One-Pot Polymerization of Dopamine as an Additive to Enhance Permeability and Antifouling Properties of Polyethersulfone Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Filtration and Antifouling Experiments
3. Results and Discussions
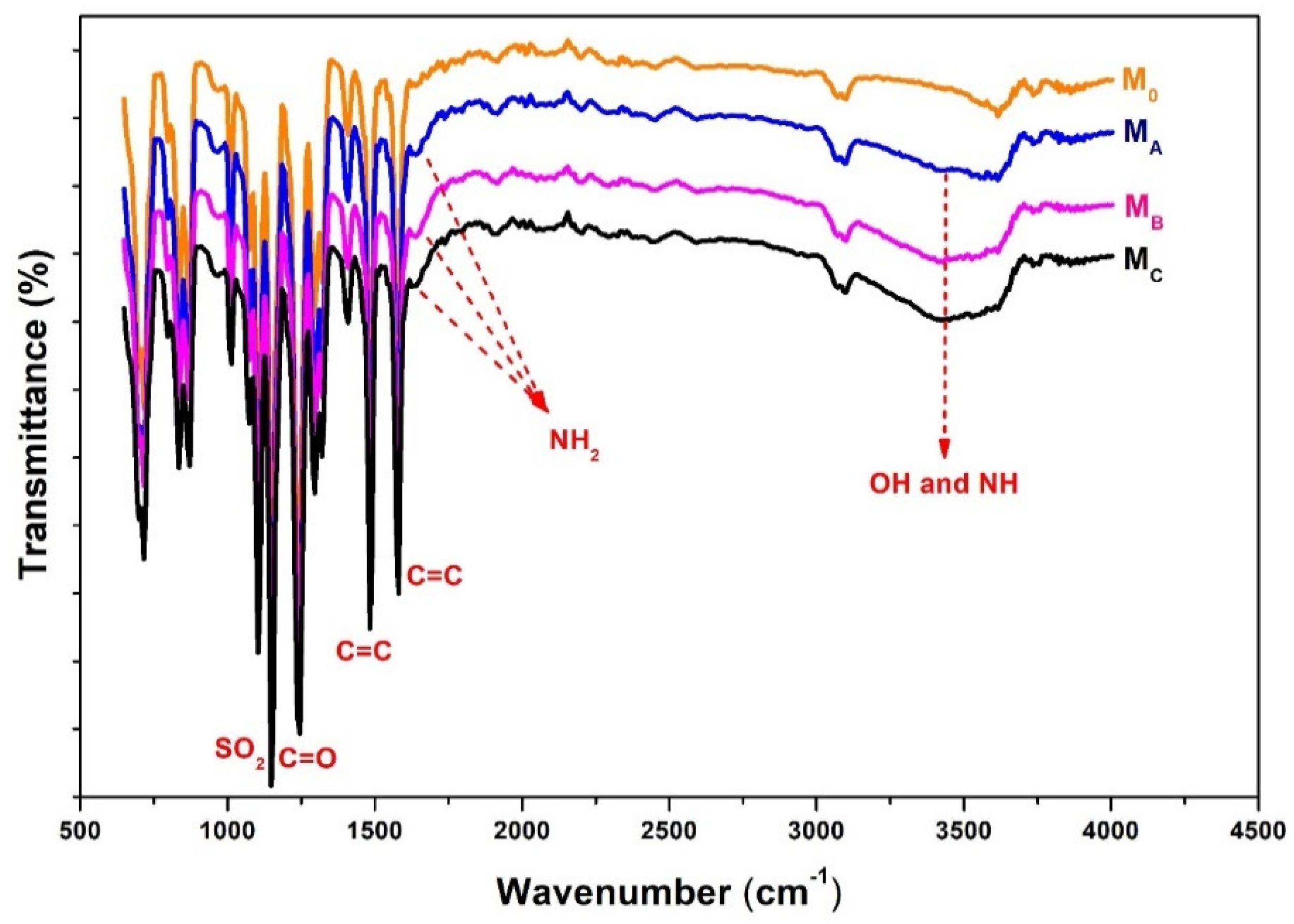
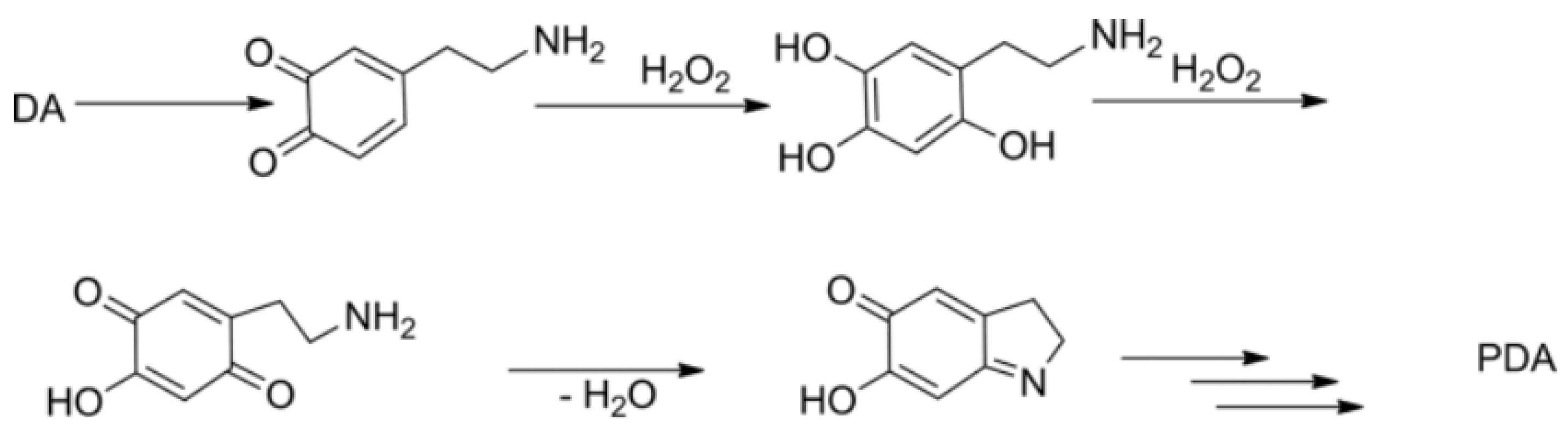
3.1. Membrane Chemical Composition
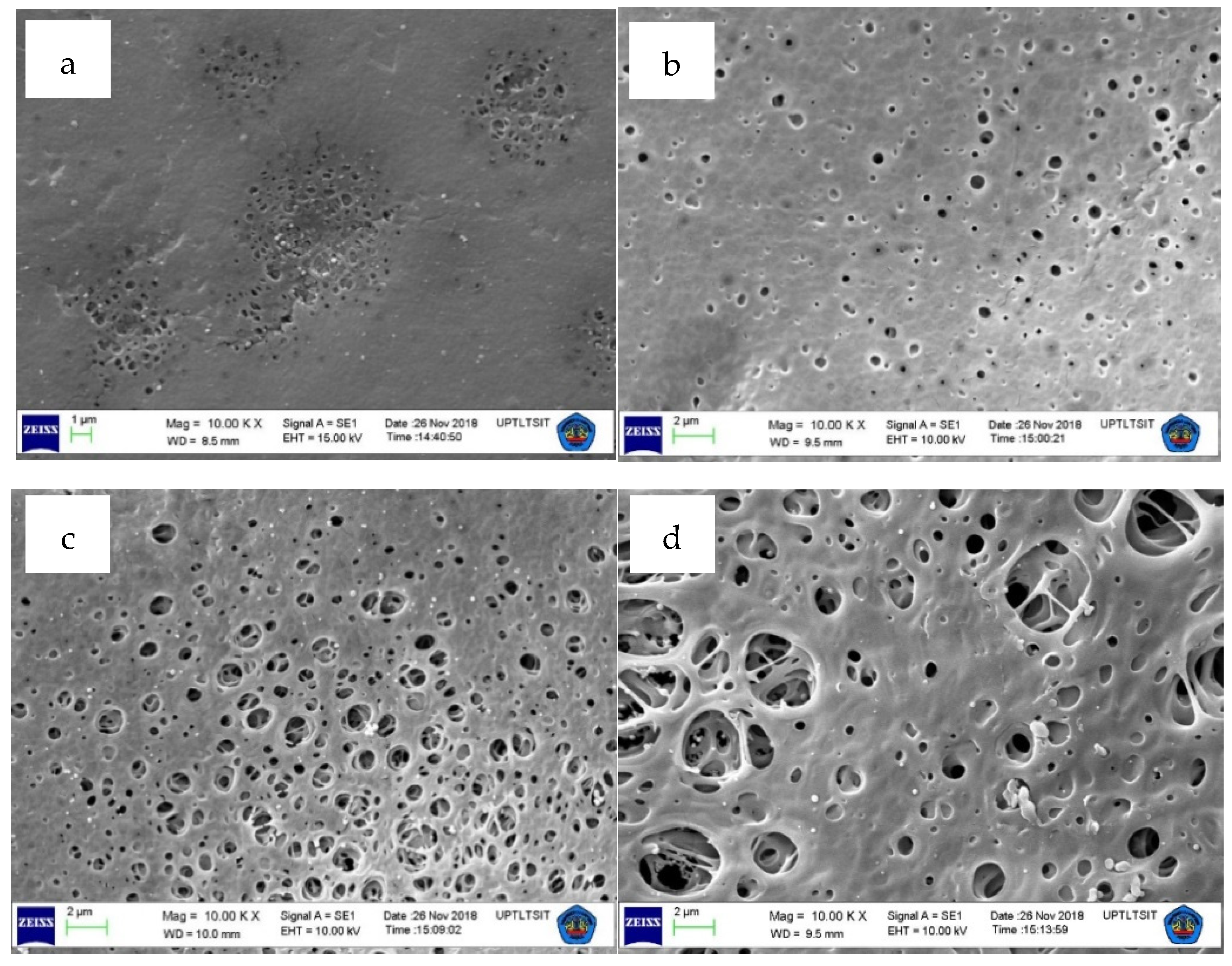
3.2. Surface Morphology and Surface Pore Size
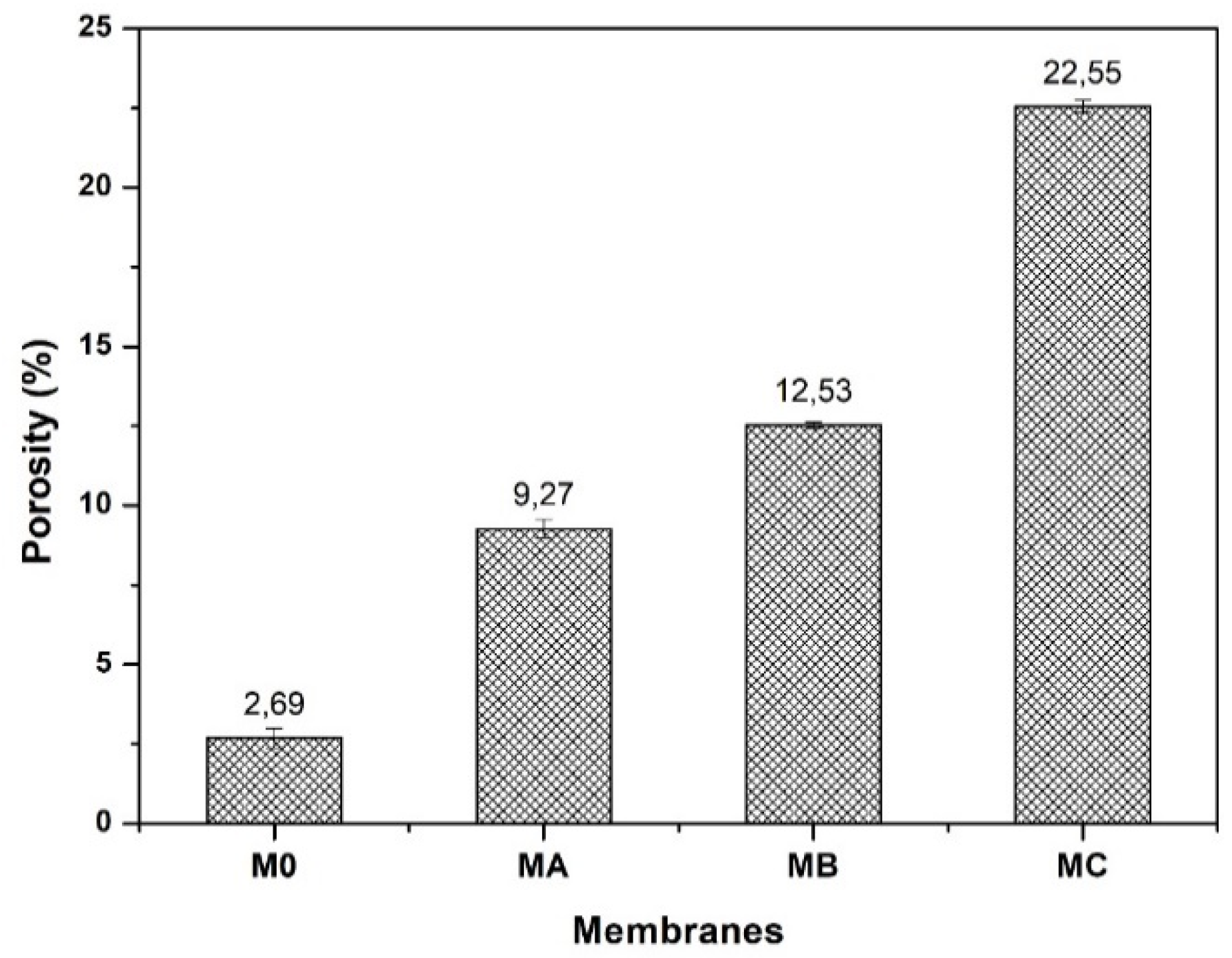
3.3. Porosity
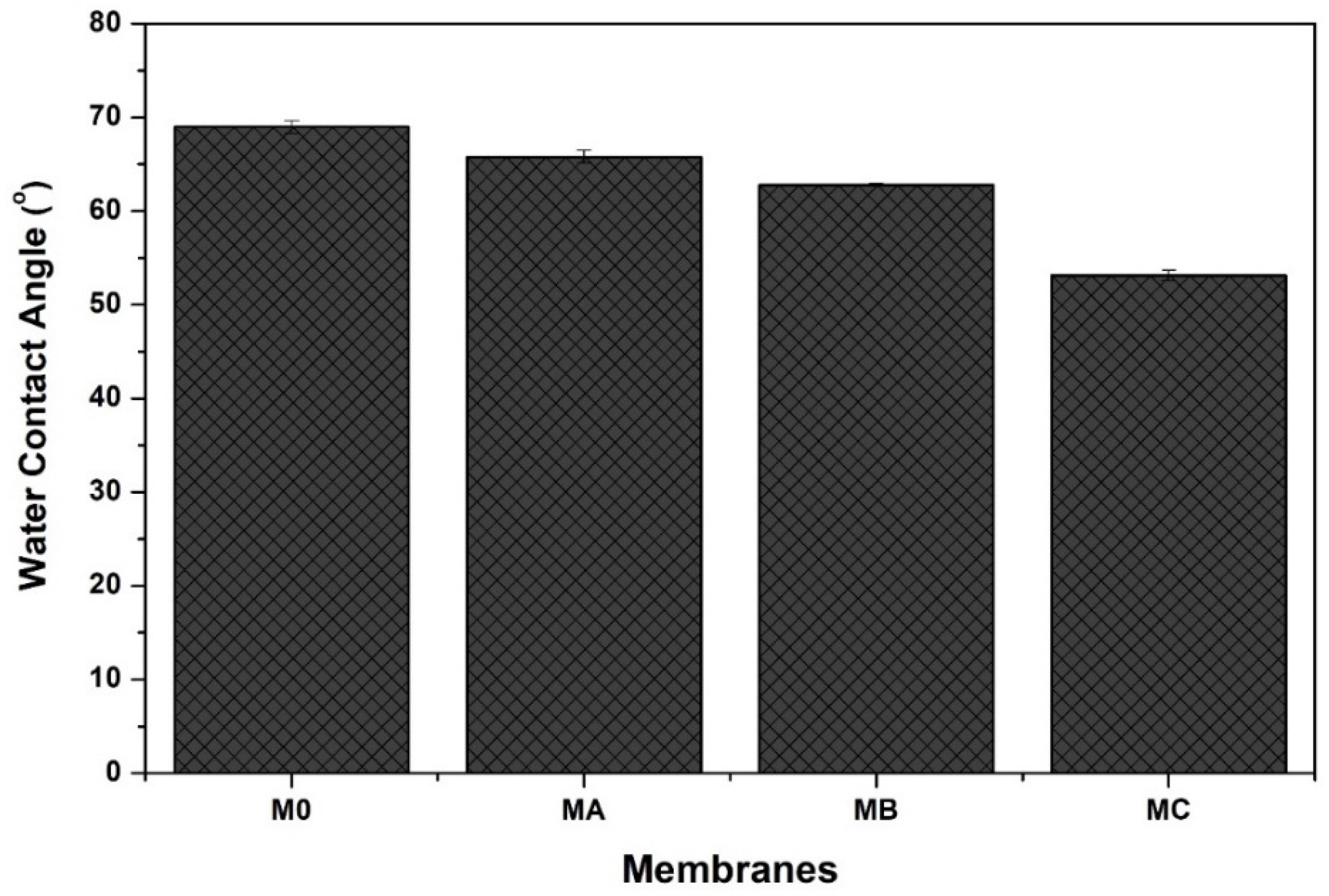
3.4. Hydrophilicity
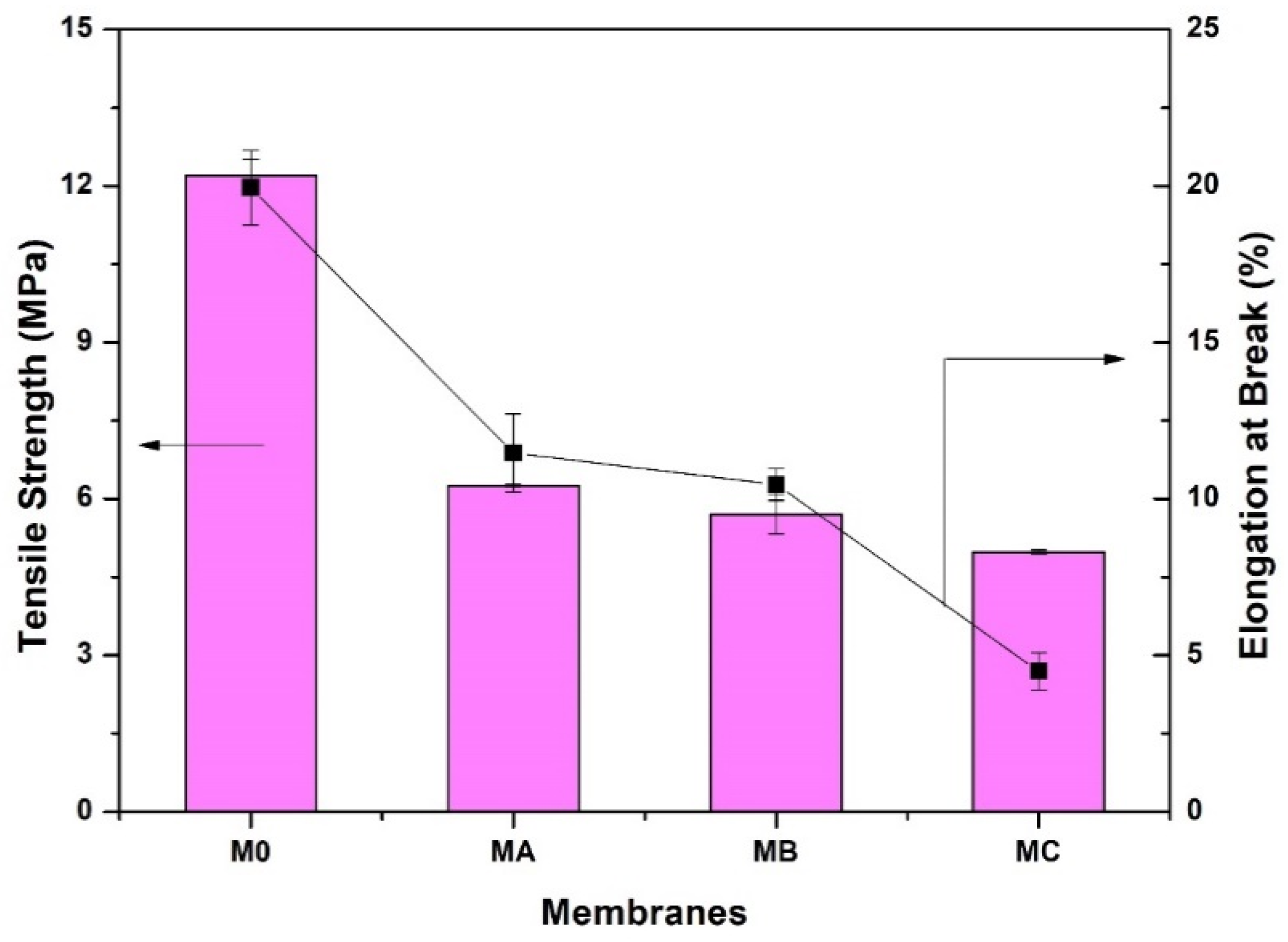
3.5. Membrane Mechanical Properties
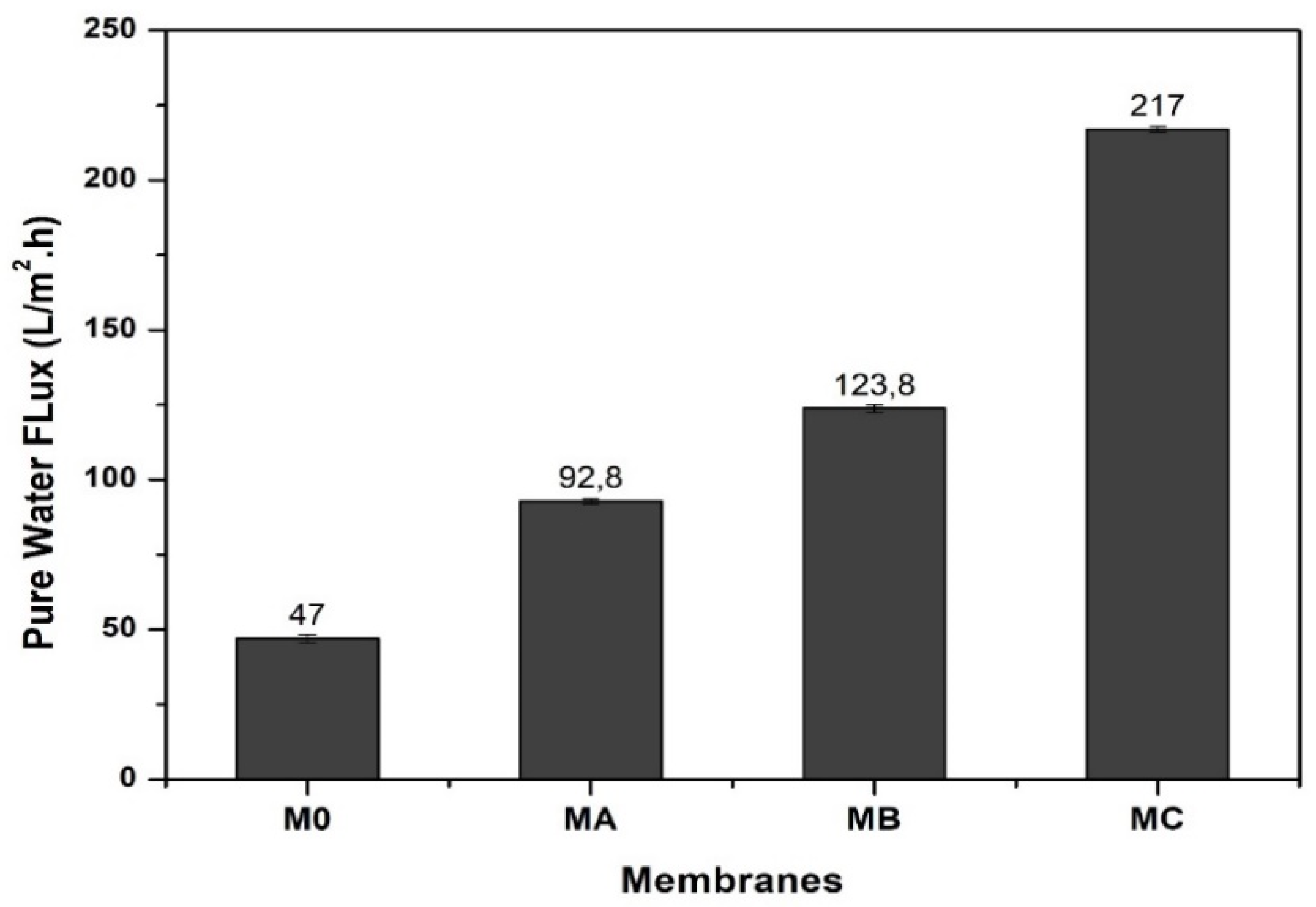
3.6. Hydraulic Performance
3.7. Fouling Endurance Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saleem, H.; Trabzon, L.; Kilic, A.; Zaidi, S.J. Recent advances in nanofibrous membranes: Production and applications in water treatment and desalination. Desalination 2020, 478, 114178. [Google Scholar] [CrossRef]
- Zuo, H.-R.; Shi, P.; Duan, M. A review on thermally stable membranes for water treatment: Material, fabrication, and application. Sep. Purif. Technol. 2020, 236, 116223. [Google Scholar] [CrossRef]
- Nabeel, F.; Rasheed, T.; Bilal, M.; Iqbal, H.M. Supramolecular membranes: A robust platform to develop separation strategies towards water-based applications. Sep. Purif. Technol. 2019, 215, 441–453. [Google Scholar] [CrossRef]
- Ibrahim, N.; Wirzal, M.; Nordin, N.; Abd Halim, N. Development of Polyvinylidene fluoride (PVDF)-ZIF-8 Membrane for Wastewater Treatment. In Proceedings of the 4th International Conference on Civil and Environmental Engineering for Sustainability (IConCEES 2017), IOP Conference Series: Earth and Environmental Science, Langkawi, Malaysia, 4–5 December 2017; p. 012021. [Google Scholar]
- Zeng, K.; Zhou, J.; Cui, Z.; Zhou, Y.; Shi, C.; Wang, X.; Zhou, L.; Ding, X.; Wang, Z.; Drioli, E. Insight into fouling behavior of poly (vinylidene fluoride)(PVDF) hollow fiber membranes caused by dextran with different pore size distributions. Chin. J. Chem. Eng. 2018, 26, 268–277. [Google Scholar] [CrossRef]
- Shoparwe, N.F.; Otitoju, T.A.; Ahmad, A.L. Fouling evaluation of polyethersulfone (PES)/sulfonated cation exchange resin (SCER) membrane for BSA separation. J. Appl. Polym. Sci. 2018, 135, 45854. [Google Scholar] [CrossRef]
- Sakinah, A.M.; Ismail, A.; Illias, R.M.; Hassan, O. Fouling characteristics and autopsy of a PES ultrafiltration membrane in cyclodextrins separation. Desalination 2007, 207, 227–242. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Abd Halim, N.S.; Wirzal, M.D.H.; Bilad, M.R.; Md Nordin, N.A.H.; Adi Putra, Z.; Sambudi, N.S.; Mohd Yusoff, A.R. Improving Performance of Electrospun Nylon 6, 6 Nanofiber Membrane for Produced Water Filtration via Solvent Vapor Treatment. Polymers 2019, 11, 2117. [Google Scholar] [CrossRef] [PubMed]
- Abd Halim, N.S.; Wirzal, M.D.H.; Bilad, M.R.; Md Nordin, N.A.H.; Adi Putra, Z.; Mohd Yusoff, A.R.; Narkkun, T.; Faungnawakij, K. Electrospun Nylon 6, 6/ZIF-8 Nanofiber Membrane for Produced Water Filtration. Water 2019, 11, 2111. [Google Scholar] [CrossRef]
- Goh, P.; Lau, W.; Othman, M.; Ismail, A. Membrane fouling in desalination and its mitigation strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Bagheri, M.; Akbari, A.; Mirbagheri, S.A. Advanced control of membrane fouling in filtration systems using artificial intelligence and machine learning techniques: A critical review. Process Saf. Environ. Prot. 2019, 123, 229–252. [Google Scholar] [CrossRef]
- Mulyati, S.; Muchtar, S.; Yusuf, M.; Arahman, N.; Sofyana, S.; Rosnelly, C.M.; Fathanah, U.; Takagi, R.; Matsuyama, H.; Shamsuddin, N. Production of High Flux Poly (Ether Sulfone) Membrane Using Silica Additive Extracted from Natural Resource. Membranes 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Younas, H.; Zhou, Y.; Li, X.; Li, X.; Sun, Q.; Cui, Z.; Wang, Z. Fabrication of high flux and fouling resistant membrane: A unique hydrophilic blend of polyvinylidene fluoride/polyethylene glycol/polymethyl methacrylate. Polymer 2019, 179, 121593. [Google Scholar] [CrossRef]
- Mu, K.; Zhang, D.; Shao, Z.; Qin, D.; Wang, Y.; Wang, S. Enhanced permeability and antifouling performance of cellulose acetate ultrafiltration membrane assisted by L-DOPA functionalized halloysite nanotubes. Carbohydr. Polym. 2017, 174, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, R.; Liu, Y.; He, M.; Su, Y.; Gao, C.; Jiang, Z. Antifouling membrane surface construction: Chemistry plays a critical role. J. Membr. Sci. 2018, 551, 145–171. [Google Scholar] [CrossRef]
- Halim, N.; Wirzal, M.; Bilad, M.; Yusoff, A.; Nordin, N.; Putra, Z.; Jaafar, J. Effect of solvent vapor treatment on electrospun nylon 6, 6 nanofiber membrane. In Proceedings of the International Conference on Advanced Manufacturing and Industry Applications, IOP Conference Series: Materials Science and Engineering, Sarawak, Malaysia, 15–17 August 2018; p. 012019. [Google Scholar]
- Mat Nawi, N.I.; Abd Halim, N.S.; Lee, L.C.; Wirzal, M.D.H.; Bilad, M.R.; Nordin, N.A.H.; Putra, Z.A. Improved nylon 6, 6 nanofiber membrane in a tilted panel filtration system for fouling control in microalgae harvesting. Polymers 2020, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Arahman, N.; Maimun, T.; Bilad, M. Fabrication of polyethersulfone membranes using nanocarbon as additive. Int. J. Geomate 2018, 15, 51–57. [Google Scholar] [CrossRef]
- Wahab, M.Y.; Muchtar, S.; Jeon, S.; Fang, L.F.; Rajabzadeh, S.; Takagi, R.; Arahman, N.; Mulyati, S.; Riza, M.; Matsuyama, H. Synergistic effects of organic and inorganic additives in preparation of composite poly (vinylidene fluoride) antifouling ultrafiltration membranes. J. Appl. Polym. Sci. 2019, 136, 47737. [Google Scholar] [CrossRef]
- Al-Husaini, I.S.; Yusoff, A.R.M.; Lau, W.-J.; Ismail, A.F.; Al-Abri, M.Z.; Wirzal, M.D.H. Iron oxide nanoparticles incorporated polyethersulfone electrospun nanofibrous membranes for effective oil removal. Chem. Eng. Res. Des. 2019, 148, 142–154. [Google Scholar] [CrossRef]
- Al-Husaini, I.; Yusoff, A.; Lau, W.; Ismail, A.; Al-Abri, M.; Al-Ghafri, B.; Wirzal, M. Fabrication of polyethersulfone electrospun nanofibrous membranes incorporated with hydrous manganese dioxide for enhanced ultrafiltration of oily solution. Sep. Purif. Technol. 2019, 212, 205–214. [Google Scholar] [CrossRef]
- Marbelia, L.; Bilad, M.R.; Vankelecom, I.F. Gradual PVP leaching from PVDF/PVP blend membranes and its effects on membrane fouling in membrane bioreactors. Sep. Purif. Technol. 2019, 213, 276–282. [Google Scholar] [CrossRef]
- Arahman, N.; Mulyati, S.; Fahrina, A.; Muchtar, S.; Yusuf, M.; Takagi, R.; Matsuyama, H.; Nordin, N.A.H.; Bilad, M.R. Improving Water Permeability of Hydrophilic PVDF Membrane Prepared via Blending with Organic and Inorganic Additives for Humic Acid Separation. Molecules 2019, 24, 4099. [Google Scholar] [CrossRef]
- Muchtar, S.; Wahab, M.Y.; Fang, L.F.; Jeon, S.; Rajabzadeh, S.; Takagi, R.; Mulyati, S.; Arahman, N.; Riza, M.; Matsuyama, H. Polydopamine-coated poly (vinylidene fluoride) membranes with high ultraviolet resistance and antifouling properties for a photocatalytic membrane reactor. J. Appl. Polym. Sci. 2019, 136, 47312. [Google Scholar] [CrossRef]
- Foong, C.Y.; Wirzal, M.D.H.; Bustam, M.A. A review on nanofibers membrane with amino-based ionic liquid for heavy metal removal. J. Mol. Liq. 2020, 297, 111793. [Google Scholar] [CrossRef]
- Vatanpour, V.; Zoqi, N. Surface modification of commercial seawater reverse osmosis membranes by grafting of hydrophilic monomer blended with carboxylated multiwalled carbon nanotubes. Appl. Surf. Sci. 2017, 396, 1478–1489. [Google Scholar] [CrossRef]
- Yang, H.-C.; Luo, J.; Lv, Y.; Shen, P.; Xu, Z.-K. Surface engineering of polymer membranes via mussel-inspired chemistry. J. Membr. Sci. 2015, 483, 42–59. [Google Scholar] [CrossRef]
- Li, B.; Liu, W.; Jiang, Z.; Dong, X.; Wang, B.; Zhong, Y. Ultrathin and stable active layer of dense composite membrane enabled by poly (dopamine). Langmuir 2009, 25, 7368–7374. [Google Scholar] [CrossRef]
- Liebscher, J.R.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hadade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of polydopamine: A never-ending story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, J.; Liu, M.; Huang, H.; Zhang, X.; Wei, Y. Polydopamine-based functional materials and their applications in energy, environmental, and catalytic fields: State-of-the-art review. Chem. Eng. J. 2020, 387, 124019. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.-C.; He, F.; Peng, S.; Li, Y.; Shao, L.; Darling, S.B. Mussel-inspired surface engineering for water-remediation materials. Matter 2019, 1, 115–155. [Google Scholar] [CrossRef]
- Jiang, J.-H.; Zhu, L.-P.; Zhang, H.-T.; Zhu, B.-K.; Xu, Y.-Y. Improved hydrodynamic permeability and antifouling properties of poly (vinylidene fluoride) membranes using polydopamine nanoparticles as additives. J. Membr. Sci. 2014, 457, 73–81. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, F.; Li, J.; Li, B.; Zhao, C. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym. Chem. 2010, 1, 1430–1433. [Google Scholar] [CrossRef]
- Gao, Z.F.; Wang, X.Y.; Gao, J.B.; Xia, F. Rapid preparation of polydopamine coating as a multifunctional hair dye. RSC Adv. 2019, 9, 20492–20496. [Google Scholar] [CrossRef]
- Muchtar, S.; Wahab, M.Y.; Mulyati, S.; Arahman, N.; Riza, M. Superior fouling resistant PVDF membrane with enhanced filtration performance fabricated by combined blending and the self-polymerization approach of dopamine. J. Water Process Eng. 2019, 28, 293–299. [Google Scholar] [CrossRef]
- Zhu, J.; Tsehaye, M.T.; Wang, J.; Uliana, A.; Tian, M.; Yuan, S.; Li, J.; Zhang, Y.; Volodin, A.; Van der Bruggen, B. A rapid deposition of polydopamine coatings induced by iron (III) chloride/hydrogen peroxide for loose nanofiltration. J. Colloid Interface Sci. 2018, 523, 86–97. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef]
- Manini, P.; Panzella, L.; Napolitano, A.; d’Ischia, M. A novel hydrogen peroxide-dependent oxidation pathway of dopamine via 6-hydroxydopamine. Tetrahedron 2003, 59, 2215–2221. [Google Scholar] [CrossRef]
- Liebscher, J. Chemistry of Polydopamine–Scope, Variation, and Limitation. Eur. J. Org. Chem. 2019, 2019, 4976–4994. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Mao, L.; Jiang, C.; Ang, J.; Lu, X. Doping polysulfone ultrafiltration membrane with TiO2-PDA nanohybrid for simultaneous self-cleaning and self-protection. J. Membr. Sci. 2017, 532, 20–29. [Google Scholar] [CrossRef]
- Smolders, K.; Franken, A. Terminology for membrane distillation. Desalination 1989, 72, 249–262. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Borovanska, I.; Todorov, P.; Ivanov, E.; Menseidov, D.; Chakraborty, S.; Bhattacharjee, C. Tensile and surface mechanical properties of polyethersulphone (pes) and polyvinylidene fluoride (PVDF) membranes. J. Theor. Appl. Mech. 2018, 48, 85–99. [Google Scholar] [CrossRef]
- Howe, K.J.; Clark, M.M. Fouling of microfiltration and ultrafiltration membranes by natural waters. Environ. Sci. Technol. 2002, 36, 3571–3576. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ismail, A. Fouling control on microfiltration/ultrafiltration membranes: Effects of morphology, hydrophilicity, and charge. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Mao, Z.; Zhang, Y.; Tan, W.S.; Chong, T.H.; Wu, B.; Chew, J.W. Enhancing fouling mitigation of submerged flat-sheet membranes by vibrating 3D-spacers. Sep. Purif. Technol. 2019, 215, 70–80. [Google Scholar] [CrossRef]
- Sun, W.; Liu, J.; Chu, H.; Dong, B. Pretreatment and membrane hydrophilic modification to reduce membrane fouling. Membranes 2013, 3, 226–241. [Google Scholar] [CrossRef]




| Membrane ID | PES (wt %) | Additive (wt %) | Solvent (wt %) | |
|---|---|---|---|---|
| Dopamine | H2O2 | NMP | ||
| MO | 15 | - | - | 85 |
| MA | 13.5 | 1 | 0.5 | 85 |
| MB | 12 | 2 | 1 | 85 |
| MC | 10.5 | 3 | 1.5 | 85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulyati, S.; Muchtar, S.; Arahman, N.; Meirisa, F.; Syamsuddin, Y.; Zuhra, Z.; Rosnelly, C.M.; Shamsuddin, N.; Mat Nawi, N.I.; Wirzal, M.D.H.; et al. One-Pot Polymerization of Dopamine as an Additive to Enhance Permeability and Antifouling Properties of Polyethersulfone Membrane. Polymers 2020, 12, 1807. https://doi.org/10.3390/polym12081807
Mulyati S, Muchtar S, Arahman N, Meirisa F, Syamsuddin Y, Zuhra Z, Rosnelly CM, Shamsuddin N, Mat Nawi NI, Wirzal MDH, et al. One-Pot Polymerization of Dopamine as an Additive to Enhance Permeability and Antifouling Properties of Polyethersulfone Membrane. Polymers. 2020; 12(8):1807. https://doi.org/10.3390/polym12081807
Chicago/Turabian StyleMulyati, Sri, Syawaliah Muchtar, Nasrul Arahman, Friska Meirisa, Yanna Syamsuddin, Zuhra Zuhra, Cut Meurah Rosnelly, Norazanita Shamsuddin, Normi Izati Mat Nawi, Mohd Dzul Hakim Wirzal, and et al. 2020. "One-Pot Polymerization of Dopamine as an Additive to Enhance Permeability and Antifouling Properties of Polyethersulfone Membrane" Polymers 12, no. 8: 1807. https://doi.org/10.3390/polym12081807
APA StyleMulyati, S., Muchtar, S., Arahman, N., Meirisa, F., Syamsuddin, Y., Zuhra, Z., Rosnelly, C. M., Shamsuddin, N., Mat Nawi, N. I., Wirzal, M. D. H., Bilad, M. R., Takagi, R., & Matsuyama, H. (2020). One-Pot Polymerization of Dopamine as an Additive to Enhance Permeability and Antifouling Properties of Polyethersulfone Membrane. Polymers, 12(8), 1807. https://doi.org/10.3390/polym12081807










