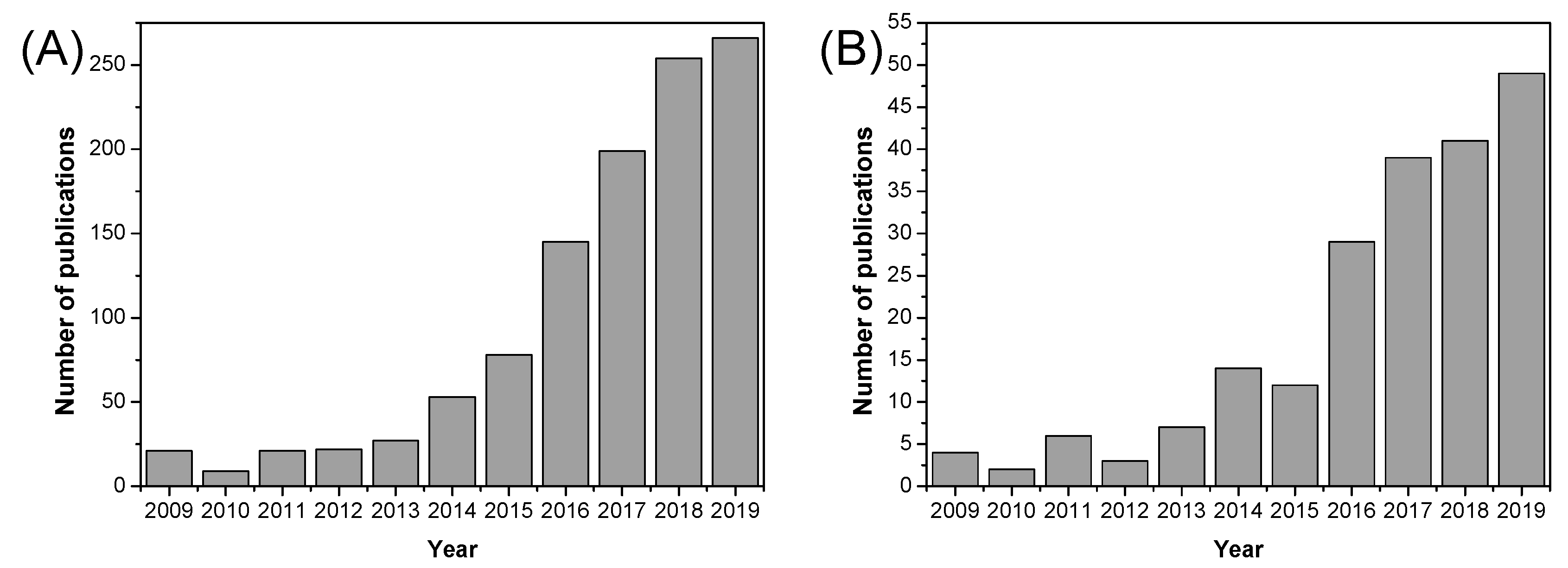
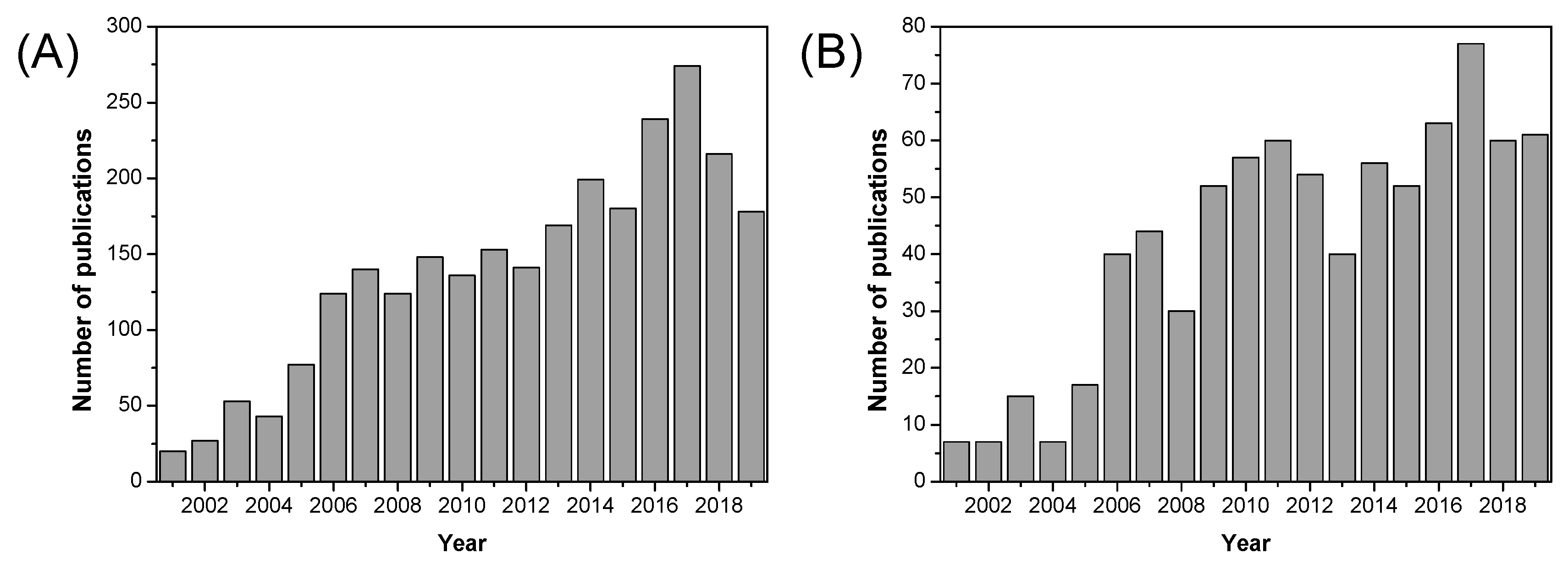
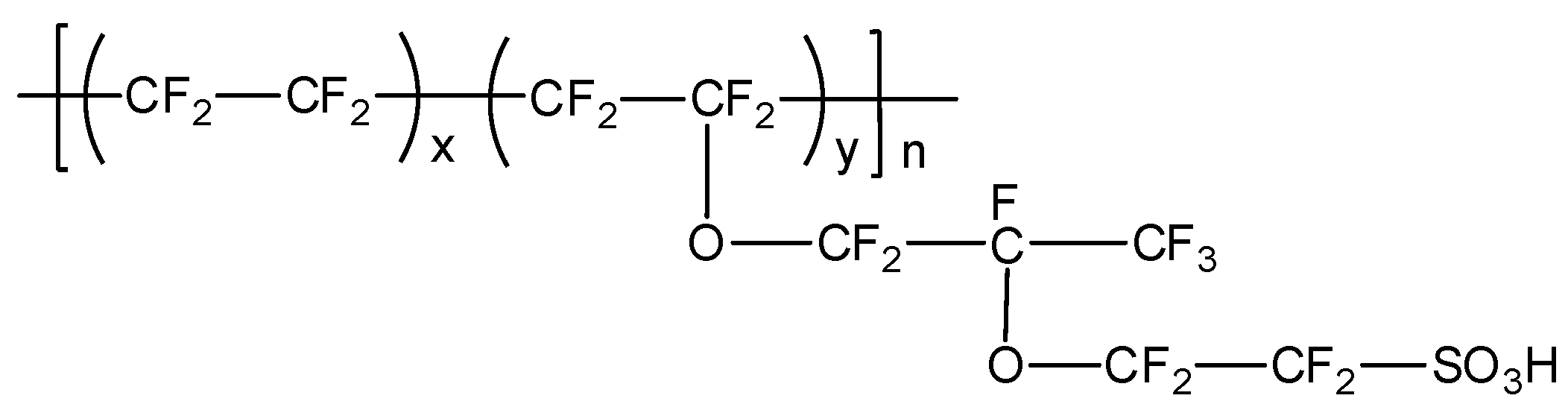1. Introduction
Proton conductivity has received intense attention owing to its application in chemical sensors, electrochemical devices, and power generation [
1,
2]. Proton conducting membranes form an important part in fuel cells (FCs), batteries, and supercapacitors [
3]. Fuel cells based on proton exchange membranes (PEMs) are one of the most promising alternative energy sources because of their high efficiency, high power density, low emissions, and energy supply [
4,
5]. These alternatives provide the possibility of receiving energy from hydrogen, synthetic, or bio-synthetic fuels and can operate with greater efficiency and environmental sustainability compared with thermal motors [
6,
7]. Fuel cells can be used for a wide variety of technological devices such as vehicles, mobile phones, portable electronics, and power generators [
8,
9,
10]; they are generally classified by the kind of electrolyte they use. Common classifications include alkaline fuel cells [
11], direct methanol fuel cells [
12], polymer electrolyte membrane fuel cells [
13], phosphoric acid fuel cells [
14], molten carbonate fuel cells [
15], solid oxide fuel cells [
16], and reversible fuel cells (also called unitized regenerative fuel cells) [
17].
In a typical proton exchange membrane fuel cell (PEMFC), the polymer electrolyte membrane is responsible for proton conductivity, which allows the transport of protons from the anode to the cathode, constituting the essential component of the electrochemical device [
18]. PEMFCs constitute a promising alternative to fossil fuels as they generate water as a byproduct and use only hydrogen and oxygen as reactants (
Figure 1). According to their range of operating temperatures, PEMFCs can be classified into three main categories: (a) low temperature PEMFCs (LT-PEMFCs), which operate around 50–80 °C [
19]; (b) intermediate temperature (IT-PEMFCs), which operate in the 80–120 °C range [
20,
21,
22]; and (c) high temperature (HT-PEMFCs), which operate from 140 °C up to 200 °C [
23,
24], generally under anhydrous conditions. Among the numerous types of PEMs, membranes based on perfluorosulfonic acid polymers, such as Nafion
® (
Figure 2), have wide acceptance as they have been demonstrated to possess good conductivity as well as chemical and mechanical properties, have been used at temperatures below 90 °C, and have endured conditions of high relative humidity [
25].
Nafion
®, developed by DuPont in the late 1960s, still remains as the state-of-the-art membrane for low temperature PEMFCs (LT-PEMFCs). The main drawbacks of Nafion
® membranes are mainly their costly manufacturing process, the destruction of the polymer structure at higher temperatures, and the strong decrease in proton conductivity at temperatures above 90 °C when low hydration conditions are reached [
26,
27,
28,
29,
30]. Consequently, Nafion
®-based membranes are limited to operate as LT-PEMFCs. These practical limitations have promoted the development of membranes that may be applied in HT-PEMFCs operating temperatures in the range of 140–200 °C and, hence, in the absence of water [
31,
32,
33,
34,
35].
Among the benefits of working at high temperatures, it is worth mentioning a decrease in the catalyst contamination by CO and CO
2 poisoning, as the kinetics in the electrodes are faster and have a simpler thermal and water handling, low dependency on cooling systems, a high amount of reusable heat energy, as well as a lower cost of the membrane-electrode assemblies (MEAs) in comparison with LT-PEMFCs based on Nafion
® [
36,
37,
38]. The high CO tolerance of the anode catalyst makes it possible for an FC to use hydrogen directly from a simple methanol reformer, thus the selective oxidant and/or CO separator device can be removed from the processing system. Consequently, the size of an FC is importantly reduced, improving its performance, responsiveness, and reliability, which ultimately allows to bring down system maintenance and operating costs [
39]. In order to optimize the performance of FCs, a lot of work has been done in the development of HT-PEMs, particularly on those based on polybenzimidazoles (PBIs), which have emerged as promising candidates to operate at high temperatures [
40,
41,
42,
43,
44].
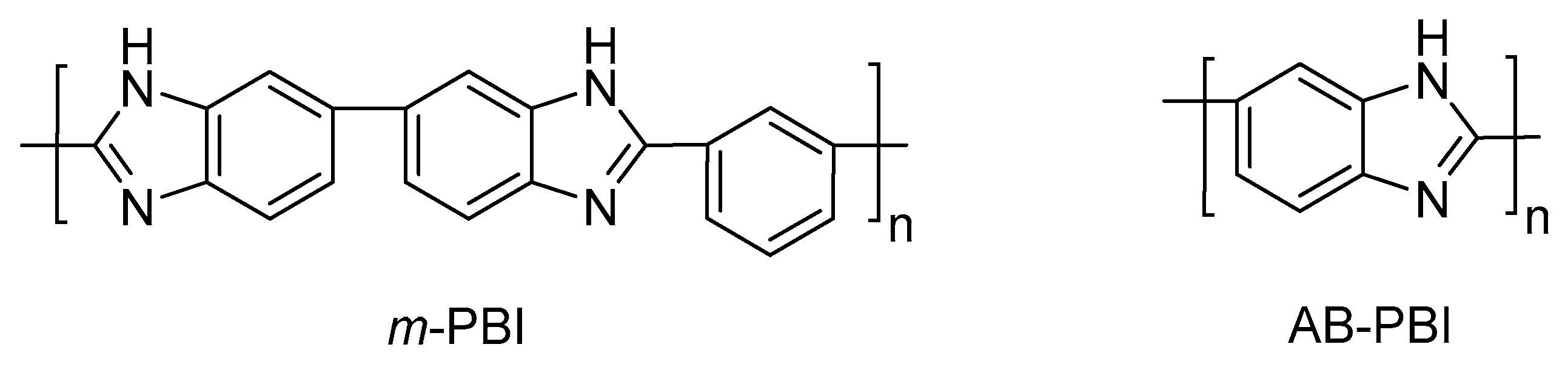
PBIs are aromatic linear heterocyclic macromolecules that belong to the class of high performance polymers; they are highly resistant to acids and bases; have high glass transition temperatures (425–436 °C); and possess excellent thermal and mechanical stability, low flammability, and high energy radiation resistance. Aromatic polybenzimidazoles were firstly synthesized by H. Vogel and C. S. Marvel in 1961 [
45], and as a consequence of their exceptional thermal and oxidative stability, they were used on aerospace and defense applications by NASA and the Air Force Materials Laboratory (AFML), respectively. The most widely studied polybenzimidazole is one commercialized by the corporation Celanese, poly [2,2′-(
m-phenylene)-5,5′-bibenzimidazole], or
m-PBI (also known as simply PBI). This polymer can be synthesized by a polycondensation reaction using 3,3′-diaminobenzidine (DAB) and isophthalic acid (IPA) [
46,
47]. Another available polybenzimidazole is poly (2,5-benzimidazole) (or AB-PBI), which was also carefully studied as membrane for HT–PEMFCs. The AB-PBI is prepared by condensation of 3,4-diaminobenzoic acid (DABA) [
48]. The chemical structures of both
m-PBI and AB-PBI are shown in
Figure 3.
However, to achieve the high proton conductivity levels required for HT-PEMFCs, these simple PBIs need to be doped with acid because their intrinsic conductivity is very low (around 10
−12 S/cm). In particular, phosphoric acid (PA)-doped PBI membranes demonstrated good thermal and mechanical stability, low gas permeability, and conductivity values between 0.07 and 0.2 S/cm at approximately 200 °C without any additional humidification [
49,
50]. As the ionic conductivity of PA is low at low temperatures, PBI embedded in PA operating temperature range is about 150–250 °C. The hydrogen oxidized at the anode splits into protons and electrons. The electrons pass through the external electrical circuit, whereas the protons are transferred through the electrolyte. On the cathode side, the redox reaction between positive hydrogen ions, electrons, and oxygen gas results in water formation [
51]. The main advantage of phosphoric fuel cells is their capacity to generate and separate electricity and useful heat at the same time. However, the use of this acid-doping method presents various inconveniences, limiting the applications; particularly, the pyrolysis of PA above 190 °C [
50], the migration of PA resulting in loss of transition metal catalyst and reducing proton conductivities, acid leaching problems, and reduced mechanical properties under HT-PEMFCs’ operation conditions.
Another efficient method to increase conductivity is the introduction of the covalently bound sulfonic and/or phosphoric groups into the backbone chains, but multi-step complex syntheses are generally needed for the formation of such PBIs [
52,
53] Additionally, the aggressive polymerization conditions may affect the side-groups, resulting in a crosslinked polymer gel instead of a linear structure. Consequently, the possible structural variations in the polymers are limited and PBIs containing functional groups in their main chain are scarce. PBIs obtained by changing one of the monomers allow for the modification of the physico-chemical properties (e.g., basicity), simply by playing with the number of the nitrogen atoms in the monomer and their distribution along the polymer [
54]; in this manner, acid leaching can be minimized [
55].
The doping strategies for PBI-type membranes consist of an incorporation of significant amounts of phosphoric acid to the polymer matrix to achieve sufficient proton conductivity. The acid doping can be performed in various ways. The simplest and most efficient method consists of directly immersing the PBI-type membrane sheet into hot phosphoric acid. The doping level of the membrane depends on the immersion time and temperature. As an example, the AB-PBI based-membrane doped at 120 °C for 24 h can absorb phosphoric acid up to 2.5 times its own weight, which corresponds to the chemical formula AB-PBI·5H
3PO
4. On the other hand, the thickness practically doubles during the doping process. For example, the thickness changed from 50 μm for a pristine PBI membrane to almost 100 μm when it was fully doped [
56]. The interaction between acid and the polymer matrix occurs via the N-imidazole sites. The basic N-sites of PBI act as proton acceptors like in a standard acid–base reaction, creating the ion pairs in this process as shown in
Figure 4. Therefore, the polymer bearing more basic N-sites forms stronger bonds with acids.
The conductivity depends not only on the doping level, but also on the acid distribution within the membrane. In this sense, the membranes should ensure that the acid percolates throughout the polymer network and interacts with nearly every basic N-site of the polymer matrix. Finally, the conductivity of composite membranes based on PBIs is also affected by the dehydration reaction of phosphoric acid. It was noticed that, under anhydrous conditions, the conductivity of phosphoric acid decreases for temperatures above 140 °C owing to a condensation reaction of PA affording pyrophosphoric acid (H
4P
2O
7) and water. Therefore, at temperatures between 140 and 180 °C, the cell resistance of MEA based on PBIs under open circuit conditions is significantly higher than that with an electrical load and, additionally, it allows to produce water through the fuel cell reaction. The water formed permits the rehydration of the membrane in the MEA and results in a better conducting phosphoric acid [
57].
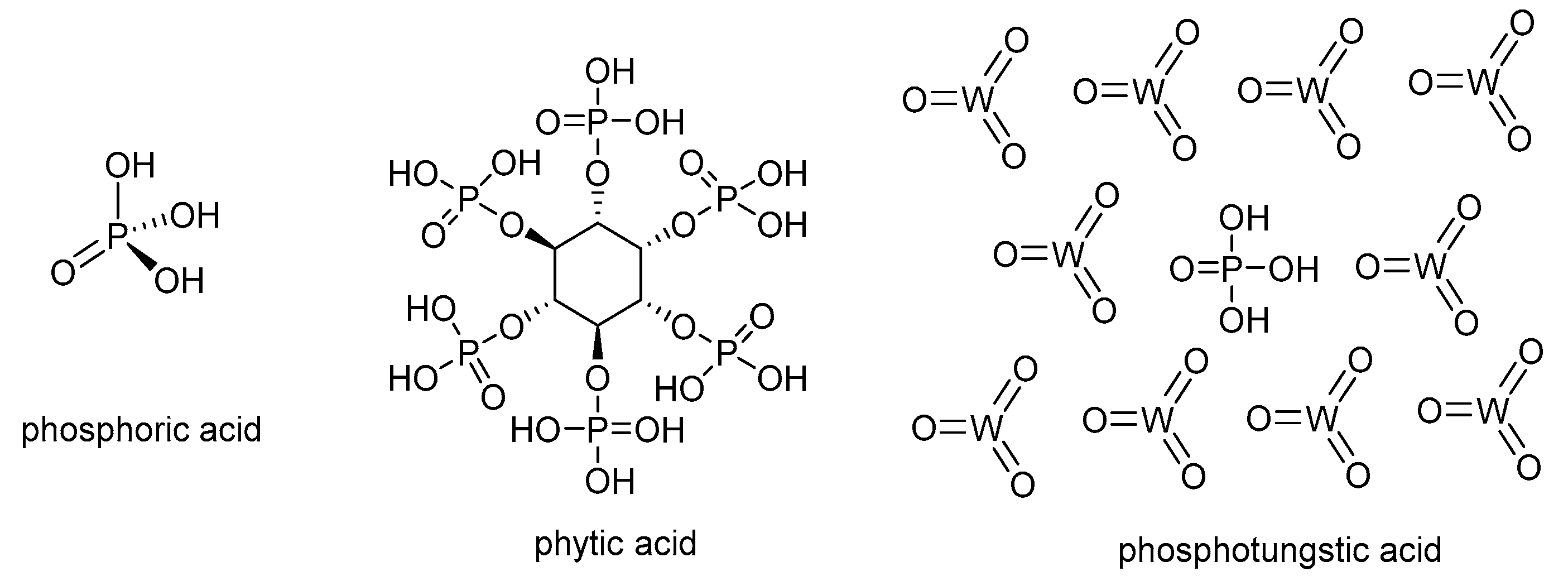
Despite the wide use of PA as acid doping for PBI-based membranes, other acids have been used in the past years to overcome the drawbacks associated with the use of PA, mainly acid leaching and corrosion, which can seriously affect the long-term stability of fuel cells. Among other doping acids containing phosphonate groups, phytic acid (myoinositol hexakisphosphate) is considered a sustainable alternative to phosphoric acid (
Figure 4). Phytic acid is a phosphorus-containing organic acid that can be found in plants, especially in seeds and fibers. This acid has been used as a doping agent in combination with metal organic frameworks (MOFs) for polymer electrolyte membranes based on Nafion
®, reaching high proton conductivities [
58,
59]. Because of its molecular size, this natural acid can be encapsulated into cavities, reducing the leaching from the membrane. Another type of alternative acid is heteropolyacids, which are a particular class of acids made up of a combination of hydrogen and oxygen with metals and non-metals. Among this family of heteropolyacids, phosphotungstic acid (HPW), with molecular formula H
3P
4W
12O
40, has been efficiently applied as a proton carrier in proton exchange membrane fuel cells [
60,
61]. Some representative literature on heteropolyacid doped polymer as PEM should be consulted [
62,
63]. In a recent work, we have evaluated the long-term stability of PBI membranes doped with different concentrations of the widely used PA, and an important acid leaching and subsequent conductivity drop was observed [
64].
2. Proton Conduction and Transport Mechanism
As has been previously commented, the proton conductivity of acid-doped PBI membranes is strongly dependent on the physico-chemical parameters such as temperature, relative humidity (RH), as well as the acid doping level measured in molar concentration of the doping solution [
65,
66]. Such conductivity is generally accepted to occur by two different mechanisms [
67]: the Grotthuss mechanism (also known as hopping mechanism) and the vehicle mechanism. The prevalence of either the hopping or vehicle mechanism depends on the hydration level of the membrane. On the other hand, the mechanism of proton transport in composite and hybrid membranes is much more complex as it involves both the surface and chemical properties of the inorganic and organic phases present in the composite membrane.
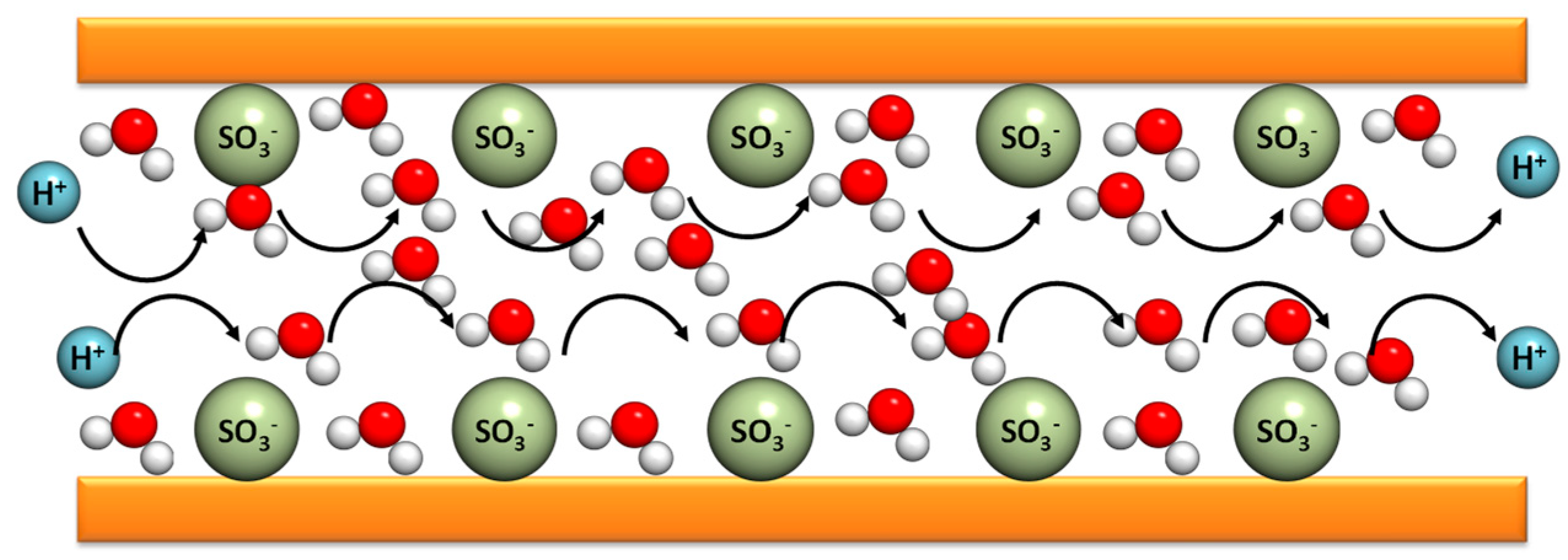
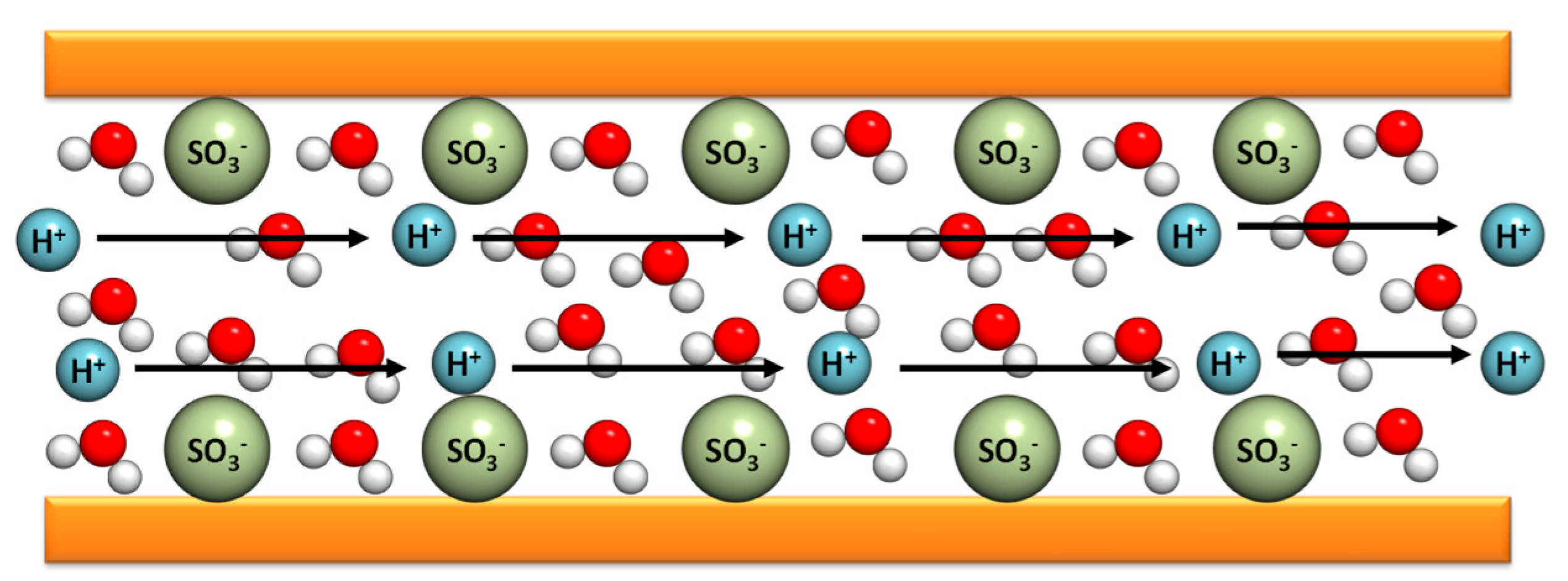
The Grotthuss mechanism is characterized by the protons’ jumping from one site to another along a hydrogen-bonding (HB) chain. In the case of PBI composite membranes, the protons are transferred from the nitrogen benzimidazole sites (N–H) to the phosphoric acid anions inside of the polymer matrix. This contribution is relevant for n = [H
3PO
4]/[PBI] < 2. When
n > 2, there is a possibility of a proton hopping along the phosphoric acid anions. This mechanism is relevant in the case of non-doped PBI. The original idea was proposed by Theodore von Grotthuss in 1806 to explain a mechanism for proton (H
+) transport between water molecules [
68]. As shown in
Figure 5, the protons jump from a protonic species such as H
3O
+ to another protonic species in the membrane [
69].
The second mechanism, namely the vehicle mechanism, was proposed in 1982 by Kreuer, Rabenau, and Weppner [
70] and rationalizes that the motion of protons is assisted by carrying molecules that, in general, are associated to the doping acidic groups. This last mechanism accounts for the major contribution for the conductivity increment of the polymeric membranes. Finally, a combination of the two described mechanisms can be observed in the case of diffusion of protons as part of polymeric structures involving molecules of water such as H
3O
+, H
5O
2+ (Zundel cation), H
9O
4+ (Eigen cation), or some other species, such as NH
4+, which may also be present in the polyelectrolyte, in combination with the diffusion of vehicles as uncharged molecules. As illustrated in
Figure 6, the protons attach themselves to a vehicle site such as water, which is diffused through the medium, and thus carry the protons along.
When the protons are moving through a hopping mechanism, the conductivity of the acid-doped PBI is governed by an activation mechanism that obeys the Arrhenius law [
71], where the relation between the conductivity and temperature is governed by the expression.
where σ (S/cm) is the proton conductivity at a certain RH, A is a pre-factor, E
a (kJ/mol) is the activation energy at a certain RH, R (8.314 J/mol K) is the gas constant, and T (K) is the absolute temperature.
The values of Ea for n > 2 are in the range 15–25 kJ/mol, very near to that of concentrated H3PO4 aqueous solution. In this situation, PBI-based membranes doped with acidic groups show high proton conductivity if properly doped with strong acids such as H3PO4 depending on the amount of PA absorbed into the porous of its matrix. In fact, PBI is a basic polymer, which dissociates PA releasing protons, as sketched by the following reaction: H3PO4 + PBI → H2PO4− + PBI−H+, where the equilibrium constant is about K = 1.17 × 103 and H+ ions can migrate through the polymer backbone by means of hydrogen bonds, in this case, assisted by phosphate anions by means of the Grotthuss mechanism, as we previously mentioned.
In some cases, such as PA-doped composite membranes of PBI containing ionic liquids, the dependence of the conductivity versus temperature presents a different behavior than the typical Arrhenius behavior and the activation energy associated with the conductivity mechanism is not constant for the entire the range of temperatures. In such a situation, the conductivity of the membranes exhibits a Vogel–Tammann–Fulcher (VTF) conduction behavior, where proton hopping is coupled with the segmental motion of the polymer chains and the activation mechanism is given according to the equation.
where
B is the fitting parameter related with the curvature of the experimental data plot;
T0 is the Vogel temperature, which is considered as the one at which the relaxation time would diverge; and
σ∞ is the pre-factor related with the conductivity limit at high temperatures.
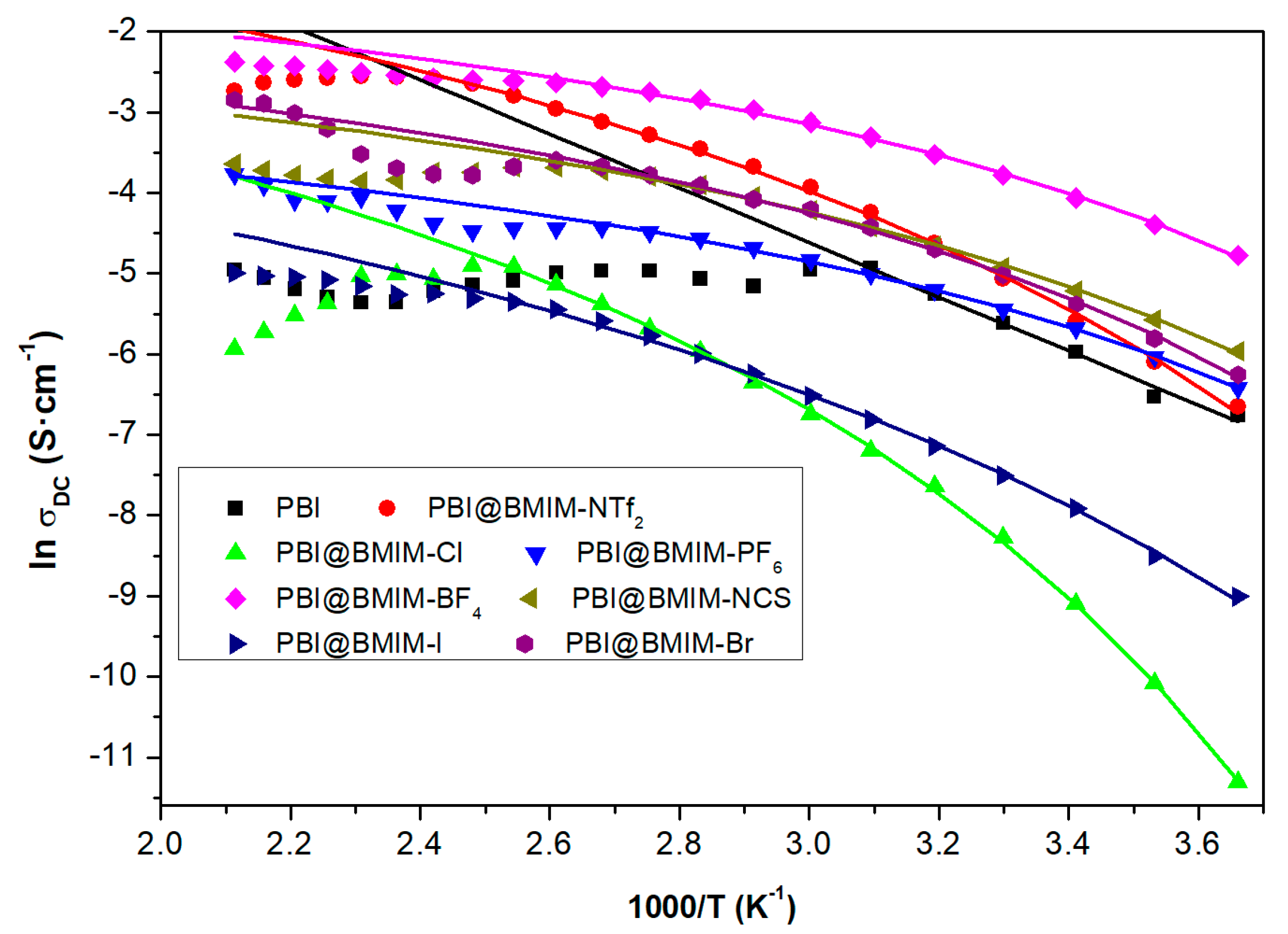
Figure 7 displays the results found for the conductivity of some composite membranes of PBI filled with ionic liquids (ILs), where a behavior different from linear is observed depending on the IL type. The activation energy associated with this kind of composite membrane is much higher for lower temperatures than the one associated with higher temperatures, in agreement with the experimental results. The values for the activation energy obtained from the fit of Equation (2) to the experimental data plotted in
Figure 7 follow the trend [Cl]
− (6.33 kJ/mol) > [I]
− (5.80 kJ/mol) > [NTf
2]
− (5.35 kJ/mol) > [Br]
− (3.04 kJ/mol) > [NCS]
− (2.91 kJ/mol) > [BF
4]
− (2.53 kJ/mol) ≈ [PF
6]
− (2.51 kJ/mol). A close inspection of
Figure 7 reveals a change in the slopes. For PBI@BMIM-NTf2 and PBI@BMIM-Cl, a negative slope is observed at high temperatures, in contrast with a slightly positive slope for PBI@BMIM-NCS and PBI@BMIM-BF4. This behavior can be associated with the variation in Debye’s length, which is related to the effective dissociation energy and the measured dielectric permittivity in the absence of electrode polarization as well as of orientational polarization of dipolar ions, as previously reported [
72,
73].
There are many examples of PBI composite membranes with values of proton conductivity and its corresponding activation energy. As an example, Wang and et al. [
74] prepared an insoluble sulfonated polyphosphazene (SPOP) with 117% degree of sulfonation; the SPOP was used as the proton conductor in the PBI as HT-PEM, and the polyfunctional triglycidyl isocyanurate (TGIC) was used as a covalent cross-linking agent to inhibit swelling at low degree of cross linking of the PBI-TGIC/SPOP composite membranes obtained. The proton conduction activation energy was calculated at a specific set of RH and is summarized in
Table 1. The conductivity of PBI-TGIC(5%)/SPOP(50%) at 100% RH, 50% RH, and 0% RH is 0.143, 0.076, and 0.044 S/cm at 180 °C, respectively. The authors described that the proton conduction activation energy increased with the increasing degree of crosslinking. This inhibited the water absorption of the composite membrane and hindered the passage in the membrane of the proton conduction through the vehicle mechanism. On the other hand, the increase of the doping amount of SPOP reduced the proton activation energy, because it promoted water absorption of the membrane and improved the connectivity of the hydrophilic regions in the membrane. When the RH% was lowered, the efficiency of the vehicle mechanism for conducting protons was reduced, thereby causing a decrease in proton conductivity and an increase in proton conduction activation energy. At 0% RH, the proton conduction mechanism of the PBI-TGIC/SPOP composite membrane is the hopping mechanism.
Recently, Rajabi et al. prepared composite membranes by introducing melamine-based dendrimer amines (MDA) with mesoporous silica (SBA-15), and 1,3-di(3-methylimidazolium) propane dibromide dicationic ionic liquid (pr(mim)
2Br
2) (DIL), the membranes were doped with PA and were used to improve the proton conductivity [
75]. The APBI-DIL
4.5-MDA
1.5 composite membranes exhibit a proton conductivity of 0.22 S/cm at 180 °C under dry conditions. With the increasing temperature, the interaction between polymer chains decreases, leading to more mobility of proton carriers. The high mobility of protons and their access to the active sites can also have a positive effect on the E
a required for proton conduction through the Grotthuss and vehicular mechanisms in the membranes. According to the results displayed in
Table 2, APBI-DIL
4.5-MDA
1.5 composite membranes require a lower E
a to conduct the proton compared with the other membranes.
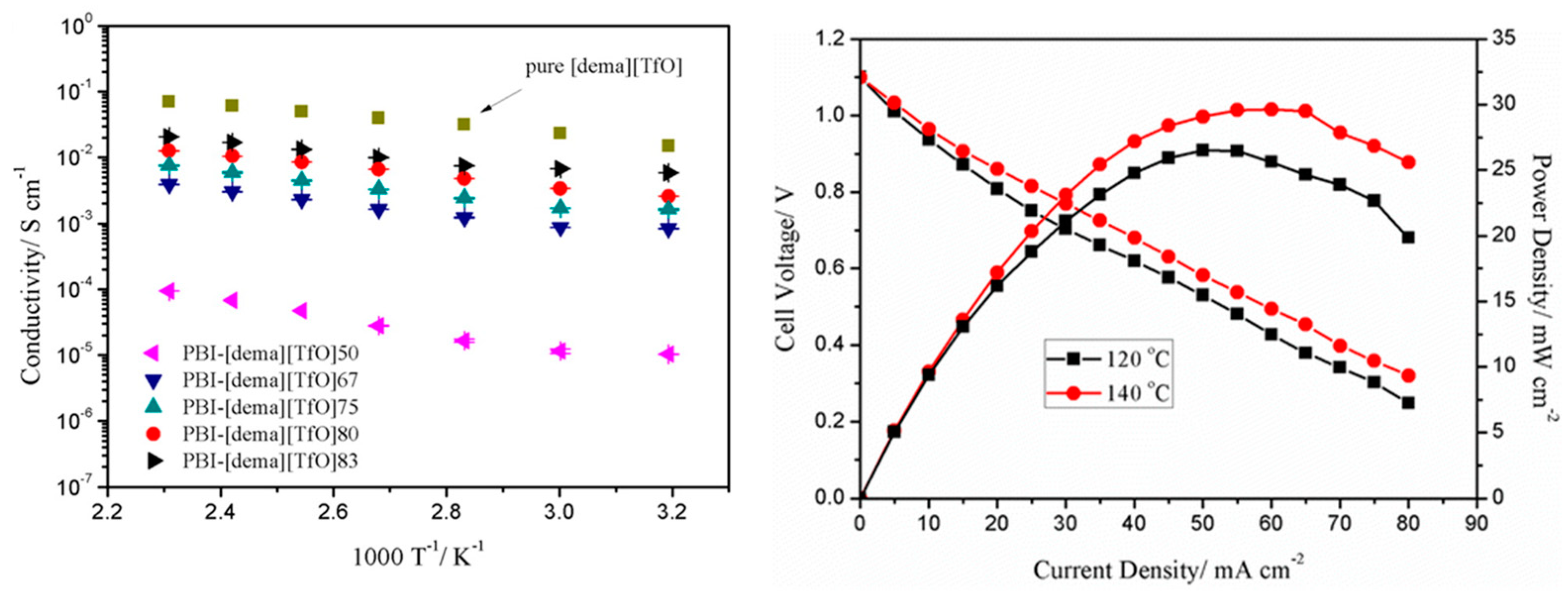
Compañ’s working group [
64] presents a systematic study of the physico-chemical properties and proton conductivity of PBI membranes doped with PA and other acids such as phytic acid and phosphotungstic acid (HPW). To further study the proton conduction mechanism of the membranes, the Arrhenius plots of the membranes and their proton conduction activation energy values (E
a) were analyzed; the results are presented in
Table 3. Proton conductivity increases for all membranes from 20 to 180 °C, following typical Arrhenius behavior. The obtained values followed the trend: E
a (PBI–PA 14 M) < (PBI–PA 1 M) ≈ (PBI–phytic) < (PBI–PA 0.1 M) < (PBI–HPW). These results indicate that proton mobilities increased with the amount of PA, and then PA formed channels in the organic phase of porous PBI. Similar activation energies were obtained for PBI–PA 1 M, PBI–PA 0.1 M, and PBI–phytic acid membranes, but PBI–PA 14 M displayed a lower value as the PA concentration was much higher and, therefore, the proton transport was more favored. According to these results, the Grotthuss mechanism dominates the proton transport in acid doped PBI membranes.
In a recent publication of the group of Compañ, SiO
2 nanofiber mats were used as fillers in the preparation of composite PBI membranes for high temperature PEMFC applications [
76]. In this work, SiO
2 nanofibers were fabricated through an electrospinning process and later functionalized using silane chemistry to introduce different polar groups, namely, −OH (neutral), −SO
3H (acidic), and −NH
2 (basic). The modified nanofiber mats were embedded in PBI to fabricate mixed matrix membranes. Proton conduction measurements show that PBI composite membranes containing nanofiber mats with basic groups showed higher proton conductivities. The E
a values of the composite membranes under wet conditions were lower than that for the pure PBI membrane, which is associated with an enhanced proton mobility, as the nanofiber acts as a carrier-bridge for protons and, consequently, the process demands less energy. On the one hand, the Grotthuss mechanism explains the conductivity by means of the interaction of protons through the jump between a hydrogen bond network of N−H groups, both from the PBI and from the functionalized groups of the nanofiber mats. On the other hand, protons can move via the vehicle mechanism through the hydroxyl, amine, or sulfonic groups from the different nanofiber functionalization and imidazole groups present in the PBI, which may interact with water molecules, promoting the proton conductivity. Additionally, activation energies under dry conditions improve proton conduction, which is mainly owing to a vehicular mechanism, as inferred from the calculated activation energies, with values higher than 55 kJ/mol (see
Table 4).
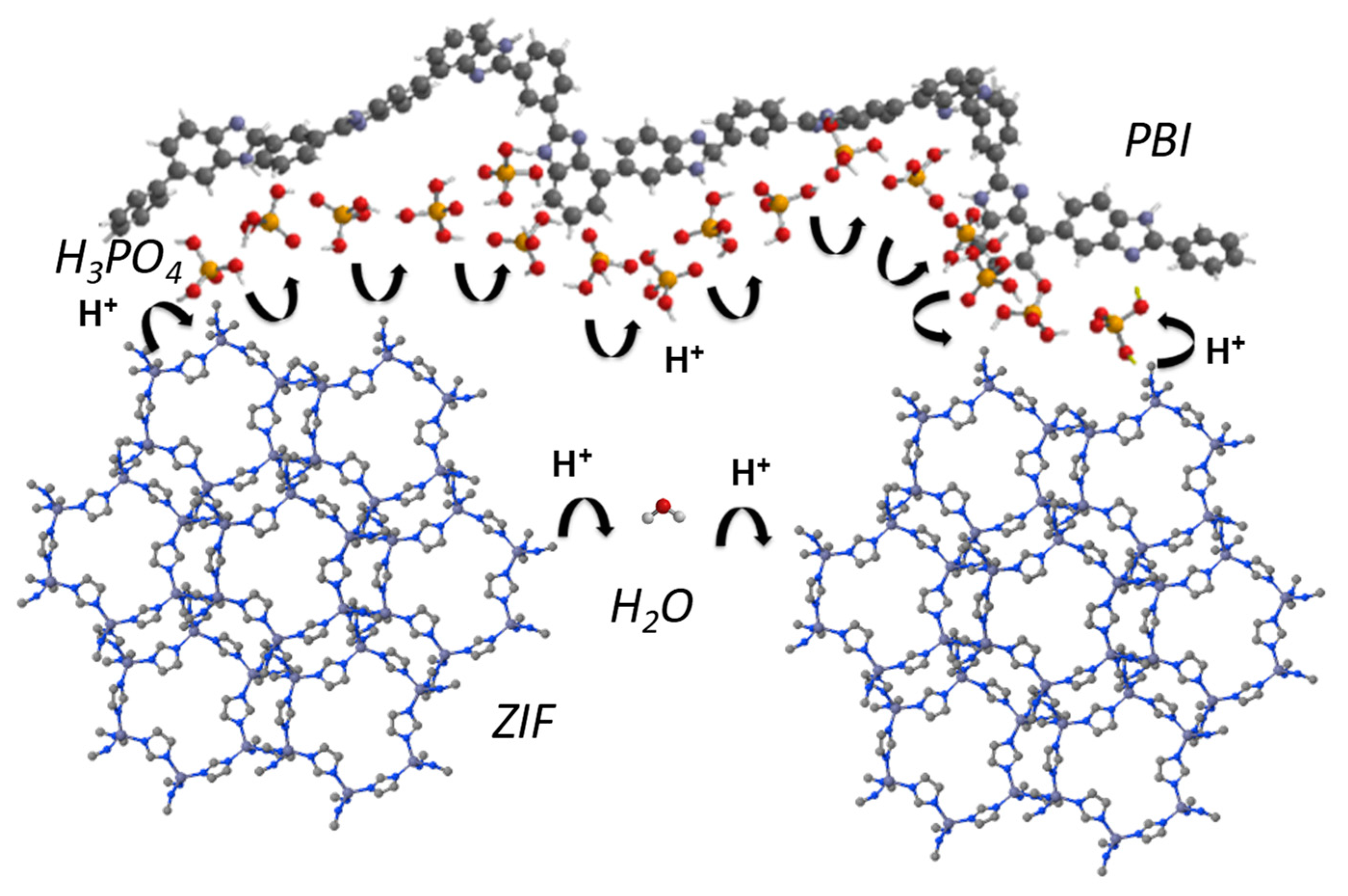
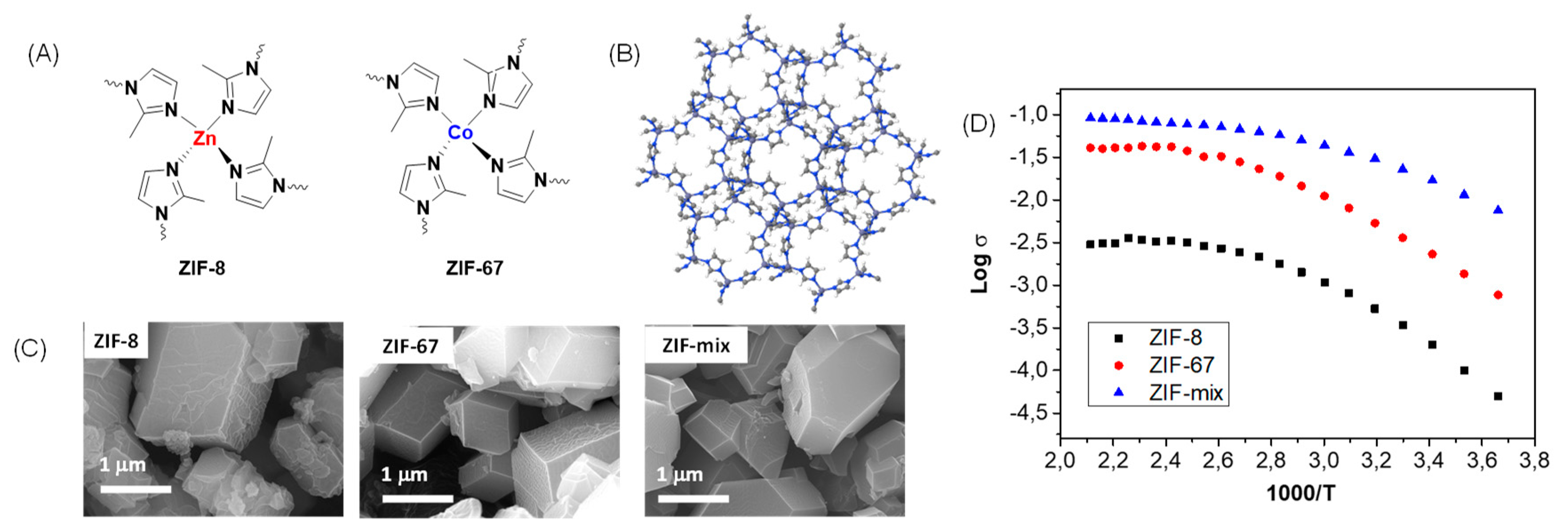
In 2018, our research group described the preparation and characterization of composite PBI membranes containing zeolitic imidazolate framework (ZIF-8) and (ZIF-67) [
77]. The calculated E
a associated with the proton transport in the PBI−ZIF-8 and PBI−ZIF-67 and PBI−ZIF−mix are shown in
Table 5. Considering that the E
a for the PBI-ZIF membranes is lower than that for pure PBI membrane, it is suggested that the ZIFs clearly favor the conductivity of the membrane at moderate and high temperatures.
According to the calculated activation energies, the proton conductivity could be rationalized by means of the Grotthuss mechanism by proton hopping through a network of hydrogen bonds along the polymeric matrix involving polybenzimidazole polymeric chains, phosphoric acid networks, and imidazolate rings from the ZIF (
Figure 8).
Abouzari-Loft and co-workers used the 2,6-pyridine functionalized polybenzimidazole (Py-PBI) as substrate for hosting PA moiety. Phosphonated graphene oxide (PGO) was added to Py-PBI substrate at different levels prior to acid doping [
78]. They found that the conductivity is more stable in the Py-PBI-PGO membranes. The proton conductivity of the membranes showed a temperature dependence, that is, an Arrhenius-type of behavior in the whole range of temperatures. The activation energy for the Py-PBI based membrane was lower for the composite membranes with 1.0% and 1.5% PGO contents, as shown in
Table 6. The authors proposed that such a reduction in the E
a for the PGO containing membranes may indicate the synergic effect of the phosphonated PGO filler in boosting the membrane proton conductivity. The presence of PGO with an exfoliated structure may have disrupted the crystalline structure of PyPBI, which results in more available and stronger sites for PA trapping and provided additional diffusion pathways for proton hopping across the membrane via the Grotthuss mechanism.
3. Influence of the PBI Structure and Synthetic Methods on Its Conductivity
As mentioned above, PBIs, owing to their excellent mechanical properties and high chemical resistance, are considered as the most important polymers for the fabrication of membranes to work at high temperatures in the construction of stacks for HT-PEMFCs. The PBI membranes are generally prepared by casting from polymer solutions and then doped with acid, although a direct method for casting the PBI together with acid has also been developed [
79,
80].
The structure of PBI has an important effect on many of its properties such as solubility, stability, and proton conductivity. PBIs have a poor solubility owing to their rigid structure. Another important factor affecting the solubility is the molecular weight of the polymer. In this regard, PBIs with molecular weights ranging from 23 to 37 kDa have relatively good solubility, but polymers with lower molecular weights are not strong enough to ensure fuel cell requirements. However, polymers with higher molecular weights are found to be poorly soluble in polar organic solvents. Therefore, many approaches have been used to improve both the strength and solubility of PBI [
81,
82].
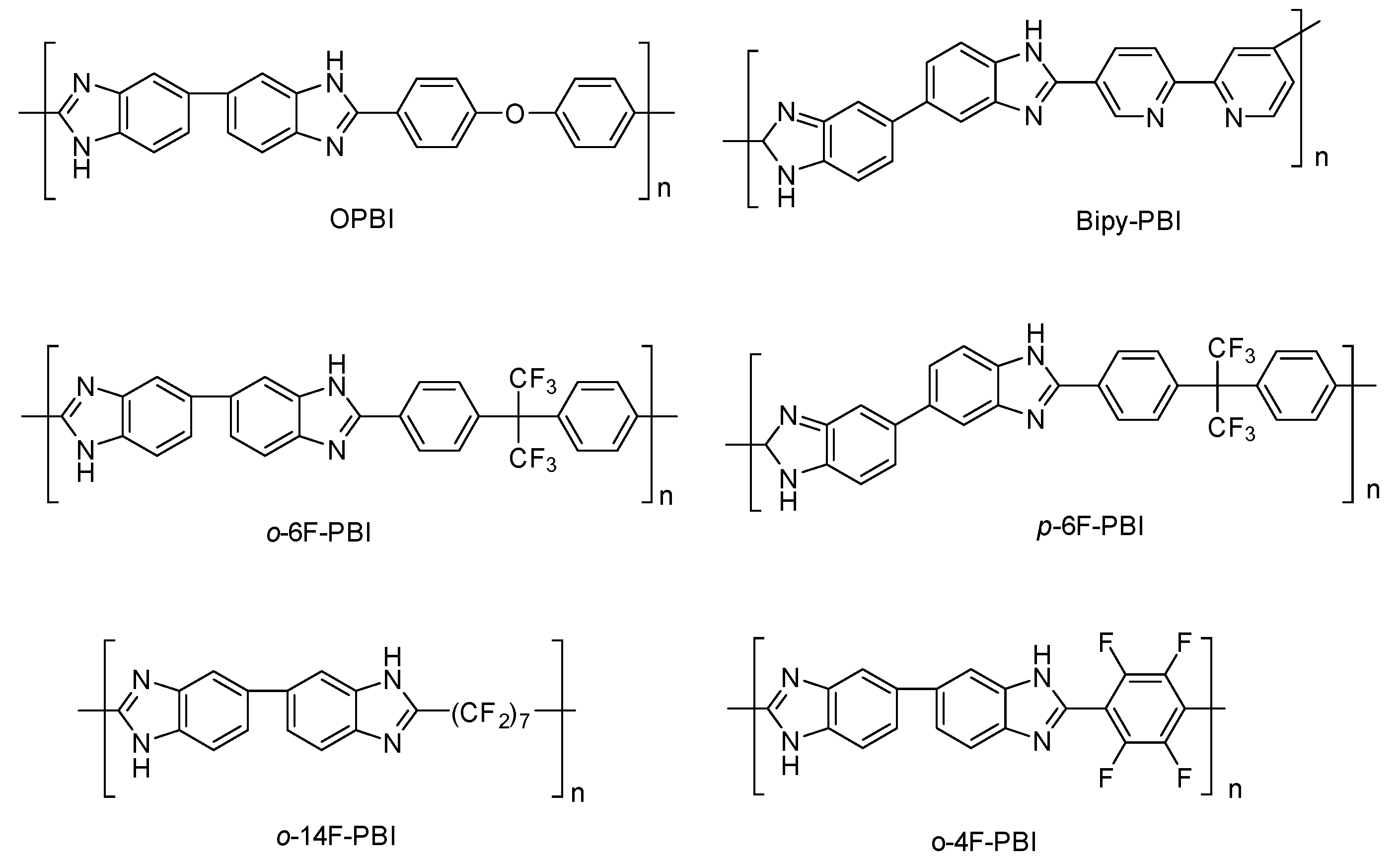
One strategy to strengthen and make PBI more soluble consists of introducing a flexible group in the backbone, such as ether linkages, that facilitates the chain mobility, and consequently solubility, and simultaneously enhances the molecular weight of the PBI and its strength. PBIs containing ether units have been synthesized and applied as polymeric electrolytes for HT-PEMFCs. Furthermore, the introduction of ether units in the PBI has been reported an effective method of increasing the proton conductivity of the membranes. So, the synthesis of poly [2,2′-(
p-oxydiphenylene)-5,5′-benzimidazole] (OPBI) obtained by polycondensation of 3,3′-diaminobenzidine (DAB) and 4,4′-oxybisbenzoic acid (OBBA) was reported (
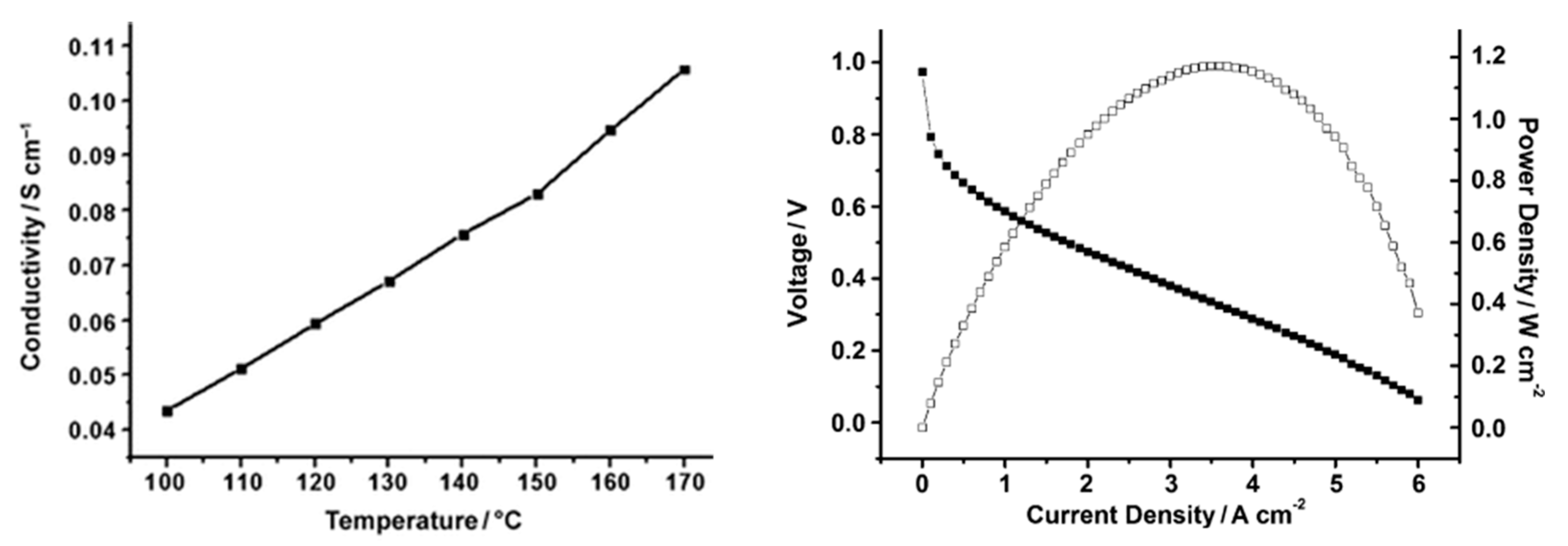
Figure 9), and the membrane prepared from this polymer, after PA doping, which showed values of proton conductivity up to 0.083 S/cm at 150 °C under anhydrous conditions [
83]. The fabricated PBI/PA MEA was tested for 100 h without any degradation in voltage noticed, reaching maximum power and current densities of 1.17 W/cm
2 and 6.0 A/cm
2, respectively (
Figure 10).
In 2019, Berber and Nakashima described the synthesis of bipyridine-based polybenzimidazole (Bipy-PBI) of various molecular weights and it was concluded that the molecular weight significantly affected thermal stability, mechanical properties, proton conductivity, and FC performance of the membranes [
84]. The conductivity of the 141 kDa Bipy-PBI membrane reached 0.037 S/cm at 120 °C and anhydrous conditions, with a maximum power density of 0.78 W/cm
2 and a current density at 0.5 V of 1.6 A/cm
2.
An alternative synthesis of OPBI by microwave irradiation was also reported by Kang and coworkers [
85]. The proton conductivity of the H
2SO
4-doped membranes prepared from this PBI showed values up to 0.190 S/cm at 160 °C and anhydrous conditions. The synthesis of porous and asymmetric OPBI without the use of any porogenic additive was also developed by Ou and coworkers; the conductivity of 0.072 S/cm at 180 °C for such OPBI membrane was reported and a peak power density of 0.4 W/cm
2 at 160 °C under anhydrous conditions was reached in the fuel cell test [
86].
The incorporation of hexafluoroisopropylidene groups in the polymeric structure has been efficiently used to improve the proton conductivity of polyimides and polyamides [
87]. Consequently, such an approach was tried on PBI membranes. In this regard, Qian and Benicewicz prepared a hexafluoroisopropylidene-containing
o-polybenzimidazole (
o-6F-PBI) membrane with a proton conductivity of 0.09 S/cm at 180 °C after phosphoric acid doping and a fuel cell performance with a maximum power density of 0.58 W/cm
2 0.2 A/cm
2 was described [
88]. A year later, another hexafluoroisopropylidene-containing PBI (
m-6F-PBI) was synthesized and the PA-doped membrane exhibited a proton conductivity of 0.02 S/cm at 180 °C, and a peak power density of 0.574 W/cm
2 at 0.2 A/cm
2 at 180 °C. The synthesis of other fluorinated PBIs, such as poly (2,2′-(tetrafluoro-
p-phenylene)-5,5′-bibenzimidazole) (
o-4F-PBI) and poly(2,2′-tetradecafluoroheptylene-5,5′-bibenzimidazole) (
o-14F-PBI), has been described, and their PA-doped membranes reached conductivity values up to 0.03 S/cm at 150 °C. The crosslinked
m-6F-PBI membranes using epoxide cross-linkers were prepared. The membranes showed high acid doping level, enhanced mechanical strength, and oxidation stability in comparison with the pure
m-6F-PBI. Additionally, the proton conductivity in these cross-linked PA-doped membranes reached values up to 0.060 S/cm at 160 °C and a current density of 0.634 A/cm
2 at 0.51 V [
89].
Chemical modification of PBI by means of click chemistry reactions [
90] and by the alternative and enviromental friendly metal-free click chemistry reactions [
91], such as the strain-promoted azide–alkyne cycloaddition (SPAAC) [
92], the thiol-ene [
93,
94] and thiol-yne coupling [
95,
96], the inverse electron−demand Diels−Alder (IEDDA) reaction [
97], the strain-promoted alkyne−nitrone cycloaddition (SPANC) [
98,
99], and the strain-promoted oxidation-controlled cyclooctyne–1,2–quinone cycloaddition (SPOCQ) [
100,
101,
102], can afford an interesting variety of PBI structures with potential applications in PEM for fuel cell applications. The main advantages of these reactions include their fast reaction kinetics, versatililty and regiospecificity, high product yields, and easy purification of the products. In this regard, these methodologies have been efficiently used along the past decade in the preparation of polymers [
103], but their application in fuel cells remains almost unexplored.
4. Copolymers
Another commonly used alternative to fabricate PBI-based polymers with desirable properties (high proton conductivity in combination with high chemical and thermal stability and adequate mechanical properties) is based on the preparation of PBI copolymers [
104]. On these lines, copolymerization has attracted researchers’ interest in the last decade as it allows a fine tuning of the polymer properties by simply selecting the monomer concentration/monomer ratio in the copolymer (
Figure 11) [
105].
Aili, Javakhishvili, and coworkers have synthesized an amino-functional benzimidazole copolymer by condensation of isophthalic acid, 3,3′-diaminobenzidine, and new a hexamine,
N,
N′-bis(2,4-diaminophenyl)-1,3-diaminopropane (
Figure 11). Blend membranes prepared with conventional PBI showed proton conductivities up to 0.12 S/cm at 160 °C after phosphoric acid doping [
106], which was in accordance with the observed high acid uptakes. Zhao et al. synthesized a series of grafted polybenzimidazole copolymers containing polyhedral oligosilsesquioxane (POSS) pendant moieties, which are a family of well-defined cage-like molecules (
Figure 11) [
107]. These copolymers exhibited improved mechanical properties over pristine PBI, with a Young’s modulus of ∼5 GPa and tensile strength of 85 MPa; whereas for pristine PBI, the values were of 1.36 GPa and 71 MPa, respectively. Another copolymerization approach described by McGrath and coworkers was based on multiblock copolymers containing different block lengths. These copolymers were prepared by coupling carboxyl functional aromatic poly (arylene ethers) with ortho diamino functional PBI oligomers in different poly(arylene ether sulfone) and PBI ratios [
108]. Using a similar approach, Lee and coworkers described the synthesis of poly (aryl ether benzimidazole) copolymers containing different contents of aryl ether linkages by condensation of 4,4-dicarboxydiphenyl ether (DCPE) and terephthalic acid (TA) by varying the DCPE/TA ratio (
Figure 11). The authors concluded that the optimal content of aryl ether linkages was 10–30 mol %. With this approach and after PA doping, membranes displayed a proton conductivity of 0.1 S/cm at 180 °C [
109].
The group of Benicewicz has deeply explored the synthesis of new compositions to improve the chemical, thermal, and mechanical stability, as well as proton conductivity, of PBI-based membranes. In 2005, a series of
meta/
para-PBI random copolymer membranes were fabricated by Benicewicz and coworkers via a phosphoric acid sol–gel process. These copolymers exhibited a maximum proton conductivity of 0.26 S/cm at 180 °C with a high acid doping level, which was efficiently retained in the membrane [
110]. Fuel cell tests displayed a current density of 0.2 A/cm
2 and a voltage around 0.65 V at temperatures above 150 °C without any feed gas humidification, and were stable for more than 1000 h. In 2011, Mader and Benicewicz reported the preparation of PBI containing sulphonic acid groups by copolymerization of
p-PBI with polyphosphoric acid (PPA), which displayed excellent proton conductivities with values close to 0.3 S/cm at 180 °C [
111]. The authors used different segmented block copolymers containing p-PBI and sulphonated PBI (s-PBI) in different ratios for the preparation of membranes, which displayed fuel cell performances at 160 °C with voltages around 0.74 V at 0.2 A/cm
2. The highest conductivity was found for the 25:75 s-PBI/p-PBI membrane (0.376 S/cm). The same group has also described the preparation of polyphenylquinoxaline-based PBI copolymers via the polyphosphoric acid (PPA) process with proton conductivities up to 0.26 S/cm [
112]. Using the PPA process, a series of pyridine-containing
m-PBI copolymers were used for the preparation of membranes with enhanced mechanical properties, which displayed a proton conductivity of 0.16 S/cm at 160 °C [
113]. Fuel cell performances of these membranes were similar to those of
para-PBI and long-term stability showed these copolymers maintained a consistent fuel cell voltage of 0.6 V at 0.2 A/cm
2 for over 2300 h. In a later study, the long-term stability was extended to more than 8000 h [
114]. A series of PBI-based block copolymers consisting of phosphophilic PBI and phosphophobic non-PBI segments were synthesized via coupling of ortho-diamino terminated
meta-PBI telechelic macromonomers and carboxylic acid end-capped poly (arylene ether) telechelic macromonomers (
Figure 12). The block copolymer PBI-fluorinated poly (arylene ether) displayed a conductivity up to 0.11 S/cm after doping with concentrated PA, but having low mechanical properties [
115]. The fuel cell performance showed a 20 μV/h degradation rate when operating at 0.2 A/cm
2 over 2000 h with an initial 0.58 V at 0.2 A/cm
2.
Maity and Jana reported the synthesis of a series of
meta-PBI-block-
para-PBI segmented block copolymers with different block lengths by the condensation reaction of diamine-terminated
meta-PBI and acid-terminated
para-PBI oligomers [
116]. The maximum proton conductivity for this block copolymer was 0.11 S/cm at 160 °C, displaying a block structural influence on the proton conductivity. Pan and coworkers synthesized a series of sulfonated polybenzimidazole multiblock copolymers containing pyridine rings via the condensation polymerization of a dicarboxyl monomer containing pyridine, 3,3′,4,4′-tetraaminobiphenyl, and 4-aminobenzoic acid in polyphosphoric acid. Next, membranes were prepared via the generally used solution casting method and subsequently doped with phosphoric acid [
117]. The prepared membranes displayed an improved thermal and oxidative stability, reaching proton conductivities up to 0.23 S/cm at 180 °C.
Co-polymers of ABPBIs containing phenoxy in the main chain were synthesized by Wang and coworkers via the solution casting method. The copolymers were synthesized by copolymerization of 3,4-diaminobenzoic acid (DABA) and 4-(3,4-diaminophenoxy)benzoic acid (DPBA) under microwave irradiation. The polymeric membranes displayed an improved stability in organic solvents with proton conductivities up to 0.05 S/cm at 160 °C for the PA-doped membranes (
Figure 13); however, a low tensile strength of ∼2 MPa was reached after acid doping [
118]. Kim et al. prepared cross-linked copolymer membranes of polybenzimidazole and polybenzoxazine [
119]. The copolymerized membranes showed a maximum proton conductivity of 0.12 S/cm at 150 °C under anhydrous conditions. Fuel cell tests showed operating voltages of 0.71 V at 0.2 A/cm
2, with an excellent long-term durability up to 2000 operating cycles.
6. Conclusions
In this review, an extensive outlook of the recent developments on composite membranes based on PBI for HT–PEMFC applications is given. The most common approach to increase proton conductivity in PBI membranes is based on the phosphoric acid doping, which enhances proton conductivity of these polymeric membranes up to 0.2 S/cm. However, degradation of the membrane at high temperatures and acid leaching are drawbacks that hamper their use as HT-PEMFCs. The use of alternatives based on the addition of fillers into the polymeric matrix can help to overcome these problems. As shown, proton conductivity values close to those of the commercially used Nafion® membranes can be reached using a wide diversity of materials used as fillers in PBI membranes. These fillers include carbon-based materials such as graphene oxide and carbon nanotubes, nanofibers obtained by electrospinning methods, inorganic fillers such as metal oxides and heteropolyacids, and organic fillers such as ionic liquids or metal organic frameworks. Other alternatives are based on the modification of PBI structure by synthetic methods or co-polymerization strategies, which can increase the proton conductivity and retain phosphoric acid more efficiently. Despite that high conductivities have been reported by these methods, phosphoric acid leaching remains a problem to be solved in the next decade.
Fuel cell technology is currently receiving much attention from researchers as well as from the industry because it can reduce the costs of the organic and inorganic fillers such as metal organic frameworks, ionic liquids, nanofibers, and carbon-based materials. The introduction of inorganic fillers into the polymer matrix of PBI in order to form novel composite membranes was reported to improve the dimensional stability, mechanical properties, and gas permeability of PBI composite membranes.
The effective design of composite membranes based-PBI doped with phosphoric acid for high performance PEMs for fuel cells has been demonstrated with higher performance and durability. The composite membranes based on PBI formed with nanofibers and organic and inorganic fillers show good mechanical properties and solvent-resistance, proving the latter to be effective additives for the preparation of reinforced PBI composite membranes through a solution process enhancing the formation of long-range proton-conductive pathways in the PBI membranes. The acid-uptake levels, dimensional stability upon acid-doping, and proton conductivity of the PBI-based membranes through retention of phosphoric acid have been significantly enhanced by the formation of composite membranes with cross-linked PBI. Cross-linking plays an important role in forming additional networks in PBI, despite that it generally weakens the interaction between the filler and polymer chain; however, cross-linking reinforces the polymer chain with additives producing more novel rigid materials. On the other hand, plasticizers can form composites membranes with high flexibility, reducing hydrophilic or hydrophobic properties (depending on the nature of the plasticizers), and even increasing the proton conductivity. The addition of plasticizers has also increased the amorphous phase in PBI composites.
The use of additives involves a wide variety of technologies in polymer technology, with a great field of materials engineering to be applied in energy existing today. The focus of this review has been the use of additives in PBI membranes to facilitate PA doping to be durable over prolonged cyclic usage and, in the long term, especially to obtain high performance and durability in HT-PEMFC with low costs. The reported membranes have been studied from their physical structure and morphology as well as their chemical and electrochemical performance. As per our findings, most of the previous work found in the literature reported that the presence of additive material in a polymer matrix enhances the properties compared with the neat polymer. However, most of the synthesized membranes display poor performance in a single fuel cell in the presence of additives with a diminution in terms of proton conductivity and mechanical strength owing to some problems such as the agglomeration, swelling, and interaction filler polymeric PBI matrix, among others. Consequently, there is a strong need to overcome these drawbacks and reach an equilibrium between proton conductivity and thermal and mechanical stability in the composite membrane.
The challenges and opportunities of composite membranes of PBI are still growing as their impact on the research and development (R&D) industry is under continuous growth, as the demand increases every year following that of devices operating at moderate and high temperatures. Thus, the design of novel materials is of crucial interest when providing major durability and strength of PBI. However, a crucial requirement they need to fulfill to be applied as fuel cells and as super capacitors is the need for high conductivity and long-term durability. For this purpose, as mentioned above, the use of PA doped membranes can be a strong limitation and, at the same time, an advantage, if the natural route is applied when synthesizing the additive. Nonetheless, there are still some aspects that need to be improved on these composite membranes based on PBI, such as reducing the degradation rates of the polymeric membranes present owing to the operation at high temperatures. It is also desirable to design new PEMs with enhanced chemical stability towards peroxide and radical attacks, as well as increase the retention factor of phosphoric acid to reduce the loss of the electrolyte, and maintain the proton conductivity for extended periods of time.
The market for polymer electrolyte membrane fuel cells is expected to grow at a compound annual growth rate (CAGR) of 15.28% during the next five years. Major factors driving the market are increasing R&D activities for energy applications, which has driven to various technological advantages, such as high power density, decrease in the time to refuel, longer storage durability, and increase of life-cycles of PEM fuel cells over alternatives such as Li-ion batteries; in addition, efforts have been focused on the design of PEM fuel cell-powered vehicles with the help of government incentives and policies. However, the current cost of PEMFC technology is still relatively high, a substantial limitation to overcome. In the next years, attempts have to be oriented towards getting around this important barrier, which can help to successfully implement this green technology in commercial usage for stationary and transportation, among others [
244].
In the past few years, considerable progress has been made through the commercialization of HT-PEMFC technology, and it has emerged as an attractive alternative to other kinds. As an example, commercially available Advent PBI MEAs, based on PBI, possess excellent thermal and oxidative stability, and can operate at 120 to 180 °C using phosphoric acid as the electrolyte. Among the advantages, it is worth mentioning that they do not need water for conductivity and can reach proton conductivities up to 0.1 S/cm with a proven lifetime of 20,000 h [
245]. The elevated operating temperature leads to important advantages, making them potential candidates as a near-future environmentally friendly technology.