Modifying the Catalyst Layer Using Polyvinyl Alcohol for the Performance Improvement of Proton Exchange Membrane Fuel Cells under Low Humidity Operations
Abstract
1. Introduction
2. Experimental
2.1. MEA Fabrication
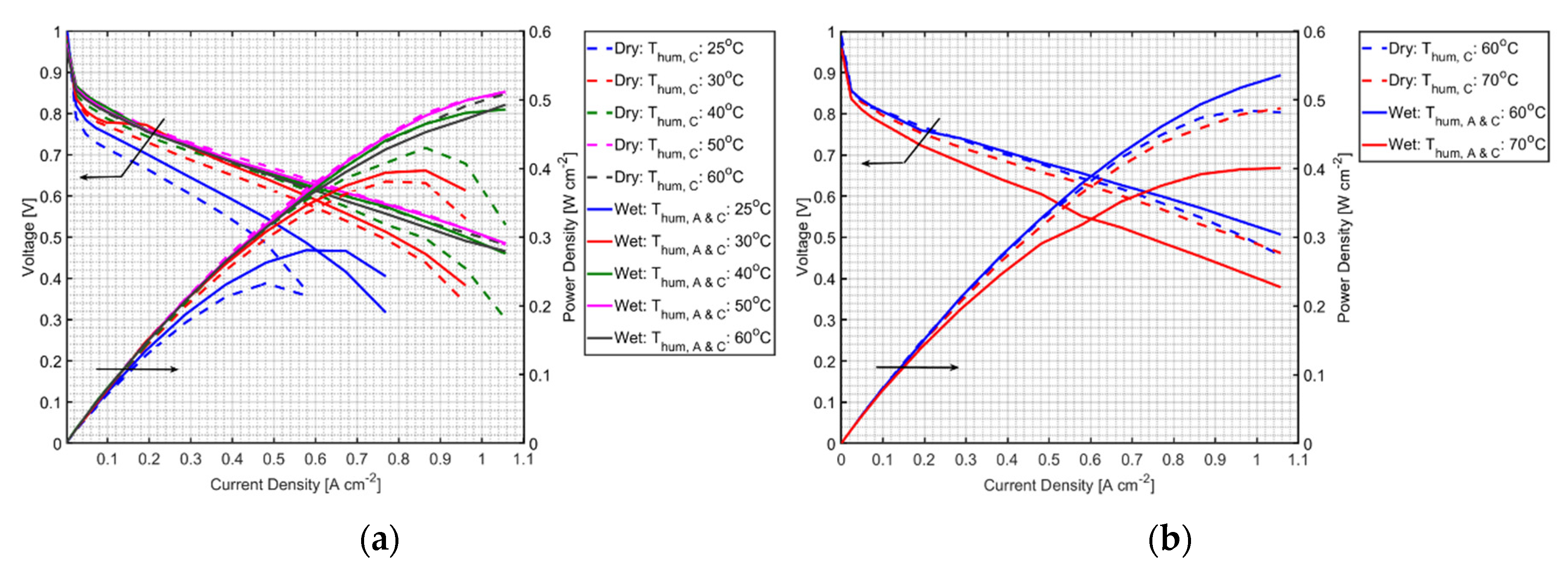
2.2. Performance Test
3. Results and Discussion
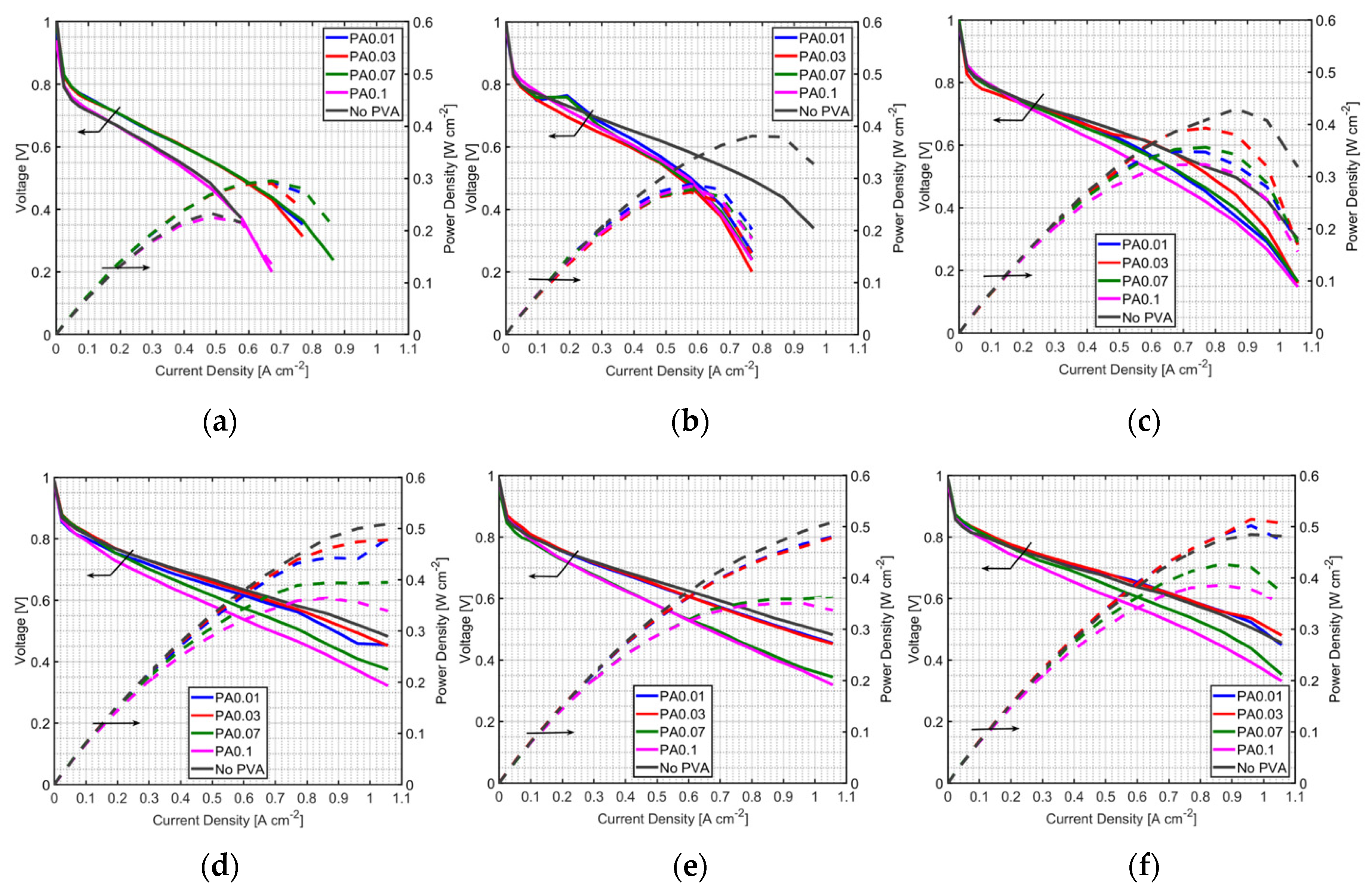
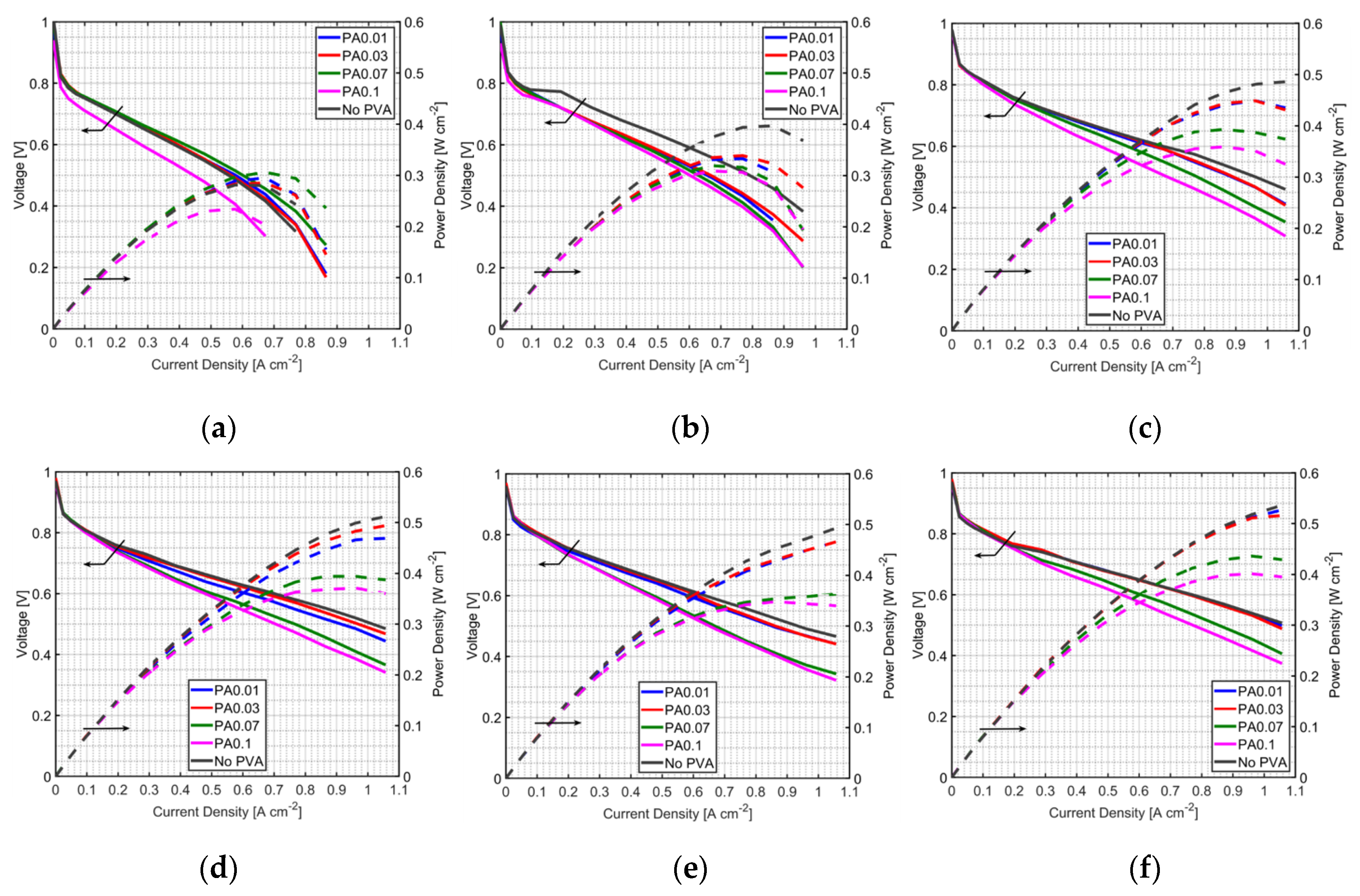
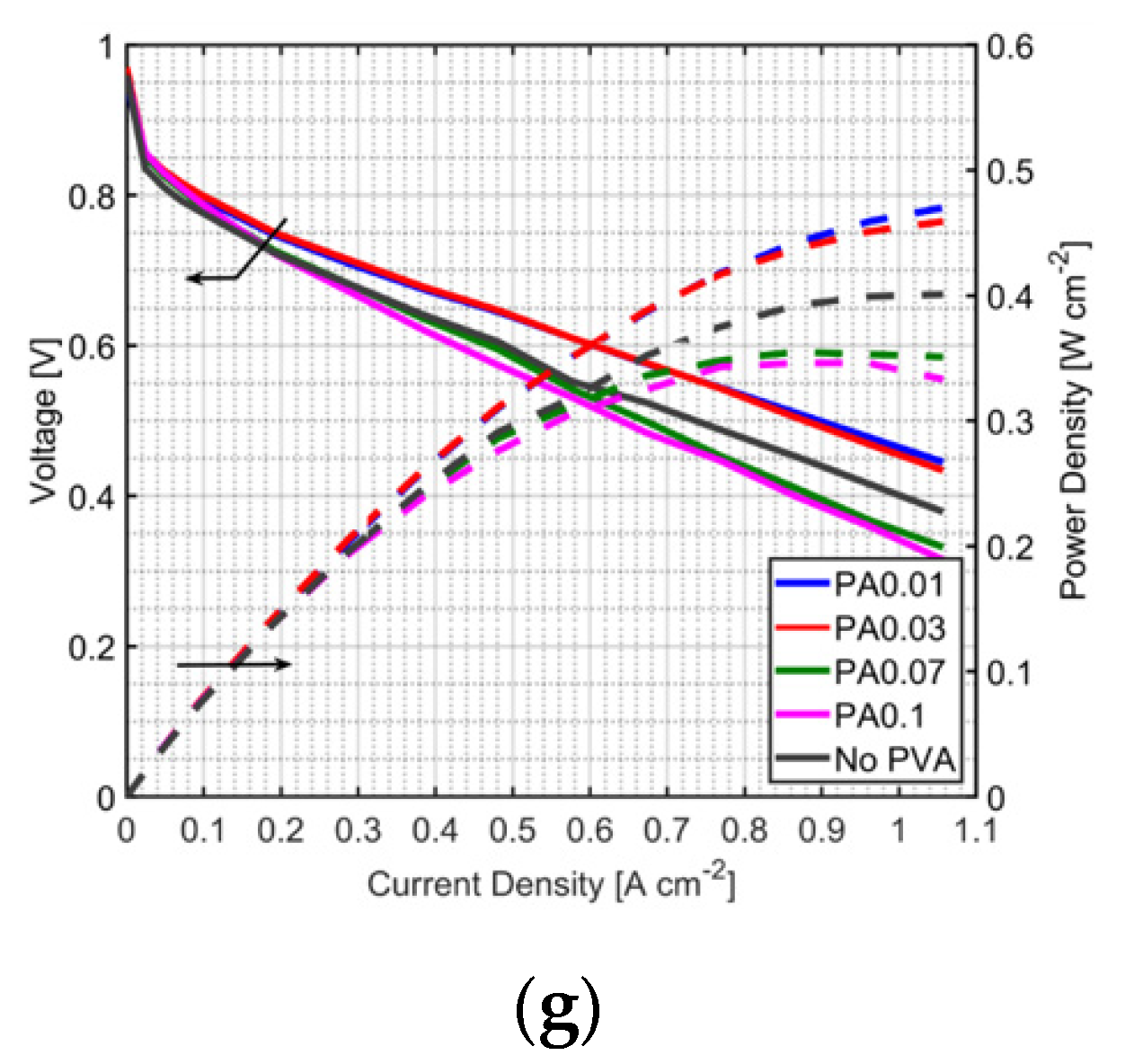
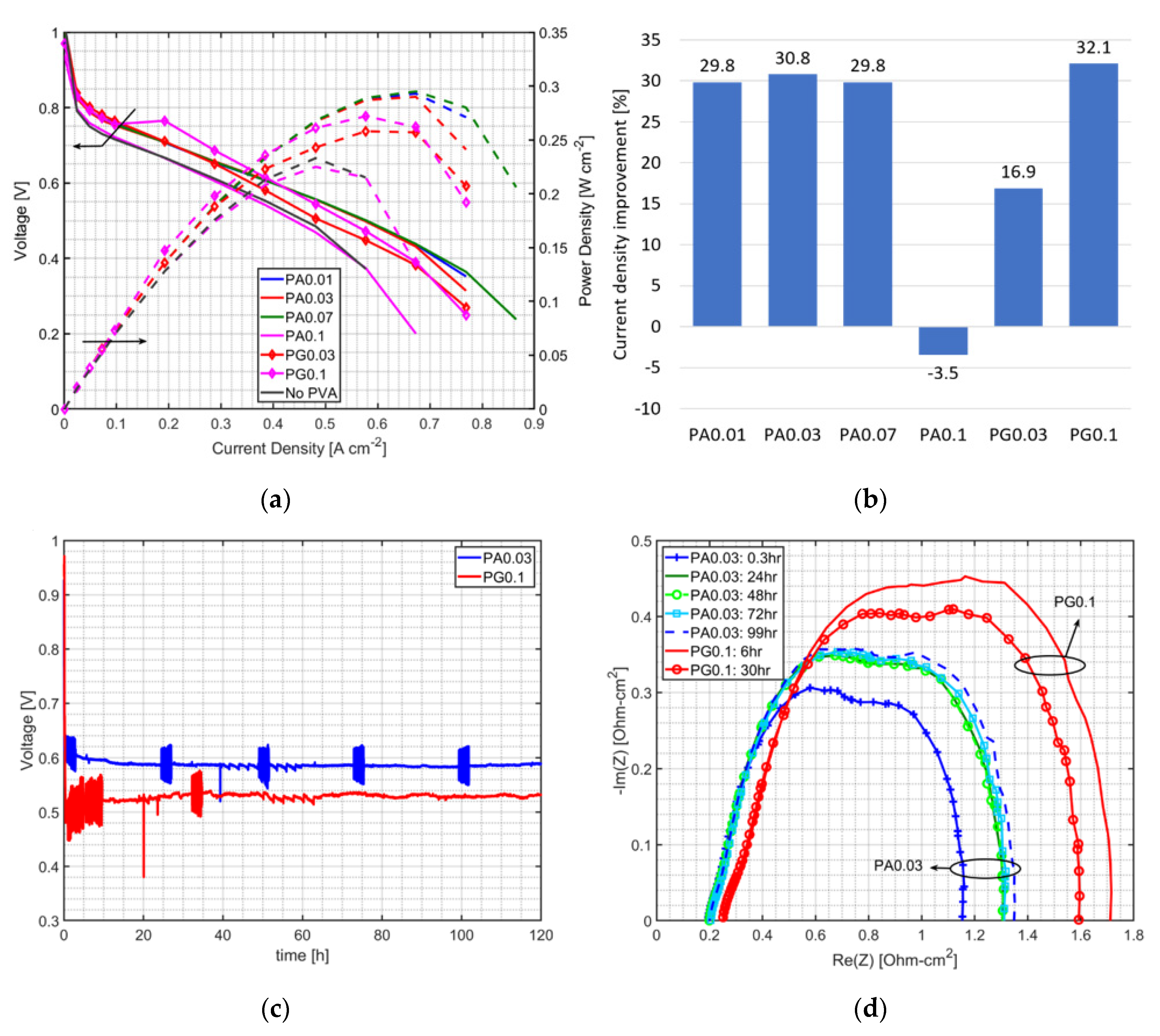
3.1. Effect of PVA on MEA Performance
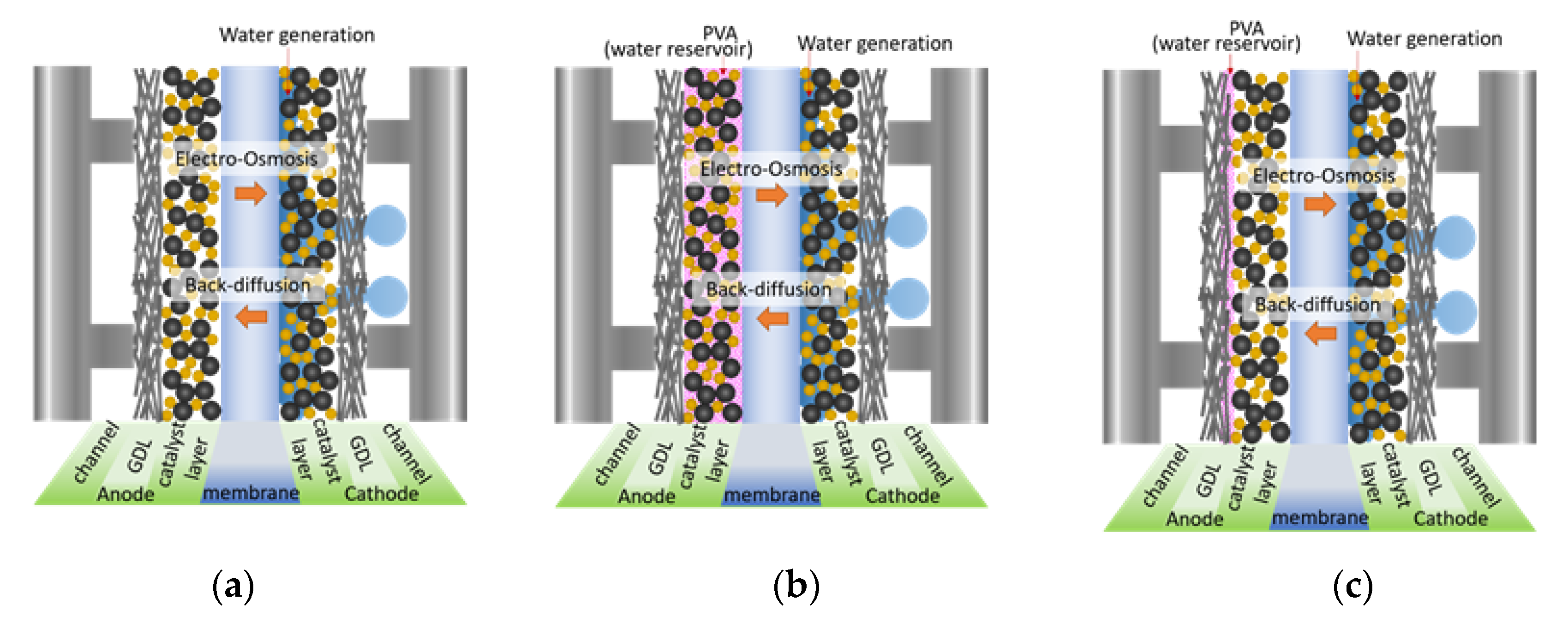
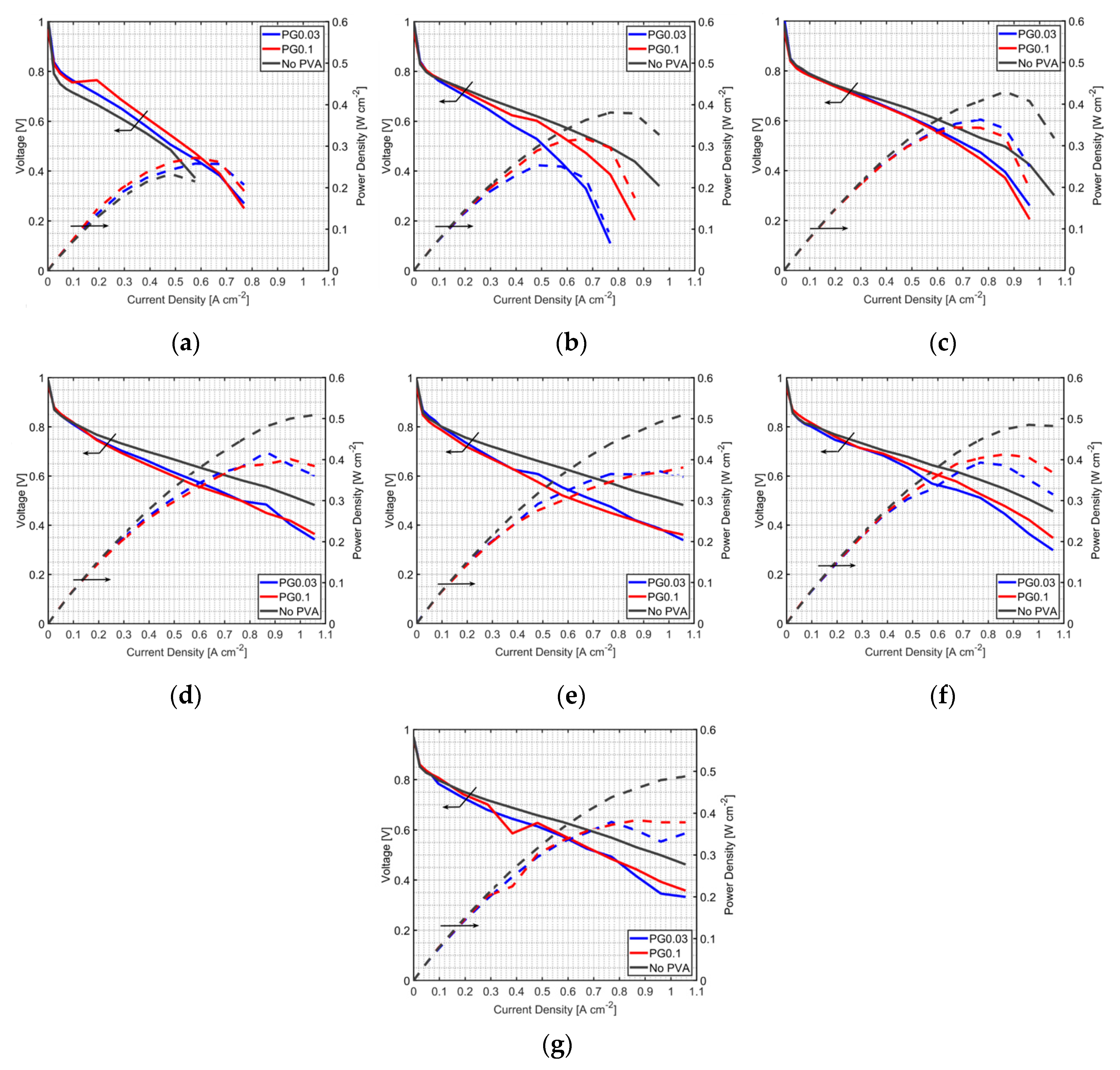
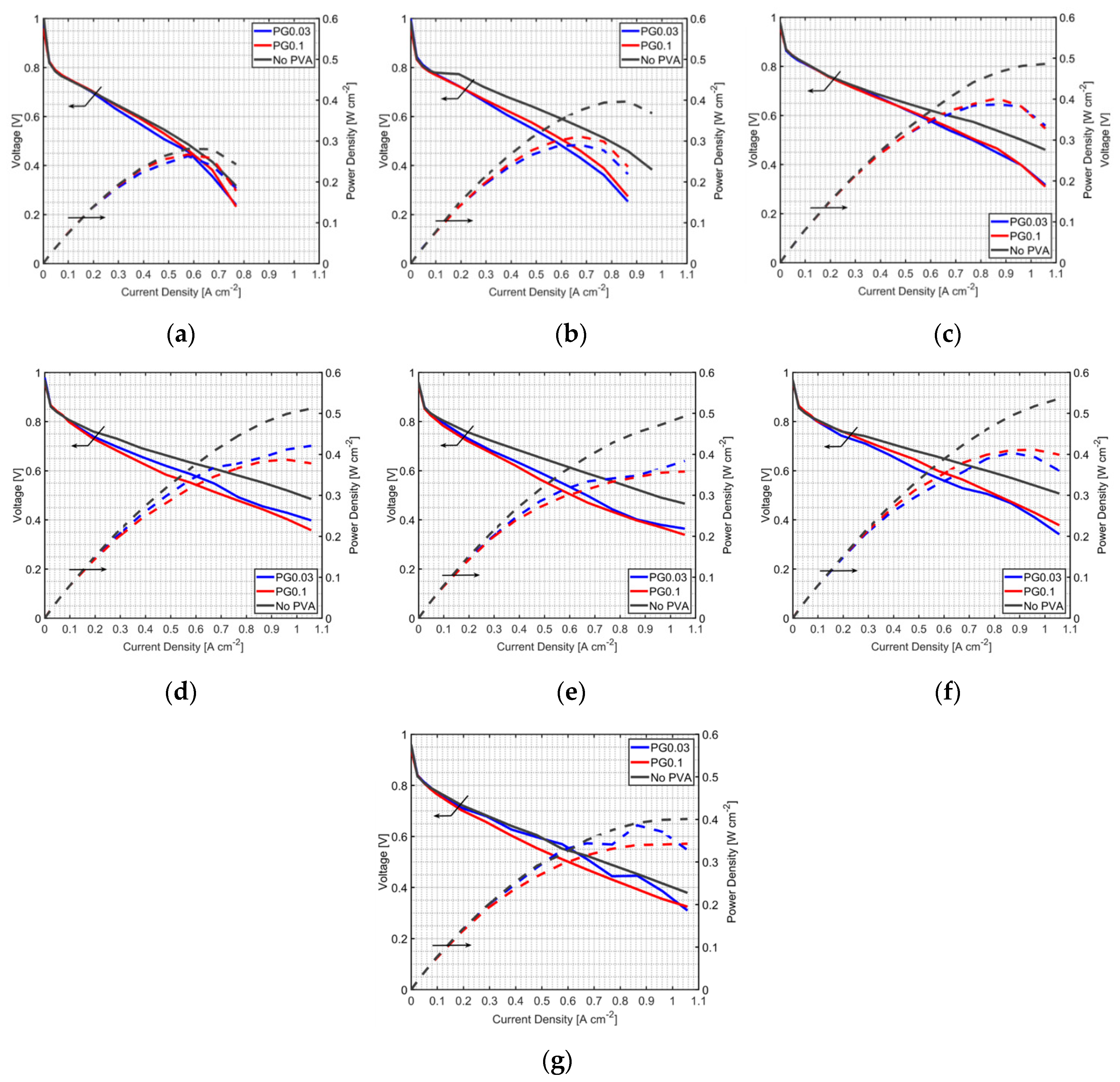
3.2. Effect of PVA Location in the MEA
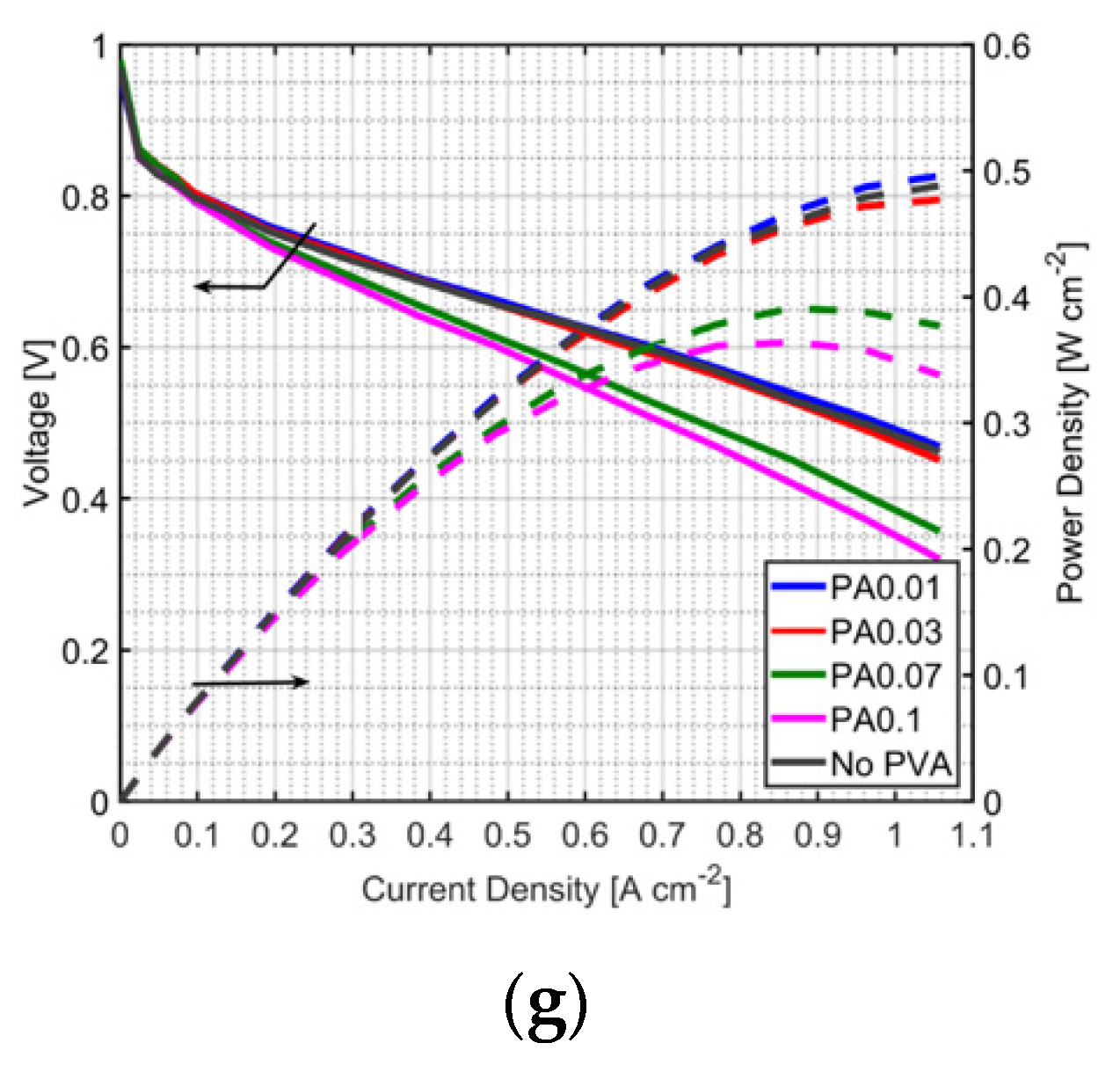
3.3. Durability Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oh, T.H. Conceptual design of small unmanned aerial vehicle with proton exchange membrane fuel cell system for long endurance mission. Energy Convers. Manag. 2018, 176, 349–356. [Google Scholar] [CrossRef]
- Teixeira, F.C.; de Sá, A.I.; Teixeira, A.P.S.; Rangel, C.M. Nafion phosphonic acid composite membranes for proton exchange membranes fuel cells. Appl. Surf. Sci. 2019, 487, 889–897. [Google Scholar] [CrossRef]
- Yang, T.-H.; Yoon, Y.-G.; Kim, C.-S.; Kwak, S.-H.; Yoon, K.-H. A novel preparation method for a self-humidifying polymer electrolyte membrane. J. Power Sources 2002, 106, 328–332. [Google Scholar] [CrossRef]
- Choi, I.; Lee, H.; Lee, K.G.; Ahn, S.H.; Lee, S.J.; Kim, H.-J.; Lee, H.-N.; Kwon, O.J. Characterization of self-humidifying ability of SiO2-supported Pt catalyst under low humidity in PEMFC. Appl. Catal. B 2015, 168–169, 220–227. [Google Scholar] [CrossRef]
- Tian, J.; Gao, P.; Zhang, Z.; Luo, W.; Shan, Z. Preparation and performance evaluation of a Nafion-TiO2 composite membrane for PEMFCs. Int. J. Hydrogen Energy 2008, 33, 5686–5690. [Google Scholar] [CrossRef]
- Mollá, S.; Compañ, V.; Gimenez, E.; Blazquez, A.; Urdanpilleta, I. Novel ultrathin composite membranes of Nafion/PVA for PEMFCs. Int. J. Hydrogen Energy 2011, 36, 9886–9895. [Google Scholar] [CrossRef]
- Ahn, M.-K.; Lee, S.-B.; Min, C.-M.; Yu, Y.-G.; Jang, J.; Gim, M.-Y.; Lee, J.-S. Enhanced proton conductivity at low humidity of proton exchange membranes with triazole moieties in the side chains. J. Membr. Sci. 2017, 523, 480–486. [Google Scholar] [CrossRef]
- Kim, K.; Heo, P.; Ko, T.; Kim, K.-H.; Kim, S.-K.; Pak, C.; Lee, J.-C. Poly (arlyene ether sulfone) based semi-interpenetrating polymer network membranes containing cross-linked poly (vinyl phosphonic acid) chains for fuel cell applications at high temperature and low humidity conditions. J. Power Sources 2015, 293, 539–547. [Google Scholar] [CrossRef]
- Patel, H.A.; Mansor, N.; Gadipelli, S.; Brett, D.J.L.; Guo, Z. Superacidity in Nafion/MOF hybrid membranes retains water at low humidity to enhance proton conduction for fuel cells. ACS Appl. Mater. Interfaces 2016, 8, 30687–30691. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, J.; Chang, Y.; Zhu, Y.; Xie, X.; Qin, Y.; Zhang, J.; Jiao, K.; Du, Q.; Guiver, M.D. Design of Pt-C/Fe-N-S-C cathode dual catalyst layers for proton exchange membrane fuel cells under low humidity. Electrochim. Acta 2019, 296, 450–457. [Google Scholar] [CrossRef]
- Guo, X.; Shao, Z.; Xiao, Y.; Zeng, Y.; Liu, S.; Wang, X.; Yi, B. Improvement of the proton exchange membrane fuel cell (PEMFC) performance at low-humidity conditions by exposing anode in ultraviolet light. Electrochem. Commun. 2014, 44, 16–18. [Google Scholar] [CrossRef]
- Roh, C.-W.; Choi, J.; Lee, H. Hydrophilic-hydrophobic dual catalyst layers for proton exchange membrane fuel cells under low humidity. Electrochem. Commun. 2018, 97, 105–109. [Google Scholar] [CrossRef]
- Su, H.; Xu, L.; Zhu, H.; Wu, Y.; Yang, L.; Liao, S.; Song, H.; Liang, Z.; Birss, V. Self-humidification of a PEM fuel cell using a novel Pt/SiO2/C anode catalyst. Int. J. Hydrogen Energy 2010, 35, 7874–7880. [Google Scholar] [CrossRef]
- Han, M.; Chan, S.H.; Jiang, S.P. Investigation of self-humidifying anode in polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2007, 32, 385–391. [Google Scholar] [CrossRef]
- Leimin, X.; Shijun, L.; Lijun, Y.; Zhenxing, L. Investigation of a novel catalyst coated membrane method to prepare low-platinum-loading membrane electrode assemblies for PEMFCs. Fuel Cells 2009, 9, 101–105. [Google Scholar] [CrossRef]
- Chen, F.; Liu, P. High electrically conductive polyaniline/partially phosphorylated poly (vinyl alcohol) composite films via aqueous dispersions. Macromol Res 2011, 19, 883. [Google Scholar] [CrossRef]
- El-Toony, M.M.; Abdel-Hady, E.E.; El-Kelsh, N.A. Casting of poly hydroxybutarate/poly (vinyl alcohol) membranes for proton exchange fuel cells. Electrochim. Acta 2014, 150, 290–297. [Google Scholar] [CrossRef]
- Attaran, A.M.; Javanbakht, M.; Hooshyari, K.; Enhessari, M. New proton conducting nanocomposite membranes based on poly vinyl alcohol/poly vinyl pyrrolidone/BaZrO3 for proton exchange membrane fuel cells. Solid State Ion. 2015, 269, 98–105. [Google Scholar] [CrossRef]
- Liang, H.; Zheng, L.; Liao, S. Self-humidifying membrane electrode assembly prepared by adding PVA as hygroscopic agent in anode catalyst layer. Int. J. Hydrogen Energy 2012, 37, 12860–12867. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Water transport in polymer electrolyte membrane fuel cells. Prog. Energy Combust. Sci. 2011, 37, 221–291. [Google Scholar] [CrossRef]
- Zhang, G.; Jiao, K. Multi-phase models for water and thermal management of proton exchange membrane fuel cell: A review. J. Power Sources 2018, 391, 120–133. [Google Scholar] [CrossRef]
| Case | Fuel Cell Temperature (°C) | Humidifier Temperature (°C) (Relative Humidity RH %) | ||
|---|---|---|---|---|
| Anode | Cathode | |||
| Wet | 1. | 60 | 25 (15.9) | 25 (15.9) |
| 2. | 60 | 30 (21.3) | 30 (21.3) | |
| 3. | 60 | 40 (37.0) | 40 (37.0) | |
| 4. | 60 | 50 (61.9) | 50 (61.9) | |
| 5. | 60 | 60 (100) | 60 (100) | |
| 6. | 70 | 60 (63.9) | 60 (63.9) | |
| 7. | 70 | 70 (100) | 70 (100) | |
| Dry | 8. | 60 | non-humidified | 25 (15.9) |
| 9. | 60 | non-humidified | 30 (21.3) | |
| 10. | 60 | non-humidified | 40 (37.0) | |
| 11. | 60 | non-humidified | 50 (61.9) | |
| 12. | 60 | non-humidified | 60 (100) | |
| 13. | 70 | non-humidified | 25 (10.2) | |
| 14. | 70 | non-humidified | 70 (100) | |
| Case | Description | PVA Loading (μg cm−2) |
|---|---|---|
| PA0.01 | PVA 0.01 wt.% in anode CL | 0.03274 |
| PA0.03 | PVA 0.03 wt.% in anode CL | 0.09823 |
| PA0.07 | PVA 0.07 wt.% in anode CL | 0.22931 |
| PA0.1 | PVA 0.1 wt.% in anode CL | 0.32762 |
| PG0.03 | PVA 0.5 cycles * on GDL which has PVA loading close to PA0.03 case | 0.09183 |
| PG0.1 | PVA 1.75 cycles * on GDL which has PVA loading close to PA0.1 case | 0.32140 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jienkulsawad, P.; Chen, Y.-S.; Arpornwichanop, A. Modifying the Catalyst Layer Using Polyvinyl Alcohol for the Performance Improvement of Proton Exchange Membrane Fuel Cells under Low Humidity Operations. Polymers 2020, 12, 1865. https://doi.org/10.3390/polym12091865
Jienkulsawad P, Chen Y-S, Arpornwichanop A. Modifying the Catalyst Layer Using Polyvinyl Alcohol for the Performance Improvement of Proton Exchange Membrane Fuel Cells under Low Humidity Operations. Polymers. 2020; 12(9):1865. https://doi.org/10.3390/polym12091865
Chicago/Turabian StyleJienkulsawad, Prathak, Yong-Song Chen, and Amornchai Arpornwichanop. 2020. "Modifying the Catalyst Layer Using Polyvinyl Alcohol for the Performance Improvement of Proton Exchange Membrane Fuel Cells under Low Humidity Operations" Polymers 12, no. 9: 1865. https://doi.org/10.3390/polym12091865
APA StyleJienkulsawad, P., Chen, Y.-S., & Arpornwichanop, A. (2020). Modifying the Catalyst Layer Using Polyvinyl Alcohol for the Performance Improvement of Proton Exchange Membrane Fuel Cells under Low Humidity Operations. Polymers, 12(9), 1865. https://doi.org/10.3390/polym12091865








