Blowing Kinetics, Pressure Resistance, Thermal Stability, and Relaxation of the Amorphous Phase of the PET Container in the SBM Process with Hot and Cold Mold. Part I: Research Methodology and Results
Abstract
1. Introduction
- free blow of the preform (without blow mold)—enables observations of changes of the shape of the blown bottle during the time of blowing, but only for a limited range of blow pressure (due to cracking of the blown preform) and without capturing the effect of “back pressure” (that is, the air between the blown bottle and the wall of the blow mold) [8,9],
- free blowing of preforms with simultaneous stretching—enables observations of changes of the shape of the blown bottle during the time of blowing with the inclusion of stretching with a stretching rod [8],
- blowing in a transparent blow mold—enables observations of changes of the shape of the blown bottle during the time of blowing, but only for simple bottle shapes, because possible significant curvatures of the transparent mold strengthen the visual distortions [10],
- define a methodology for determining the number of measurement repetitions so that the measurement error is within acceptable limits relative to the actual value of the measured characteristic,
- define a methodology for calculating the power of ANOVA tests,
- define a methodology for determining the blow kinetics in aluminum blow molds—shear phenomena along the wall thickness of the blown bottle have been noticed, and it has been deduced that the air temperature between the blow mold and the wall of the blown bottle has an impact on the kinetics of blowing the bottle,
- define a methodology for determining the relaxation of the amorphous phase—the occurrence of microcavitation phenomena has been deduced in the PET material of the blown bottle.
2. Purpose of Research and Methodology of Experimental Research
- bottle thickness profile,
- thermal shrinkage of the bottle (as a macroscopic indicator of the bottle’s thermal stability),
- bottle burst pressure together with the place where the bottle starts to crack (as a macroscopic indicator of the bottle’s pressure resistance),
- blowing kinetics coefficients (as indicators of preform material displacement during the SBM process),
- degree of relaxation of the amorphous phase (as a microscopic indicator of relaxation of the oriented amorphous phase).
- D—General power of heating lamps in the preform heating furnace: 65%,
- E—General power of heating heaters in a hot blow mold for hot mold: 60%, or water cooling temperature blow mold for cold mold: 10 °C,
- F—Time of annealing the bottle in a hot blow mold, or the time of bottle staying in a cold blow mold: 1.5 s,
- G—Time from opening a blow mold to starting filling process: 120 s,
- H—Water filling temperature: 86 °C,
- I—Annealing time with hot water: 30 s,
- Hot water heating method: Lack (A series), free (B series), bath (C series).
| Features | Dependent Variables | |||||
|---|---|---|---|---|---|---|
| Thickness Profile | Blow Kinetics Coefficients | Bottle Weight | Pressure Resistance | Degree of Crystallinity 1 | Density 1 | |
| Method | Measurement of bottle thickness at selected point (Figure 5) | Measurement of the dimensions of the measuring points shown in Figure 3, Figure 4, Figure 6 | Measuring the weight of an empty bottle and filled with maximum fill level water | Bottles burst test with water pressure | DSC analysis at a rate of 10 °C/min | Measurement in accordance with ASTM D 1505-85 norm |
| Measurement tool | Inductive sensor FH4 [29] | Electronic altimeter, an electronic caliper | Electronic scale | CMC KUHNKE ABT-3100-PET [30] | TA Inst Q20 microcalorimeter | Gradient column 2 |
| Meter type | digital meter | digital meter | digital meter | digital meter | digital meter | analog meter |
| Sample | bottle | bottle | bottle | bottle | 1 × 1 cm square sample cut out from base area IV-2 point (Figure 5) | 1 × 1 cm square sample cut out from base area IV-2 point (Figure 5) |
| Maximum measurement error | altimeter caliper | |||||
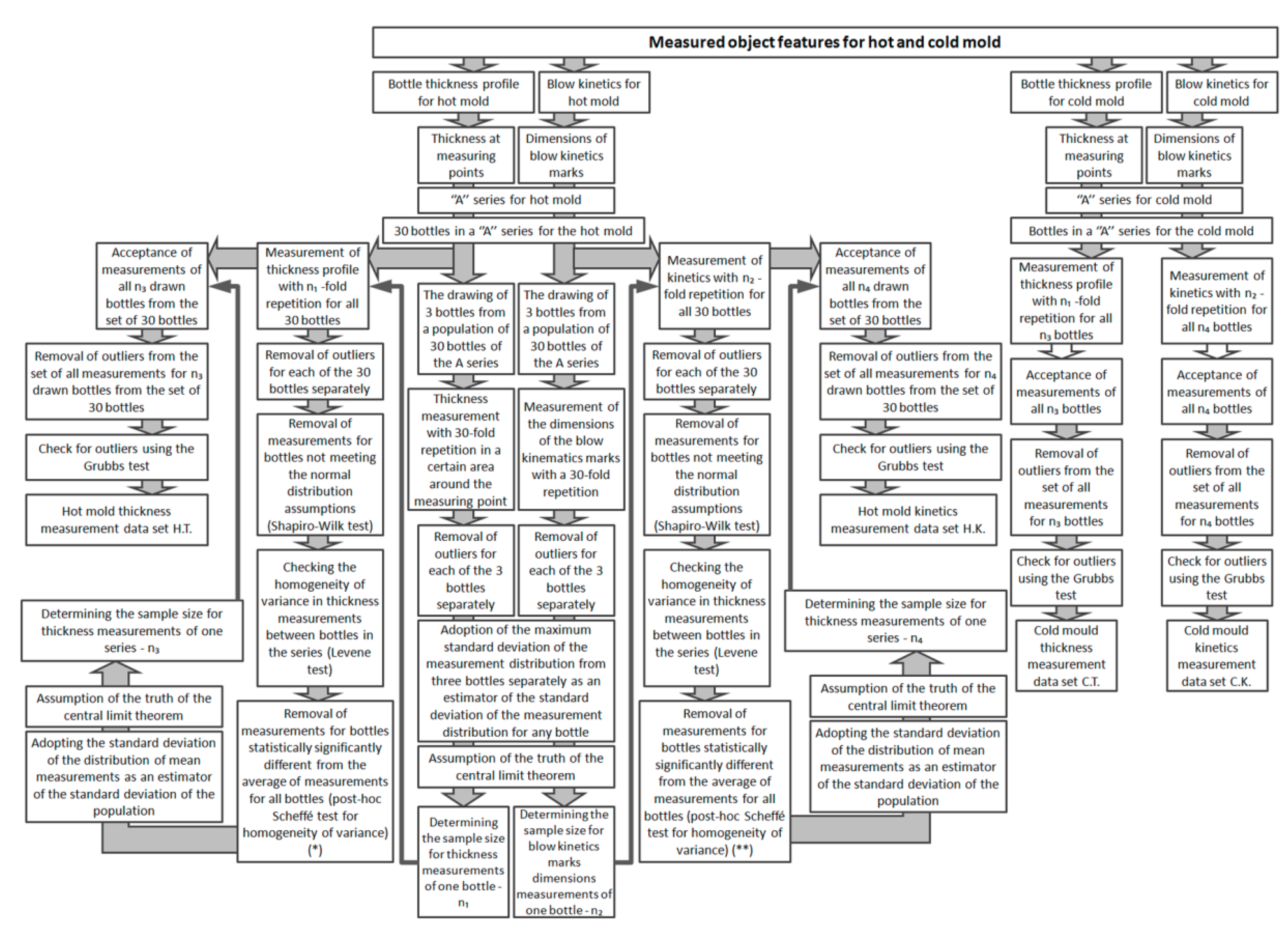
3. Preliminary Statistical Research
4. ANOVA Test Power Calculation
- the adopted level of the first type of error (the higher this is, the higher is the quality of the analysis, but also the likelihood of making the first type of error increases, as a result of which the certainty of rejecting the null hypothesis decreases);
- the variance of the distribution of measurements in the test sample, which is influenced by the number of measurement repetitions, error from the measuring instrument, error from the researcher (the higher the variance of the distribution, the lower the quality of the analysis);
- maximum difference between main effect means;
- number of factor levels in the model.
5. Plan of Experiments and Result
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Gomes, T.S.; Visconte, L.L.Y.; Pacheco, E.B.A.V. Life cycle assessment of polyethylene terephthalate packaging: An overview. J. Polym. Environ. 2019, 27, 533–548. [Google Scholar] [CrossRef]
- Kouloumpis, V.; Pell, R.S.; Correa-Cano, M.E.; Yan, X. Potential trade-offs between eliminating plastics and mitigating climate change: An LCA perspective on Polyethylene Terephthalate (PET) bottles in Cornwall. Sci. Total Environ. 2020, 727, 138681. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, P.; Datta, J. Characteristics of the blowing stages of poly (ethylene terephthalate) preforms in the blowing process with simultaneous stretching. Przem. Chem. 2015, 94, 1114–1118. [Google Scholar] [CrossRef]
- Wawrzyniak, P.; Datta, J. Stretch blow molding machines used for manufacturing PET bottles. Przem. Chem. 2015, 94, 1110–1113. [Google Scholar] [CrossRef]
- Wawrzyniak, P.; Karaszewski, W. A literature survey of the influence of preform reheating and stretch blow molding with hot mold process parameters on the properties of PET containers. Part I. Polimery 2020, 5, 346–356. [Google Scholar] [CrossRef]
- Wawrzyniak, P.; Karaszewski, W. A literature survey of the influence of preform reheating and stretch blow molding with hot mold process parameters on the properties of PET containers. Part II. Polimery 2020, 6, 437–448. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Agassant, J.F.; Bellet, M. Experimental study and numerical simulation of the injection stretch/blow molding process. Polym. Eng. Sci. 1998, 38, 1399–1412. [Google Scholar] [CrossRef]
- Menary, G.; Tan, C.; Armstrong, C.G.; Salomeia, Y.; Picard, M.; Billon, N.; Harkin-Jones, E. Validating injection stretch-blow molding simulation through free blow trials. Polym. Eng. Sci. 2010, 50, 1047–1057. [Google Scholar] [CrossRef]
- Luo, Y.; Chevalier, L.; Monteiro, E.; Yan, S.; Menary, G. Simulation of the injection stretch blow molding process: An anisotropic visco-hyperelastic model for Polyethylene Terephthalate behavior. Polym. Eng. Sci. 2020, 60, 823–831. [Google Scholar] [CrossRef]
- Huang, H.X.; Yin, Z.S.; Liu, J.H. Visualization study and analysis on preform growth in polyethylene terephthalate stretch blow molding. J. Appl. Polym. Sci. 2007, 103, 564–573. [Google Scholar] [CrossRef]
- Salomeia, Y.M.; Menary, G.H.; Armstrong, C.G. Experimental investigation of stretch blow molding, Part 2: Analysis of process variables, blowing kinematics, and bottle properties. Adv. Polym. Technol. 2013, 32, E436–E450. [Google Scholar] [CrossRef]
- Salomeia, Y.M.; Menary, G.H.; Armstrong, C.G. Experimental investigation of stretch blow molding, Part 1: Instrumentation in an industrial environment. Adv. Polym. Technol. 2013, 32, E771–E783. [Google Scholar] [CrossRef]
- Firas, A.; Dumitru, P. Statistical models for optimisation of properties of bottles produced using blends of reactive extruded recycled PET and virgin PET. Eur. Polym. J. 2005, 41, 2097–2106. [Google Scholar] [CrossRef]
- Ghada, Z.; Tamer, S. Concentrations of several phthalates contaminants in Egyptian bottled water: Effects of storage conditions and estimate of human exposure. Sci. Total Environ. 2018, 618, 142–150. [Google Scholar] [CrossRef]
- Reimann, C.; Birke, M.; Filzmoser, P. Bottled drinking water: Water contamination from bottle materials (glass, hard PET, soft PET), the influence of colour and acidification. Appl. Geochem. 2010, 25, 1030–1046. [Google Scholar] [CrossRef]
- Dombre, C.; Rigou, P.; Wirth, J.; Chalier, P. Aromatic evolution of wine packed in virgin and recycled PET bottlers. Food Chem. 2015, 176, 376–387. [Google Scholar] [CrossRef]
- Piqueras-Fiszman, B.; Spence, C. The weight of the bottle as a possible extrinsic cue with which to estimate the price (and quality) of the wine? Observed correlations. Food Qual. Prefer. 2012, 25, 41–45. [Google Scholar] [CrossRef]
- Rizzo, V.; Torri, L.; Licciardello, F.; Piergiovanni, L.; Muratore, G. Quality changes of extra virgin olive oil packaged in coloured Polyethylene Terephthalate bottles stored under different lighting conditions. Packag. Technol. Sci. 2014, 27, 437–448. [Google Scholar] [CrossRef]
- Chapa-Martínez, C.; Hinojosa-Reyes, L.; Hernandez-Ramirez, A.; Ruiz-Ruiz, E.; Maya-Treviño, M.D.L.; Guzmán-Mar, J.L. An evaluation of the migration of antimony from polyethylene terephthalate (PET) plastic used for bottled drinking water. Sci. Total Environ. 2016, 565, 511–518. [Google Scholar] [CrossRef]
- Greifenstein, M.; White, D.W.; Stubner, A.; Hout, J.; Whelton, A. Impact of temperature and storage duration on the chemical and odor quality of military packaged water in polyethylene terephthalate bottles. Sci. Total Environ. 2013, 456–457, 376–383. [Google Scholar] [CrossRef]
- Badía, J.D.; Strömberg, E.; Ribes-Greus, A.; Karlsson, S. A statistical design of experiments for optimizing the MALDI-TOF-MS sample preparation of polymers. An application in the assessment of the thermo-mechanical degradation mechanisms of poly (ethylene terephthalate). Anal. Chim. Acta 2011, 692, 85–95. [Google Scholar] [CrossRef]
- Demirel, B.; Daver, F. The effects on the properties of PET bottles of changes to bottle-base geometry. J. Appl. Polym. Sci. 2009, 114, 3811–3818. [Google Scholar] [CrossRef]
- Demirel, B. Optimisation of mould surface temperature and bottle residence time in mould for the carbonated soft drink PET containers. Polym. Test. 2017, 60, 220–228. [Google Scholar] [CrossRef]
- Luo, Y.-M.; Chevalier, L.; Monteiro, E.; Utheza, F. Numerical simulation of self heating during stretch blow moulding of PET: Viscohyperelastic modelling versus experimental results. Int. J. Mater. 2020. [Google Scholar] [CrossRef]
- Blueline Hitech 8 Cavities—Modern Stretch Blow Molding Machine. Available online: https://tes.com.pl/news/blueline-hitech-8-cavities-modern-stretch-blow-molding-machine/ (accessed on 24 May 2020).
- Kuśmierek, Z.; Kalus-Jęcek, B. Wzorce Wielkości Elektrycznych i Ocena Niepewności Pomiaru; Wydawnictwo Politechniki Łódzkiej: Łódz, Poland, 2006. [Google Scholar]
- Allegra, G.; Corradini, P.; Elias, H.-G.; Geil, P.H.; Keith, H.D.; Wunderlich, B. Definitions of terms relating to crystalline polymers (Recommendations 1988). Pure Appl. Chem. 1988, 61, 769–785. [Google Scholar] [CrossRef]
- Płonka, S. Multi-Criteria Optimization of Machine Manufacturing Processes; Wydawnictwo Naukowo Techniczne: Warsaw, Poland, 2010. [Google Scholar]
- Elektrophysik FH4-1MM (80-174-0300) Magnetic Wall Thickness Probe (0–6 mm/0.236”) for Use with FH7200 or FH7400 Gauge Body. Available online: http://jayamakmurteknik.com/elektrophysik-80-174-0300 (accessed on 24 May 2020).
- ABT-3100 PET Bottle Burst Pressure. Available online: https://www.cmc-kuhnke.com/page/products-18/product/pet-bottle-burst-tester-47.html (accessed on 24 May 2020).
- Israel, G.D. Determining Sample Size. Fact Sheet PEOD-6. 1992. Available online: https://www.tarleton.edu/academicassessment/documents/Samplesize.pdf (accessed on 24 May 2020).
- Pokarowski, P.; Prochenka, A. Statystyka II Wykłady; Uniwersytet Warszawski: Warszawa, Poland, 2012; Available online: http://mst.mimuw.edu.pl/lecture.php?lecture=st2&part=Ch8 (accessed on 24 May 2020).
- Stata Software. Available online: https://www.stata.com/manuals/pss-2powertwoway.pdf (accessed on 24 May 2020).
- Rozdział 26. Regresja Liniowa. Available online: https://algolytics.com/download/AdvancedMiner_documentation/userdoc/bk10pt02ch26s02.html (accessed on 24 May 2020).
- Bahçecitapar, M.K.; Karadağ, Ö.; Aktaş, S. Estimation of sample size and power for general full factorial designs. J. Stat. Actuar. Sci. 2016, 2, 79–86. [Google Scholar]
- Excel Software. Available online: http://www.real-statistics.com/chi-square-and-f-distributions/noncentral-f-distribution/ (accessed on 24 May 2020).
- Lawton, E.L.; Ringwald, E.L. Physical constants of poly (oxyethylene–oxyterephthaloyl) (polyethylene terephthalate). In Polymer Handbook, 3rd ed.; Brandrup, J., Immergut, E.H., Eds.; John Wiley & Sons: Hoboken, NY, USA; Chichester, UK; Brisbane, Australia; Toronto, ON, Canada; Singapore, 1989; pp. V101–V106. [Google Scholar] [CrossRef]






| Designation | Explanation |
|---|---|
| I | Preform heating process and SBM process. |
| II | Hot filling process—carried out at a specially designed stand (Figure 2). |
| 1 | Delivery of the bottle on the waiting table (marked as 1 in Figure 2)—time from opening blow molds to starting the filling of hot water120 s; 90 manufactured bottles are numbered consecutively with a permanent marker, divided into three measuring series A, B, and C of 30 bottles each and put on the appropriate waiting table, with the first bottle for the A series, the second bottle for the B series, the third bottle for the C series, the fourth bottle for the A series, the fifth bottle for the B series, the sixth bottle for the C series, the seventh bottle for the A series, etc. |
| 2 | Rapid cooling of the bottle by spraying with cold water at 15 °C for 60 s (marked as 2 in Figure 2). |
| 3 | The maximum volume of a bottle measured by the gravimetric method (in accordance with PN-O-79782: 1996). |
| 4 | Taking pictures of the bottle. |
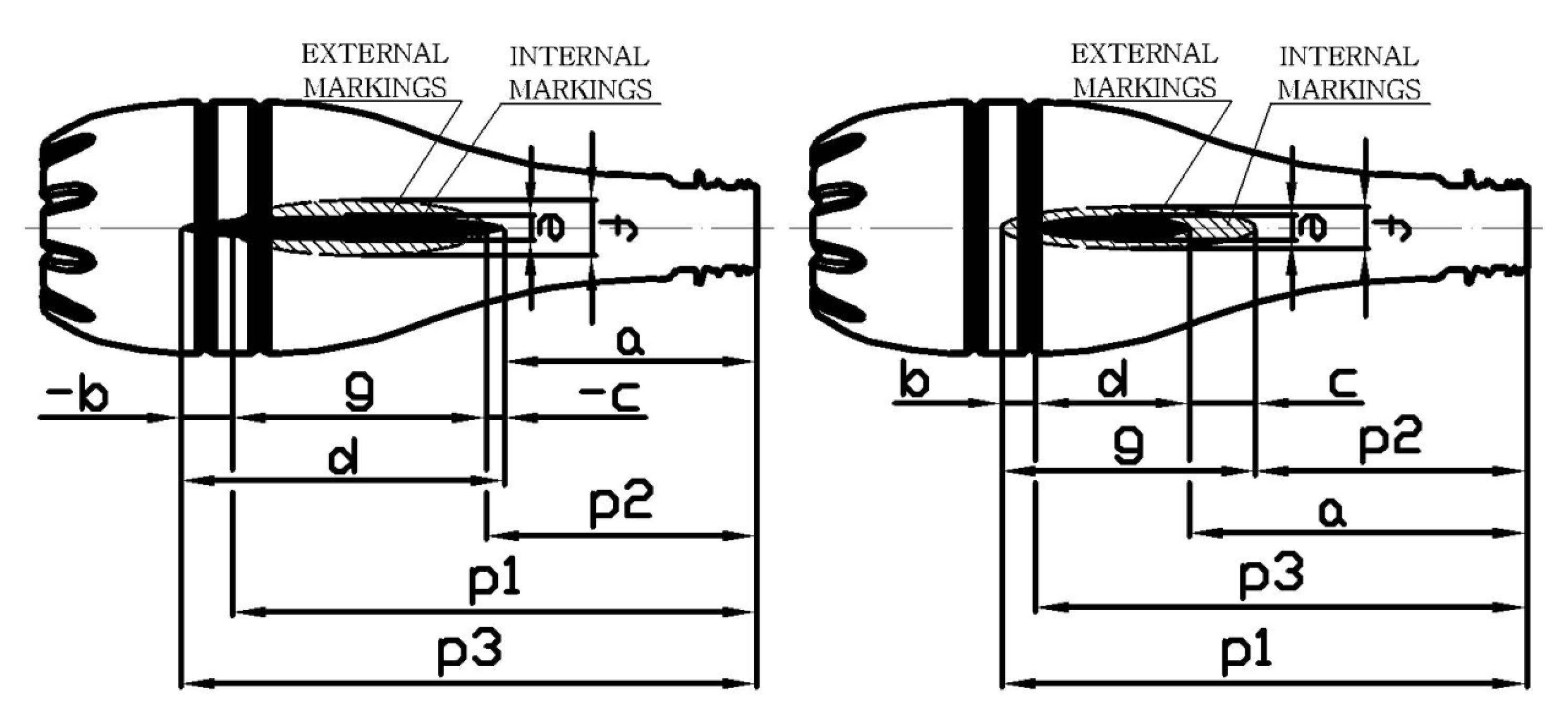
| 5 | Measurement of the position and shape of external and internal bottle markings applied to the preform-blow kinetics parameters (explained in Figure 6): a, p1, p2, p3, e, f. |
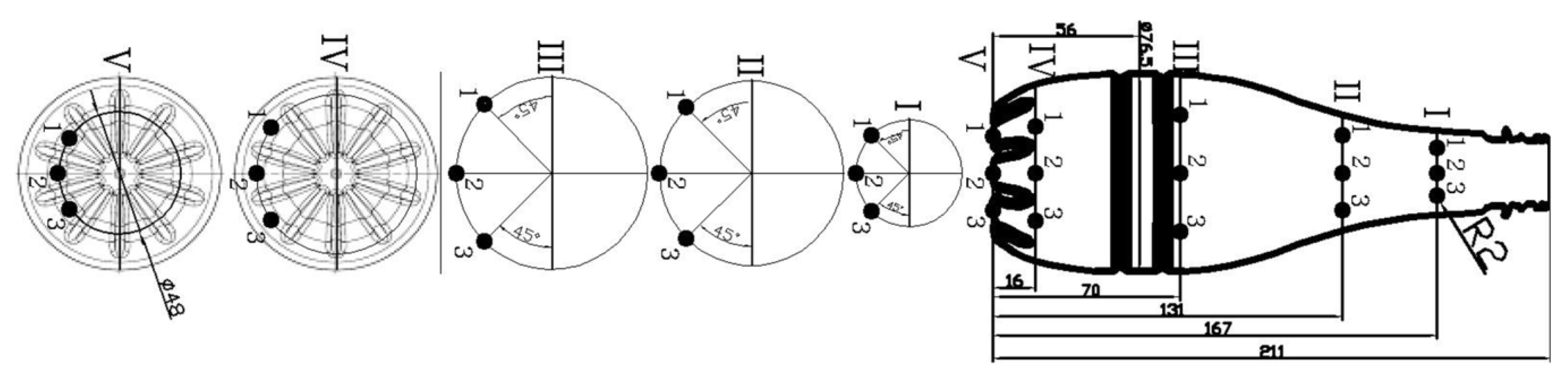
| 6 | Measurement of the bottle thickness profile by the FH4 inductive sensor (according to points arranged as in Figure 5). |
| 7 | Measurement of the bottle pressure resistance-using the CMC KUHNKE ABT-3100-PET pressure strength testing machine. |
| 8 | Cutting the sample out of bottles at the IV-2 thickness measurement point (base part of the bottle shown in Figure 5) for measuring the amount of oriented amorphous phase in accordance with formula (A36). The dimensions of the cut sample should be approximately 1 cm × 1 cm. |
| 9 | Filling the bottles with hot water at a temperature of 86 °C to the nominal volume level; hot water was delivered from a large tank with 86 °C water previously prepared in the tank (marked as 9 in Figure 2). |
| 10 | Free annealing (B series)—the bottles were filled to the nominal volume with hot water on the free heating place (marked as 10 in Figure 2) for 30 s. |
| 11 | Bath annealing (C series)—the filled bottles were placed in the 86 °C hot water bath (marked as 11 in Figure 2), the water level in the bath tank was the same as the water level in the bottle (elimination of pressure from the water filled inside the bottle reducing the shrinkage of the bottle because of microstructure changes). |
| 12 | Annealing time in the bath with hot water: 30 s. |
| 13 | Removing the bottles from the water bath. |
| 14 | Pouring out hot water from the bottles. |
| A | Bottles not annealed with hot water (A series). |
| B | Bottles annealed with hot water by free annealing (B series). |
| C | Bottles annealed with hot water by bath annealing (C series). |
| D | Technological parameters—general power of heating lamps in the preform heating oven. |
| E | Technological parameters—general power of electric heaters in blow mold. |
| F | Technological parameters—toughing time of the bottle surface in a hot blow mold. |
| G | Technological parameters—time from opening blow molds to start of filling process. |
| H | Technological parameters—temperature of hot water filled inside the bottle. |
| I | Technological parameters—annealing time of the bottle filled with hot water. |
| SBM Process Parameters | Heating Power of Individual Heating Lamps in the Heating Oven | ||
|---|---|---|---|
| Intrinsic viscosity of the raw material | 0.7 dL/g | 01: 55.0% 02: 18.0% 03: 12.0% 04: 13.0% 05: 21.5% 06: 0.0% 07: 0.0% 08: 0.0% 09: 0.0% General power of the oven: 65.0% | |
| Stretching rod speed | 1.2 m/s | ||
| Initial blow start delays relative to the position of the stretching rod | 55 mm | ||
| Pre-blow air pressure | 8.0 bars | ||
| Pre-blow time | 0.08 s | ||
| Main blow air pressure | 35 bar | ||
| Main blow time | 0.72 s | ||
| Post-mold bottles cooling air temperature | 19 °C | ||
| Post-mold bottles cooling air pressure | 2.5 bars | ||
| Hot mold temperature profile (medium values) | thread area | 21 °C | |
| label area | 125 °C | ||
| base area | 62 °C | ||
| Cold mold temperature | 10 °C | ||
| NoE | Main Factors | Interaction Factors | Mean Response | |
|---|---|---|---|---|
| A | B | A∗B | ||
| 1 | -1 | -1 | 1 | |
| 2 | -1 | 1 | -1 | |
| 3 | 1 | -1 | -1 | |
| 4 | 1 | 1 | 1 | |
| NoE | Factor—SBM Process (Blow Mold Temperature) | Responses for Bottles in “A” Series | |||||
|---|---|---|---|---|---|---|---|
| Thickness Profile | Blow Kinetics Coefficients | ||||||
| Point | Mean Value [mm] | Measurement Uncertainty [mm] | Coefficients | Mean Value | Measurement Uncertainty | ||
| 1 | -1 (cold mold) | I-1 | 0.23 | 0.03 | I-w.a | 1.46 | 0.03 |
| I-2 | 0.22 | 0.03 | I-w.b | −0.59 mm | 2.11 mm | ||
| I-3 | 0.25 | 0.07 | I-w.c | 0.26 mm | 1.23 mm | ||
| II-1 | 0.16 | 0.01 | I-w.d | 3.16 | 0.79 | ||
| II-2 | 0.16 | 0.01 | I-w.e | 1.46 | 0.45 | ||
| II-3 | 0.16 | 0.01 | I-w.f | 1.69 | 0.42 | ||
| III-1 | 0.17 | 0.01 | I-w.g | 3.03 | 0.59 | ||
| III-2 | 0.17 | 0.01 | II-w.a | 2.02 | 0.03 | ||
| III-3 | 0.17 | 0.01 | II-w.b | −0.56 mm | 0.84 mm | ||
| IV-1 | 0.19 | 0.01 | II-w.c | 0.61 mm | 0.69 mm | ||
| IV-2 | 0.19 | 0.01 | II-w.d | 3.17 | 0.17 | ||
| IV-3 | 0.19 | 0.01 | II-w.e | 1.81 | 0.33 | ||
| V-1 | 0.20 | 0.04 | II-w.f | 2.84 | 0.44 | ||
| V-2 | 0.21 | 0.04 | II-w.g | 3.19 | 0.20 | ||
| V-3 | 0.21 | 0.05 | III-w.a | 2.56 | 0.02 | ||
| - | - | - | III-w.b | 0.14 mm | 0.57 mm | ||
| - | - | - | III-w.c | 0.29 mm | 0.60 mm | ||
| - | - | - | III-w.d | 3.12 | 0.19 | ||
| - | - | - | III-w.e | 2.77 | 0.23 | ||
| - | - | - | III-w.f | 3.88 | 0.24 | ||
| - | - | - | III-w.g | 3.30 | 0.13 | ||
| - | - | - | IV-w.a | 2.78 | 0.02 | ||
| - | - | - | IV-w.b | 0.35 mm | 0.48 mm | ||
| - | - | - | IV-w.c | 0.19 mm | 0.33 mm | ||
| - | - | - | IV-w.d | 2.94 | 0.13 | ||
| - | - | - | IV-w.e | 2.69 | 0.21 | ||
| - | - | - | IV-w.f | 3.72 | 0.22 | ||
| - | - | - | IV-w.g | 3.15 | 0.24 | ||
| 1 (hot mold) | I-1 | 0.27 | 0.03 | I-w.a | 1.60 | 0.03 | |
| I-2 | 0.25 | 0.03 | I-w.b | 0.19 mm | 2.66 mm | ||
| I-3 | 0.25 | 0.03 | I-w.c | −0.55 mm | 1.99 mm | ||
| II-1 | 0.16 | 0.01 | I-w.d | 3.25 | 0.86 | ||
| II-2 | 0.16 | 0.01 | I-w.e | 1.48 | 0.36 | ||
| II-3 | 0.16 | 0.01 | I-w.f | 1.82 | 0.91 | ||
| III-1 | 0.18 | 0.01 | I-w.g | 3.11 | 1.01 | ||
| III-2 | 0.18 | 0.01 | II-w.a | 2.02 | 0.03 | ||
| III-3 | 0.18 | 0.01 | II-w.b | 0.16 mm | 1.05 mm | ||
| IV-1 | 0.20 | 0.01 | II-w.c | 0.07 mm | 0.98 mm | ||
| IV-2 | 0.19 | 0.01 | II-w.d | 4.00 | 0.53 | ||
| IV-3 | 0.19 | 0.01 | II-w.e | 2.04 | 0.27 | ||
| V-1 | 0.20 | 0.02 | II-w.f | 2.71 | 0.24 | ||
| V-2 | 0.19 | 0.02 | II-w.g | 4.09 | 0.34 | ||
| V-3 | 0.19 | 0.03 | III-w.a | 2.58 | 0.02 | ||
| - | - | - | III-w.b | −0.65 mm | 0.93 mm | ||
| - | - | - | III-w.c | −0.39 mm | 1.26 mm | ||
| - | - | - | III-w.d | 3.65 | 0.34 | ||
| - | - | - | III-w.e | 3.44 | 0.28 | ||
| - | - | - | III-w.f | 4.33 | 0.32 | ||
| - | - | - | III-w.g | 3.23 | 0.56 | ||
| - | - | - | IV-w.a | 2.76 | 0.02 | ||
| - | - | - | IV-w.b | −0.29 mm | 1.13 mm | ||
| - | - | - | IV-w.c | 0.44 mm | 0.88 mm | ||
| - | - | - | IV-w.d | 3.11 | 0.39 | ||
| - | - | - | IV-w.e | 3.06 | 0.31 | ||
| - | - | - | IV-w.f | 4.04 | 0.48 | ||
| - | - | - | IV-w.g | 3.17 | 0.42 | ||
| NoE | Factor—SBM Process with Hot Fill Process | Results for Bottle Material | |||||||
|---|---|---|---|---|---|---|---|---|---|
| First Value (-1) | Second Value (1) | Density | DSC Crystallite | Relaxation of Amorphous Phase | |||||
| SBM Process (Blow Mold Temperature) | Hot Fill Process | Mean [g/cm3] | Measurement Uncertainty [g/cm3] | Mean [%] | Measurement Uncertainty [%] | Mean [-] | Measurement Uncertainty [-] | ||
| 2 | preform | cold | A—lack | 1.3578 | 0.0016 | 27.6 | 4.0 | 1.008 | 0.009 |
| 3 | preform | cold | B—free | 1.3589 | 0.0017 | 29.7 | 4.4 | 1.010 | 0.010 |
| 4 | preform | cold | C—bath | 1.3562 | 0.0017 | 30.8 | 4.1 | 1.014 | 0.010 |
| 5 | preform | hot | A—lack | 1.3664 | 0.0014 | 27.3 | 6.0 | 0.999 | 0.012 |
| 6 | preform | hot | B—free | 1.3660 | 0.0030 | 29.8 | 4.5 | 1.003 | 0.011 |
| 7 | preform | hot | C—bath | 1.3662 | 0.0033 | 29.1 | 4.6 | 1.002 | 0.011 |
| Results for preform material | 1.3385 | 0.0006 | 3.5 | 4.8 | 1.000 | 0.007 | |||
| NoE | Factor—SBM Process (Blow Mold Temperature) | Factor—Hot Fill Process | ||
|---|---|---|---|---|
| 8 | -1 (cold mold) | 1 (hot mold) | A | |
| 9 | -1 (cold mold) | 1 (hot mold) | B | |
| 10 | -1 (cold mold) | 1 (hot mold) | C | |
| 11 | cold mold | -1 (A) | 1 (B) | |
| 12 | hot mold | -1 (A) | 1 (B) | |
| 13 | cold mold | -1 (A) | 1 (C) | |
| 14 | hot mold | -1 (A) | 1 (C) | |
| 15 | cold mold | -1 (B) | 1 (C) | |
| 16 | hot mold | -1 (B) | 1 (C) | |
| Hot Fill Combination | Factors | Response | ||||||
|---|---|---|---|---|---|---|---|---|
| Hot Fill | NoE | Mold Temperature | Hot Filling | Interaction Factor | Pressure Resistance | Shrinkage | ||
| Mean [bar] | Measurement Uncertainty [bar] | Mean [-] | Measurement Uncertainty [-] | |||||
| A-B | 17 | -1 (cold) | -1 (A) | 1 | 12.73 | 0.15 | - | - |
| 18 | -1 (cold) | 1 (B) | -1 | 12.64 | 0.28 | - | - | |
| 19 | 1 (hot) | -1 (A) | -1 | 9.93 | 0.29 | - | - | |
| 20 | 1 (hot) | 1 (B) | 1 | 10.13 | 0.33 | - | - | |
| A-C | 21 | -1 (cold) | -1 (A) | 1 | 12.73 | 0.15 | - | - |
| 22 | -1 (cold) | 1 (C) | -1 | 12.75 | 0.24 | - | - | |
| 23 | 1 (hot) | -1 (A) | -1 | 9.93 | 0.29 | - | - | |
| 24 | 1 (hot) | 1 (C) | 1 | 10.21 | 0.18 | - | - | |
| B-C | 25 | -1 (cold) | -1 (B) | 1 | 12.64 | 0.28 | 0.224 | 0.004 |
| 26 | -1 (cold) | 1 (C) | -1 | 12.75 | 0.24 | 0.286 | 0.009 | |
| 27 | 1 (hot) | -1 (B) | -1 | 10.13 | 0.33 | 0.114 | 0.016 | |
| 28 | 1 (hot) | 1 (C) | 1 | 10.21 | 0.18 | 0.158 | 0.022 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyniak, P.; Karaszewski, W. Blowing Kinetics, Pressure Resistance, Thermal Stability, and Relaxation of the Amorphous Phase of the PET Container in the SBM Process with Hot and Cold Mold. Part I: Research Methodology and Results. Polymers 2020, 12, 1749. https://doi.org/10.3390/polym12081749
Wawrzyniak P, Karaszewski W. Blowing Kinetics, Pressure Resistance, Thermal Stability, and Relaxation of the Amorphous Phase of the PET Container in the SBM Process with Hot and Cold Mold. Part I: Research Methodology and Results. Polymers. 2020; 12(8):1749. https://doi.org/10.3390/polym12081749
Chicago/Turabian StyleWawrzyniak, Paweł, and Waldemar Karaszewski. 2020. "Blowing Kinetics, Pressure Resistance, Thermal Stability, and Relaxation of the Amorphous Phase of the PET Container in the SBM Process with Hot and Cold Mold. Part I: Research Methodology and Results" Polymers 12, no. 8: 1749. https://doi.org/10.3390/polym12081749
APA StyleWawrzyniak, P., & Karaszewski, W. (2020). Blowing Kinetics, Pressure Resistance, Thermal Stability, and Relaxation of the Amorphous Phase of the PET Container in the SBM Process with Hot and Cold Mold. Part I: Research Methodology and Results. Polymers, 12(8), 1749. https://doi.org/10.3390/polym12081749





