Sonication-Assisted Method for Decellularization of Human Umbilical Artery for Small-Caliber Vascular Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
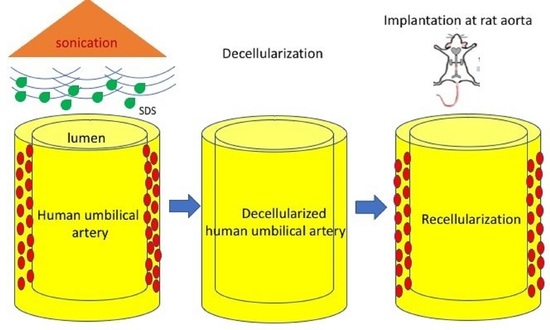
2.1. Recovery of the Human Umbilical Artery
2.2. Decellularization of HUA
2.3. Histology of HUAs and DHUAs
2.4. DNA Quantification
2.5. ECM Component Quantification
2.6. Mechanical Property Test
2.7. Cell Seeding Test
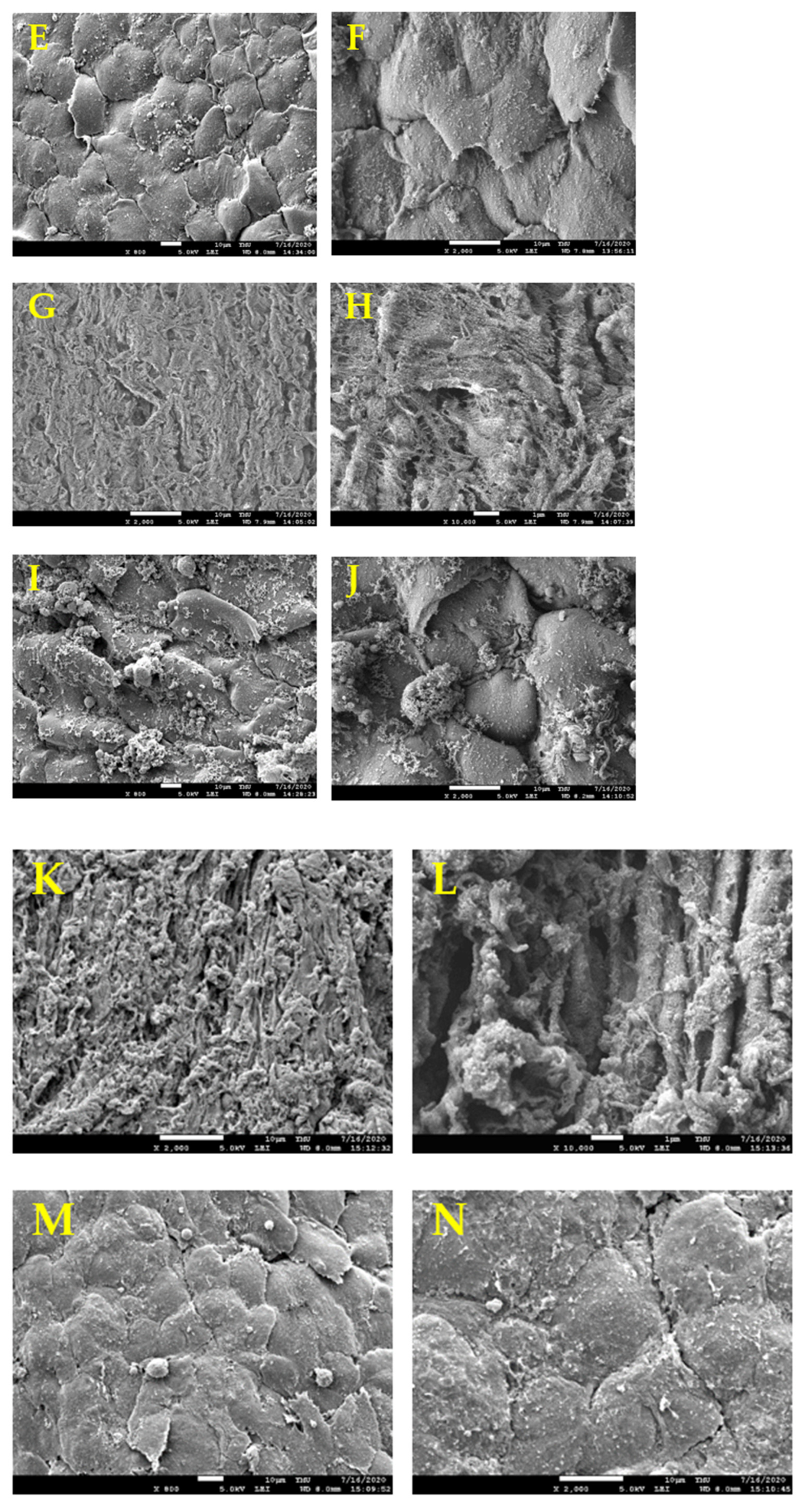
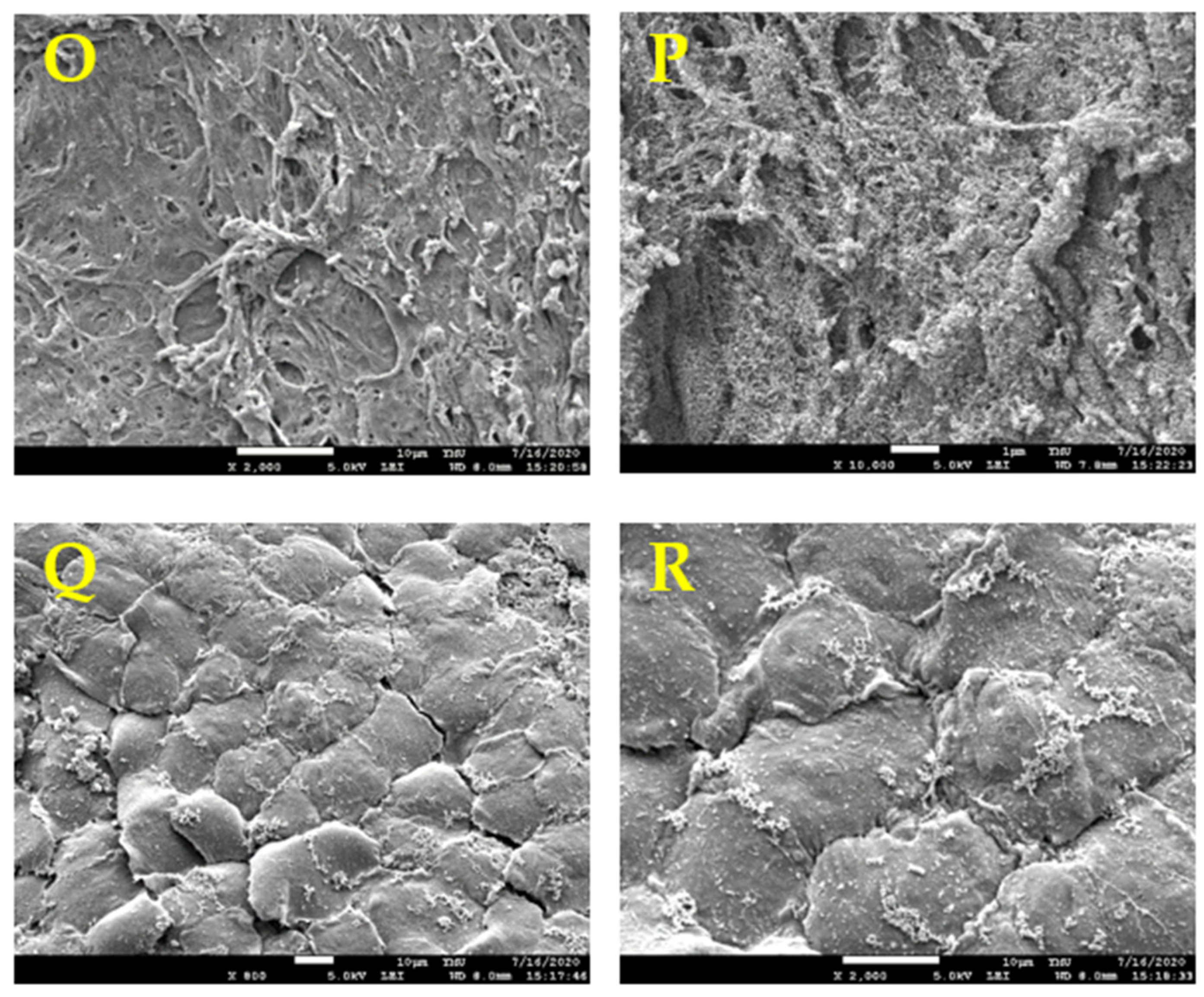
2.8. Scanning Electron Microscopy (SEM)
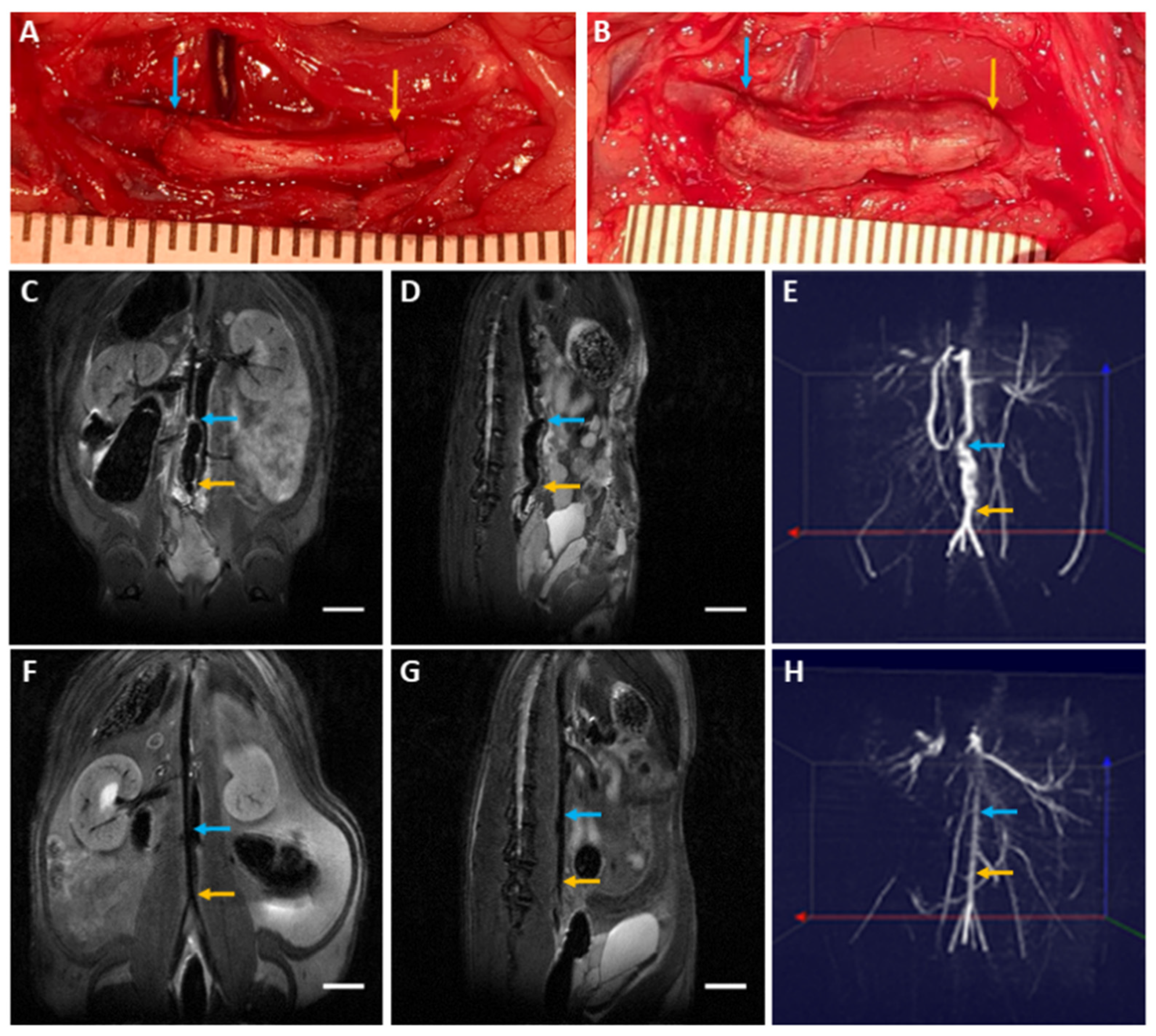
2.9. Biocompatibility Evaluation in a Rat Abdominal Aorta Implantation Model
2.10. Magnetic Resonance Angiography and Histological Examination
2.11. Statistical Analysis
3. Results
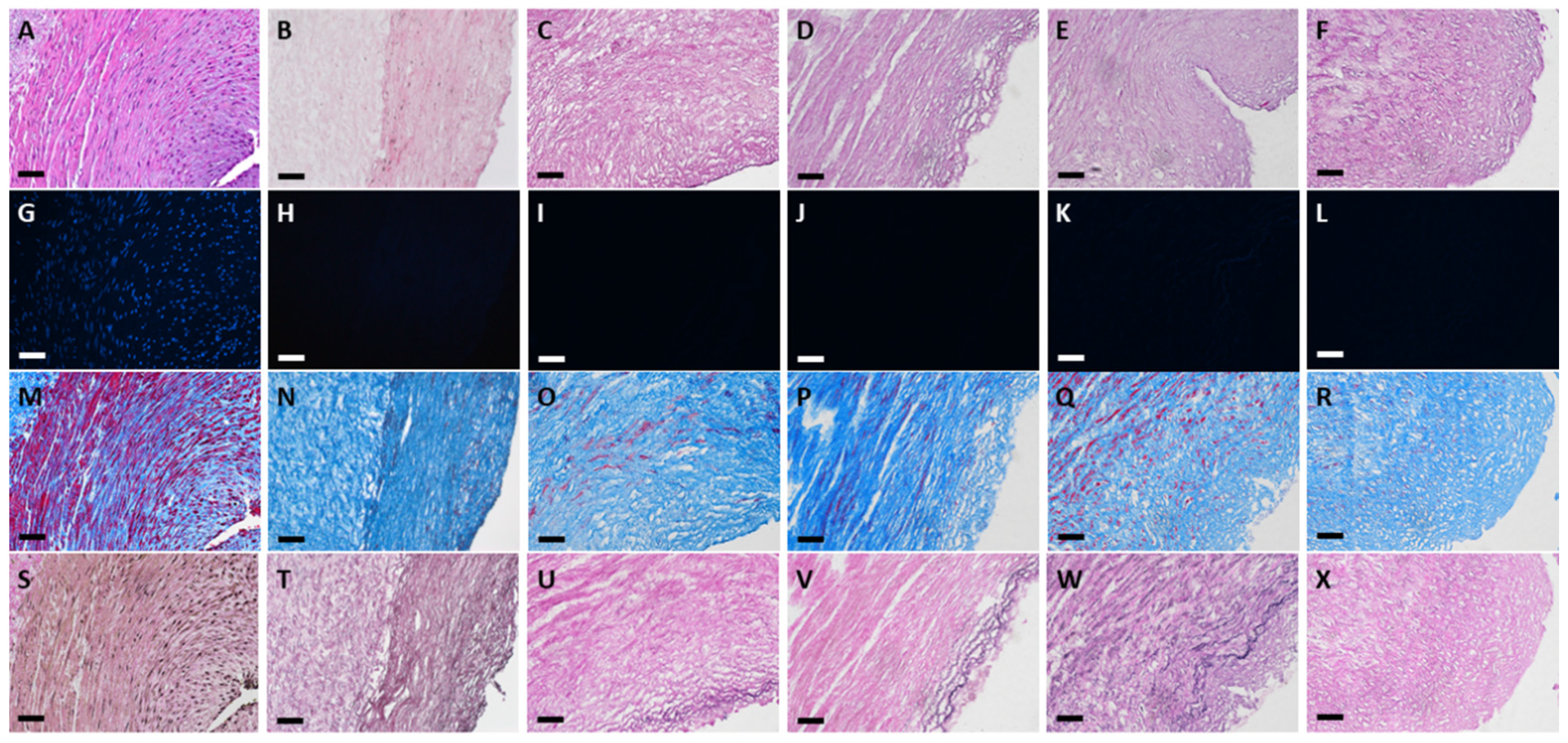
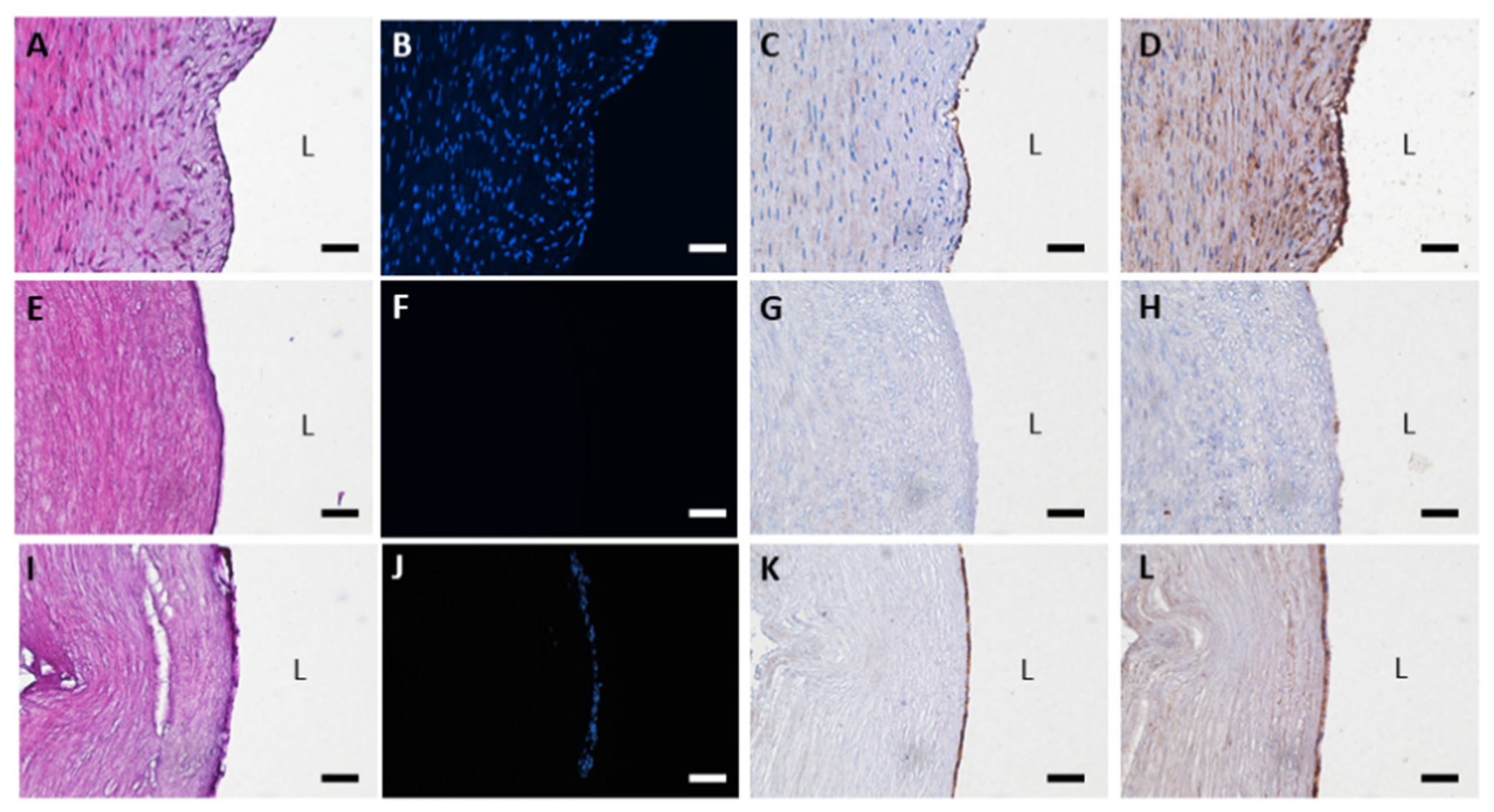
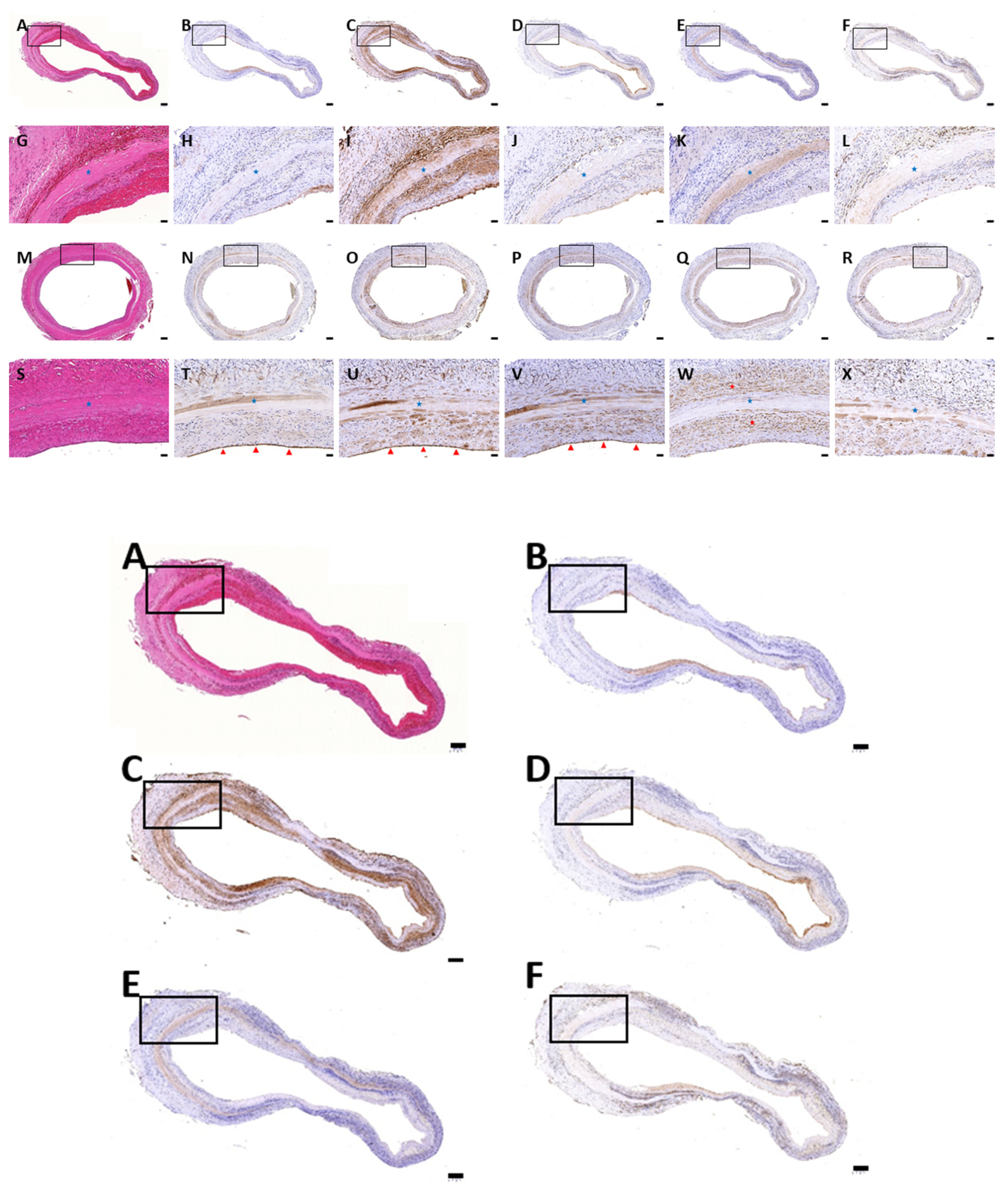
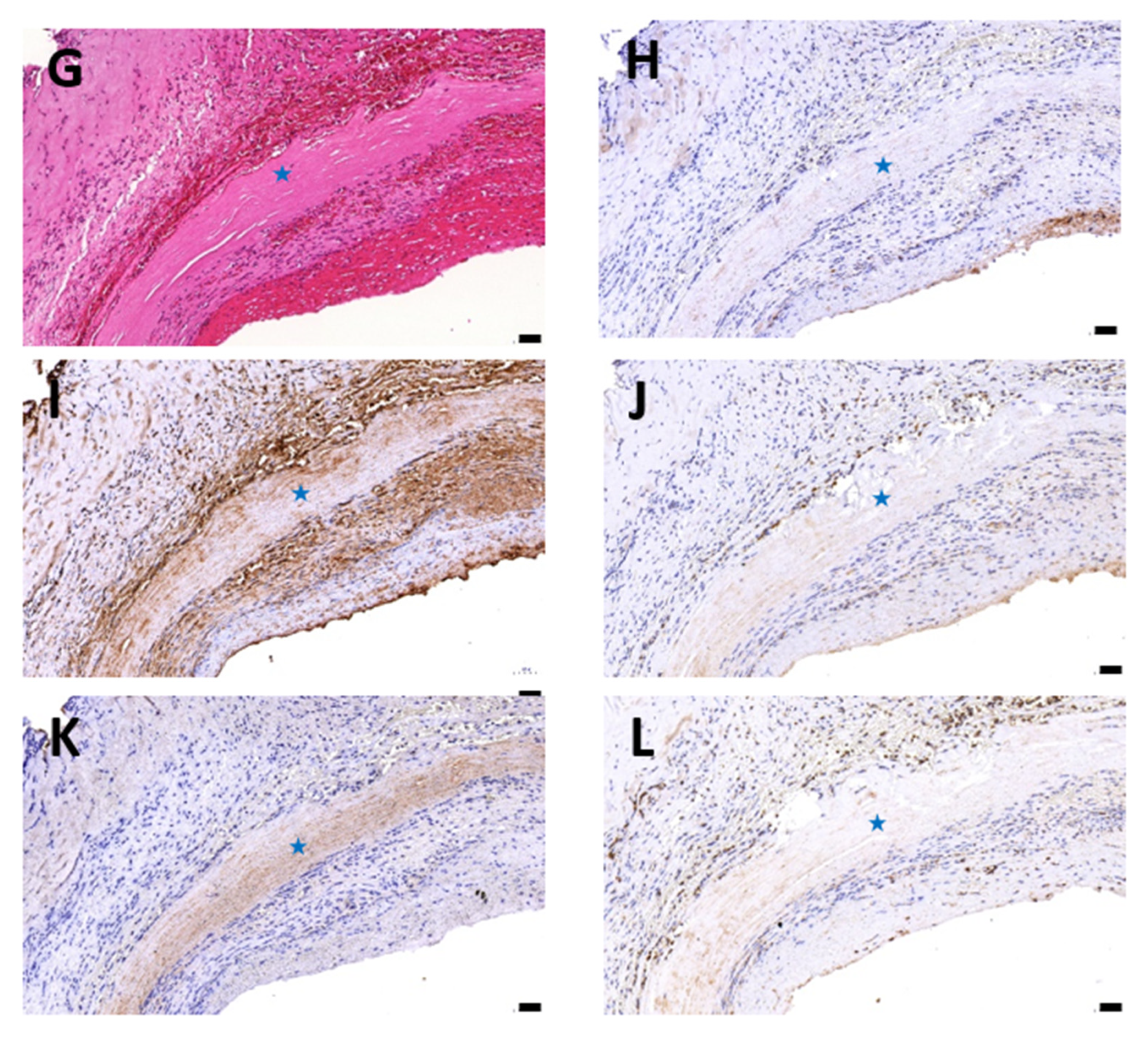
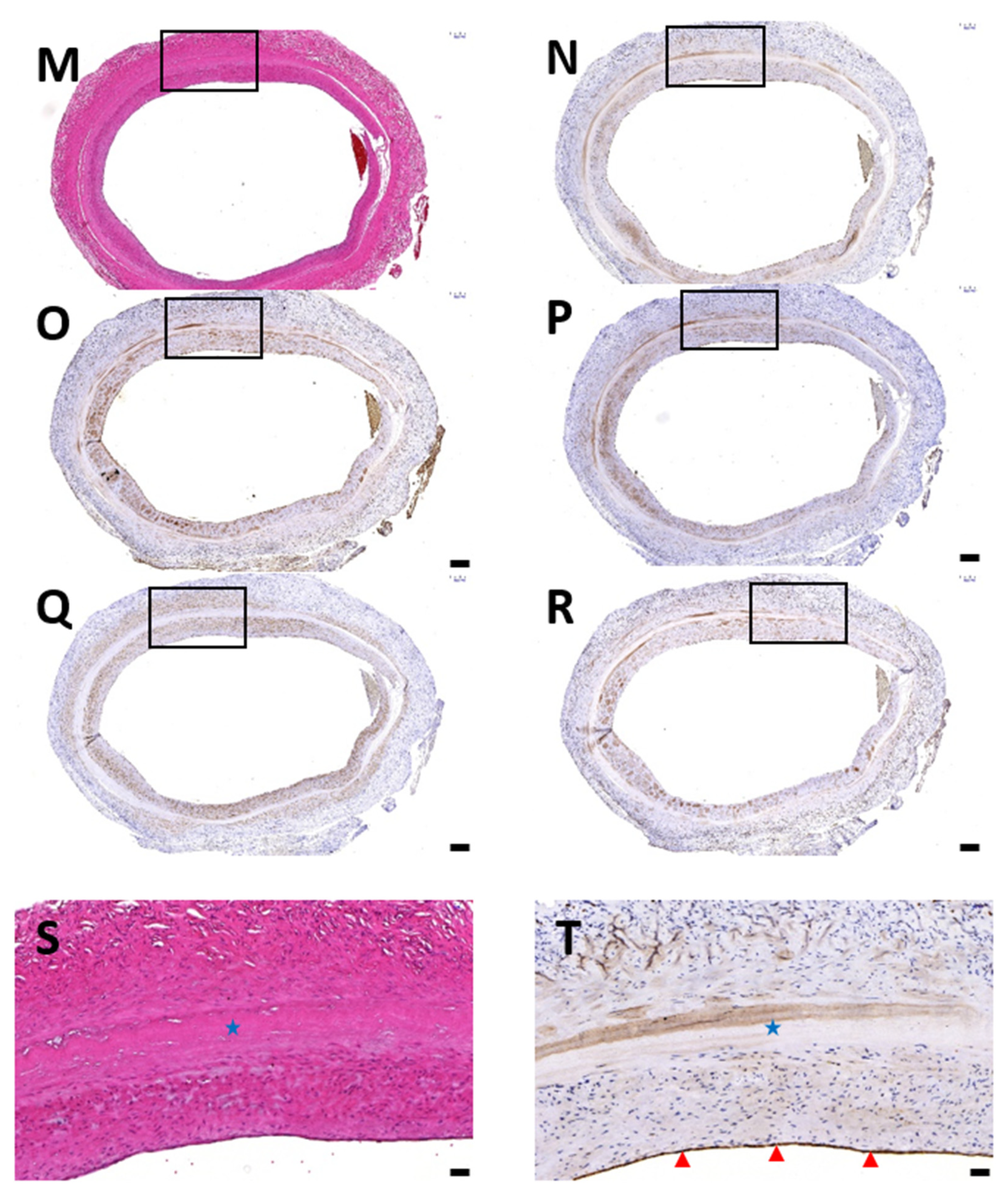
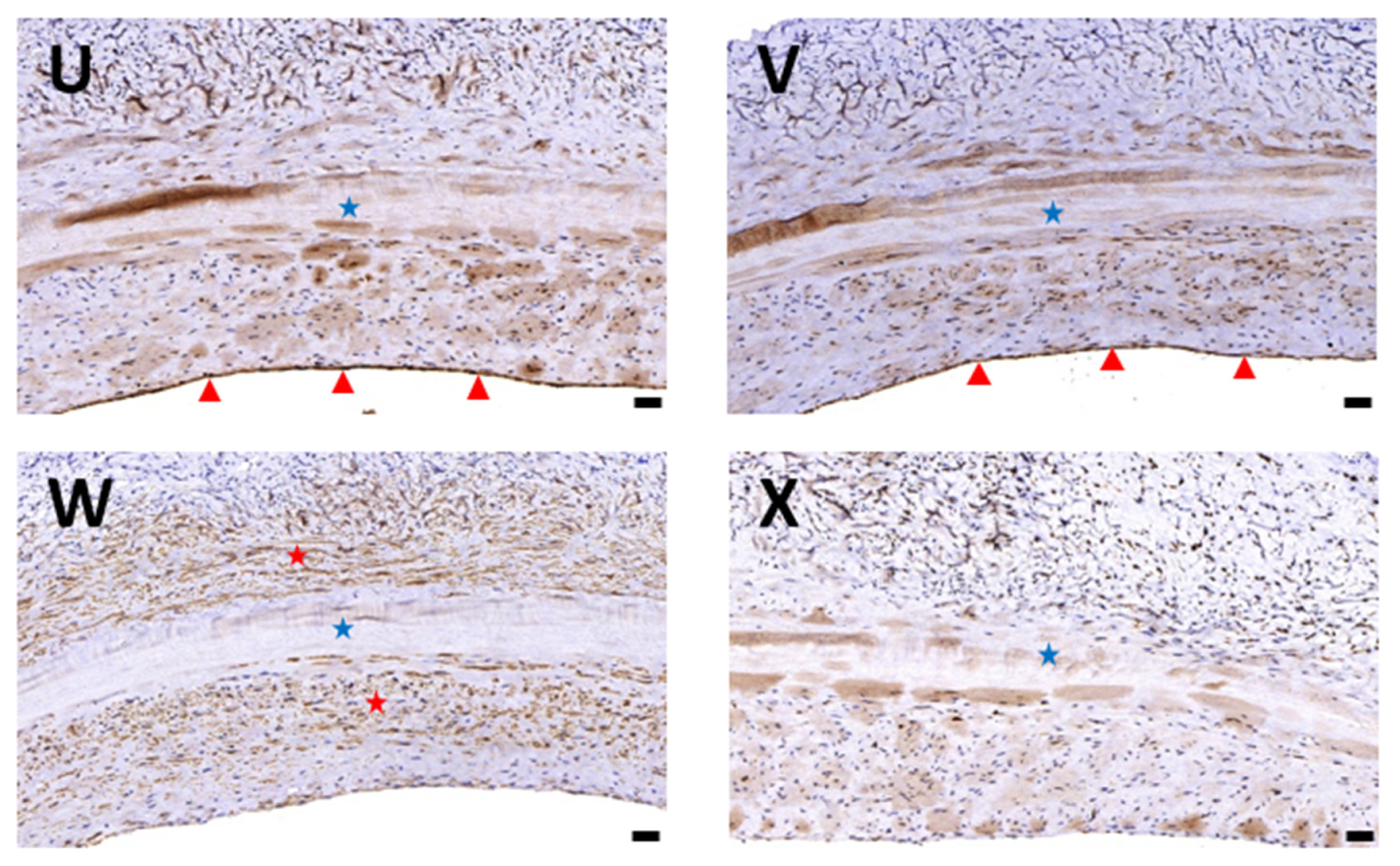
3.1. Histological Evaluation of HUAs, cDHUAs, and sDHUAS
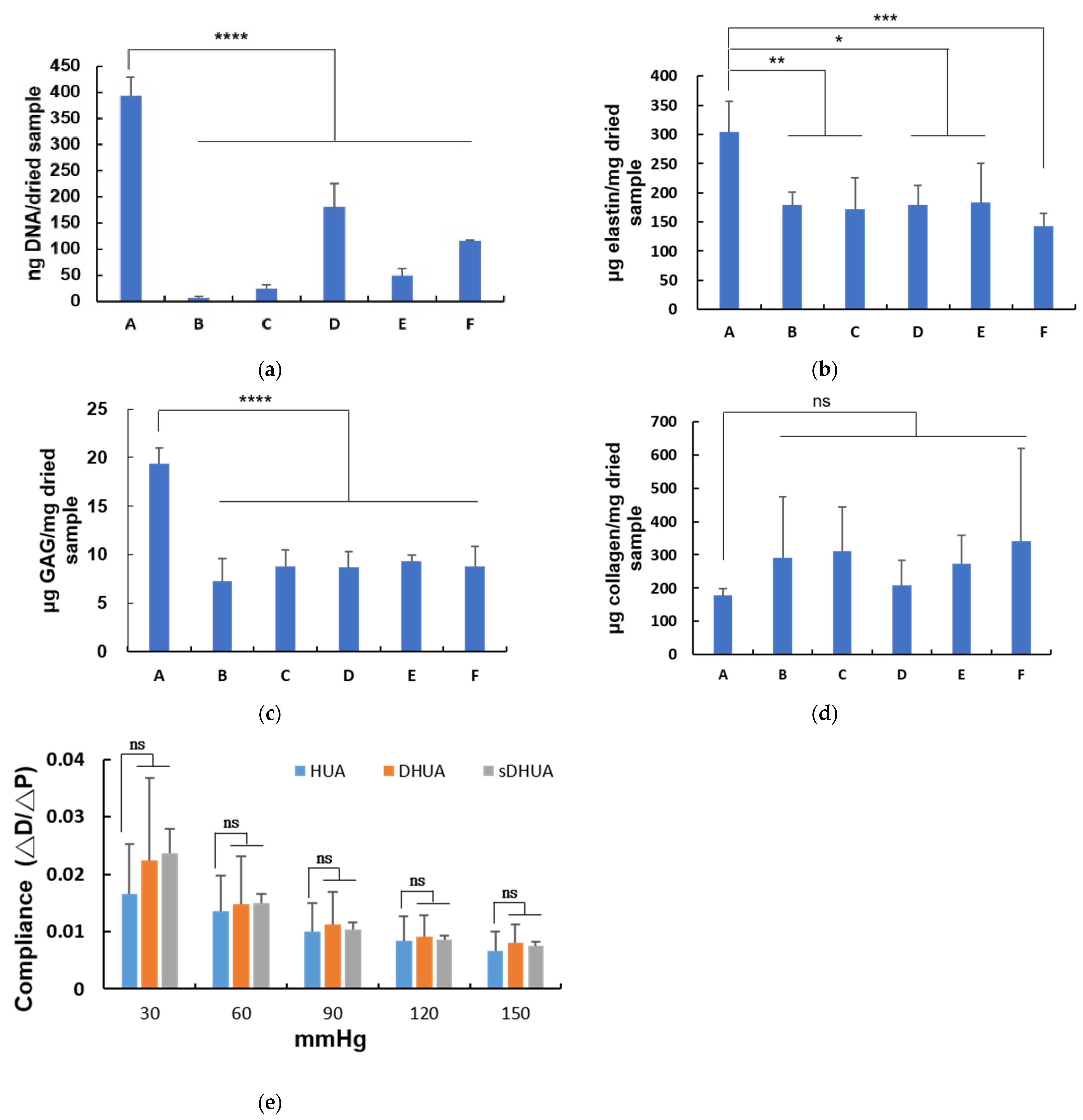
3.2. DNA Quantification of HUAs, cDHUAs, and sDHUAS
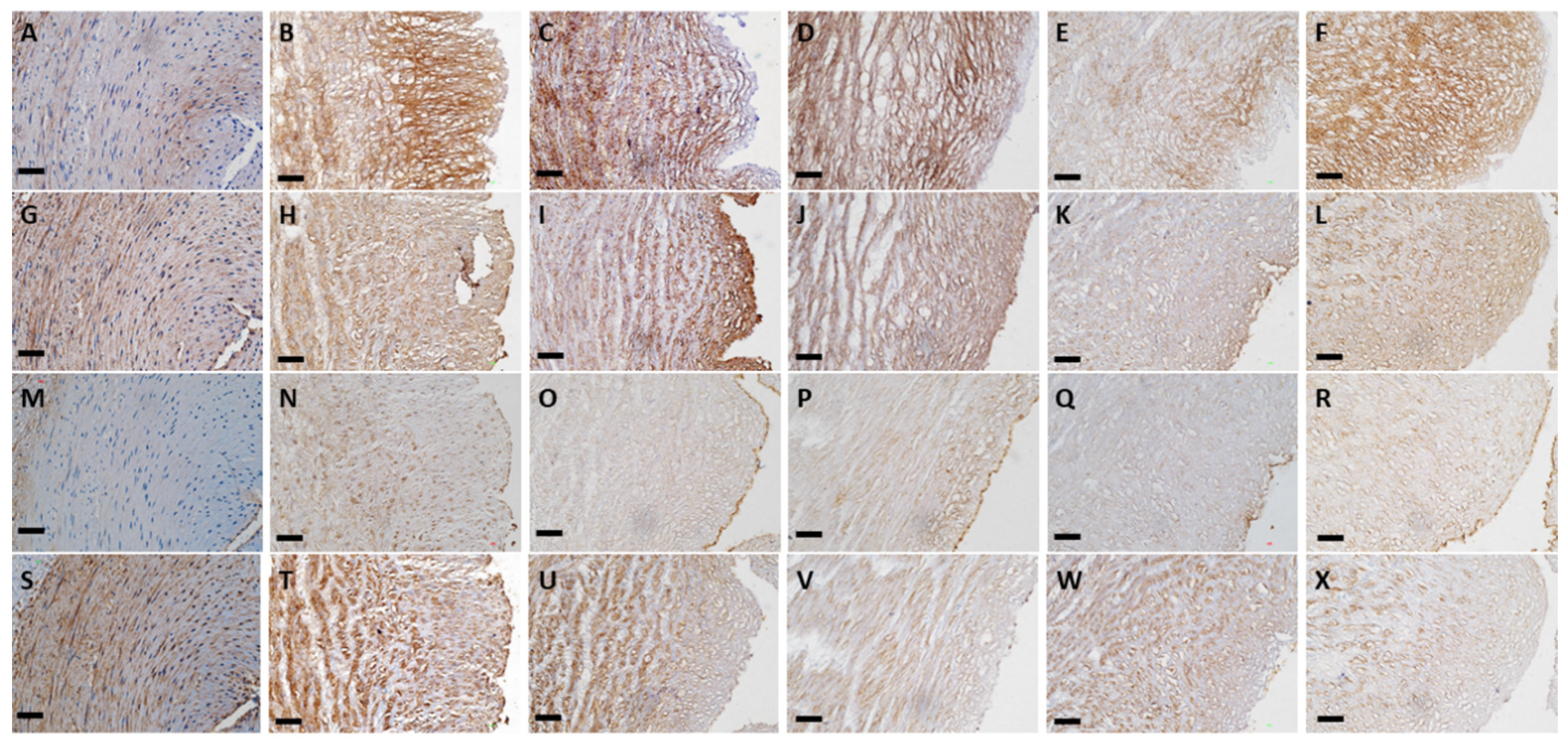
3.3. ECM Component Quantification
3.4. Evaluation of the Mechanical Properties of HUA With Sonication-Assisted Decellularization
3.5. HUVECs Seeded on Optimized Scaffolds
3.6. Biocompatibility Evaluation in a Rat Abdominal Aorta Implantation Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salama, S.; Erin, C.M. A systemic analysis for the global burden of disease study 2017. Lancet 2017, 393, 1958–1972. [Google Scholar]
- Rashid, S.T.; Fuller, B.; Hamilton, G.; Seifalian, A.M. Tissue engineering of a hybrid bypass graft for coronary and lower limb bypass surgery. FASEB J. 2008, 22, 2084–2089. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Butler, P.E.; Hamilton, G.; Seifalian, A.M. Current status of prosthetic bypass graft: A review. J. Biomed. Mater. Res. Part B 2005, 74, 570–581. [Google Scholar] [CrossRef]
- Bennion, R.S.; Williams, R.A.; Stabile, B.E.; Fox, M.A.; Owens, M.L.; Wilson, S.E. Patency of autogenous saphenous vein versus polytetrafluoroethylene grafts in femoropopliteal bypass for advanced ischemia of the extremity. Surg. Gynecol. Obstet. 1985, 160, 239–242. [Google Scholar]
- Abbott, W.M.; Callow, A.; Moore, W.; Rutherford, R.; Veith, F.; Weinberg, S. Evaluation and performance standards for arterial prostheses. J. Vasc. Surg. 1993, 17, 746–756. [Google Scholar] [CrossRef]
- Yokota, T.; Ichikawa, H.; Matsumiya, G.; Kuratani, T.; Sakaguchi, T.; Iwai, S.; Shirakawa, Y.; Torikai, K.; Saito, A.; Uchimura, E.; et al. In situ tissue regeneration using a novel tissue-engineered, small-caliber vascular graft without cell seeding. J. Thorac. Cardiovasc. Surg. 2008, 136, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Kostakis, A.; Stavropoulos-Giokas, C.; Michalopoulos, E. Future perspectives in small-diameter vascular graft engineering. Bioengineering 2020, 7, 160. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Londono, R.; Turner, N.J.; Badylak, S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012, 33, 1771–1781. [Google Scholar] [CrossRef]
- Hawkins, J.A.; Hillman, N.D.; Lambert, L.M.; Jones, J.; Di Russo, G.B.; Profaizer, T.; Fuller, T.C.; Minich, L.L.; Williams, R.V.; Shaddy, R.E. Immunogenicity of decellularized cryopreserved allografts in pediatric cardiac surgery: Comparison with standard cryopreserved allografts. J. Thorac. Cardiovasc. Surg. 2003, 126, 247–252. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Terada, D.; Yamaoka, T.; Kitamura, S.; Fujisato, T. Cell removal with supercritical carbon dioxide for acellular artificial tissue. J. Chem. Technol. Biotechnol. Int. Res. Process.Environ. Clean Technol. 2008, 83, 943–949. [Google Scholar] [CrossRef]
- Sasaki, S.; Funamoto, S.; Hashimoto, Y.; Kimura, T.; Honda, T.; Hattori, S.; Kobayashi, H.; Kishida, A.; Mochizuki, M. In vivo evaluation of a novel scaffold for artificial corneas prepared by using ultrahigh hydrostatic pressure to decellularize porcine corneas. Mol. Vis. 2009, 15, 2022. [Google Scholar] [PubMed]
- Funamoto, S.; Nam, K.; Kimura, T.; Murakoshi, A.; Hashimoto, Y.; Niwaya, K.; Kitamura, S.; Fujisato, T.; Kishida, A. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials 2010, 31, 3590–3595. [Google Scholar] [CrossRef] [PubMed]
- Azhim, A.; Yamagami, K.; Muramatsu, K.; Morimoto, Y.; Furukawa, K.S.; Tanaka, M.; Fukui, Y.; Ushida, T. The use of sonication treatment to completely decellularize aorta tissue. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Beijing, China, 26–31 May 2012; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1987–1990. [Google Scholar]
- Syazwani, N.; Azhim, A.; Morimoto, Y.; Furukawa, K.S.; Ushida, T. Decellularization of aorta tissue using sonication treatment as potential scaffold for vascular tissue engineering. J. Med. Biol. Eng. 2015, 35, 258–269. [Google Scholar] [CrossRef]
- Say, S.; Dugos, N.; Roces, S.; Mondragon, J.M. Effect of sonication power on perfusion decellularization of cadaveric porcine kidney. In MATEC Web of Conferences, 5–7 June 2019; EDP Sciences: Sibiu, Romania, 2019; p. 01009. [Google Scholar]
- Azhim, A.; Ono, T.; Fukui, Y.; Morimoto, Y.; Furukawa, K.; Ushida, T. Preparation of decellularized meniscal scaffolds using sonication treatment for tissue engineering. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 6953–6956. [Google Scholar]
- Azhim, A.; Shafiq, M.; Rasyada, A.R.; Furukawa, K.; Ushida, T. The impact of acoustic intensity on solution parameters and decellularization using sonication treatment. J. Biomater. Tissue Eng. 2015, 5, 195–203. [Google Scholar] [CrossRef]
- Niemczewski, B. Chemical activation of ultrasonic cavitation. Ultrason. Sonochemistry 1999, 6, 211–216. [Google Scholar] [CrossRef]
- Sun, D.-W. Emerging Technologies for Food Processing, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Hung, S.H.; Su, C.H.; Lee, F.P.; Tseng, H. Larynx decellularization: Combining freeze-drying and sonication as an effective method. J. Voice 2013, 27, 289–294. [Google Scholar] [CrossRef]
- Lin, C.H.; Lu, J.H.; Hsia, K.; Lee, H.; Yao, C.L.; Ma, H. The Antithrombotic Function of Sphingosine-1-Phosphate on Human Adipose-Stem-Cell-Recellularized Tissue Engineered Vascular Graft In Vitro. Int. J. Mol. Sci. 2019, 20, 5218. [Google Scholar] [CrossRef]
- Kawecki, M.; Łabuś, W.; Klama-Baryla, A.; Kitala, D.; Kraut, M.; Glik, J.; Misiuga, M.; Nowak, M.; Bielecki, T.; Kasperczyk, A. A review of decellurization methods caused by an urgent need for quality control of cell-free extracellular matrix’ scaffolds and their role in regenerative medicine. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2018, 106, 909–923. [Google Scholar] [CrossRef]
- Gui, L.; Muto, A.; Chan, S.A.; Breuer, C.K.; Niklason, L.E. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng. Part. A 2009, 15, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L. Effects of a high-amplitude 1-MHz standing ultrasonic field on the algae hydrodictyon. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1986, 33, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Mosher, D. Fibronectin and other adhesive glycoproteins. The Extracellular Matrix: An Overview; Springer: Berlin/Heidelberg, Germany, 2011; pp. 41–75. [Google Scholar]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Chan, S.A.; Breuer, C.K.; Niklason, L.E. Novel utilization of serum in tissue decellularization. Tissue Eng. Part. C Methods 2010, 16, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Woods, T.; Gratzer, P.F. Effectiveness of three extraction techniques in the development of a decellularized bone–anterior cruciate ligament–bone graft. Biomaterials 2005, 26, 7339–7349. [Google Scholar] [CrossRef] [PubMed]
- Deeken, C.R.; White, A.K.; Bachman, S.L.; Ramshaw, B.J.; Cleveland, D.S.; Loy, T.S.; Grant, S.A. Method of preparing a decellularized porcine tendon using tributyl phosphate. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2011, 96, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Maibach, H.I. The sodium lauryl sulfate model: An overview. Contact Dermat. 1995, 33, 1–7. [Google Scholar] [CrossRef]
- Gratzer, P.F.; Harrison, R.D.; Woods, T. Matrix alteration and not residual sodium dodecyl sulfate cytotoxicity affects the cellular repopulation of a decellularized matrix. Tissue Eng. 2006, 12, 2975–2983. [Google Scholar] [CrossRef]
- Azhim, A.; Yamagami, K.; Muramatsu, K.; Morimoto, Y.; Tanaka, M. The use of sonication treatment to completely decellularize blood arteries: A pilot study. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 2468–2471. [Google Scholar]
- Chattopadhyay, A.; Harikumar, K. Dependence of critical micelle concentration of a zwitterionic detergent on ionic strength: Implications in receptor solubilization. Febs Lett. 1996, 391, 199–202. [Google Scholar] [CrossRef]
- Daniel, J.; Abe, K.; McFetridge, P.S. Development of the human umbilical vein scaffold for cardiovascular tissue engineering applications. Asaio, J. 2005, 51, 252–261. [Google Scholar] [CrossRef]
- Rodriguez, M.; Juran, C.; McClendon, M.; Eyadiel, C.; McFetridge, P.S. Development of a mechanically tuneable 3D scaffold for vascular reconstruction. J. Biomed. Mater. Res. Part. A 2012, 100, 3480–3489. [Google Scholar] [CrossRef] [PubMed]
- Barnwal, M.; Rathi, S.; Chhabra, S.; Nanda, S. Histomorphometry of umbilical cord and its vessels in pre-eclampsia as compared to normal pregnancies. Nepal, J. Obstet. Gynaecol. 2012, 7, 28–32. [Google Scholar] [CrossRef]
- Blanco, M.V.; Vega, H.R.; Guerri-Guttenberg, R.A.; Giuliano, R.; Grana, D.R.; Azzato, F.; Milei, J. Histopathology and histomorphometry of umbilical cord blood vessels. Findings in normal and high risk pregnancies. Artery Res. 2011, 5, 50–57. [Google Scholar] [CrossRef]
- Bańkowski, E. Collagen of the umbilical cord and its alteration in EPH-gestosis (preeclampsia). In Proceedings of the Indian Academy of Sciences-Chemical Sciences, 1999; Springer: Berlin/Heidelberg, Germany, 1999; pp. 207–213. [Google Scholar]
- de São José, J.F.B.; de Andrade, N.J.; Ramos, A.M.; Vanetti, M.C.D.; Stringheta, P.C.; Chaves, J.B.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control. 2014, 45, 36–50. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Mason, T.J. Sonochemistry. In Kirk-Othmer Encyclopedia of Chemical Technology; Seidel, A., Bickford, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 1–34. [Google Scholar]
- Wang, Y. Vascular biology of the placenta. In Colloquium Series on Integrated Systems Physiology: From Molecule to Function; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010; pp. 1–98. [Google Scholar]
- Wagenseil, J.E.; Mecham, R.P. Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 2009, 89, 957–989. [Google Scholar] [CrossRef] [PubMed]
- Tuan-Mu, H.Y.; Yu, C.H.; Hu, J.J. On the decellularization of fresh or frozen human umbilical arteries: Implications for small-diameter tissue engineered vascular grafts. Ann. Biomed. Eng. 2014, 42, 1305–1318. [Google Scholar] [CrossRef]
- Pennati, G. Biomechanical properties of the human umbilical cord. Biorheology 2001, 38, 355–366. [Google Scholar]
- Sánchez, P.F.; Brey, E.M.; Briceño, J.C. Endothelialization mechanisms in vascular grafts. J. Tissue Eng. Regen. Med. 2018, 12, 2164–2178. [Google Scholar] [CrossRef]
- Martin, N.D.; Schaner, P.J.; Tulenko, T.N.; Shapiro, I.M.; Dimatteo, C.A.; Williams, T.K.; Hager, E.S.; DiMuzio, P.J. In vivo behavior of decellularized vein Allograft. J. Surg. Res. 2005, 129, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Allaire, E.; Bruneval, P.; Mandet, C.; Becquemin, J.P.; Michel. J.B. The immunogenicity of the extracellular matrix in arterial xenografts. Surgery 1997, 122, 73–81. [Google Scholar] [CrossRef]
- Talacua, H.; Smits, A.I.; Muylaert, D.E.; van Rijswijk, J.W.; Vink, A.; Verhaar, M.C.; Driessen-Mol, A.; van Herwerden, L.A.; Bouten, C.V.; Kluin, J.; et al. In situ tissue engineering of functional small-diameter blood vessels by host circulating cells only. Tissue Eng. Part A 2015, 21, 2583–2594. [Google Scholar] [CrossRef]
- Badylak, S.F. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: Factors that influence the host response. Ann. Biomed. Eng. 2014, 42, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Valentin, J.E.; Stewart-Akers, A.M.; Gilbert, T.W.; Badylak, S.F. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng. Part. A 2009, 15, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Allaire, E.; Forough, R.; Clowes, M.; Starcher, B.; Clowes, A.W. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J. Clin. Investig. 1998, 102, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Ariganello, M.B.; Simionescu, D.T.; Labow, R.S.; Lee, J.M. Macrophage differentiation and polarization on a decellularized pericardial biomaterial. Biomaterials 2011, 32, 439–449. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Hsia, K.; Su, C.-K.; Chen, C.-C.; Yeh, C.-C.; Ma, H.; Lu, J.-H. Sonication-Assisted Method for Decellularization of Human Umbilical Artery for Small-Caliber Vascular Tissue Engineering. Polymers 2021, 13, 1699. https://doi.org/10.3390/polym13111699
Lin C-H, Hsia K, Su C-K, Chen C-C, Yeh C-C, Ma H, Lu J-H. Sonication-Assisted Method for Decellularization of Human Umbilical Artery for Small-Caliber Vascular Tissue Engineering. Polymers. 2021; 13(11):1699. https://doi.org/10.3390/polym13111699
Chicago/Turabian StyleLin, Chih-Hsun, Kai Hsia, Chih-Kuan Su, Chien-Chin Chen, Chang-Ching Yeh, Hsu Ma, and Jen-Her Lu. 2021. "Sonication-Assisted Method for Decellularization of Human Umbilical Artery for Small-Caliber Vascular Tissue Engineering" Polymers 13, no. 11: 1699. https://doi.org/10.3390/polym13111699
APA StyleLin, C.-H., Hsia, K., Su, C.-K., Chen, C.-C., Yeh, C.-C., Ma, H., & Lu, J.-H. (2021). Sonication-Assisted Method for Decellularization of Human Umbilical Artery for Small-Caliber Vascular Tissue Engineering. Polymers, 13(11), 1699. https://doi.org/10.3390/polym13111699