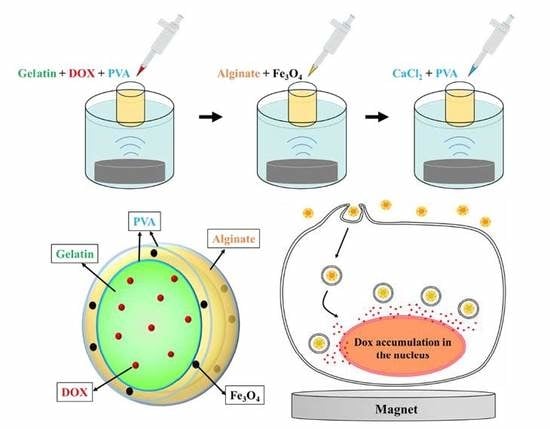
Doxorubicin–Gelatin/Fe3O4–Alginate Dual-Layer Magnetic Nanoparticles as Targeted Anticancer Drug Delivery Vehicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DOX–Gel/Fe3O4–Alginate Nanoparticles (DG/FA NPs)
2.3. Characterization of DG/FA NPs
2.4. Evaluation of DG/FA NPs Stability
2.5. Evaluation of Encapsulation Efficiency (EE)
2.6. Evaluation of In Vitro Drug Release
2.7. In Vitro Cytotoxicity Evaluation
2.8. Drug Delivery and Accumulation of DOX and DOX-Loaded MNPs
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Prepared Nanoparticles
3.1.1. Particle Size, Zeta Potential, and Encapsulated Rates
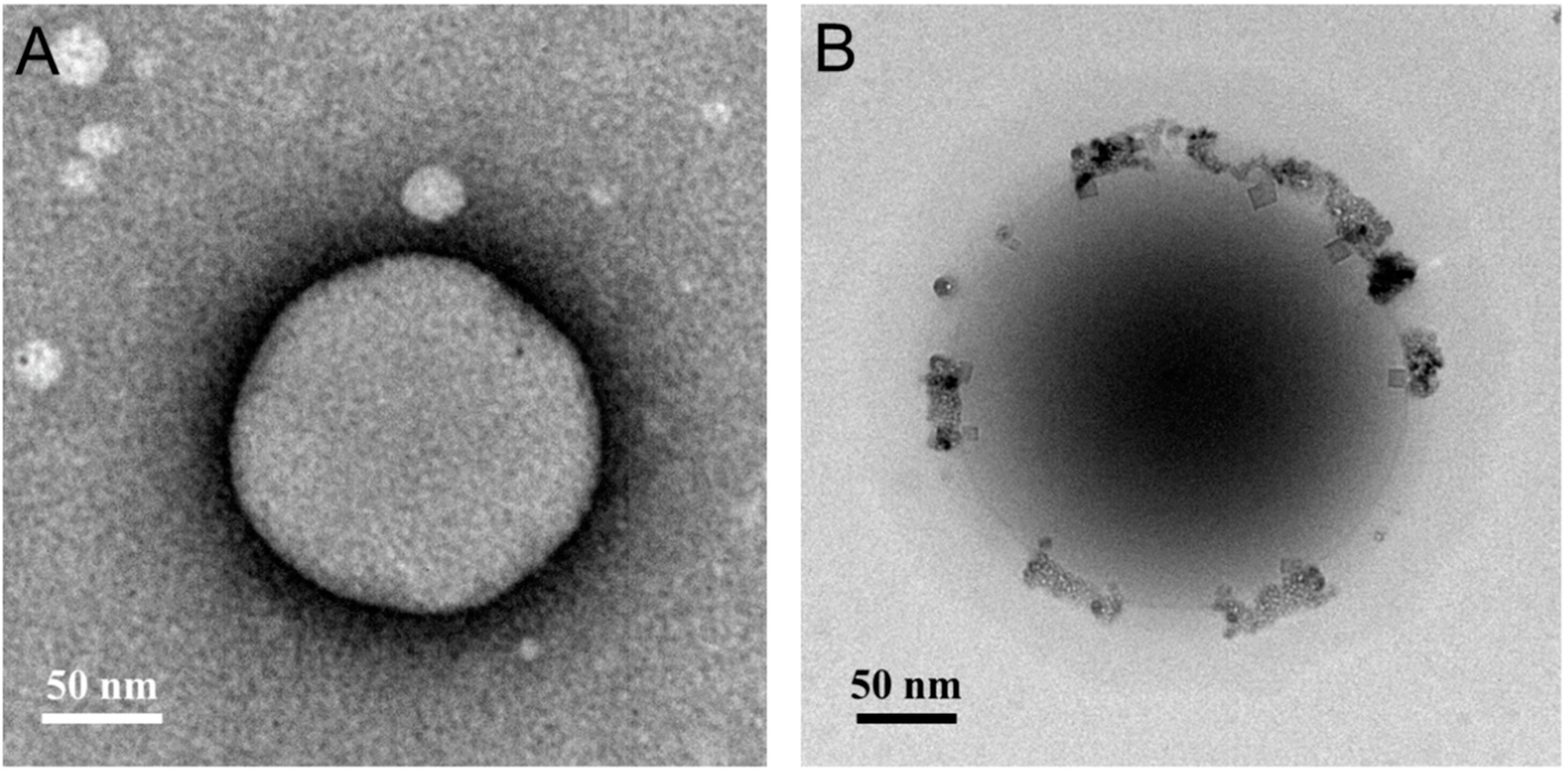
3.1.2. Transmission Electron Microscopy Analysis
3.1.3. X-ray Diffraction and Fourier-Transform Infrared Spectroscopy Analysis
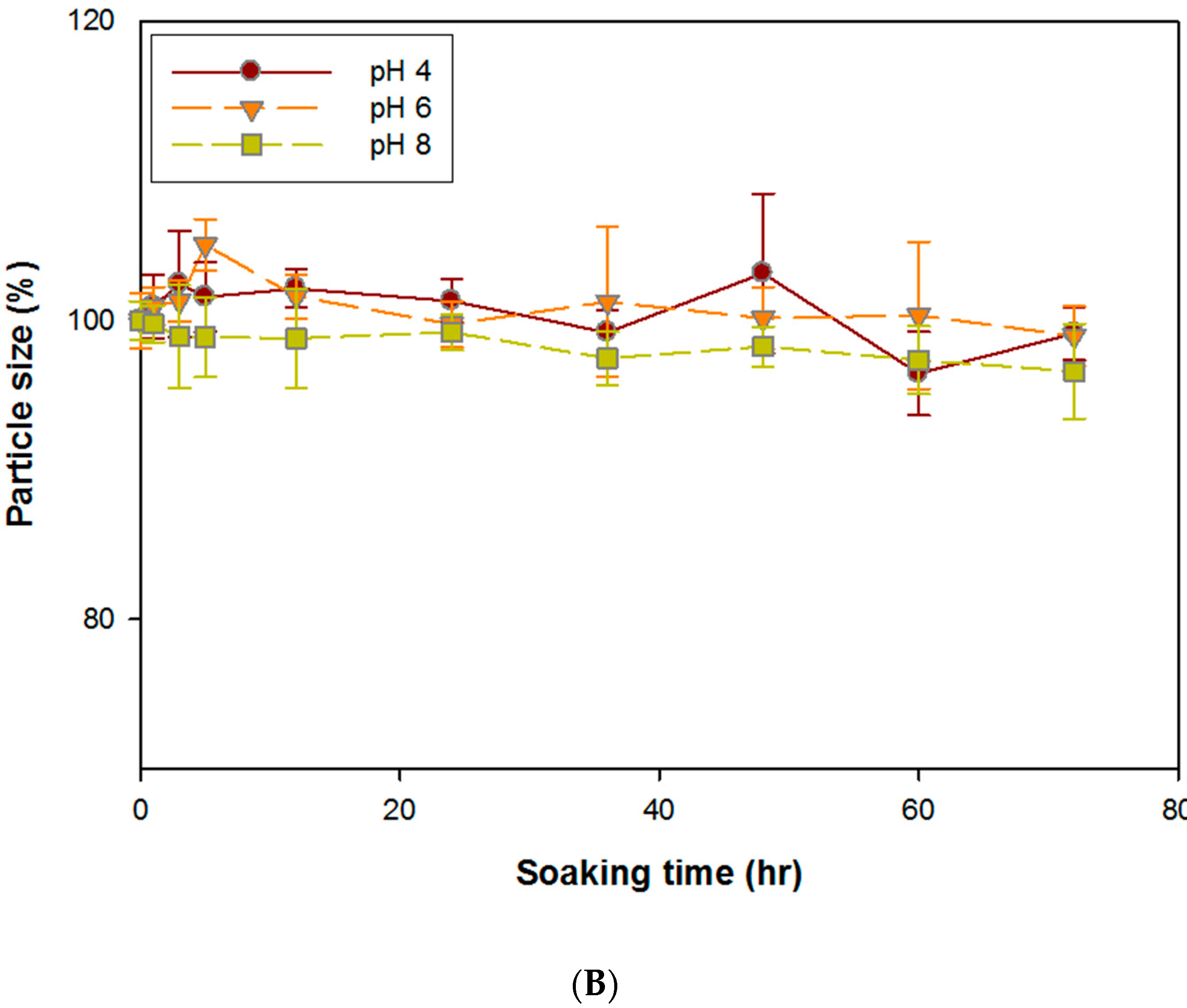
3.2. Stability of DG/FA NPs
3.3. In Vitro Drug Release Assay of DG/FA NPs
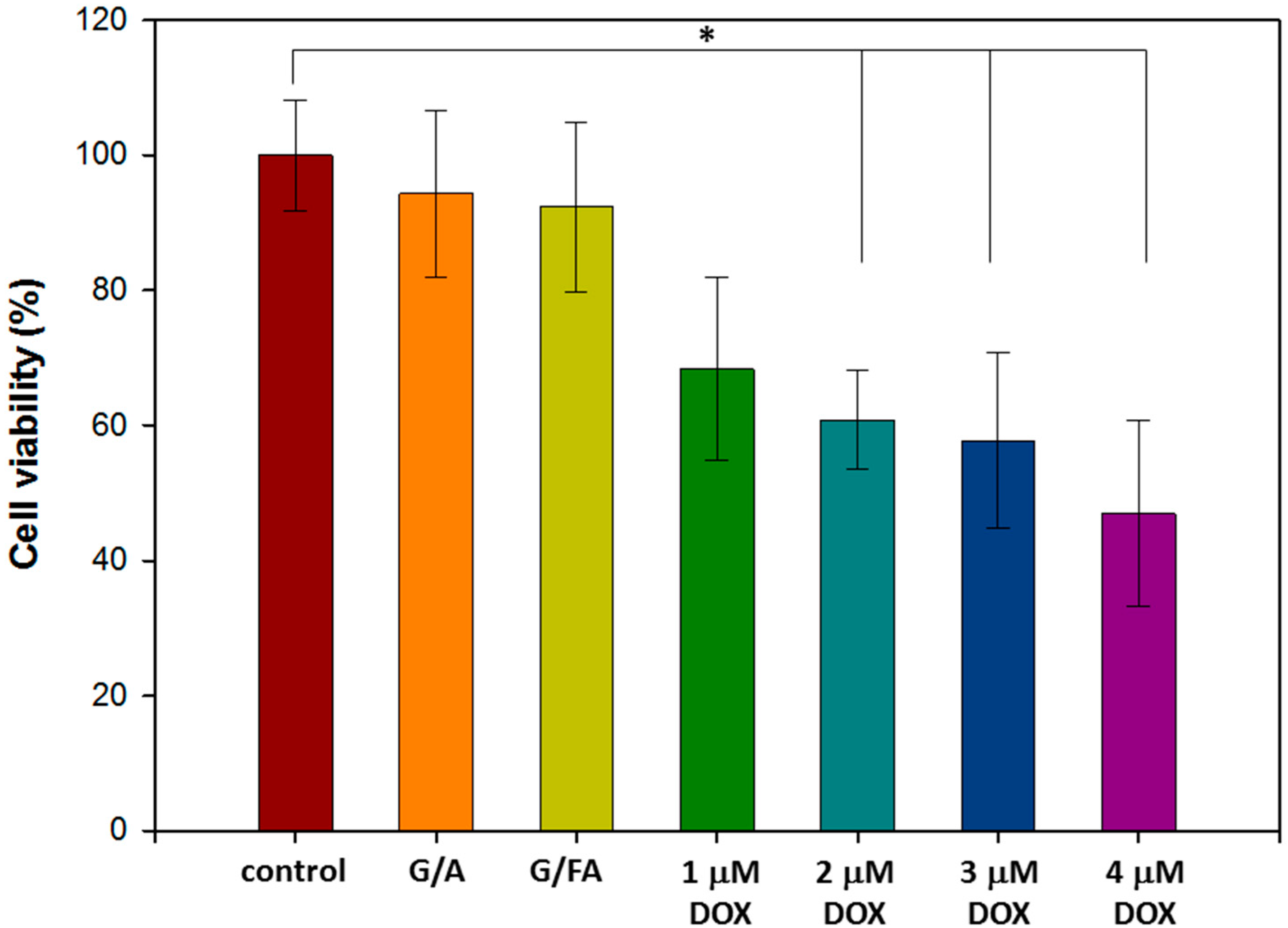
3.4. In Vitro Cytotoxicity Assay of Free DOX and NPs without DOX
3.4.1. In Vitro Biocompatibility Assay of NPs without DOX
3.4.2. Half-Maximal Inhibitory Concentration (IC50) of Free DOX
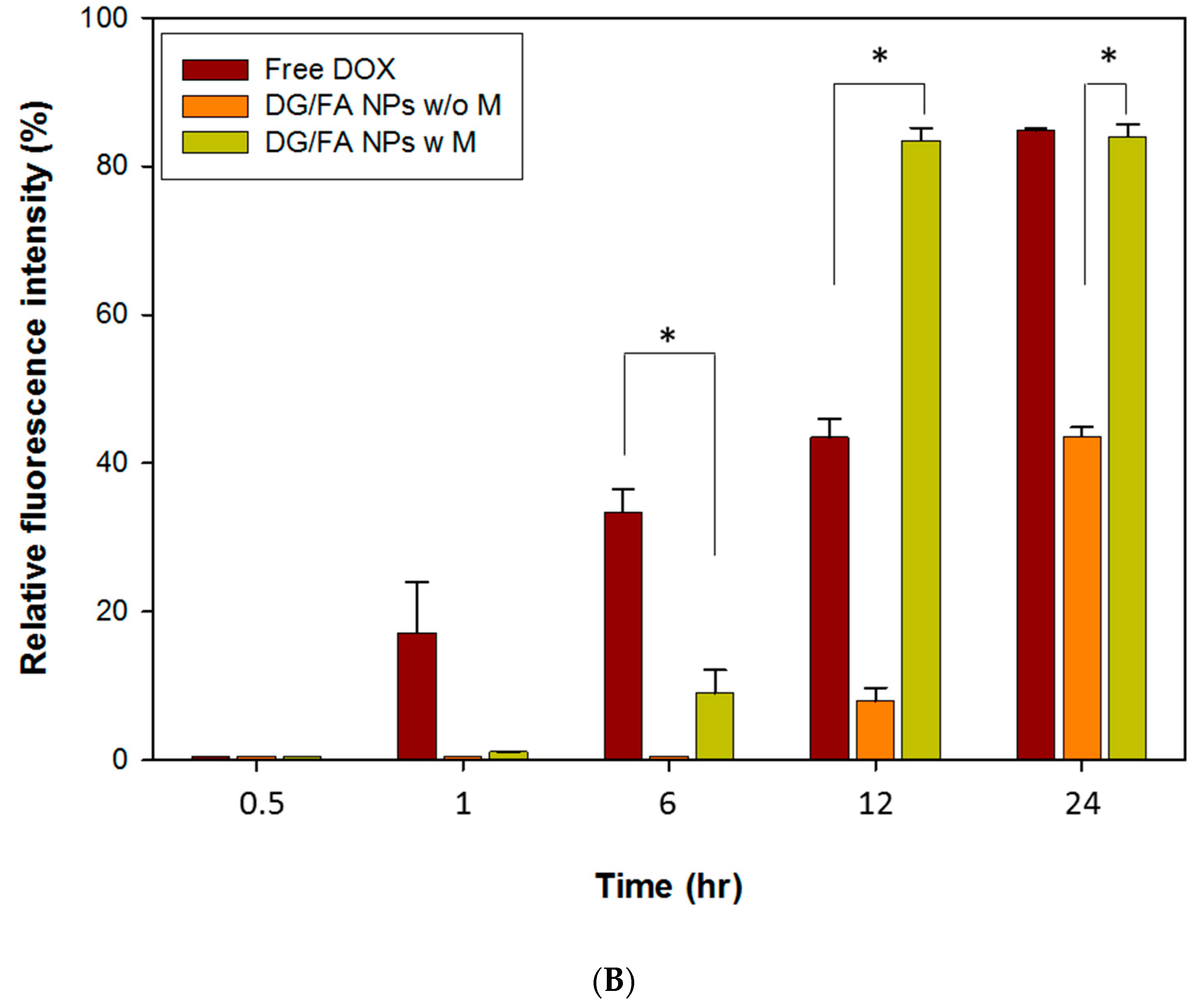
3.5. In Vitro Studies of DOX Drug Delivery and Accumulation
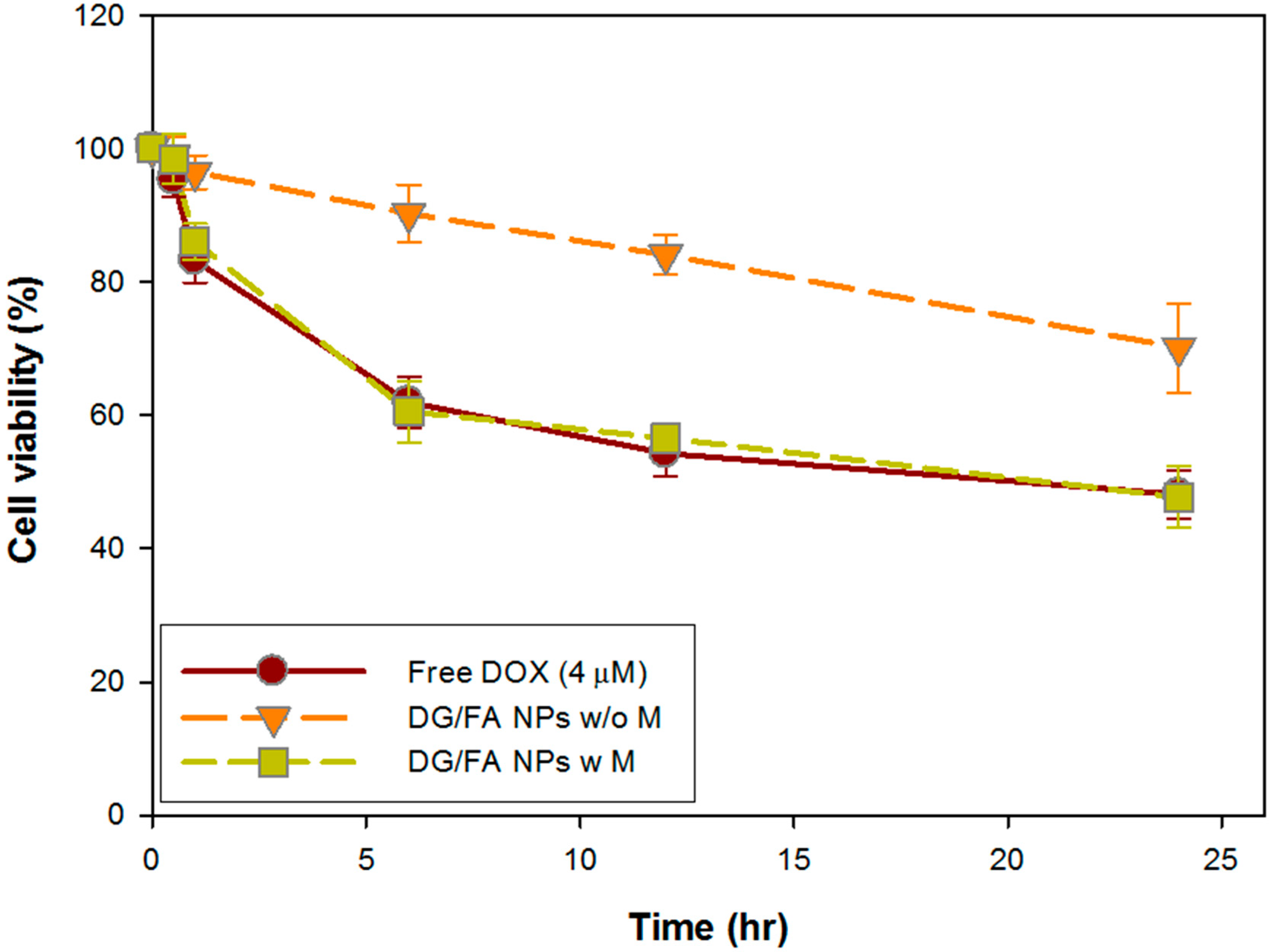
3.6. In Vitro Cytotoxicity Assay of DG/FA NPs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alexiou, C.; Tietze, R.; Schreiber, E.; Jurgons, R.; Richter, H.; Trahms, L.; Rahn, H.; Odenbach, S.; Lyer, S. Cancer therapy with drug loaded magnetic nanoparticles—Magnetic drug targeting. J. Magn. Magn. Mater. 2011, 323, 1404–1407. [Google Scholar] [CrossRef]
- Chomoucka, J.; Drbohlavova, J.; Huska, D.; Adam, V.; Kizek, R.; Hubalek, J. Magnetic nanoparticles and targeted drug delivering. Pharmacol. Res. 2010, 62, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, S. Magnetic nanoparticles in cancer diagnosis, drug Deliv. and treatment. Mol. Clin. Oncol. 2017, 7, 738–746. [Google Scholar] [PubMed]
- Amiri, S.; Shokrollahi, H. The role of cobalt ferrite magnetic nanoparticles in medical science. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.R.; Gomes, I.T.; Almeida, B.G.; Araujo, J.P.; Castanheira, E.M.; Coutinho, P.J. Magnetic liposomes based on nickel ferrite nanoparticles for biomedical applications. Phys. Chem. Chem. Phys. PCCP 2015, 17, 18011–18021. [Google Scholar] [CrossRef]
- Lee, J.H.; Chen, K.J.; Noh, S.H.; Garcia, M.A.; Wang, H.; Lin, W.Y.; Jeong, H.; Kong, B.J.; Stout, D.B.; Cheon, J.; et al. On-demand drug release system for in vivo cancer treatment through self-assembled magnetic nanoparticles. Angew. Chem. 2013, 52, 4384–4388. [Google Scholar] [CrossRef]
- Xie, L.; Jiang, R.; Zhu, F.; Liu, H.; Ouyang, G. Application of functionalized magnetic nanoparticles in sample preparation. Anal. Bioanal. Chem. 2014, 406, 377–399. [Google Scholar] [CrossRef]
- Hu, Y.; Mignani, S.; Majoral, J.P.; Shen, M.; Shi, X. Construction of iron oxide nanoparticle-based hybrid platforms for tumor imaging and therapy. Chem. Soc. Rev. 2018, 47, 1874–1900. [Google Scholar] [CrossRef]
- Gupta, A.K.; Curtis, A.S. Lactoferrin and ceruloplasmin derivatized superparamagnetic iron oxide nanoparticles for targeting cell surface receptors. Biomaterials 2004, 25, 3029–3040. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release Off. J. Control. Release Soc. 2005, 109, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Leo, E.; Arletti, R.; Forni, F.; Cameroni, R. General and cardiac toxicity of doxorubicin-loaded gelatin nanoparticles. Farmaco 1997, 52, 385–388. [Google Scholar]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.; Sagis, L.M. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Lee, E.M.; Singh, D.; Singh, D.; Choi, S.M.; Zo, S.M.; Park, S.J.; Han, S.S. Novel alginate-gelatin hybrid nanoparticle for drug delivery and tissue engineering applications. J. Nanomater. 2014, 2014, 7. [Google Scholar] [CrossRef]
- Young, R.C.; Ozols, R.F.; Myers, C.E. The anthracycline antineoplastic drugs. N. Engl. J. Med. 1981, 305, 139–153. [Google Scholar] [CrossRef]
- Seib, F.P.; Pritchard, E.M.; Kaplan, D.L. Self-assembling doxorubicin silk hydrogels for the focal treatment of primary breast cancer. Adv. Funct. Mater. 2013, 23, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, P.; Luo, L.; Cheng, H.; Li, Y.; Du, T.; Zou, B.; Gou, M. Efficient intravesical therapy of bladder cancer with cationic doxorubicin nanoassemblies. Int. J. Nanomed. 2016, 11, 4535–4544. [Google Scholar]
- Maeng, J.H.; Lee, D.H.; Jung, K.H.; Bae, Y.H.; Park, I.S.; Jeong, S.; Jeon, Y.S.; Shim, C.K.; Kim, W.; Kim, J.; et al. Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials 2010, 31, 4995–5006. [Google Scholar] [CrossRef] [PubMed]
- Escherich, G.; Zimmermann, M.; Janka-Schaub, G.; CoALL study group. Doxorubicin or daunorubicin given upfront in a therapeutic window are equally effective in children with newly diagnosed acute lymphoblastic leukemia. A randomized comparison in trial coall 07-03. Pediatr. Blood Cancer 2013, 60, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci. Rep. 2017, 7, 44735. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Abdalla, A.M.E.; Xiao, L.; Ullah, M.W.; Yu, M.; Ouyang, C.; Yang, G. Current challenges of cancer anti-angiogenic therapy and the promise of nanotherapeutics. Theranostics 2018, 8, 533–548. [Google Scholar] [CrossRef]
- Stylianopoulos, T. Epr-effect: Utilizing size-dependent nanoparticle delivery to solid tumors. Ther. Deliv. 2013, 4, 421–423. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Singh, D.; Zo, S.M.; Kumar, A.; Han, S.S. Engineering three-dimensional macroporous hydroxyethyl methacrylate-alginate-gelatin cryogel for growth and proliferation of lung epithelial cells. J. Biomater. Sci. Polym. Ed. 2013, 24, 1343–1359. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.; Sahoo, R.K.; Biswas, N.; Guha, A.; Kuotsu, K. Recent advancement of gelatin nanoparticles in drug and vaccine delivery. Int. J. Biol. Macromol. 2015, 81, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, I.; Pernal, S.; Duproz, A.; Bentley, J.; Engelhard, H.; Linninger, A. Magnetic field-enhanced cellular uptake of doxorubicin loaded magnetic nanoparticles for tumor treatment. Mater. Res. Express 2016, 3, 095010. [Google Scholar] [CrossRef]
- Mindell, J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gawlinski, E.T.; Gmitro, A.F.; Kaylor, B.; Gillies, R.J. Acid-mediated tumor invasion: A multidisciplinary study. Cancer Res. 2006, 66, 5216–5223. [Google Scholar] [CrossRef]
- Popescu, R.C.; Savu, D.; Dorobantu, I.; Vasile, B.S.; Hosser, H.; Boldeiu, A.; Temelie, M.; Straticiuc, M.; Iancu, D.A.; Andronescu, E.; et al. Efficient uptake and retention of iron oxide-based nanoparticles in hela cells leads to an effective intracellular delivery of doxorubicin. Sci. Rep. 2020, 10, 10530. [Google Scholar] [CrossRef]
- Burrow, S.M.; Phoenix, D.A.; Wainwright, M.; Tobin, M.J. Intracellular localization studies of doxorubicin and victoria blue bo in emt6-s and emt6-r cells using confocal microscopy. Cytotechnology 2002, 39, 15–25. [Google Scholar] [CrossRef] [PubMed]




| Particle Size (nm) | Zeta Potentials (mV) | Encapsulation Efficiency (%) | |
|---|---|---|---|
| G NPs | 318.5 ± 4.4 | 3.5 ± 0.7 | - |
| A NPs | 366.6 ± 4.7 | −8.5 ± 0.6 | - |
| G/A NPs | 325.0 ± 5.0 | −2.4 ± 0.5 | - |
| DG/A NPs | 370.5 ± 2.8 | −1.5 ± 0.4 | 71.5 ± 0.4 |
| DG/FA NPs | 401.8 ± 3.6 | −0.9 ± 0.8 | 64.6 ± 11.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Chuang, T.-J.; Ke, C.-J.; Yao, C.-H. Doxorubicin–Gelatin/Fe3O4–Alginate Dual-Layer Magnetic Nanoparticles as Targeted Anticancer Drug Delivery Vehicles. Polymers 2020, 12, 1747. https://doi.org/10.3390/polym12081747
Huang C-H, Chuang T-J, Ke C-J, Yao C-H. Doxorubicin–Gelatin/Fe3O4–Alginate Dual-Layer Magnetic Nanoparticles as Targeted Anticancer Drug Delivery Vehicles. Polymers. 2020; 12(8):1747. https://doi.org/10.3390/polym12081747
Chicago/Turabian StyleHuang, Chiung-Hua, Ting-Ju Chuang, Cherng-Jyh Ke, and Chun-Hsu Yao. 2020. "Doxorubicin–Gelatin/Fe3O4–Alginate Dual-Layer Magnetic Nanoparticles as Targeted Anticancer Drug Delivery Vehicles" Polymers 12, no. 8: 1747. https://doi.org/10.3390/polym12081747
APA StyleHuang, C.-H., Chuang, T.-J., Ke, C.-J., & Yao, C.-H. (2020). Doxorubicin–Gelatin/Fe3O4–Alginate Dual-Layer Magnetic Nanoparticles as Targeted Anticancer Drug Delivery Vehicles. Polymers, 12(8), 1747. https://doi.org/10.3390/polym12081747