Formulation and Characterization of Antimicrobial Edible Films Based on Whey Protein Isolate and Tarragon Essential Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Essential Oil Extraction
2.1.1. ITEX/GC-MS Analysis of Tarragon Essential Oil
2.1.2. Determination of Total Phenolic Content
2.1.3. Determination of Antioxidant Capacity
2.2. Bacterial Strains
2.2.1. Kirby-Bauer Disk Diffusion Test
2.2.2. Minimum Inhibitory Concentration (MIC) Test
2.2.3. Minimum Bactericidal Concentration (MBC) Test
2.3. Films’ Preparation
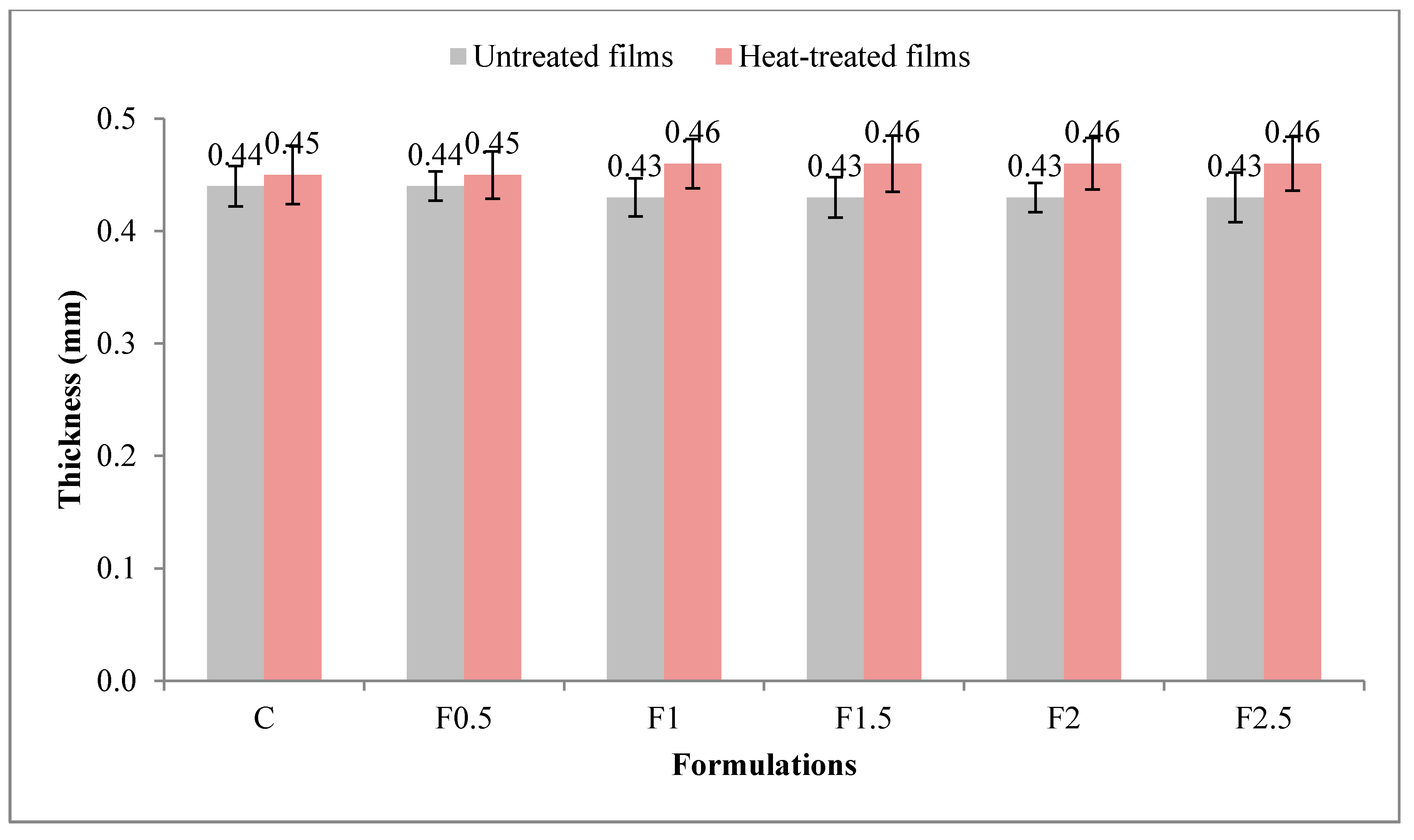
2.3.1. Measurement of Thickness
2.3.2. Determination of Moisture Content
2.3.3. Determination of Swelling Degree and Solubility in Water
2.3.4. Determination of Water Vapor Permeability (WVP)
2.3.5. Measurement of Color
2.3.6. Measurement of Light Transmittance and Transparency
2.3.7. Puncture Test
2.4. Statistical Analysis
3. Results
3.1. Chemical and Biological Properties of Tarragon Essential Oil
3.2. Physical and Mechanical Properties of Films
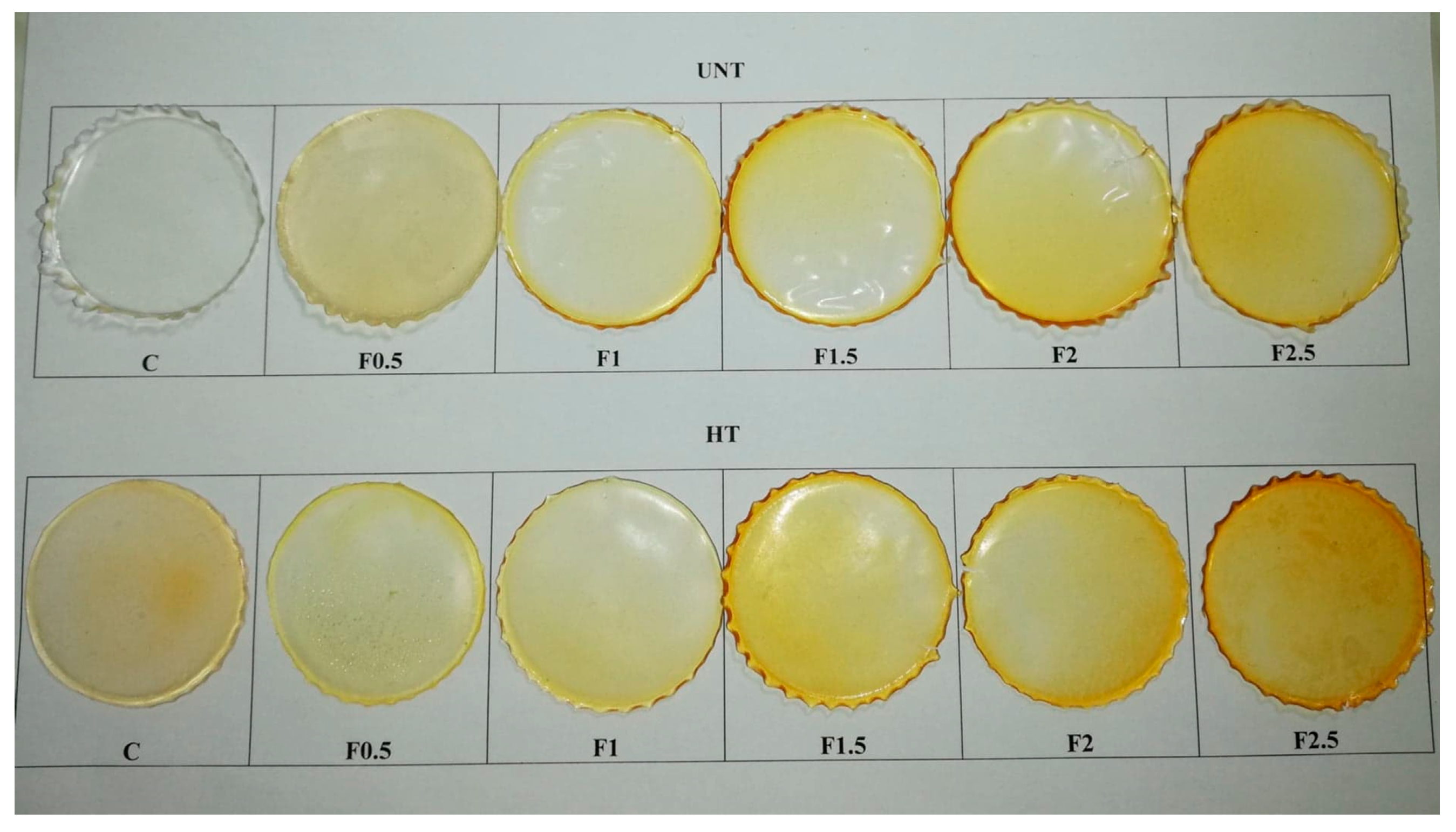
3.2.1. Films’ Appearance
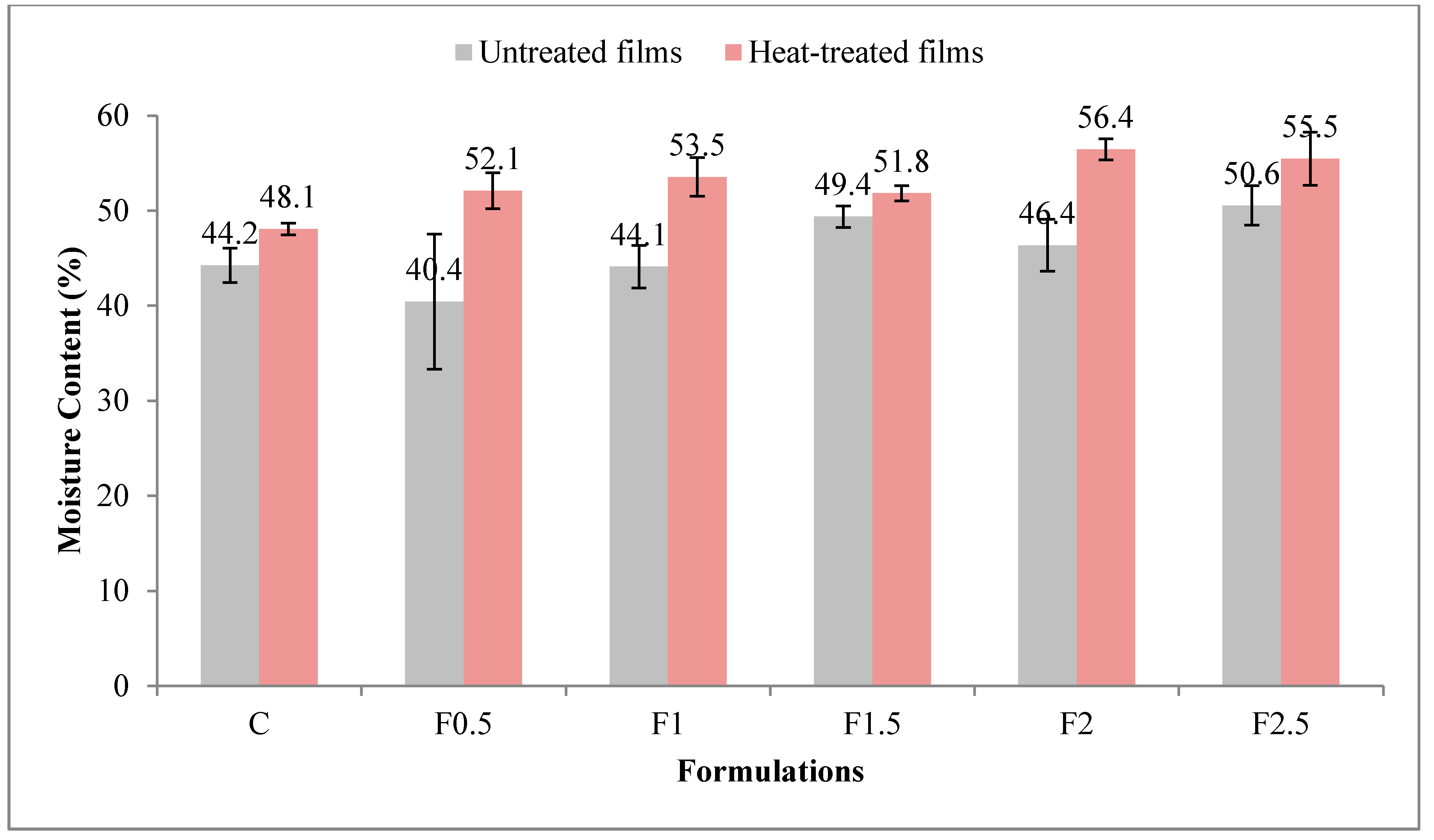
3.2.2. Moisture Content
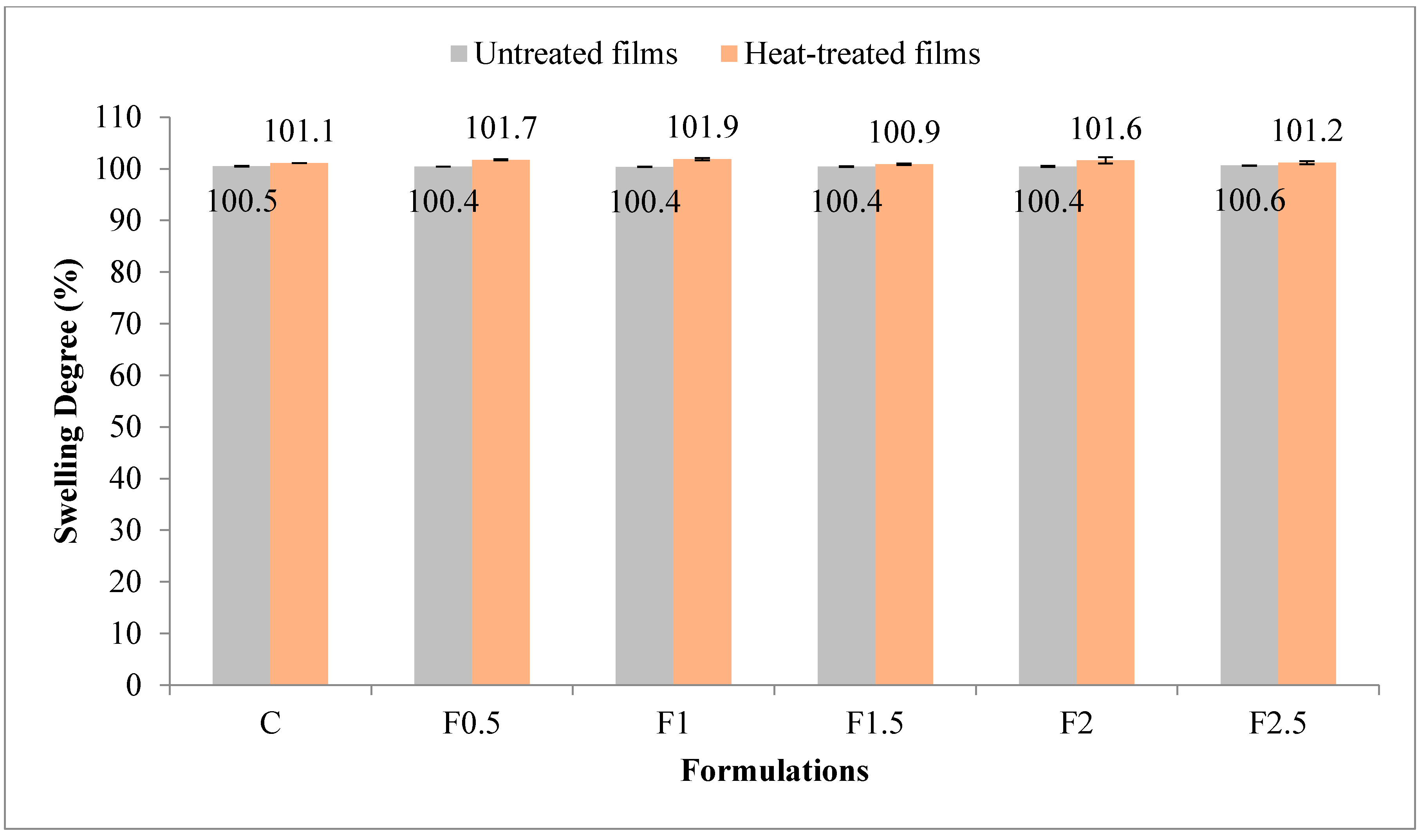
3.2.3. Swelling Degree
3.2.4. Solubility in Water
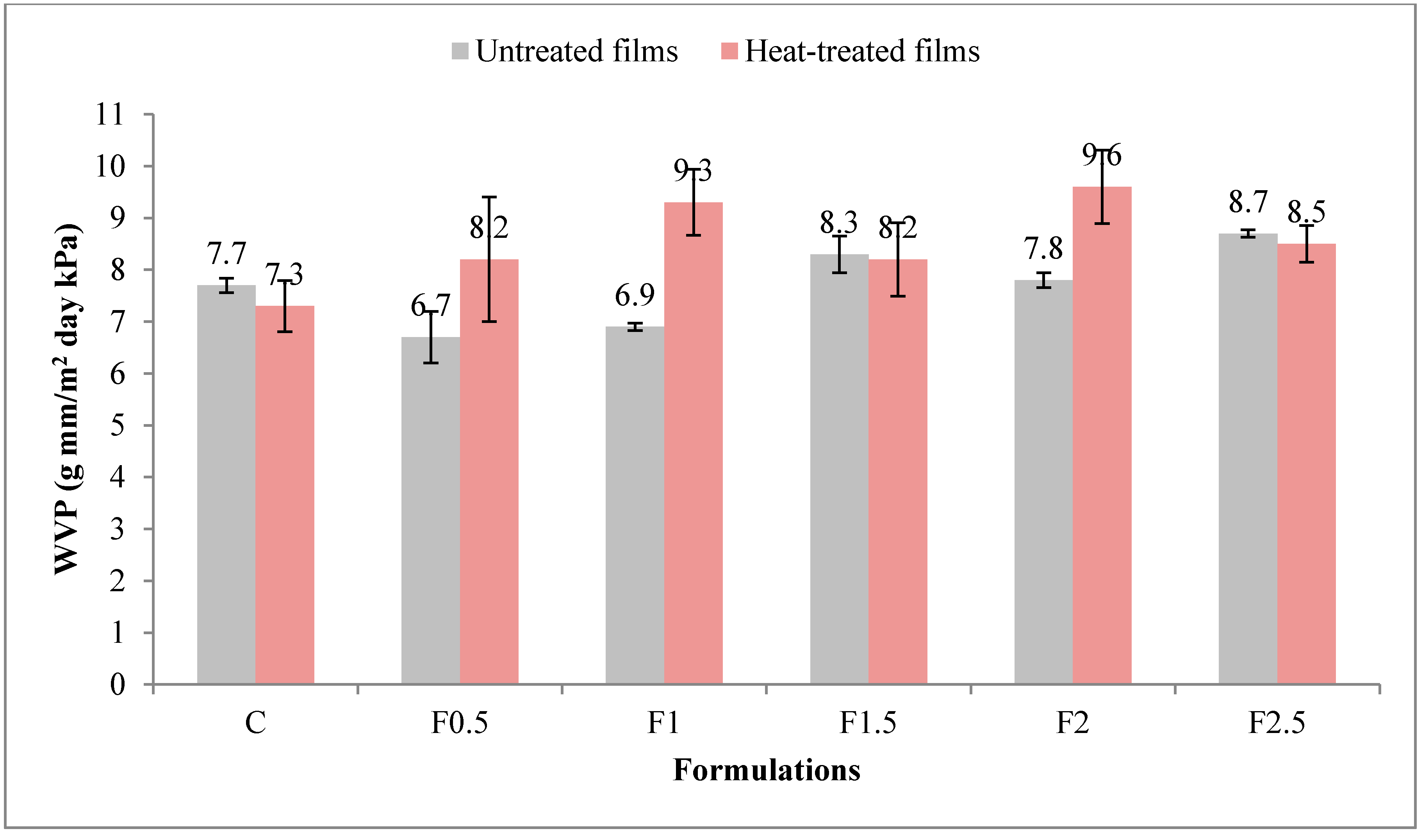
3.2.5. Water Vapor Permeability (WVP)
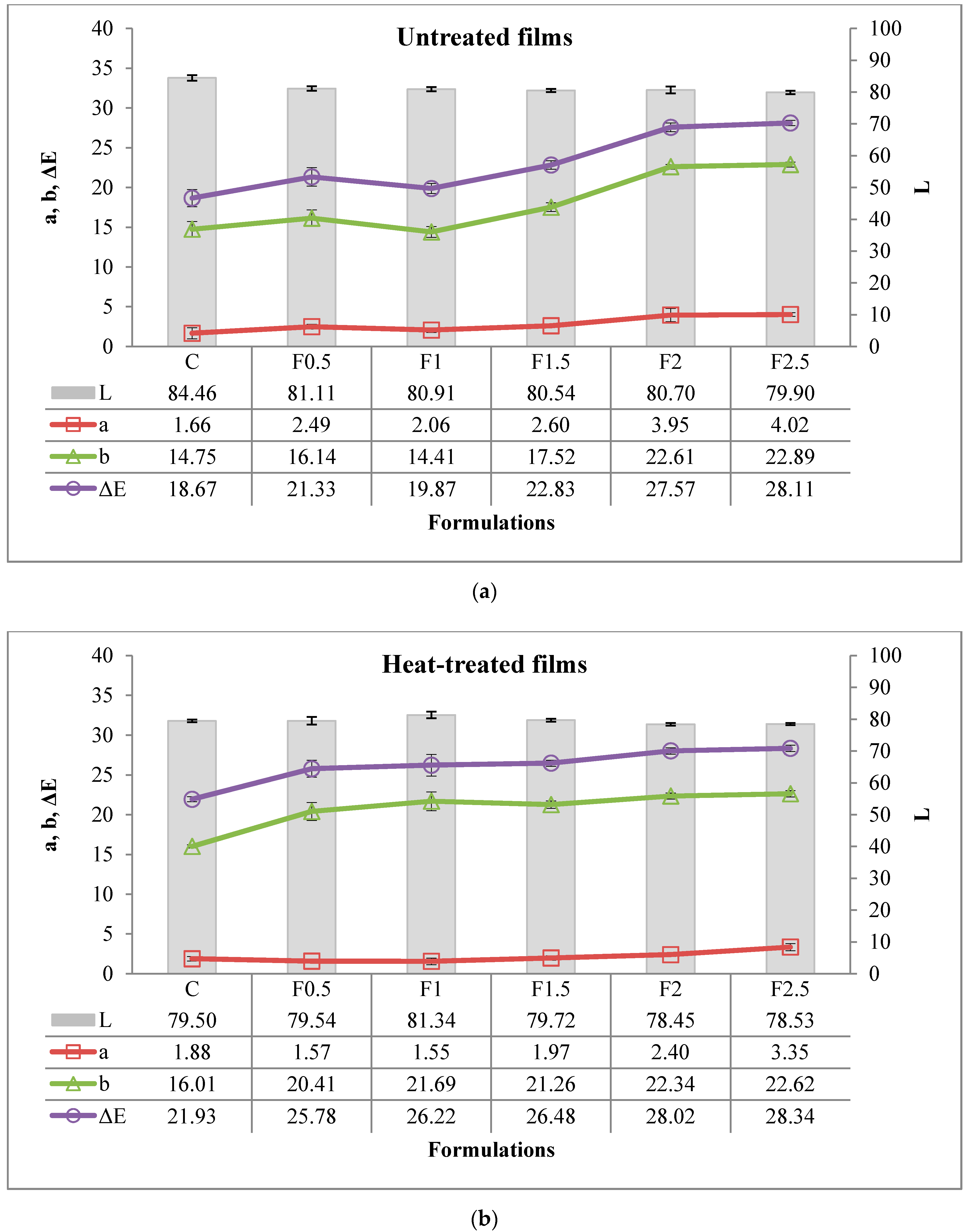
3.2.6. Color
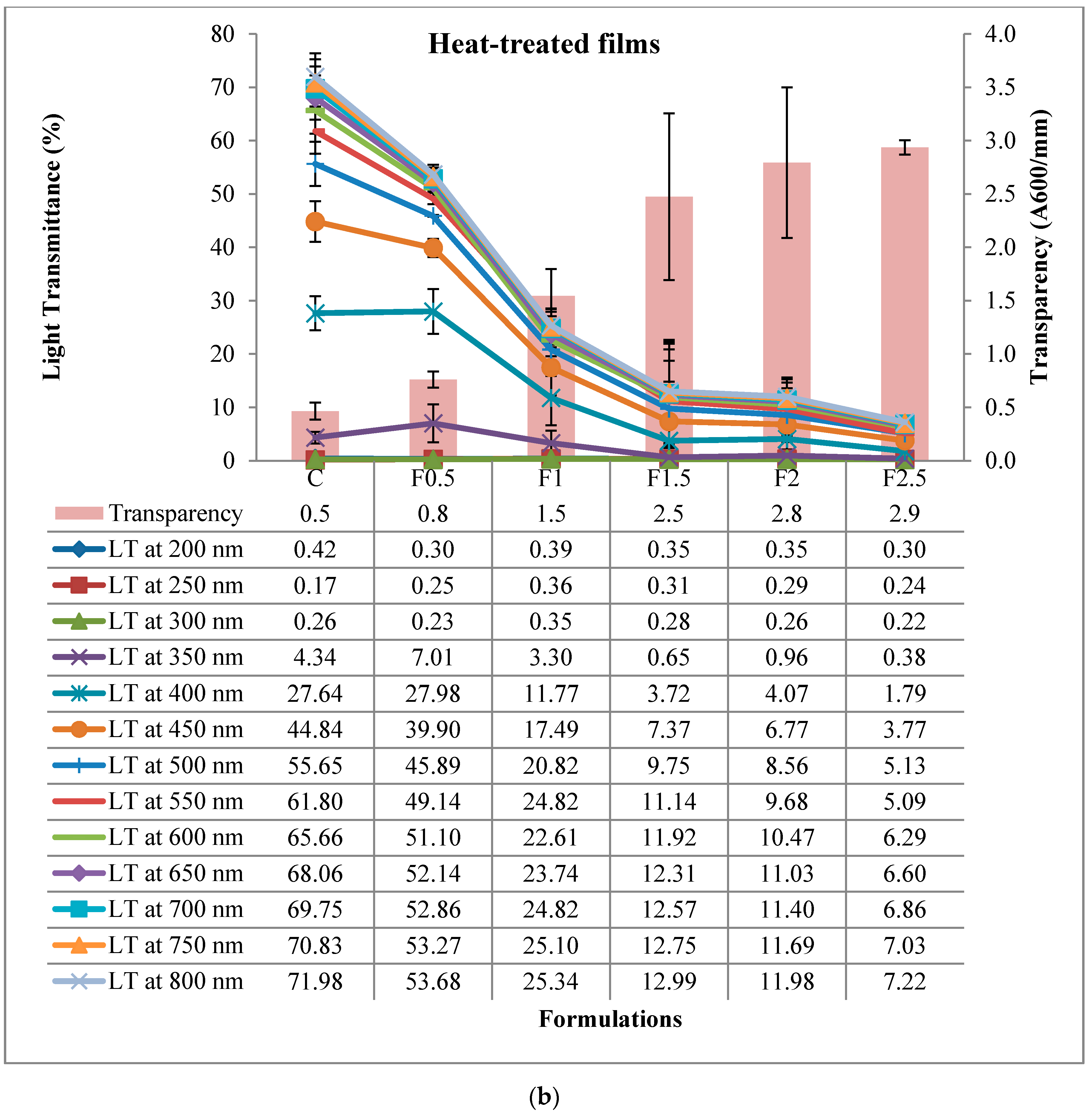
3.2.7. Light Transmittance and Transparency
3.2.8. Puncture Resistance (PR) and Puncture Deformation (PD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Socaciu, M.I.; Semeniuc, C.A.; Vodnar, D.C. Edible films and coatings for fresh fish packaging: Focus on quality changes and shelf-life extension. Coatings 2018, 8, 366. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizers in edible film and coatings. In Innovations in Food Packaging, 1st ed.; Han, J.H., Ed.; Elsevier-Academic Press: Amsterdam, The Netherlands, 2005; pp. 403–433. [Google Scholar]
- Erginkaya, Z.; Kalkan, S.; Ünal, E. Use of antimicrobial edible films and coatings as packaging materials for food safety. In Food Processing: Strategies for Quality Assessment; Malik, A., Erginkaya, Z., Ahmad, S., Erten, H., Eds.; Springer: New York, USA, 2014; pp. 261–295. [Google Scholar]
- Morar, M.I.; Fetea, F.; Rotar, A.M.; Nagy, M.; Semeniuc, C.A. Characterization of essential oils extracted from different aromatic plants by FTIR spectroscopy. Bulletin UASVM Food Science and Technology 2017, 74, 37–38. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Socaciu, M.I.; Socaci, S.A.; Mureşan, V.; Fogarasi, M.; Rotar, A.M. Chemometric comparison and classification of some essential oils extracted from plants belonging to Apiaceae and Lamiaceae families based on their chemical composition and biological activities. Molecules 2018, 23, 2261. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Kotan, R.; Mavi, A.; Cakir, A.; Ala, A.; Yildirim, A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J. Agric. Food Chem. 2005, 53, 9452–9458. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef]
- Sharafati Chaleshtori, R.; Rokni, N.; Razavilar, V.; Rafieian Kopaei, M. The evaluation of the antibacterial and antioxidant activity of tarragon (Artemisia dracunculus L.) essential oil and its chemical composition. Jundishapur J. Microbiol. 2013, 6, e7877. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Antioxidant activity and antimicrobial effect of tarragon (Artemisia dracunculus) extract and chemical composition of its essential oil. J. Food Meas. Charact. 2017, 11, 847–863. [Google Scholar] [CrossRef]
- Kıvanç, M.; Akgül, A. Antibacterial activities of essential oils from Turkish spices and citrus. Flavour Frag. J. 1986, 1, 175–179. [Google Scholar] [CrossRef]
- Juteau, F.; Jerkovic, I.; Masotti, V.; Milos, M.; Mastelic, J.; Bessière, J.M.; Viano, J. Composition and antimicrobial activity of the essential oil of Artemisia absinthium from Croatia and France. Planta Med. 2003, 69, 158–161. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Feistel, B.; Glotov, N.; Heinrich, M. Artemisia dracunculus L. (tarragon): A critical review of its traditional use, chemical composition, pharmacology, and safety. J. Agric. Food Chem. 2011, 59, 11367–11384. [Google Scholar] [CrossRef]
- Aulin, C.; Lindström, T. Biopolymer films and coatings. In Biopolymers –New Materials for Sustainable Films and Coatings, 1st ed.; Plackett, D., Ed.; John Wiley & Sons: Chichester, UK, 2011; pp. 257–276. [Google Scholar]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of protein-based films and coatings for food packaging: A review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed]
- Shendurse, A.M.; Gopikrishna, G.; Patel, A.C.; Pandya, A.J. Milk protein based edible films and coatings–preparation, properties and food applications. J. Nutr. Health Food Eng. 2018, 8, 219–226. [Google Scholar] [CrossRef]
- Sanjeev, A.; Khanal, S.N.; Marella, C. Whey and whey products. In Milk and Dairy Products in Human Nutrition: Production, Composition and Health; Park, Y.W., Haenlein, G.F.W., Eds.; John Wiley & Sons: Chichester, UK, 2013; pp. 477–497. [Google Scholar]
- Guo, M.; Wang, G. Whey protein polymerisation and its applications in environmentally safe adhesives. Int. J. Dairy Technol. 2016, 69, 481–488. [Google Scholar] [CrossRef]
- Khwaldia, K.; Perez, C.; Banon, S.; Desobry, S.; Hardy, J. Milk proteins for edible films and coatings. Crit. Rev. Food Sci. Nutr. 2004, 44, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Kokoszka, S.; Debeaufort, F.; Lenart, A.; Voilley, A. Water vapour permeability, thermal and wetting properties of whey protein isolate based edible films. Int. Dairy J. 2010, 20, 53–60. [Google Scholar] [CrossRef]
- Gurgel Adeodato Vieira, M.; Altenhofen da Silva, M.; Oliveira dos Santos, L.; Masumi Beppu, M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Suppakul, P. Packaging, active, and intelligent: Polymer application. In Encyclopedia of Polymer Applications, 1st ed.; Mishra, M., Ed.; CRC Press: Boca Raton, USA, 2019; pp. 1958–1989. [Google Scholar]
- Ullsten, N.H.; Gällstedt, M.; Hedenqvist, M.S. Plasticizers for protein-based materials. In Viscoelastic and Viscoplastic Materials; El-Amin, M., Ed.; IntechOpen: London, UK, 2016; pp. 81–101. [Google Scholar]
- The Pherobase: Database of Pheromones and Semiochemicals. Available online: www.pherobase.com (accessed on 29 April 2020).
- Flavornet and Human Odor Space. Available online: www.flavornet.org (accessed on 29 April 2020).
- McFarland, J. Nephelometer: An instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. JAMA 1907, XLIX, 1176–1178. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug. Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef]
- Badr, K.R.; Ahmed, Z.S.; ElGamal, M.S. Evaluation of the antimicrobial action of whey protein edible films incorporated with cinnamon, cumin and thyme against spoilage flora of fresh beef. Int. J. Agric. Res. 2014, 9, 242–250. [Google Scholar] [CrossRef]
- Wang, J.; Shang, J.; Ren, F.; Leng, X. Study of the physical properties of whey protein: Sericin protein-blended edible films. Eur. Food Res. Technol. 2010, 231, 109–116. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabad, S.; Keshavarz, B.; Cran, M.J.; Khaksar, R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013, 98, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.J. Food packaging materials, barrier properties, and selection. In Handbook of Food Engineering Practice; Valentas, K.J., Rotstein, E., Singh, R.P., Eds.; CRC Press: Boca Raton, USA, 1997; pp. 307–360. [Google Scholar]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.F.; Martău, G.A.; Szabo, K.; Teleky, B.E.; Mureșan, V.; Rusu, A.V.; Socol, C.T.; Vodnar, D.C. Poly(vinyl alcohol)-based biofilms plasticized with polyols and colored with pigments extracted from tomato by-products. Polymers 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Šuput, D.; Lazić, V.; Popović, S.; Hromiš, N.; Bulut, S. Biopolymer films synthesis and characterization. J. Process. Energy Agric. 2017, 21, 9–12. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Chai, Z.; Leng, X. Study of the physical properties of whey protein isolate and gelatin composite films. J. Agric. Food Chem. 2010, 58, 5100–5108. [Google Scholar] [CrossRef] [PubMed]
- Ramos, Ó.L.; Silva, S.I.; Soares, J.C.; Fernandes, J.C.; Fátima Poças, M.; Pintado, M.E.; Malcata, F.X. Features and performance of edible films, obtained from whey protein isolate formulated with antimicrobial compounds. Food Res. Int. 2012, 45, 351–361. [Google Scholar] [CrossRef]
- Pérez-Gago, M.B.; Nadaud, P.; Krochta, J.M. Water vapor permeability, solubility, and tensile properties of heat-denatured versus native whey protein films. J. Food Sci. 1999, 64, 1034–1037. [Google Scholar] [CrossRef]
- Perez, O.E.; Carrera-Sanchez, C.; Rodrıguez-Patino, J.M.; Pilosof, A.M.R. Behavior of hydroxypropylmethylcelluloses of different molecular structure and water affinity at the air–water interface. In Water Properties of Food, Pharmaceutical, and Biological Materials, 1st ed.; del Pilar Buera, M., Welti-Chanes, J., Lillford, P.J., Corti, H.R., Eds.; CRC Press: Boca Raton, USA, 2006; pp. 445–454. [Google Scholar]
- Athavale, S.P. Hand Book of Printing, Packaging and Lamination: Packaging Technology, 1st ed.; Notion Press: Chennai, India, 2018; pp. 319–388. [Google Scholar]
- González Sandoval, D.C.; Luna Sosa, B.; Martínez-Ávila, G.C.G.; Rodríguez Fuentes, H.; Avendaño Abarca, V.H.; Rojas, R. Formulation and characterization of edible films based on organic mucilage from Mexican Opuntia ficus-indica. Coatings 2019, 9, 506. [Google Scholar] [CrossRef]
- Machado Azevedo, V.; Vilela Borges, S.; Marconcini, J.M.; Yoshida, M.I.; Sena Neto, A.R.; Coelho Pereira, T.; Ferreira Gonçalves Pereira, C. Effect of replacement of corn starch by whey protein isolate in biodegradable film blends obtained by extrusion. Carbohydr. Polym. 2017, 157, 971–980. [Google Scholar] [CrossRef]



| Crt. No. | Compound | Chemical Class | Retention Time | Relative Content |
|---|---|---|---|---|
| 1 | Hexanal | A.A. | 5.451 | 0.18 |
| 2 | α-Phellandrene | M.C. | 9.695 | 2.27 |
| 3 | α-Pinene | M.C. | 9.959 | 1.35 |
| 4 | Sabinene | M.C. | 11.537 | 74.98 |
| 5 | β-Pinene | M.C. | 11.709 | 1.76 |
| 6 | β-Myrcene | M.C. | 12.224 | 3.38 |
| 7 | 4-Carene | M.C. | 13.306 | 2.58 |
| 8 | o-Cymene | M.C. | 13.627 | 1.10 |
| 9 | D-Limonene | M.C. | 13.806 | 3.69 |
| 10 | 1,8-cineole | O.M. | 13.947 | 0.10 |
| 11 | trans-β-Ocimene | M.C. | 14.119 | 0.70 |
| 12 | cis-β-Ocimene | M.C. | 14.538 | 0.36 |
| 13 | γ-Terpinene | M.C. | 14.990 | 3.80 |
| 14 | Terpinolene | M.C. | 16.083 | 0.69 |
| 15 | trans-4-Thujanol | O.M. | 19.882 | 0.89 |
| 16 | Isoeugenol methyl ether | Phe.P. | 27.898 | 2.18 |
| - | TOTAL | - | - | 100.00 |
| Bacterial Strain | Tarragon Essential Oil | Gentamicin |
|---|---|---|
| E. coli (ATCC 25922) | 12.03 ± 0.47 | 25.27 ± 0.08 |
| S. enteritidis (ATCC 13076) | 14.41 ± 0.29 | 23.39 ± 0.03 |
| S. aureus (ATCC 25923) | 13.53 ± 0.38 | 25.77 ± 0.39 |
| L. monocytogenes (ATCC 19114) | 11.42 ± 0.27 | 26.67 ± 1.68 |
| Bacterial Strain | Tarragon Essential Oil | |
|---|---|---|
| MIC | MBC | |
| E. coli (ATCC 25922) | 5.14 ± 0.0 | 5.14 ± 0.0 |
| S. enteritidis (ATCC 13076) | 10.80 ± 0.0 | 10.80 ± 0.0 |
| S. aureus (ATCC 25923) | 10.80 ± 0.0 | 22.68 ± 0.0 |
| L. monocytogenes (ATCC 19114) | 5.14 ± 0.0 | 10.80 ± 0.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socaciu, M.-I.; Fogarasi, M.; Semeniuc, C.A.; Socaci, S.A.; Rotar, M.A.; Mureşan, V.; Pop, O.L.; Vodnar, D.C. Formulation and Characterization of Antimicrobial Edible Films Based on Whey Protein Isolate and Tarragon Essential Oil. Polymers 2020, 12, 1748. https://doi.org/10.3390/polym12081748
Socaciu M-I, Fogarasi M, Semeniuc CA, Socaci SA, Rotar MA, Mureşan V, Pop OL, Vodnar DC. Formulation and Characterization of Antimicrobial Edible Films Based on Whey Protein Isolate and Tarragon Essential Oil. Polymers. 2020; 12(8):1748. https://doi.org/10.3390/polym12081748
Chicago/Turabian StyleSocaciu, Maria-Ioana, Melinda Fogarasi, Cristina Anamaria Semeniuc, Sonia Ancuţa Socaci, Mihaela Ancuţa Rotar, Vlad Mureşan, Oana Lelia Pop, and Dan Cristian Vodnar. 2020. "Formulation and Characterization of Antimicrobial Edible Films Based on Whey Protein Isolate and Tarragon Essential Oil" Polymers 12, no. 8: 1748. https://doi.org/10.3390/polym12081748
APA StyleSocaciu, M.-I., Fogarasi, M., Semeniuc, C. A., Socaci, S. A., Rotar, M. A., Mureşan, V., Pop, O. L., & Vodnar, D. C. (2020). Formulation and Characterization of Antimicrobial Edible Films Based on Whey Protein Isolate and Tarragon Essential Oil. Polymers, 12(8), 1748. https://doi.org/10.3390/polym12081748