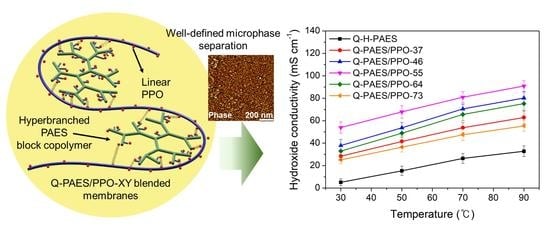
Enhanced Hydroxide Conductivity and Dimensional Stability with Blended Membranes Containing Hyperbranched PAES/Linear PPO as Anion Exchange Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
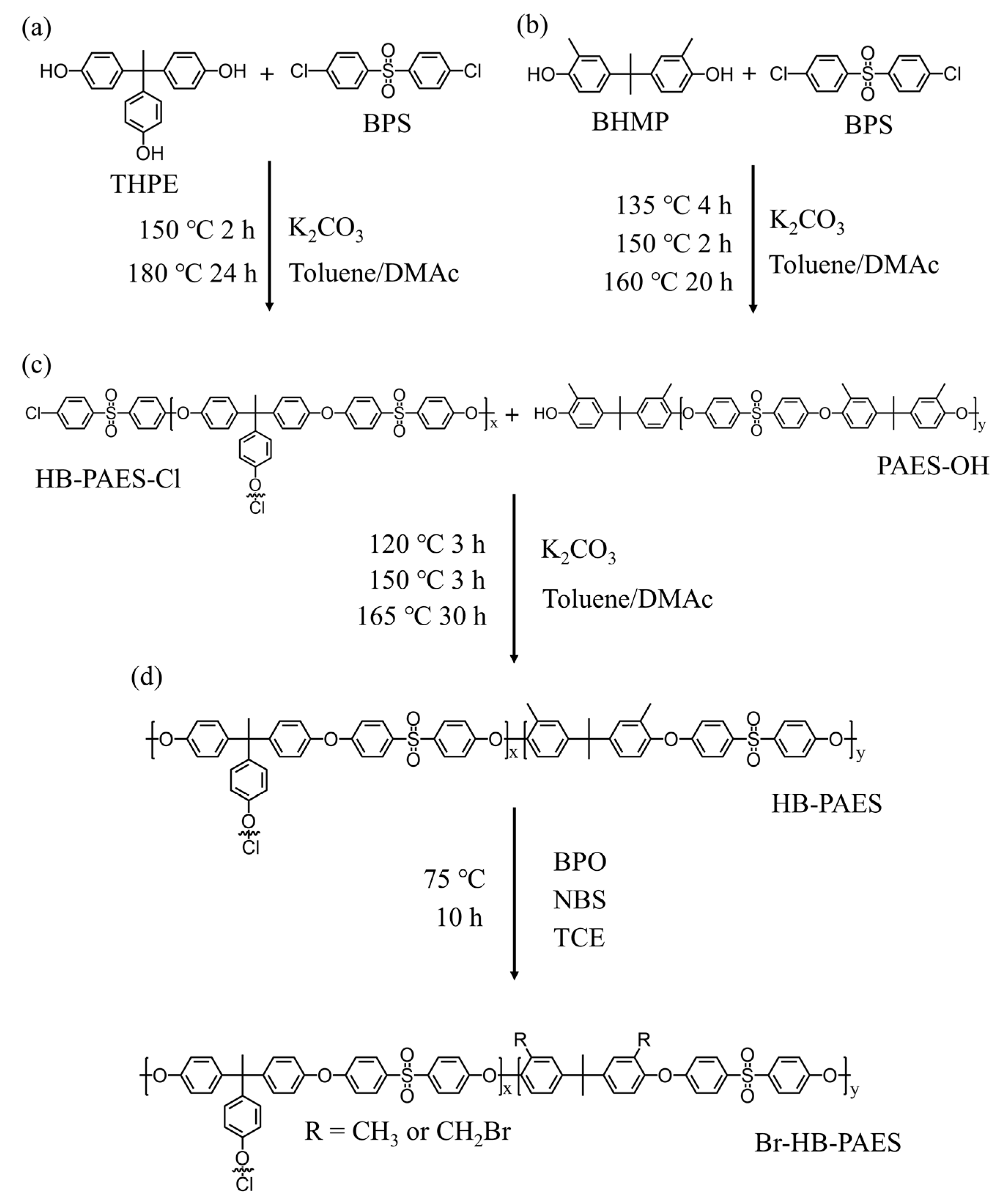
2.2. Synthesis of the Hyperbranched Hydrophobic Oligomer
2.3. Synthesis of the Hydrophilic Oligomer
2.4. Synthesis of Hyperbranched Poly(Arylene Ether Sulfone) Block Copolymer
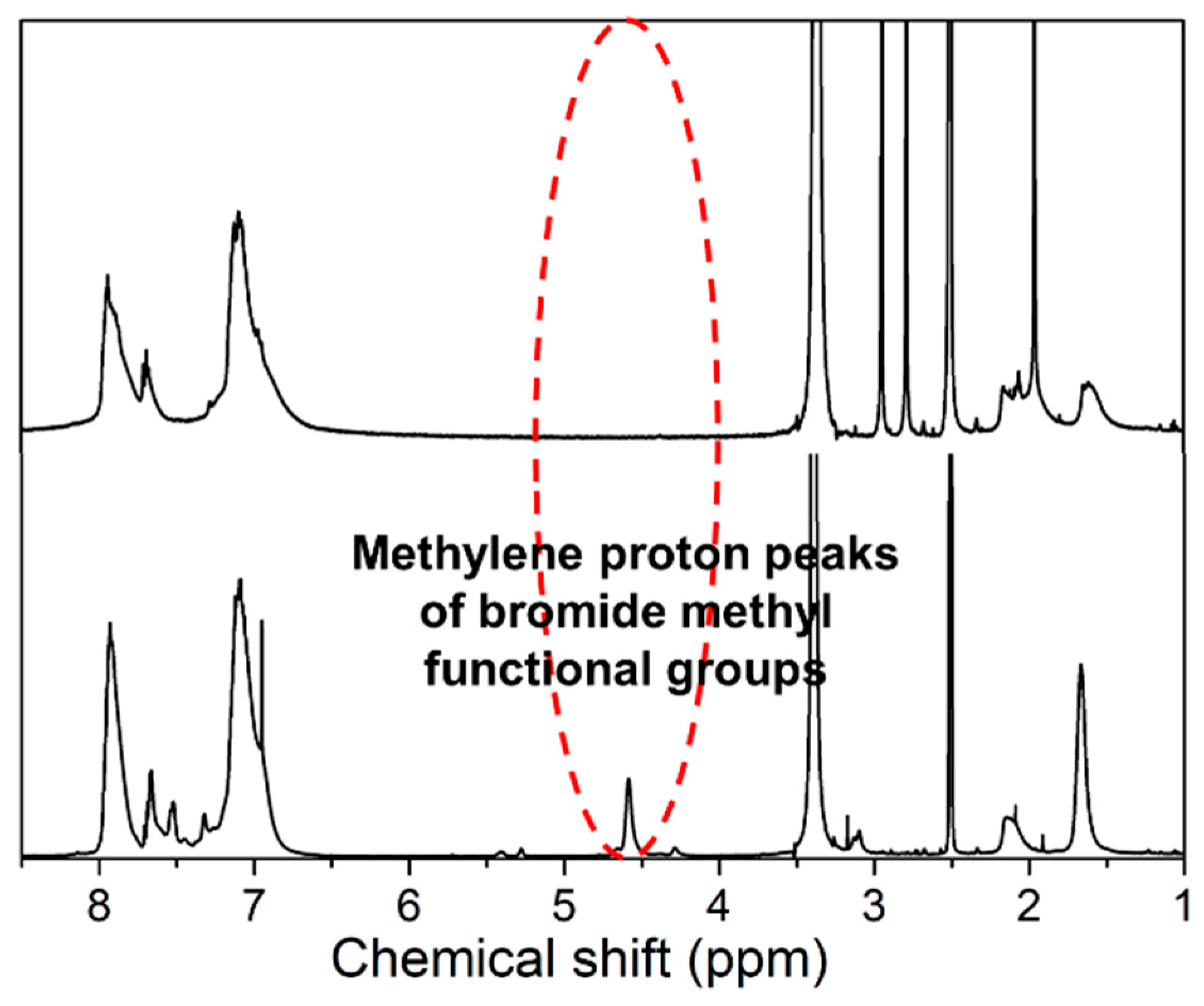
2.5. Synthesis of Brominated HB-PAES (Br-HB-PAES)
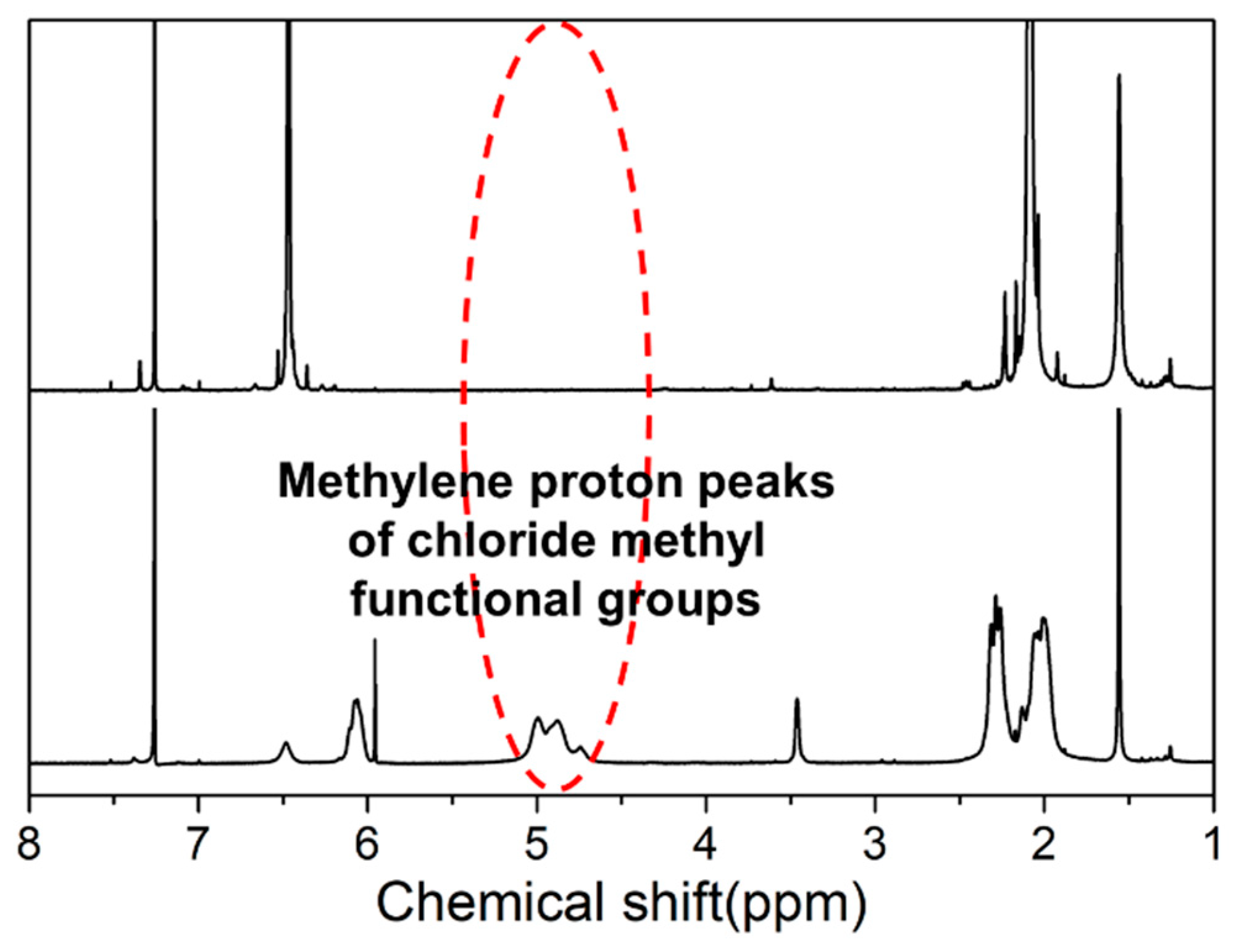
2.6. Synthesis of Chloromethylated PPO (CM-PPO)
2.7. Fabrication of the BC-PAES/PPO-XY Blended Membranes
2.8. Quaternization of BC-PAES/PPO-XY Membranes
3. Characterizations
4. Discussion
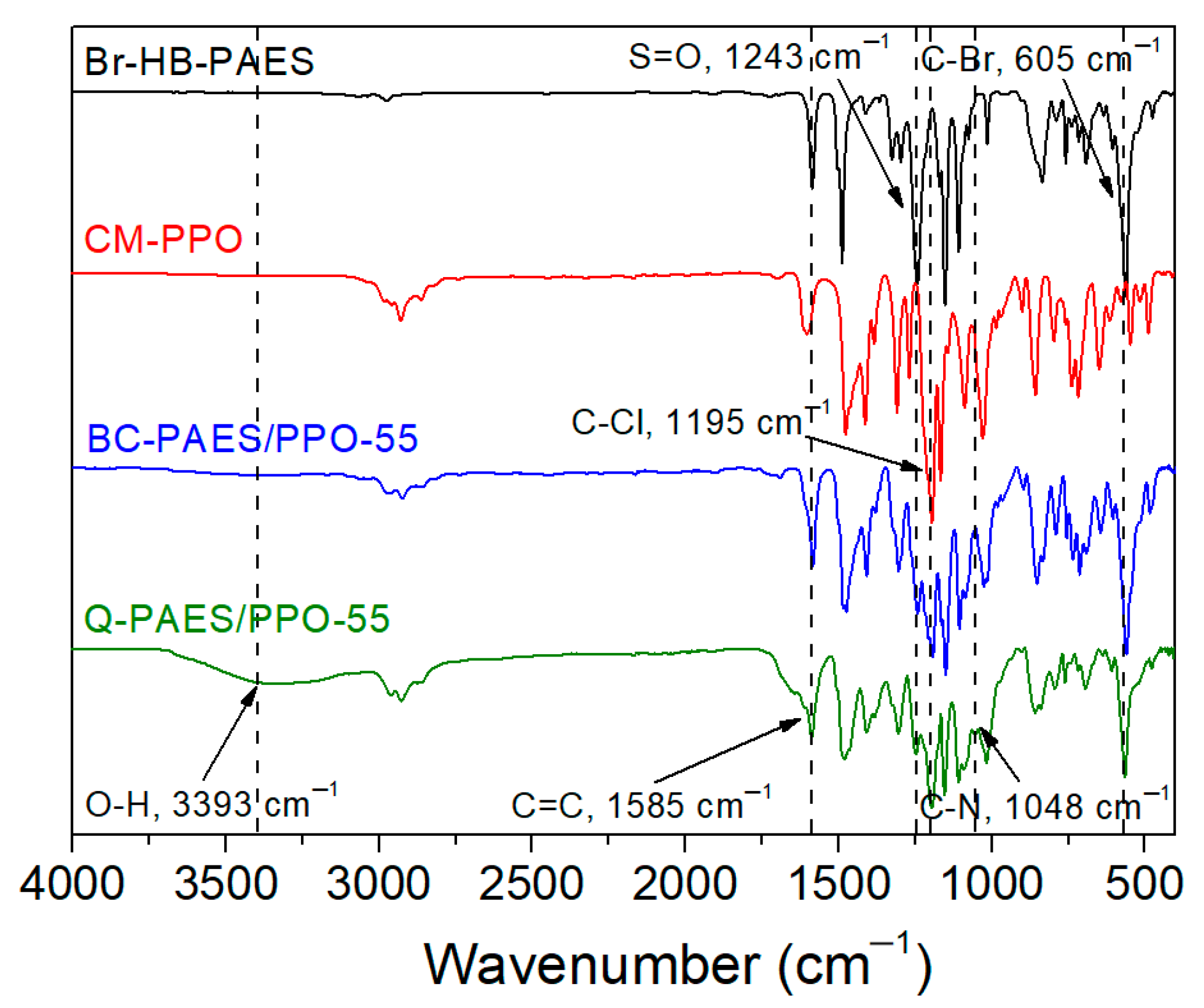
4.1. Characterization of Br-HB-PAES and CM-PPO Polymers
4.2. Fabrication and Charaterization of Blended Membranes
4.3. Morphology of Q-PAES/PPO-XY Blended Membranes
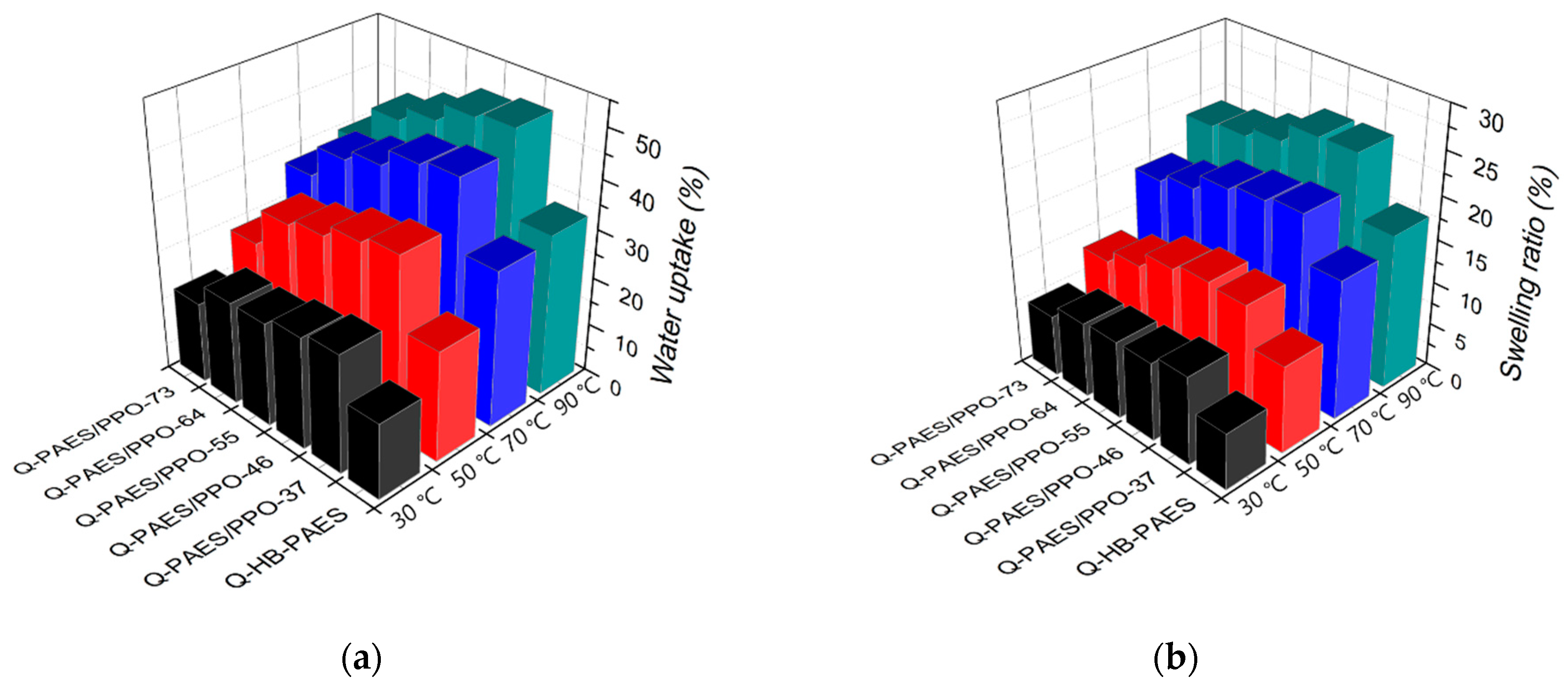
4.4. Ionic Exchange Capacity, Water Uptake, and Swelling Ratio
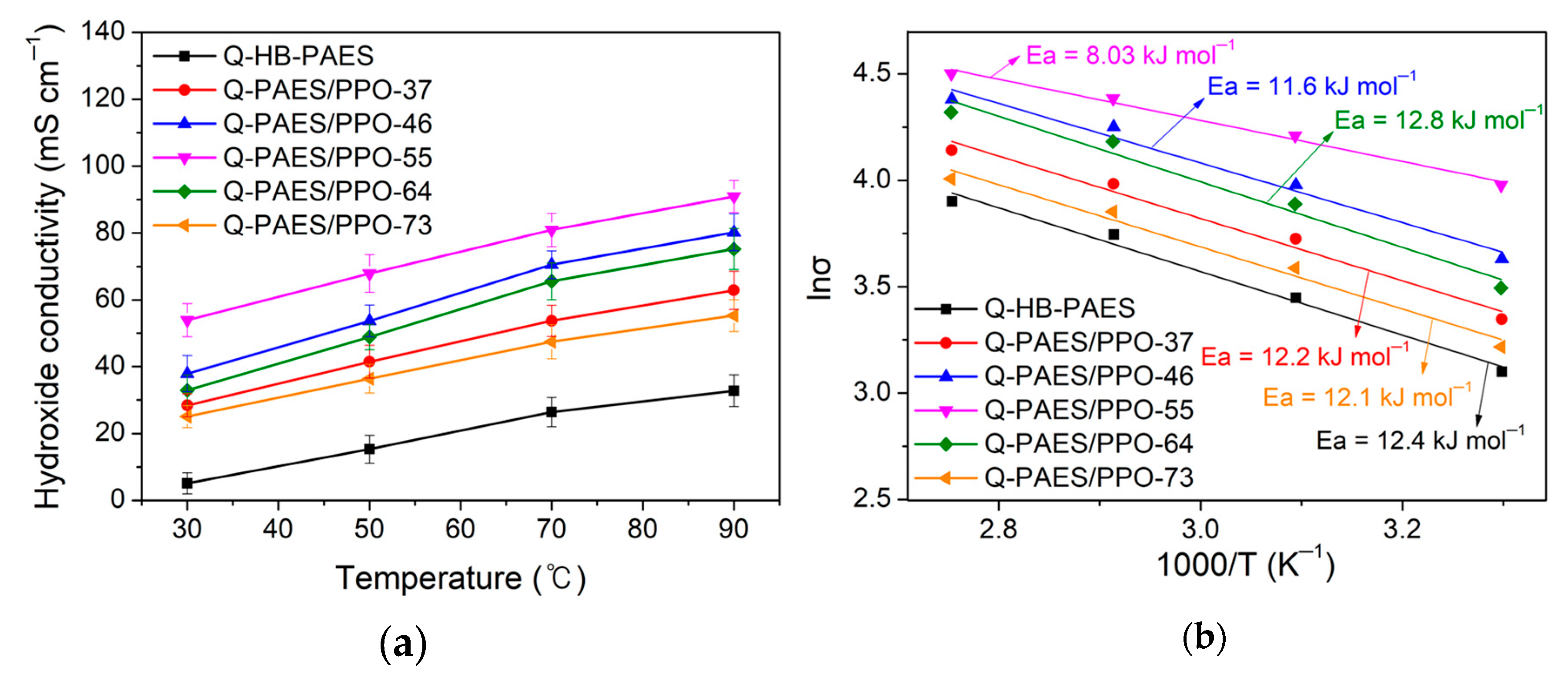
4.5. Hydroxide Conductivity and Arrhenius Plots of the Q-PAES/PPO-XY Blended Membranes
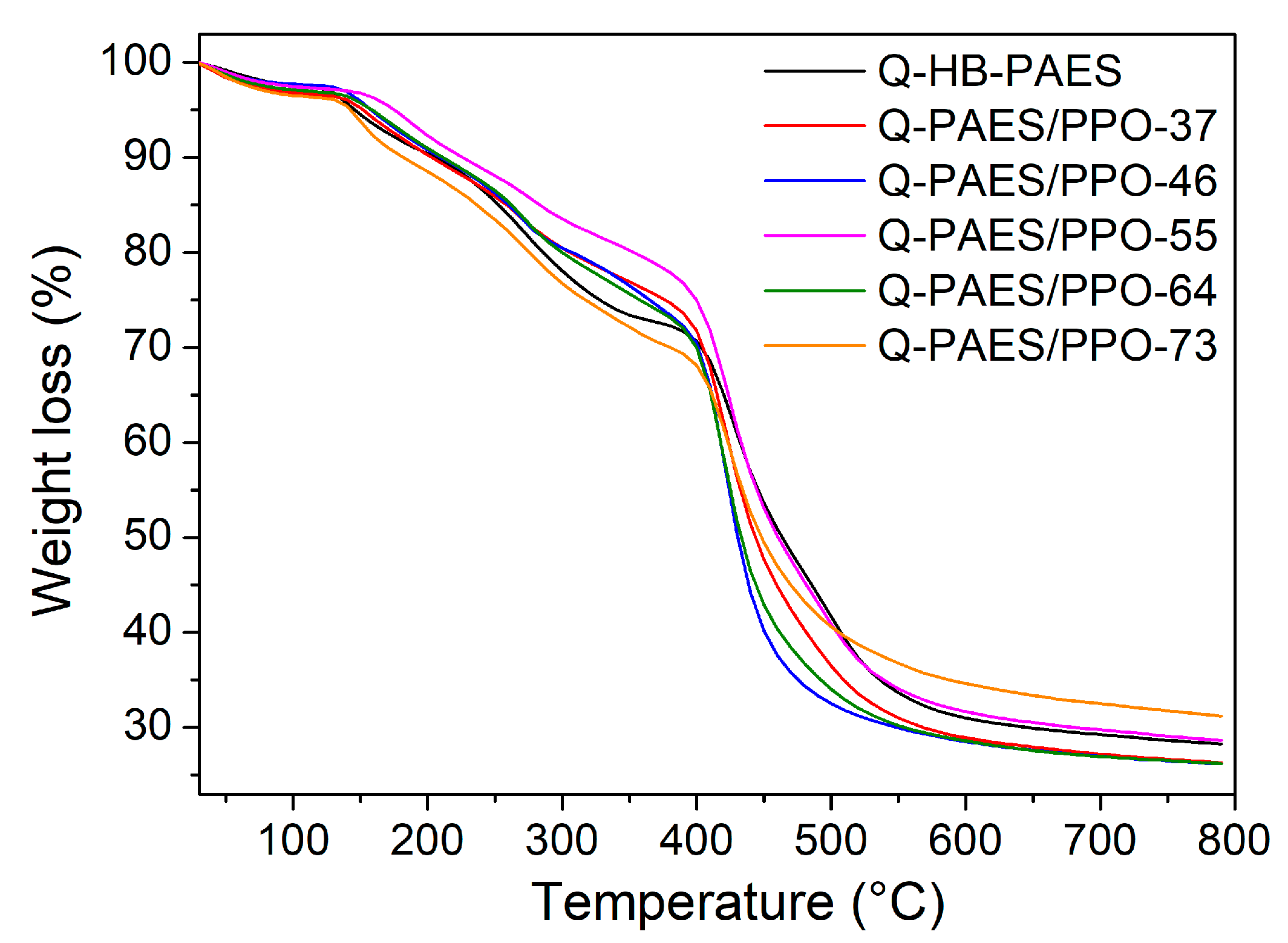
4.6. Thermal Properties
4.7. Alkaline Stability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vijayakumar, V.; Nam, S.Y. Recent advancements in applications of alkaline anion exchange membranes for polymer electrolyte fuel cells. J. Ind. Eng. Chem. 2019, 70, 70–86. [Google Scholar] [CrossRef]
- Li, N.; Guiver, M.D. Ion transport by nanochannels in ion-containing aromatic copolymers. Macromolecules 2014, 47, 2175–2198. [Google Scholar] [CrossRef] [Green Version]
- Ramanavicius, S.; Ramanavicius, A. Progress and insights in the application of MXenes as new 2D nano-materials suitable for biosensors and biofuel cell design. Int. J. Mol. Sci. 2020, 21, 9224. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Effect of functionalized SiO2 toward proton conductivity of composite membranes for PEMFC application. Int. J. Energy Res. 2019, 43, 5333–5345. [Google Scholar] [CrossRef]
- Yoo, D.J.; Hyun, S.H.; Kim, A.R.; Kumar, G.G.; Nahm, K.S. Novel sulfonated poly(arylene biphenylsulfone ether) copolymers containing bisphenylsulfonyl biphenyl moiety: Structural, thermal, electrochemical and morphological characteristics. Polym. Int. 2011, 60, 85–92. [Google Scholar] [CrossRef]
- Chu, J.Y.; Lee, K.H.; Kim, A.R.; Yoo, D.J. Improved electrochemical performance of composite anion exchange membranes for fuel cells through cross linking of the polymer chain with functionalized graphene oxide. J. Membr. Sci. 2020, 611, 118385. [Google Scholar] [CrossRef]
- Lai, A.N.; Zhuo, Y.Z.; Lin, C.X.; Zhang, Q.G.; Zhu, A.M.; Ye, M.L.; Liu, Q.L. Side-chain-type phenolphthalein-based poly(arylene ether sulfone nitrile)s anion exchange membrane for fuel cells. J. Membr. Sci. 2016, 502, 94–105. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Z.; Tian, H.; Wang, S.; Zhang, B.; Cao, Y.; He, G.; Li, Z.; Wu, H. Preparing alkaline anion exchange membrane with enhanced hydroxide conductivity via blending imidazolium-functionalized and sulfonated poly(ether ether ketone). J. Power Sources 2015, 288, 384–392. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, B.; Yan, F. Anion-exchange membranes for alkaline fuel-cell applications: The effects of cations. ChemSusChem 2018, 11, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, X.; Jin, C. Development of a high-performance anion exchange membrane using poly(isatin biphenylene) with flexible heterocyclic quaternary ammonium cations for alkaline fuel cells. J. Mater. Chem. A 2019, 7, 6883–6893. [Google Scholar] [CrossRef]
- He, S.; Liu, L.; Wang, X.; Zhang, S.; Guiver, M.D.; Li, N. Azide-assisted self-crosslinking of highly ion conductive anion exchange membranes. J. Membr. Sci. 2016, 509, 48–56. [Google Scholar] [CrossRef]
- Wei, H.; Tong, L.; Yu, S.; Zhang, J.; Dong, Y.; Li, X.; Ding, Y. Non-covalently crosslinked anion exchange membranes: Effect of urea hydrogen-bonding group position. Polymer 2019, 179, 121654. [Google Scholar] [CrossRef]
- Qaisrani, N.A.; Ma, L.; Hussain, M.; Liu, J.; Li, L.; Zhou, R.; Jia, Y.; Zhang, F.; He, G. Hydrophilic flexible ether containing, cross-linked anion-exchange membrane quaternized with DABCO. Acs Appl. Mater. Interfaces 2020, 12, 3510–3521. [Google Scholar] [CrossRef]
- Pan, J.; Zhu, L.; Han, J.; Hickner, M.A. Mechanically tough and chemically stable anion exchange membranes from rigid-flexible semi-interpenetrating networks. Chem. Mater. 2015, 27, 6689–6698. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Yoo, D.J. Fabrication of binary sulfonated poly ether sulfone and sulfonated polyvinylidene fluoride-co-hexafluoro propylene blend membrane as efficient electrolyte for proton exchange membrane fuel cells. Bull. Korean Chem. Soc. 2018, 39, 913–919. [Google Scholar] [CrossRef]
- Msomi, P.; Nonjola, P.; Ndungu, P.; Ramontja, J. Quaternized poly(2,6 dimethyl–1,4 phenylene oxide)/polysulfone blended anion exchange membrane for alkaline fuel cells application. Mater. Today Proc. 2018, 5, 10496–10504. [Google Scholar] [CrossRef]
- Morandi, C.G.; Peach, R.; Krieg, H.M.; Kerres, J. Novel morpholinium-functionalized anion-exchange PBI–polymer blends. J. Mater. Chem. A 2015, 3, 1110–1120. [Google Scholar] [CrossRef]
- Msomi, P.F.; Nonjola, P.T.; Ndungu, P.G.; Ramontja, J. Poly(2, 6-dimethyl-1, 4-phenylene)/polysulfone anion exchange membrane blended with TiO2 with improved water uptake for alkaline fuel cell application. Int. J. Hydrogen Energy 2020, 45, 29465–29476. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Song, M.H.; Lee, J.-Y.; Lee, H.-K.; Yoo, D.J. Amine functionalized carbon nanotube (ACNT) filled in sulfonated poly(ether ether ketone) membrane: Effects of ACNT in improving polymer electrolyte fuel cell performance under reduced relative humidity. Compos. Part B Eng. 2020, 188, 107890. [Google Scholar] [CrossRef]
- Nhung, L.T.; Kim, I.Y.; Yoon, Y.S. Quaternized chitosan-based anion exchange membrane composited with quaternized poly(vinylbenzyl chloride)/polysulfone blend. Polymers 2020, 12, 2714. [Google Scholar] [CrossRef]
- Li, N.; Yan, T.; Li, Z.; Thurn-Albrecht, T.; Binder, W.H. Comb-shaped polymers to enhance hydroxide transport in anion exchange membranes. Energy Environ. Sci. 2012, 5, 7888–7892. [Google Scholar] [CrossRef]
- Li, N.; Wang, L.; Hickner, M. Cross-linked comb-shaped anion exchange membranes with high base stability. Chem. Commun. 2014, 50, 4092–4095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Gao, X.; Liu, P.; Wang, X.; Zhu, X. Enhanced conductivity and stability via comb-shaped polymer anion exchange membrane incorporated with porous polymeric nanospheres. J. Membr. Sci. 2020, 597, 117750. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Du, N.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Ionomers for proton exchange membrane fuel cells with sulfonic acid groups on the end-groups: Novel branched poly(ether-ketone)s with 3,6-ditrityl-9H-carbazole end-groups. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3860–3868. [Google Scholar] [CrossRef]
- Gao, X.L.; Yang, Q.; Wu, H.Y.; Sun, Q.H.; Zhu, Z.Y.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Orderly branched anion exchange membranes bearing long flexible multi-cation side chain for alkaline fuel cells. J. Membr. Sci. 2019, 589, 117247. [Google Scholar] [CrossRef]
- Wang, K.; Wu, Q.; Yan, X.; Liu, J.; Gao, L.; Hu, L.; Zhang, N.; Pan, Y.; Zheng, W.; He, G. Branched poly(ether ether ketone) based anion exchange membrane for H2/O2 fuel cell. Int. J. Hydrogen Energy 2019, 44, 23750–23761. [Google Scholar] [CrossRef]
- Pan, J.; Li, Y.; Zhuang, L.; Lu, J. Self-crosslinked alkaline polymer electrolyte exceptionally stable at 90 °C. Chem. Commun. 2010, 46, 8597–8599. [Google Scholar] [CrossRef]
- Robertson, N.J.; Kostalik, H.A.; Clark, T.J.; Mutolo, P.F.; Abruña, H.D.; Coates, G.W. Tunable high performance cross-linked alkaline anion exchange membranes for fuel cell applications. J. Am. Chem. Soc. 2010, 132, 3400–3404. [Google Scholar] [CrossRef]
- Ge, Q.; Liu, Y.; Yang, Z.; Wu, B.; Hu, M.; Liu, X.; Hou, J.; Xu, T. Hyper-branched anion exchange membranes with high conductivity and chemical stability. Chem. Commun. 2016, 52, 10141–10143. [Google Scholar] [CrossRef]
- Lee, K.H.; Cho, D.H.; Kim, Y.M.; Moon, S.J.; Seong, J.G.; Shin, D.W.; Sohn, J.-Y.; Kim, J.F.; Lee, Y.M. Highly conductive and durable poly(arylene ether sulfone) anion exchange membrane with end-group cross-linking. Energy Environ. Sci. 2017, 10, 275–285. [Google Scholar] [CrossRef]
- Ghanem, A.F.; El-Gendi, A.; Rehim, M.H.A.; El-Khatib, K.M. Hyperbranched polyester and its sodium titanate nanocomposites as proton exchange membranes for fuel cells. RSC Adv. 2016, 6, 32245–32257. [Google Scholar] [CrossRef]
- Chu, P.P.; Wu, C.-S.; Liu, P.-C.; Wang, T.-H.; Pan, J.-P. Proton exchange membrane bearing entangled structure: Sulfonated poly(ether ether ketone)/bismaleimide hyperbranch. Polymer 2010, 51, 1386–1394. [Google Scholar] [CrossRef]
- Georgi, U.; Erber, M.; Stadermann, J.; Abulikemu, M.; Komber, H.; Lederer, A.; Voit, B. New approaches to hyperbranched poly(4-chloromethylstyrene) and introduction of various functional end groups by polymer-analogous reactions. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2224–2235. [Google Scholar] [CrossRef]
- Gong, S.; Li, L.; Ma, L.; Qaisrani, N.A.; Liu, J.; He, G.; Zhang, F. Blend anion exchange membranes containing polymer of intrinsic microporosity for fuel cell application. J. Membr. Sci. 2020, 595, 117541. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Yoo, D.J. Sulfonated-fluorinated copolymer blending membranes containing SPEEK for use as the electrolyte in polymer electrolyte fuel cells (PEFC). Int. J. Hydrogen Energy 2017, 42, 4349–4365. [Google Scholar] [CrossRef]
- Kim, A.R.; Park, C.J.; Vinothkannan, M.; Yoo, D.J. Sulfonated poly ether sulfone/heteropoly acid composite membranes as electrolytes for the improved power generation of proton exchange membrane fuel cells. Compos. Part B Eng. 2018, 155, 272–281. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; Ru, Y.; Hu, M.; Din, L.; Xu, T. Anion exchange membranes (AEMs) based on poly(2,6-dimethyl-1,4-phenylene oxide) (PPO) and its derivatives. Polym. Chem. 2015, 6, 5809–5826. [Google Scholar] [CrossRef]
- Zhu, Y.; Ding, L.; Liang, X.; Shehzad, M.A.; Wang, L.; Ge, X.; He, Y.; Wu, L.; Varcoe, J.R.; Xu, T. Beneficial use of rotatable-spacer side-chains in alkaline anion exchange membranes for fuel cells. Energy Environ. Sci. 2018, 11, 3472–3479. [Google Scholar] [CrossRef]
- Martín-Zarco, M.; Titvinidze, G.; García-Martínez, J.C.; Rodríguez-López, J. Sulfonated dendrimer- and hyperbranched polyglycerol-PBIOO® blend membranes for fuel cells. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 69–80. [Google Scholar] [CrossRef]
- Gong, S.; Bai, L.; Li, L.; Qaisrani, N.A.; Ma, L.; He, G.; Zhang, F. Block copolymer anion exchange membrane containing polymer of intrinsic microporosity for fuel cell application. Int. J. Hydrogen Energy 2020, in press. [Google Scholar] [CrossRef]
- Chu, J.Y.; Lee, K.H.; Kim, A.R.; Yoo, D.J. Study on the chemical stabilities of poly(arylene ether) random copolymers for alkaline fuel cells: Effect of main chain structures with different monomer units. ACS Sustain. Chem. Eng. 2019, 7, 20077–20087. [Google Scholar] [CrossRef]
- Ma, L.; Qaisrani, N.A.; Hussain, M.; Li, L.; Jia, Y.; Ma, S.; Zhou, R.; Bai, L.; He, G.; Zhang, F. Cyclodextrin modified, multication cross-linked high performance anion exchange membranes for fuel cell application. J. Membr. Sci. 2020, 607, 118190. [Google Scholar] [CrossRef]
- Kim, J.H.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Anion exchange membranes obtained from poly(arylene ether sulfone) block copolymers comprising hydrophilic and hydrophobic segments. Polymers 2020, 12, 325. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Liu, J.; Wang, Y.; An, L.; Guiver, M.D.; Li, N. Highly stable anion exchange membranes based on quaternized polypropylene. J. Mater. Chem. A 2015, 3, 12284–12296. [Google Scholar] [CrossRef]
- Yan, J.; Zhu, L.; Chaloux, B.L.; Hickner, M.A. Anion exchange membranes by bromination of tetramethylbiphenol-based poly(sulfone)s. Polym. Chem. 2017, 8, 2442–2449. [Google Scholar] [CrossRef]
- Becerra-Arciniegas, R.A.; Narducci, R.; Ercolani, G.; Antonaroli, S.; Sgreccia, E.; Pasquini, L.; Knauth, P.; Di Vona, M.L. Alkaline stability of model anion exchange membranes based on poly(phenylene oxide) (PPO) with grafted quaternary ammonium groups: Influence of the functionalization route. Polymer 2019, 185, 121931. [Google Scholar] [CrossRef]
- Lin, B.; Xu, F.; Chu, F.; Ren, Y.; Ding, J.; Yan, F. Bis-imidazolium based poly(phenylene oxide) anion exchange membranes for fuel cells: The effect of cross-linking. J. Mater. Chem. A 2019, 7, 13275–13283. [Google Scholar] [CrossRef]
- Alam, M.M.; Wang, Y.; Jiang, C.; Xu, T.; Liu, Y.; Xu, T. A novel anion exchange membrane for bisulfite anion separation by grafting a quaternized moiety through BPPO via thermal-induced phase separation. Int. J. Mol. Sci. 2020, 21, 5782. [Google Scholar] [CrossRef]
- Fang, J.; Shen, P.K. Quaternized poly(phthalazinon ether sulfone ketone) membrane for anion exchange membrane fuel cells. J. Membr. Sci. 2006, 285, 317–322. [Google Scholar] [CrossRef]
- McNair, R.; Cseri, L.; Szekely, G.; Dryfe, R. Asymmetric membrane capacitive deionization using anion-exchange membranes based on quaternized polymer blends. Acs Appl. Polym. Mater. 2020, 2, 2946–2956. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hu, M.; Wang, H.; Wu, X.; Gong, X.; Yan, X.; Zhen, D.; He, G. Dimensionally stable hexamethylenetetramine functionalized polysulfone anion exchange membranes. J. Mater. Chem. A 2017, 5, 15038–15047. [Google Scholar] [CrossRef]
- Chen, C.; Tse, Y.-L.S.; Lindberg, G.E.; Knight, C.; Voth, G.A. Hydroxide solvation and transport in anion exchange membranes. J. Am. Chem. Soc. 2016, 138, 991–1000. [Google Scholar] [CrossRef]
- Zhao, Y.; Yoshimura, K.; Shishitani, H.; Yamaguchi, S.; Tanaka, H.; Koizumi, S.; Szekely, N.; Radulescu, A.; Richter, D.; Maekawa, Y. Imidazolium-based anion exchange membranes for alkaline anion fuel cells: Elucidation of the morphology and the interplay between the morphology and properties. Soft Matter 2016, 12, 1567–1578. [Google Scholar] [CrossRef] [Green Version]
- Abouzari-lotf, E.; Ghassemi, H.; Nasef, M.M.; Ahmad, A.; Zakeri, M.; Ting, T.M.; Abbasi, A.; Mehdipour-Ataei, S. Phase separated nanofibrous anion exchange membranes with polycationic side chains. J. Mater. Chem. A 2017, 5, 15326–15341. [Google Scholar] [CrossRef]
- Ge, Q.; Liang, X.; Ding, L.; Hou, J.; Miao, J.; Wu, B.; Yang, Z.; Xu, T. Guiding the self-assembly of hyperbranched anion exchange membranes utilized in alkaline fuel cells. J. Membr. Sci. 2019, 573, 595–601. [Google Scholar] [CrossRef]
- Niu, M.; Zhang, C.; He, G.; Zhang, F.; Wu, X. Pendent piperidinium-functionalized blend anion exchange membrane for fuel cell application. Int. J. Hydrogen Energy 2019, 44, 15482–15493. [Google Scholar] [CrossRef]
- Zhu, Y.; He, Y.; Ge, X.; Liang, X.; Shehzad, M.A.; Hu, M.; Liu, Y.; Wu, L.; Xu, T. A benzyltetramethylimidazolium-based membrane with exceptional alkaline stability in fuel cells: Role of its structure in alkaline stability. J. Mater. Chem. A 2018, 6, 527–534. [Google Scholar] [CrossRef]
- Kang, D.H.; Das, G.; Yoon, H.H.; Kim, I.T. A composite anion conducting membrane based on quaternized cellulose and poly(phenylene oxide) for alkaline fuel cell applications. Polymers 2020, 12, 2676. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, C.; Wang, J.; Li, L.; Wei, Z. A general strategy to enhance the alkaline stability of anion exchange membranes. J. Mater. Chem. A 2017, 5, 6318–6327. [Google Scholar] [CrossRef]
- Jung, M.-s.J.; Arges, C.G.; Ramani, V. A perfluorinated anion exchange membrane with a 1,4-dimethylpiperazinium cation. J. Mater. Chem. 2011, 21, 6158–6160. [Google Scholar] [CrossRef]
- Arges, C.G.; Parrondo, J.; Johnson, G.; Nadhan, A.; Ramani, V. Assessing the influence of different cation chemistries on ionic conductivity and alkaline stability of anion exchange membranes. J. Mater. Chem. 2012, 22, 3733–3744. [Google Scholar] [CrossRef]
- Son, T.Y.; Kim, T.-H.; Nam, S.Y. Crosslinked pore-filling anion exchange membrane using the cylindrical centrifugal force for anion exchange membrane fuel cell system. Polymers 2020, 12, 2758. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Ueda, M.; Fang, Y.; Chen, S.; Hu, Z.; Zhang, X.; Wang, L. Alkaline stable anion exchange membranes based on poly(phenylene-co-arylene ether ketone) backbones. Polym. Chem. 2016, 7, 5988–5995. [Google Scholar] [CrossRef]




| Samples | Br-HB-PAES (%) | CM-PPO (%) |
|---|---|---|
| BC-PAES/PPO-37 | 30 | 70 |
| BC-PAES/PPO-46 | 40 | 60 |
| BC-PAES/PPO-55 | 50 | 50 |
| BC-PAES/PPO-64 | 60 | 40 |
| BC-PAES/PPO-73 | 70 | 30 |
| Membrane | DMSO | DMF | THF | TCE | NMP | MC | Chloroform | Acetone | Methanol |
|---|---|---|---|---|---|---|---|---|---|
| BC-PAES/PPO-37 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | - | -- |
| BC-PAES/PPO-46 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | - | -- |
| BC-PAES/PPO-55 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | - | -- |
| BC-PAES/PPO-64 | ++ | + | ++ | ++ | ++ | ++ | + | -- | -- |
| BC-PAES/PPO-73 | ++ | ++ | ++ | ++ | ++ | ++ | + | -- | -- |
| Membranes | Water Uptake (%) | Swelling Ratio (%) | IEC (mmol g−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ∆i | Δt | ||||||||
| 30 °C | 50 °C | 70 °C | 90 °C | 30 °C | 90 °C | 30 °C | 90 °C | ||
| Q-HB-PAES | 15.5 | 22.7 | 32.0 | 33.2 | 6.5 | 17.9 | 7.1 | 18.2 | 1.24 ± 0.03 |
| Q-PAES/PPO-37 | 24.3 | 37.1 | 46.1 | 50.3 | 10.2 | 25.0 | 12.1 | 27.1 | 1.97 ± 0.04 |
| Q-PAES/PPO-46 | 23.0 | 35.5 | 45.0 | 49.2 | 9.1 | 24.9 | 11.0 | 28.0 | 1.90 ± 0.03 |
| Q-PAES/PPO-55 | 21.3 | 32.9 | 41.4 | 45.4 | 9.0 | 22.7 | 10.7 | 25.7 | 1.79 ± 0.05 |
| Q-PAES/PPO-64 | 21.2 | 31.3 | 39.1 | 42.3 | 8.7 | 21.5 | 9.2 | 22.9 | 1.32 ± 0.04 |
| Q-PAES/PPO-73 | 16.7 | 23.5 | 32.5 | 34.7 | 7.1 | 20.9 | 8.6 | 21.4 | 1.27 ± 0.05 |
| Membranes | Hydroxide Conductivity (mS cm−1) | |||
|---|---|---|---|---|
| 30 °C | 50 °C | 70 °C | 90 °C | |
| Q-HB-PAES | 5.1 | 15.3 | 26.4 | 32.8 |
| Q-PAES/PPO-37 | 28.4 | 41.5 | 53.7 | 62.9 |
| Q-PAES/PPO-46 | 37.9 | 53.7 | 70.5 | 80.2 |
| Q-PAES/PPO-55 | 53.9 | 67.9 | 80.9 | 90.9 |
| Q-PAES/PPO-64 | 32.9 | 48.9 | 65.5 | 75.2 |
| Q-PAES/PPO-73 | 25.1 | 36.4 | 47.4 | 55.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Enhanced Hydroxide Conductivity and Dimensional Stability with Blended Membranes Containing Hyperbranched PAES/Linear PPO as Anion Exchange Membranes. Polymers 2020, 12, 3011. https://doi.org/10.3390/polym12123011
Kim SH, Lee KH, Chu JY, Kim AR, Yoo DJ. Enhanced Hydroxide Conductivity and Dimensional Stability with Blended Membranes Containing Hyperbranched PAES/Linear PPO as Anion Exchange Membranes. Polymers. 2020; 12(12):3011. https://doi.org/10.3390/polym12123011
Chicago/Turabian StyleKim, Sang Hee, Kyu Ha Lee, Ji Young Chu, Ae Rhan Kim, and Dong Jin Yoo. 2020. "Enhanced Hydroxide Conductivity and Dimensional Stability with Blended Membranes Containing Hyperbranched PAES/Linear PPO as Anion Exchange Membranes" Polymers 12, no. 12: 3011. https://doi.org/10.3390/polym12123011
APA StyleKim, S. H., Lee, K. H., Chu, J. Y., Kim, A. R., & Yoo, D. J. (2020). Enhanced Hydroxide Conductivity and Dimensional Stability with Blended Membranes Containing Hyperbranched PAES/Linear PPO as Anion Exchange Membranes. Polymers, 12(12), 3011. https://doi.org/10.3390/polym12123011