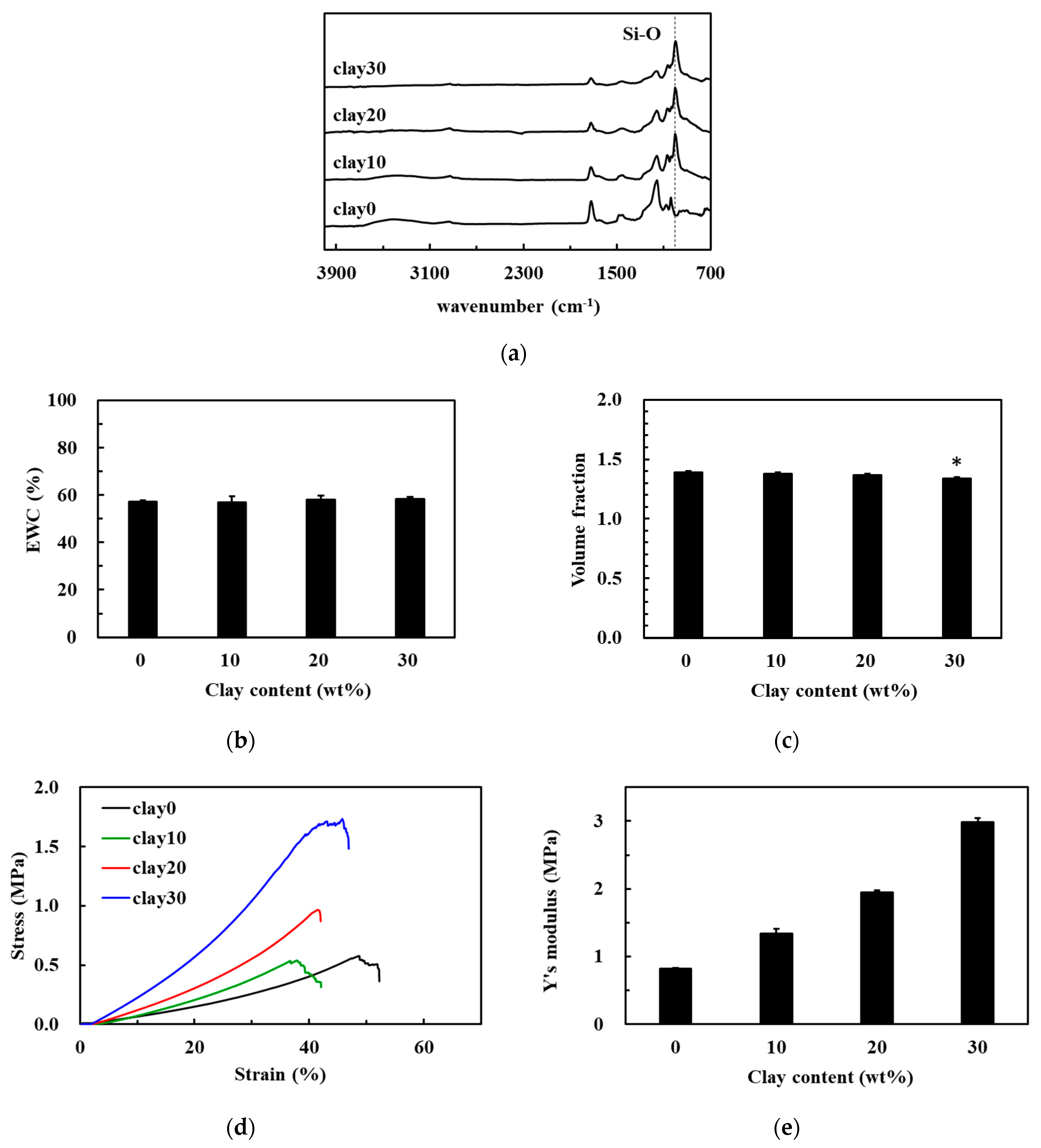
3.2. Preparation of Nanocomposite Hydrogels
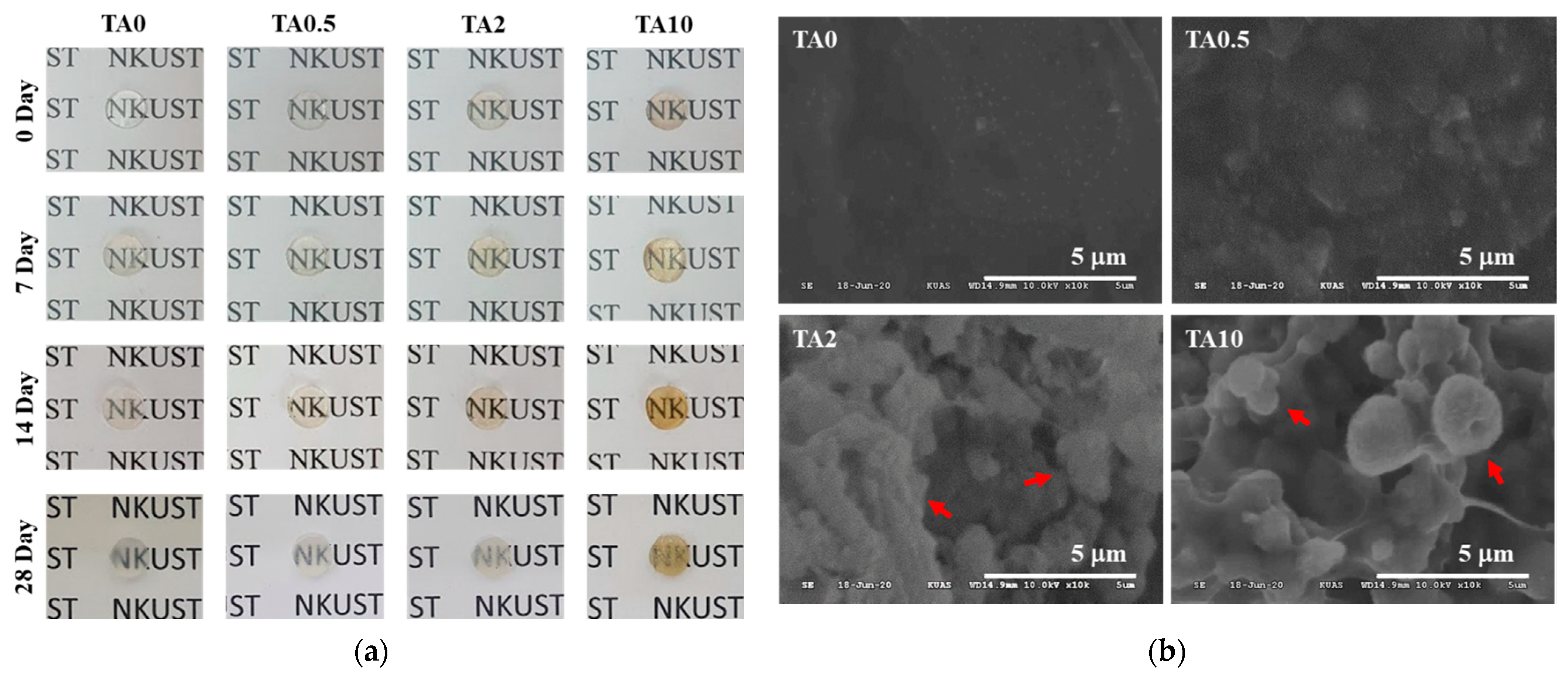
3.3. Preparation of TA-Supplemented Nanocomposite Hydrogels for Mineralization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynska, A.L.; Binias, D.; Maniukiewicz, W.; Modrzejewska, Z.; Douglas, T.E.L. The mineralization effect on chitosan hydrogel structure containing collagen and alkaline phosphatase. J. Mol. Struct. 2019, 1187, 86–97. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Huang, P.; Jiang, G.; Zhang, M.; Yu, F.; Zhang, W.; Fu, G.; Wang, Y.; Li, W.; et al. A novel mineralized high strength hydrogel for enhancing cell adhesion and promoting skull bone regeneration in situ. Compos. Part B. Eng. 2020, 197, 108183. [Google Scholar] [CrossRef]
- Gkioni, K.; Leeuwenburgh, S.C.G.; Douglas, T.E.L.; Mikos, A.G.; Jansen, J.A. Mineralization of hydrogels for bone regeneration. Tissue Eng. Part. B Rev. 2010, 16, 577. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Galante, R.; Pinto, T.J.A.; Colaço, R.; Serro, A.P. Sterilization of hydrogels for biomedical applications: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2472–2492. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, D.; Zheng, M.; Kissel, T.; Agarwal, S. Biocompatible and degradable poly(2-hydroxyethyl methacrylate) based polymers for biomedical applications. Polym. Chem. 2012, 3, 2752–2759. [Google Scholar] [CrossRef]
- Çetin, D.; Kahraman, A.S.; Gümüşderelioğlu, M. Novel scaffolds based on poly(2-hydroxyethyl methacrylate) superporous hydrogels for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2011, 22, 1157. [Google Scholar]
- Lee, J.H.; Youm, J.S.; Ju, H.T.; Kim, J.C. Influence of N-vinyl-2-pyrrolidone and methacrylic acid on thermal curing process of 2-hydroxyethyl methacrylate hydrogel. J. Appl. Polym. Sci. 2020, 137, 48622. [Google Scholar] [CrossRef]
- Bhat, A.; Smith, B.; Dinu, C.-Z.; Guiseppi-Elie, A. Molecular engineering of poly(HEMA-co-PEGMA)-based hydrogels: Role of minor AEMA and DMAEMA inclusion. Mater. Sci. Eng. C 2019, 98, 89–100. [Google Scholar] [CrossRef]
- Kim, S.; Shin, B.H.; Yang, C.; Jeong, S.; Shim, J.H.; Park, M.H.; Choy, Y.B.; Heo, C.Y.; Lee, K. Development of poly(HEMA-Am) polymer hydrogel filler for soft tissue reconstruction by facile polymerization. Polymers 2018, 10, 772. [Google Scholar] [CrossRef]
- Del Grosso, C.A.; Leng, C.; Zhang, K.; Hung, H.-C.; Jiang, S.; Chen, Z.; Wilker, J.J. Surface hydration for antifouling and bio-adhesion. Chem. Sci. 2020, 11, 10367–10377. [Google Scholar] [CrossRef]
- Chien, H.-W.; Yu, J.; Li, S.T.; Chen, H.Y.; Tsai, W.-B. An in situ poly(carboxybetaine) hydrogel for tissue engineering applications. Biomater. Sci. 2017, 5, 322. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-W.; Tsai, W.-B.; Jiang, S. Direct cell encapsulation in biodegradable and functionalizable carboxybetaine hydrogels. Biomaterials 2012, 33, 5706–5712. [Google Scholar] [CrossRef]
- Liu, P.; Song, J. Sulfobetaine as a zwitterionic mediator for 3D hydroxyapatite mineralization. Biomaterials 2013, 34, 2442. [Google Scholar] [CrossRef]
- Carr, L.; Cheng, G.; Xue, H.; Jiang, S. Engineering the polymer backbone to strengthen nonfouling sulfobetaine hydrogels. Langmuir 2010, 26, 14793–14798. [Google Scholar] [CrossRef]
- Hua, J.; Ng, P.F.; Fei, B. High-strength hydrogels: Microstructure design, characterization and applications. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1325–1335. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, J.; Lee, C.-S.; Kim, S.; Chen, C.; Lee, M. Supramolecular hydrogels based on nanoclay and guanidine-rich chitosan: Injectable and moldable osteoinductive carriers. ACS Appl. Mater. Interfaces 2020, 12, 16088–16096. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Schexnailder, P.J.; Kline, B.P.; Schmidt, G. Assessment of using Laponite® cross-linked poly(ethylene oxide) for controlled cell adhesion and mineralization. Acta Biomater. 2011, 7, 568–577. [Google Scholar] [CrossRef]
- Hlushko, R.; Hlushko, H.; Sukhishvili, S.A. A family of linear phenolic polymers with controlled hydrophobicity, adsorption and antioxidant properties. Polym. Chem. 2018, 9, 506–516. [Google Scholar] [CrossRef]
- Rahim, M.A.; Ejima, H.; Cho, K.L.; Kempe, K.; Müllner, M.; Best, J.P.; Caruso, F. Coordination-driven multistep assembly of metal–polyphenol films and capsules. Chem. Mater. 2014, 26, 1645–1653. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, R.; Gung, B.W.; Tindall, S.; Gonzalez, J.M.; Halvorson, J.J.; Hagerman, A.E. Polyphenol–aluminum complex formation: Implications for aluminum tolerance in plants. J. Agric. Food Chem. 2016, 64, 3025–3033. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, J.; Tan, G.; Liao, J.; Zhou, L.; Chen, J.; Yu, P.; Wang, Q.; Ning, C. Incorporating catechol into electroactive polypyrrole nanowires on titanium to promote hydroxyapatite formation. Bioact. Mater. 2018, 3, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-W.; Kuo, C.-J. Preparation, material properties and antimicrobial efficacy of silicone hydrogel by modulating silicone and hydrophilic monomer. J. Biomater. Sci. Polym. Ed. 2019, 30, 1050. [Google Scholar] [CrossRef]
- Marques, P.A.A.P.; Serro, A.P.; Saramago, B.J.; Fernandes, A.C.; Magalhães, M.C.F.; Correia, R.N. Mineralisation of two calcium phosphate ceramics in biological model fluids. J. Mater. Chem. 2003, 13, 1484–1490. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Liao, C.-C.; Chen, Y.-C.; Ou, S.-F.; Chiu, C.-Y. The feasibility of eco-friendly electrical discharge machining for surface modification of Ti: A comparison study in surface properties, bioactivity, and cytocompatibility. Mater. Sci. Eng. C 2020, 108, 110192. [Google Scholar] [CrossRef]
- Shao, Q.; He, Y.; White, A.D.; Jiang, S. Difference in hydration between carboxybetaine and sulfobetaine. J. Phys. Chem. B 2010, 114, 16625–16631. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Chang, C.-W.; van Spreeuwel, A.; Zhang, C.; Varghese, S. PEG/clay nanocomposite hydrogel: A mechanically robust tissue engineering scaffold. Soft Matter 2010, 6, 5157–5164. [Google Scholar] [CrossRef]
- Xiang, H.; Xia, M.; Cunningham, A.; Chen, W.; Sun, B.; Zhu, M. Mechanical properties of biocompatible clay/P(MEO2MA-co-OEGMA) nanocomposite hydrogels. J. Mech. Behav. Biomed. Mater. 2017, 72, 74–81. [Google Scholar] [CrossRef]
- Huang, K.-T.; Fang, Y.-L.; Hsieh, P.-S.; Li, C.-C.; Dai, N.-T.; Huang, C.-J. Zwitterionic nanocomposite hydrogels as effective wound dressings. J. Mater. Chem. B 2016, 4, 4206–4215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, M.-Q.; Chen, T.-T.; Zhang, H.; Hu, D.-F.; Wu, B.-H.; Ji, J.; Xu, Z.-K. Dopamine-triggered one-step polymerization and codeposition of acrylate monomers for functional coatings. ACS Appl. Mater. Interfaces 2017, 9, 34356–34366. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-W.; Lin, H.-Y.; Tsai, C.-Y.; Chen, T.-Y.; Chen, W.-N. Superhydrophilic coating with antibacterial and oil-repellent properties via NaIO4-triggered polydopamine/sulfobetaine methacrylate Polymerization. Polymers 2020, 12, 2008. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Sun, W.-B.; Yang, X. First detection, characterization, and application of amorphous calcium phosphate in dentistry. J. Dent. Sci. 2012, 7, 316–323. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Hoff, B.; Suvarnapathaki, S.; Lantigua, D.; McCarthy, C.; Wu, B.; Camci-Unal, G. Mineralized hydrogels induce bone regeneration in critical size cranial defects. Adv. Healthc. Mater. 2021, 10, 2001101. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Regitsky, A.U.; Song, J.; Ilavsky, J.; McKinley, G.H.; Holten-Andersen, N. In situ mechanical reinforcement of polymer hydrogels via metal-coordinated crosslink mineralization. Nat. Commun. 2021, 12, 667. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, L.; Zhang, F.M.; Jiang, Y.; Cai, M.; Dai, C.; Luo, Y.A.; Tu, L.J.; Zhou, Z.N.; Li, X.J.; et al. Hybrid gelatin/oxidized chondroitin sulfate hydrogels incorporating bioactive glass nanoparticles with enhanced mechanical properties, mineralization, and osteogenic differentiation. Bioact. Mater. 2021, 6, 890. [Google Scholar] [CrossRef]
- Sarker, B.; Li, W.; Zheng, K.; Detsch, R.; Boccaccini, A.R. Designing porous bone tissue engineering scaffolds with enhanced mechanical properties from composite hydrogels composed of modified alginate, gelatin, and bioactive glass. ACS Biomater. Sci. Eng. 2016, 2, 2240–2254. [Google Scholar] [CrossRef]
- Goel, H.; Gupta, N.; Santhiya, D.; Dey, N.; Bohidar, H.B.; Bhattacharya, A. Bioactivity reinforced surface patch bound collagen-pectin hydrogel. Int. J. Biol. Macromol. 2021, 174, 240–253. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, P.; Gao, F.; Wang, W.; Zhang, H.; Zhang, X.; Feng, X.; Liu, W. A mineralized high strength and tough hydrogel for skull bone regeneration. Adv. Funct. Mater. 2017, 27, 1604327. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Skwarczynska, A.; Modrzejewska, Z.; Balcaen, L.; Schaubroeck, D.; Lycke, S.; Vanhaecke, F.; Vandenabeele, P.; Dubruel, P.; Jansen, J.A.; et al. Acceleration of gelation and promotion of mineralization of chitosan hydrogels by alkaline phosphatase. Int. J. Biol. Macromol. 2013, 56, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Rauner, N.; Meuris, M.; Zoric, M.; Tiller, J.C. Enzymatic mineralization generates ultrastiff and tough hydrogels with tunable mechanics. Nature 2017, 543, 407–410. [Google Scholar] [CrossRef] [PubMed]

| Elemental Composition % | |||||||
|---|---|---|---|---|---|---|---|
| C | O | Na | Cl | Ca | P | Ca/P | |
| TA0 | 54.77 | 42.28 | 1.90 | 0.70 | 0.35 | 0 | - |
| TA0.5 | 58.32 | 38.16 | 2.98 | 0 | 0.22 | 0.31 | 0.71 |
| TA2 | 40.64 | 48.56 | 2.50 | 0.8 | 4.69 | 2.81 | 1.67 |
| TA10 | 40.62 | 41.4 | 2.03 | 1.14 | 8.93 | 5.88 | 1.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-Y.; Ou, S.-F.; Chien, H.-W. Biomimetic Mineralization of Tannic Acid-Supplemented HEMA/SBMA Nanocomposite Hydrogels. Polymers 2021, 13, 1697. https://doi.org/10.3390/polym13111697
Chen T-Y, Ou S-F, Chien H-W. Biomimetic Mineralization of Tannic Acid-Supplemented HEMA/SBMA Nanocomposite Hydrogels. Polymers. 2021; 13(11):1697. https://doi.org/10.3390/polym13111697
Chicago/Turabian StyleChen, Tai-Yu, Shih-Fu Ou, and Hsiu-Wen Chien. 2021. "Biomimetic Mineralization of Tannic Acid-Supplemented HEMA/SBMA Nanocomposite Hydrogels" Polymers 13, no. 11: 1697. https://doi.org/10.3390/polym13111697
APA StyleChen, T.-Y., Ou, S.-F., & Chien, H.-W. (2021). Biomimetic Mineralization of Tannic Acid-Supplemented HEMA/SBMA Nanocomposite Hydrogels. Polymers, 13(11), 1697. https://doi.org/10.3390/polym13111697






