1. Introduction
Carbon fibers are new high-performance materials with high tensile strength (2–7 GPa), high elastic modulus values (200–700 GPa), low density (1.5–2.0 g/cm
2), small linear expansion coefficients, and excellent conductivity, and they exhibit good corrosion resistance to acids and alkali and organic solvents [
1,
2]. To make use of their low weight and high strength, carbon fibers are often combined with polymers to form carbon-fiber-reinforced plastics (CFRPs), which can be used to improve the mechanical and fatigue resistance properties of other materials. Researchers have discovered and developed many excellent properties of CFRPs, such as high toughness, high corrosion resistance, and low weight, and CFRPs are favored by many industries and production fields, including aerospace [
3,
4,
5], automotive [
6,
7], civil construction [
8,
9], and sporting goods industries [
10,
11]. As the application of CFRP materials continues to increase, the amount of carbon fiber waste generated becomes staggering [
12,
13]. For both environmental protection and sustainable economic development, CFRP waste must be recycled [
14,
15].
The main purpose of CFRP recycling is to degrade the epoxy resin and obtain high-value carbon fibers. The existing CFRP recovery methods mainly include mechanical recovery [
16,
17], pyrolysis [
18,
19], fluidized bed method [
20,
21], super/subcritical fluid decomposition [
22,
23], etc. The mechanical recovery has the advantages low cost and simple operation, but this method needs to crush the size of CFRP waste to 5~10 mm, which reduces the reuse value of the recovered fiber [
16,
17]. Through pyrolysis, the recovered carbon fiber, which accounts for 50–80% of the strength of the virgin fiber, can be recovered, but poisonous gases will be released [
18,
19]. The strength of carbon fiber recovered by the fluidized bed method is 25–50% of the strength of the virgin fiber, and the length of the fiber is 5–10 mm [
20,
21]. The strength of carbon fiber recovered by super/subcritical fluid decomposition is 80–85% of the virgin fiber, and the length can reach 10–50 mm. However, toxic organic solvents are needed and energy consumption is high in this method [
22,
23]. The use of water as a solvent has many advantages, such as a widely available raw material source, low cost, no pollution, and easily handled reaction products [
23,
24,
25]. With the increasing demand for environmental protection, the decomposition of composite materials by the aqueous phase method will be the main trend in the future. Oliveux [
26] recycled glass fibers from unsaturated polyester resin under subcritical conditions. Their study revealed that the surface conditions of the recycled glass fibers were well maintained, enabling full reuse. Yuan [
27] also successfully recycled glass fibers from a poly(hexahydrotriazine) resin matrix.
In our previous research, a green, energy-saving, and efficient CFRP waste electrocatalytic decomposition recovery method (EHD) based on the degradation mechanism of CFRP was invented [
28,
29,
30]. Sun [
29] placed CFRP in 3%, 10%, and 20% NaCl aqueous solutions, respectively, and applied a current of 4–25 mA for carbon fiber recovery with a cycle of 21 d. A small amount of carbon fiber was recovered, and high concentrations of NaCl resulted in severe chlorination and oxidation of the carbon fiber. The tensile strength of the recovered carbon fiber ranged from 32% to 80% of that of the virgin carbon fiber (VCF). Microstructure analysis showed that some epoxy resin residues remained on the carbon fiber surface. The study verified the feasibility of the electrochemical recycling method of carbon fiber under the condition of atmospheric pressure. The utilization of conventional non-toxic chemical solvents and equipment without generating dust, harmful gases or other secondary pollution greatly reduces the recovery cost and initial investment. However, the recovery time needs to be shortened and the tensile strength of the carbon fiber removal rate of epoxy resin needs to be improved. Zhu [
30] investigated the effects of varying the applied current density, electrolyte concentration, catalyst concentration, and temperature on the mechanical properties and microstructures of the recycled CFs. With the optimization of parameters, the removal rate of epoxy resin was close to 100% and the average residual tensile strength and interfacial shear strength were approximately 90% and 120% of those of virgin CFs, respectively. This method is a simple, environmentally friendly process with low equipment requirements and can, therefore, reduce the size limit of CFRP waste and be used on a large scale.
Although previous research on water-phase CFRP recovery has led to a series of achievements, it shows that the applied current density strongly influences the quality of recycled carbon fibers [
28,
29,
30,
31]. The current density must be precisely controlled to obtain recycled carbon fibers with the same mechanical properties, which requires precise process control. In most cases, the mechanical properties of recycled carbon fibers are normally distributed and can be applied to non-critical parts in the aeronautics, automotive, and architectural industries [
32]. Therefore, we want to explore an electrocatalyst that is less sensitive to current density to enable easier operation in practical engineering applications. Besides, the interaction between the factors and the kinetics and mechanism of epoxy resin degradation in the process of recycling needs to be solved.
Heteropoly acids (HPA) are a new class of catalytic materials that have attracted the attention of researchers [
33,
34]. Heteropoly acids have a high dissociation constant, high ionic conductance, excellent oxophilicity, and good thermodynamic stability. Extensive research on heteropoly acids has led to heteropoly anions with potential applications as electrocatalysts [
35,
36]. Phosphotungstic heteropoly acid, which is an oxygen-containing acid consisting of phosphorous and tungsten atoms linked by oxygen atoms, is one of the most widely used heteropoly acid catalysts. Phosphotungstic heteropoly acid can react in both the homogeneous phase and the heterogeneous phase and even as a phase-transfer catalyst, thereby making this acid a promising green catalyst for use in aqueous-phase systems [
37].
In this study, the electrochemical catalytic method for the depolymerization of epoxy resin and the reclamation of CFRP in phosphotungstic acid (PA) aqueous solution is proposed. Orthogonal test analysis was used to demonstrate the interactions among the reaction parameters reported in previous studies to influence the recovery efficiency: current density, PA concentration, and reaction time. The fundamental degradation mechanism of CFRPs was also investigated via thermogravimetric analysis (TGA), scanning electron microscopy (SEM), atomic force microscopy (AFM), X-ray photoelectron spectrometry (XPS), and gas chromatography–mass spectrometry (GC–MS).
2. Materials and Methods
2.1. Materials
PA (H
3[P(W
3O
10)
4]∙12H
2O), hydrochloric acid (HCl), sodium hydroxide (NaOH) and sodium chloride (NaCl) were of analytical grade and were purchased from Sigma-Aldrich (St. Louis, MO, USA). CFRPs in form of unidirectional carbon fiber pultruded plate were provided by Nanjing Hitech Composites Co., Ltd. (Nanjing, China). The main components of the epoxy resin used in the CFRPs were diglycidyl ether of bisphenol A/ethylenediamine (DGEBA/EDA). The glass-transition temperature of the initial CFRPs was measured by a NETZSCH (Selb, Bavaria, German) STA409PC comprehensive thermal analyzer and reported in
Supplementary Information. The information of the initial CFRPs was shown in
Table 1.
2.2. Test-Specimen Preparation
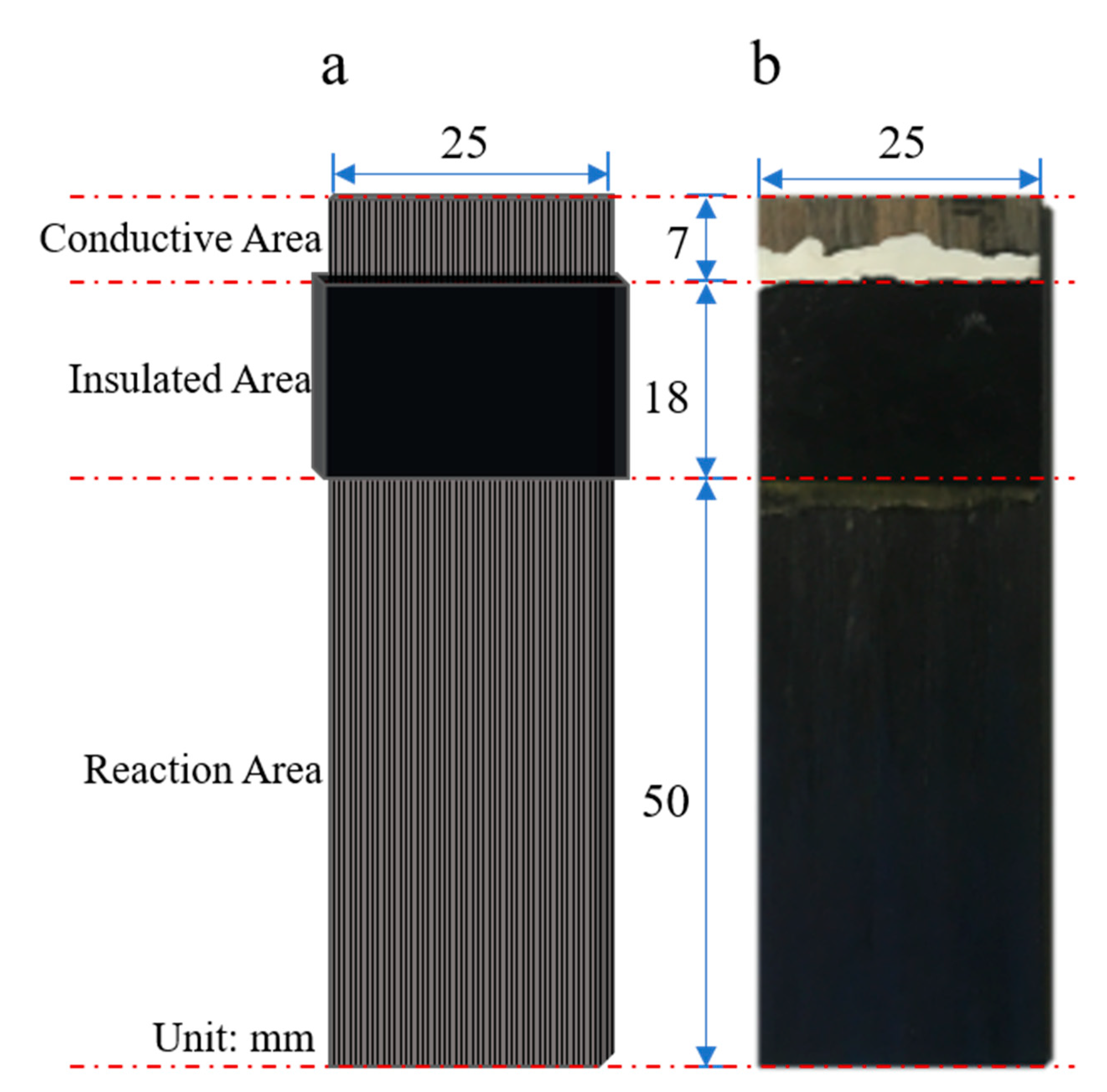
The dimensions of each CFRP test specimen were 75 mm × 25 mm × 3 mm, and each specimen was divided into three parts: the reaction area, the insulated area, and the conductive area. A schematic is shown in
Figure 1a, and an actual specimen is shown in
Figure 1b. First, the surface of the clean and dry CFRP specimen insulation layer was coated with Kraft silicone rubber with a uniform thickness of approximately 1.5 mm, which was then air-dried in the laboratory for 24 h under dry and ventilated conditions. The silicone rubber surface was then wrapped with insulating tape the same width as the insulating layer. The test specimen was then sealed with epoxy sealant and dried for 24 h under dry and ventilated conditions. The main functions of the insulation layer were to control the reaction area of the specimen and isolate the electrolyte from the non-reaction area. The sizes of the reaction area, insulated area, and conductive area were 50 mm × 25 mm, 18 mm × 25 mm, and 7 mm × 25 mm, respectively.
2.3. Electrocatalytic Procedures
The setup for the electrochemical catalytic process is shown in
Figure 2. The CFRP and stainless-steel plate were connected to an external power supply as the anode and cathode, respectively. Both the CFRP and stainless steel were placed in a container with a conductive solution composed of NaCl, PA, and deionized water. The distance between the cathode and anode was 50 mm, and the reaction temperature was 25 °C. During the electrocatalytic process, the epoxy resin in the CFRP depolymerized and dissolved in the solution; this process enables the recycling of the carbon fibers. HCl and NaOH were used to maintain a consistent pH of 4.5 since PA decomposes at pH values greater than 5.2 [
38,
39]. The byproducts in the solution may affect the conductivity and consequently change the voltage of the circuit. The electrocatalytic reaction system was controlled at a constant current. A multimeter (MS8261, MASTECH, Dongguan, China) was used to monitor the current and voltage of the system. After the completion of the electrocatalytic reaction, the carbon fibers were cleaned 3 times with deionized water and ethanol in an ultrasonic bath, where each cleaning cycle lasted for 8 min. The carbon fibers were then dried at 60 °C for 24 h to obtain the recovered carbon fiber samples.
2.4. Design of Orthogonal Experiment
To investigate the effect of electrocatalytic recycling on the recovery efficiency of CFs, three factors were considered, including current density (A), PA concentration (B), and reaction time (C). Each factor included four levels: A: 3, 4.5, 6, and 7.5 A/m
2; B: 0, 1, 2, and 3 g/L; C: 24, 48, 72, and 96 h. The L
16(3
4) matrix (
Table 2) was used to observe and optimize the impacting parameters in the assessment of the effect of the experimental error on the outcomes [
40,
41]. Each group of experiments was repeated three times.
2.5. Characterization Instruments
A STA409PC (NETZSCH, Selb, Bavaria, German) comprehensive thermal analyzer was used for TGA of the recycled carbon fibers under ambient pressure in the temperature range between 30 °C and 800 °C at a controlled heating rate of 10 °C/min. Three samples were tested for each recycling working condition, and the average was reported. Agilent T150 UTM nanomechanical tensile tester with UTM-Bionix Standard Toecomp Quasistatic test system (Palo Alto, Santa Clara, CA, USA) was used to conduct the monofilament tensile tests of the recycled carbon fibers, with a 750 μN loading, 0.2 μm/s tensile rate, 50 nN loading resolution, <0.1 nm displacement resolution, 35 nm tensile resolution, and a ±1 mm maximum displacement of the actuator. The temperature and relative humidity were kept at 20–30 °C and 40% throughout the test, respectively. Twenty replicates were conducted for each recycling working condition. A laser caliper from Changchun Industrial Photoelectric Technology Co., Ltd. (Changchun, China) was used to measure the diameter of the carbon fiber monofilament. The recycled carbon fiber monofilaments were placed on the sample holder. The diameters of the monofilaments were calculated according to the diffraction principle by using the diffraction dark stripe spacing. Twenty replicate measurements were conducted for each recycling working condition. The interfacial shear strength of the carbon fiber monofilament was measured via microdroplet test on an HM410 interfacial evaluation device from Dongrong Industrial Co., Ltd. (Hong Kong, China). The recycled carbon fiber monofilaments were fixed onto the locating hole of concave paper by gluing both ends of the fibers; the paper was then cured for 24 h in a foam plate slot. During the test, the loading speed was 0.12 mm/min and the microscope magnification was 2×. The diameter of the resin drop was measured by a microscope with high magnification before the test. Five filaments were tested for each recycling working condition, and the average was taken for evaluation. The surface morphologies of the carbon fibers were analyzed using an Quanta TM 250 FEG environmental scanning electron microscope (FEI, Hillsboro, OR, USA) with a working distance of approximately 10 mm and an acceleration voltage of 20 kV. An MFP-3d Infinity atomic force microscope from Oxford Instruments (Oxford, United Kingdom) was used to observe the surface morphologies of the carbon fibers. The scanning area was selected to be 1 μm × 1 μm, and the scanning rate was set to 1.0 Hz in tapping mode. Twenty replicate measurements were conducted for each recycling working condition to reduce the experimental deviation. A VPII X-ray photoelectron spectrometer with an aluminum target (ULVAC-PHI, Kanagawa, Japan) was used to obtain the excitation energy peaks of elements. The XPS Peak 4.1 software (Raymund W.M. Kwok, Hong Kong, China) was used to conduct curve fitting of the results.
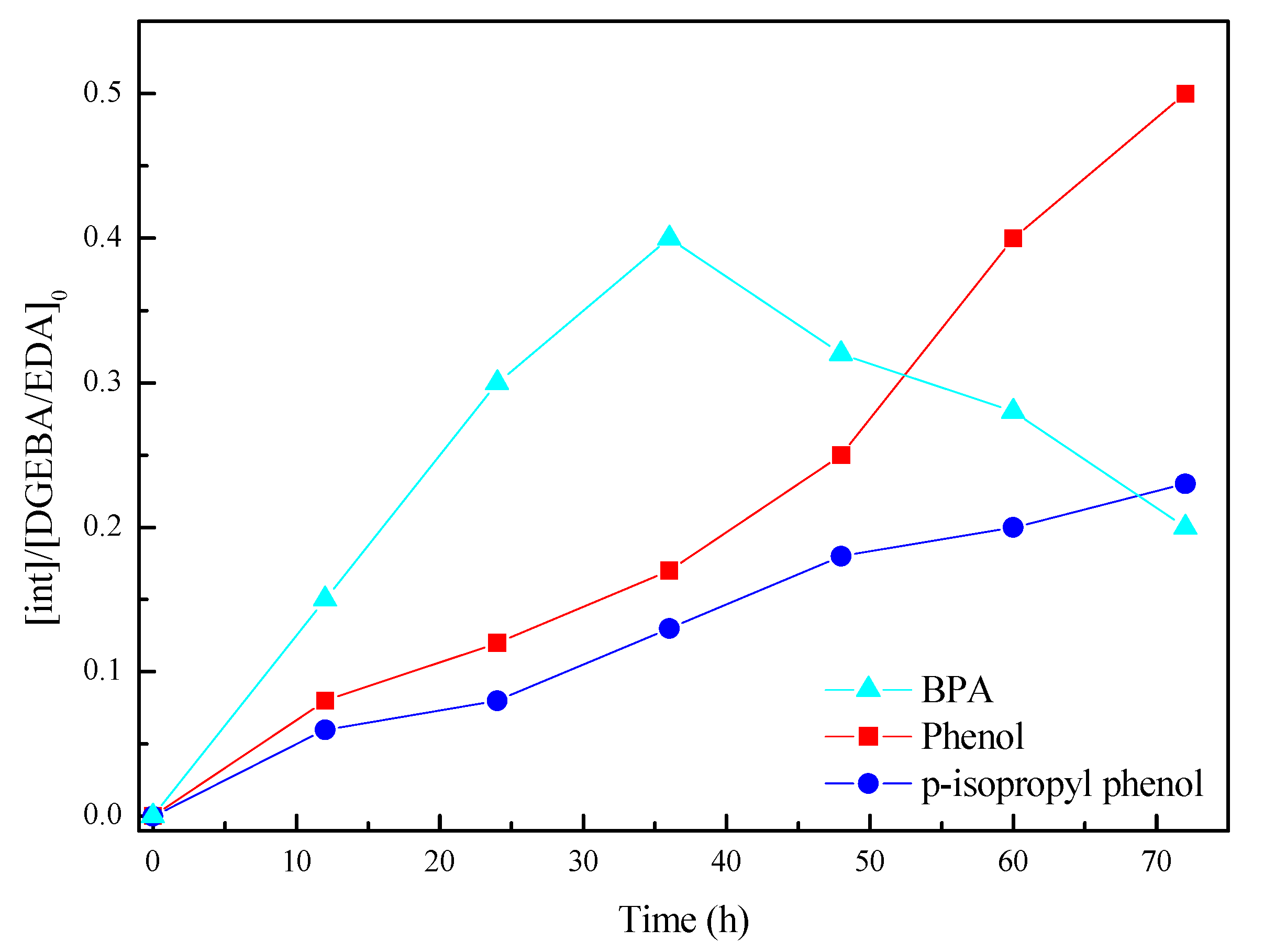
The intermediate and final products during the electrocatalytic process were qualitatively analyzed using a 7890a–5975c gas chromatograph-mass spectrometer (Agilent, Palo Alto, Santa Clara, CA, USA). The column was a 30 m × 0.25 mm capillary column with a film thickness of 0.25 µm. The flow rate of the He carrier gas was 1.0 mL/min. The temperatures of the injection port and the transfer line were 250 and 280 °C, respectively. The oven temperature was kept at 70 °C for 1 min and increased to 280 °C at a rate of 10 °C/min. Samples of 2 µL were injected in the pulsed splitless mode (pulse pressure, 25 psi; pulse time, 1 min; purge activation time, 0.9 min). The mass spectrometer was operated in the electron-impact mode at an electron energy of 70 eV. The ion source and quadrupole analyzer were maintained at 230 and 150 °C, respectively. Data were obtained in the selected ion monitoring/scan mode.