Enhanced Functional Properties of Low-Density Polyethylene Nanocomposites Containing Hybrid Fillers of Multi-Walled Carbon Nanotubes and Nano Carbon Black
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization Methods
- -
- differential scanning calorimetry (DSC) measurements were carried out using a DSC 204 F1 Phoenix (Netzsch, Germany) instrument. Measurements were proceeded under heating-cooling-the heating procedure in a temperature range from −25 °C to 200 °C under a nitrogen atmosphere at a flow-rate of 50 mL/min. Both heating and cooling rates were 10 °C/min. The temperatures and enthalpies of crystallization and melting were determined from first cooling and second heating scans, respectively. The heat fusion has been estimated by the integration of the area under normalized melting peak. The degree of crystallinity of the sample (Xc) was calculated using the following equation:where ΔHm is the melting enthalpy determined by DSC and (=293 J/g [24]) is the enthalpy of melting of fully crystalline PE, and φn is a weight content of nanofiller.
- -
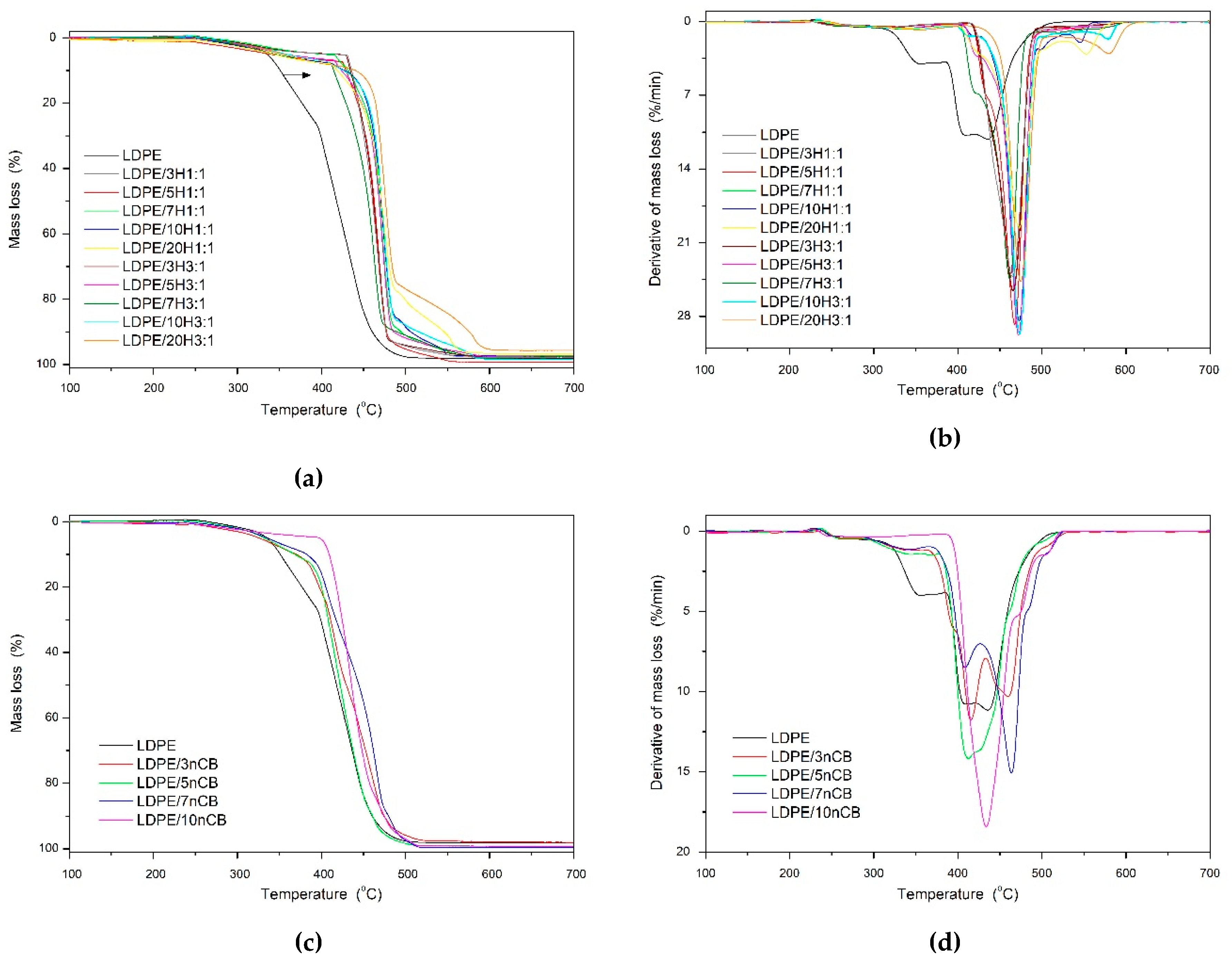
- thermo-oxidative stability of the pure LDPE and its nanocomposites was tested on the thermogravimetric analyzer (TGA 92-16.18 Setaram, Caluire-et-Cuire, France) in the temperature range from in the temperature range of 20–700 °C at the heating rate of 10 °C/min and under 20 L/min of synthetic air (N2:O2 = 80:20 vol. %).
- -
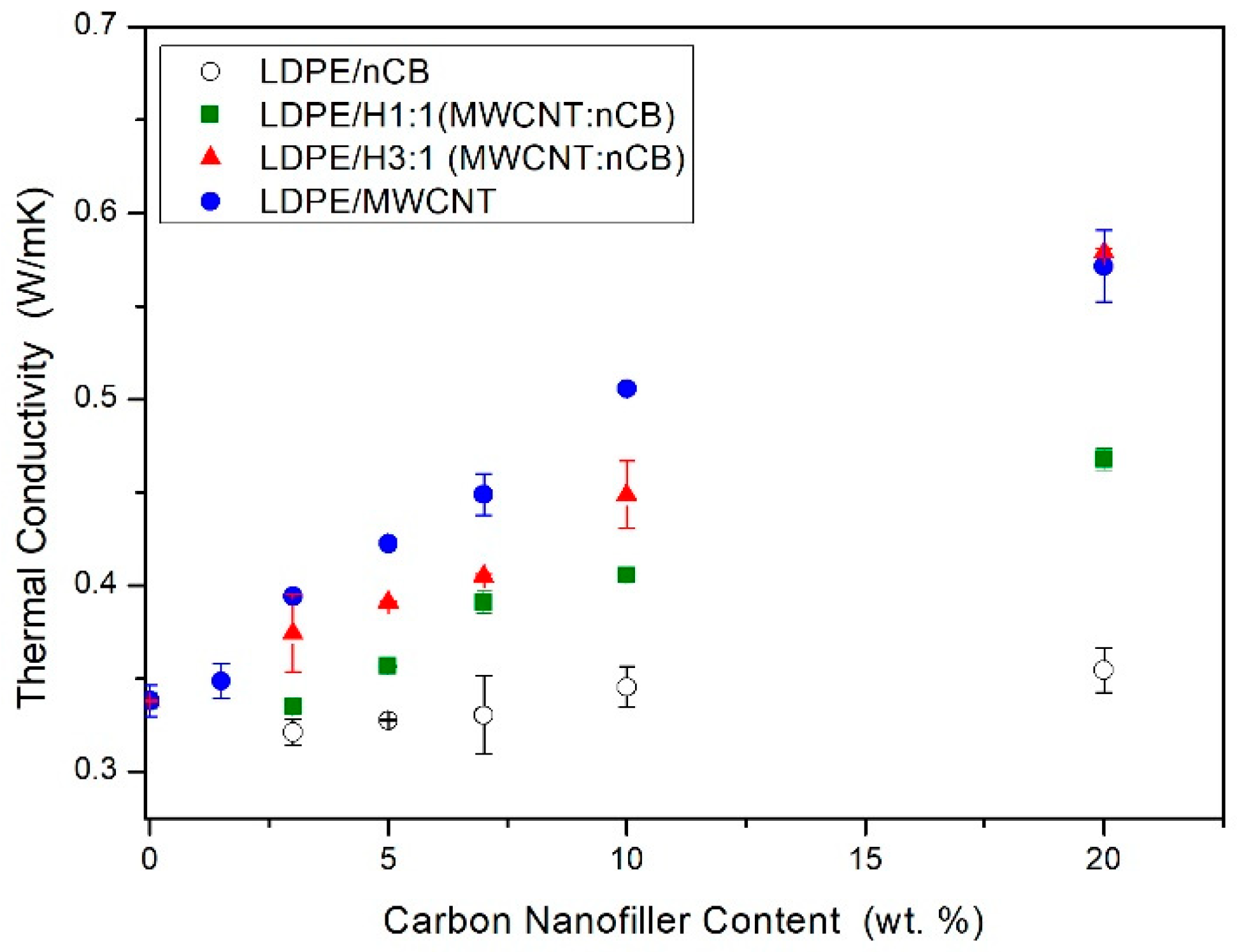
- the thermal conductivity of the neat LDPE and nanocomposites was determined using the transient plane source (TPS) technique (Hot Disk TPS 2500, Uppsala, Sweden), and the Hot Disk thermal constants analyzer. The measurements were performed according to ISO 22007-2. Three tests were conducted, and the mean values were reported for each sample.
3. Results and Discussion
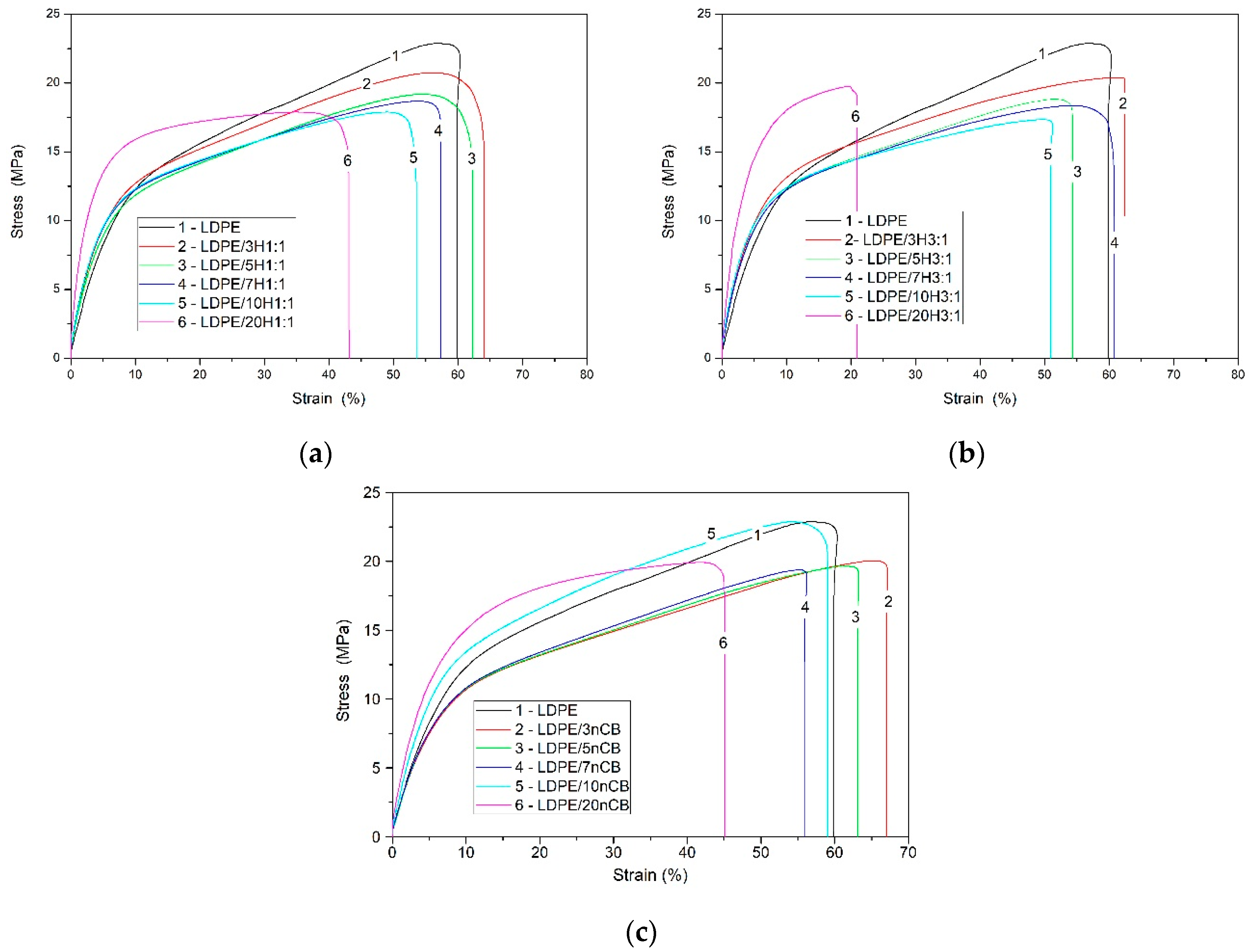
3.1. Physical Properties and Stress–Strain Behavior of the LDPE Nanocomposites
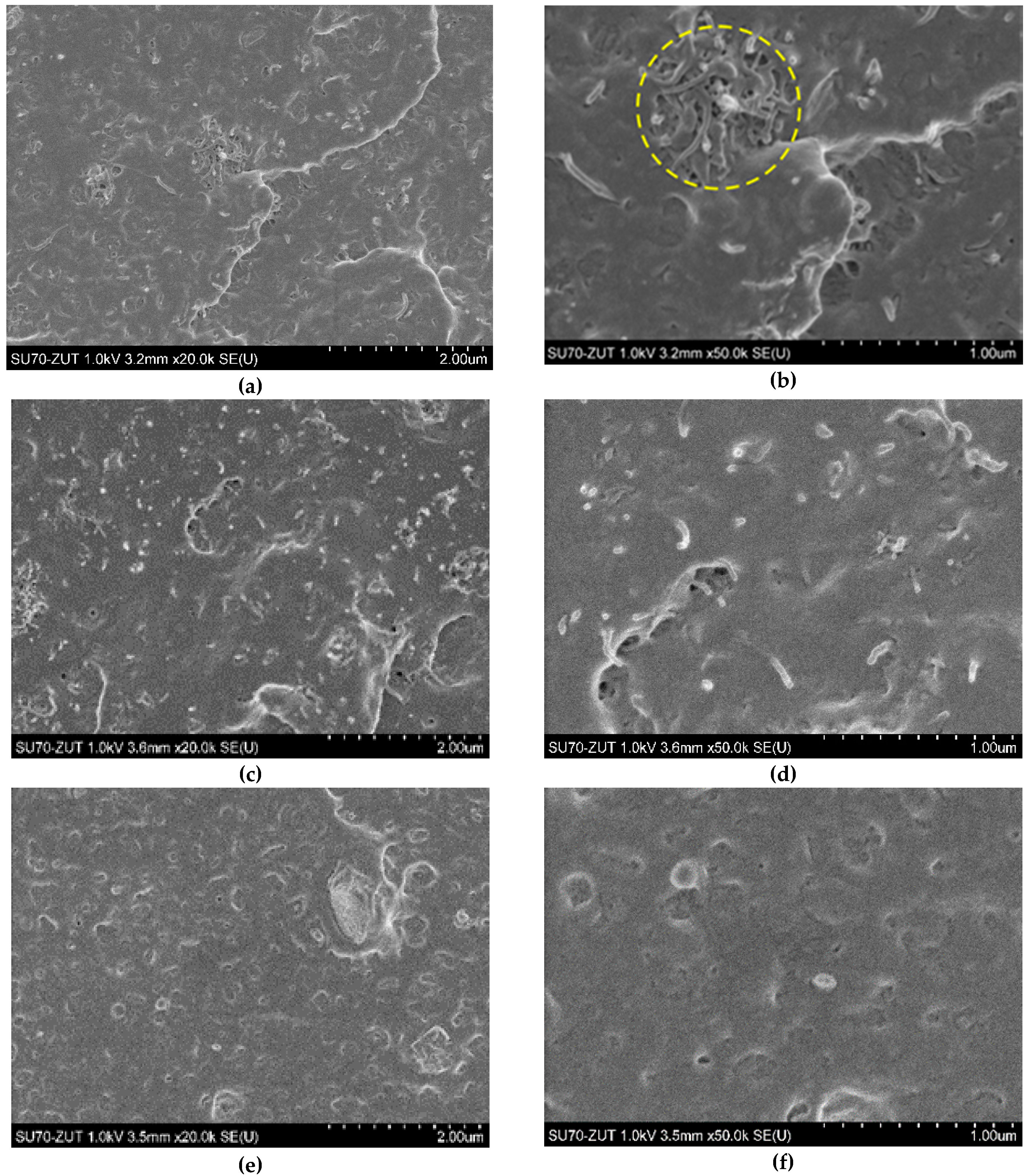
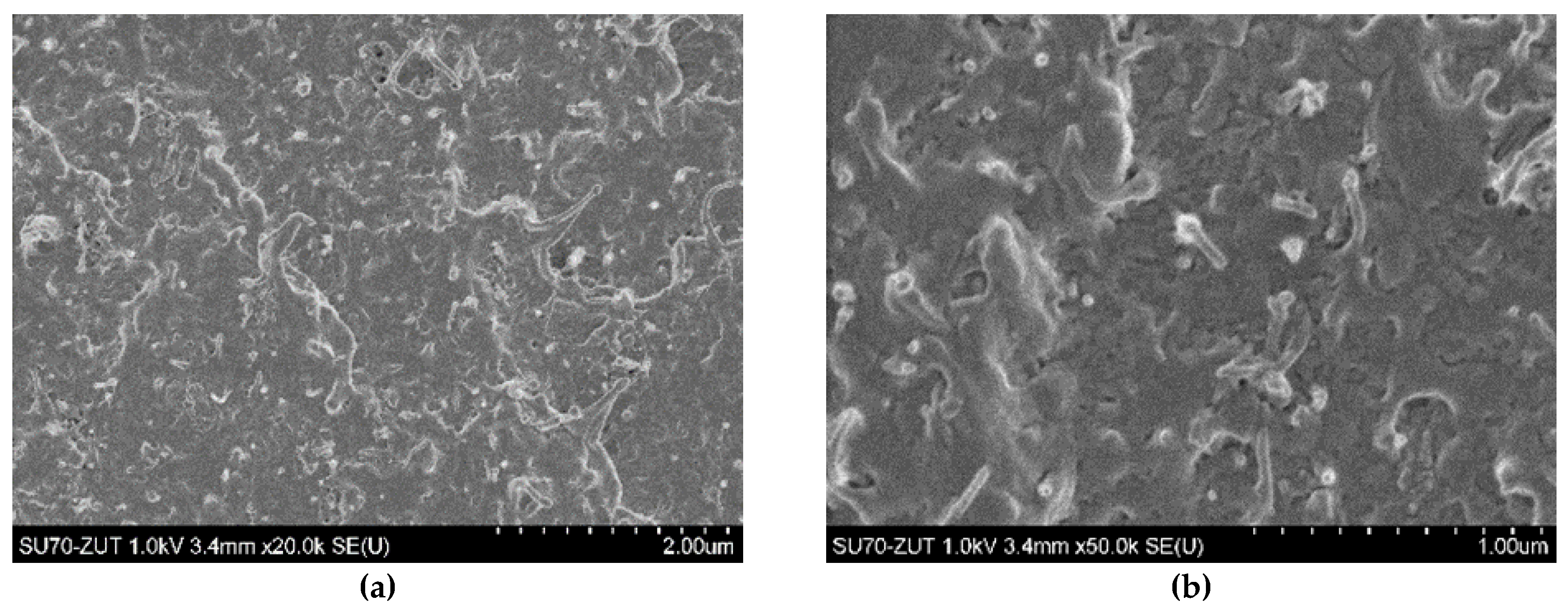
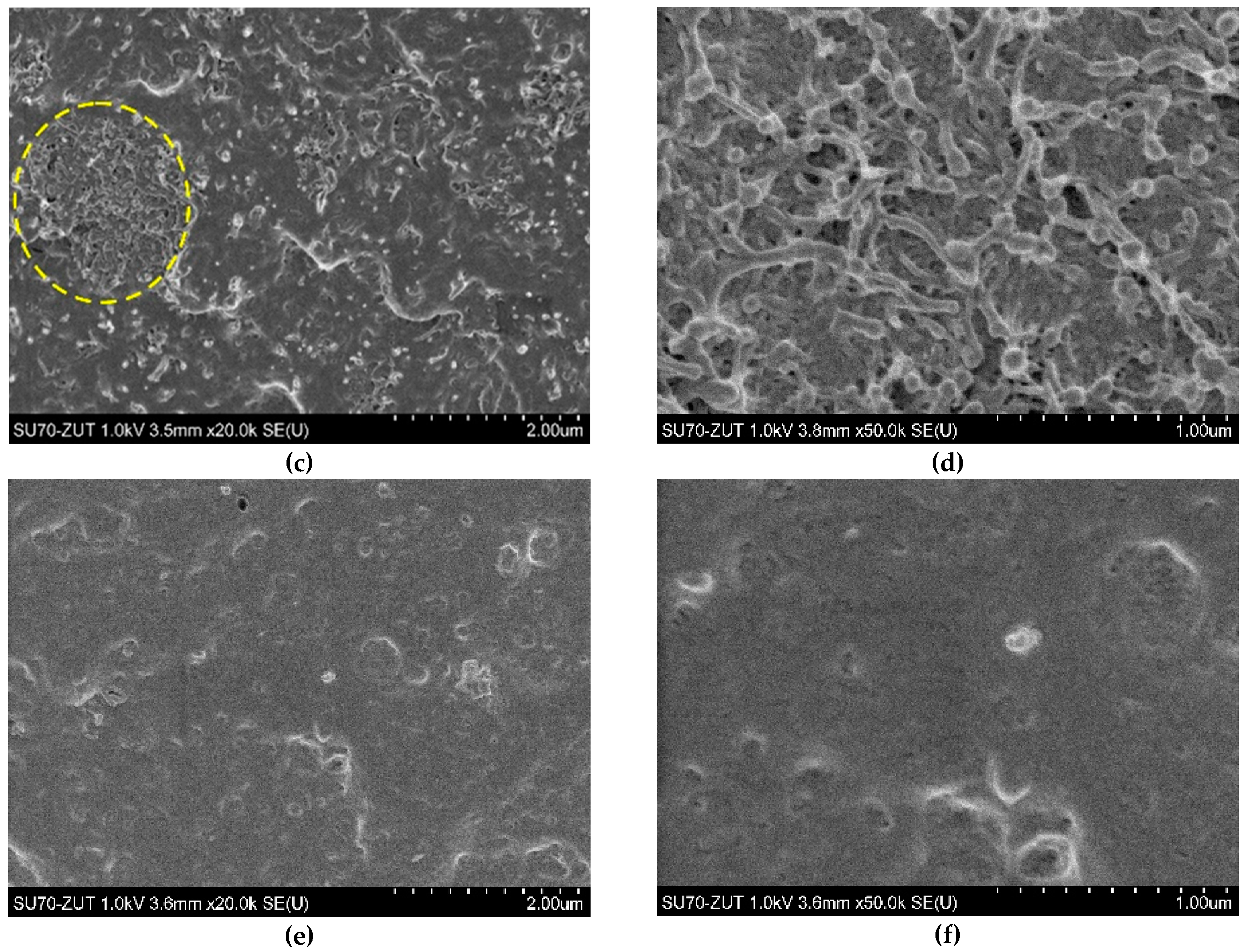
3.2. Morphological Study
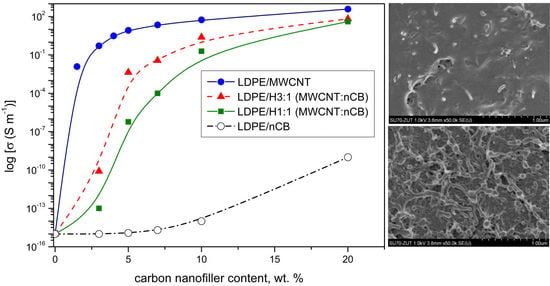
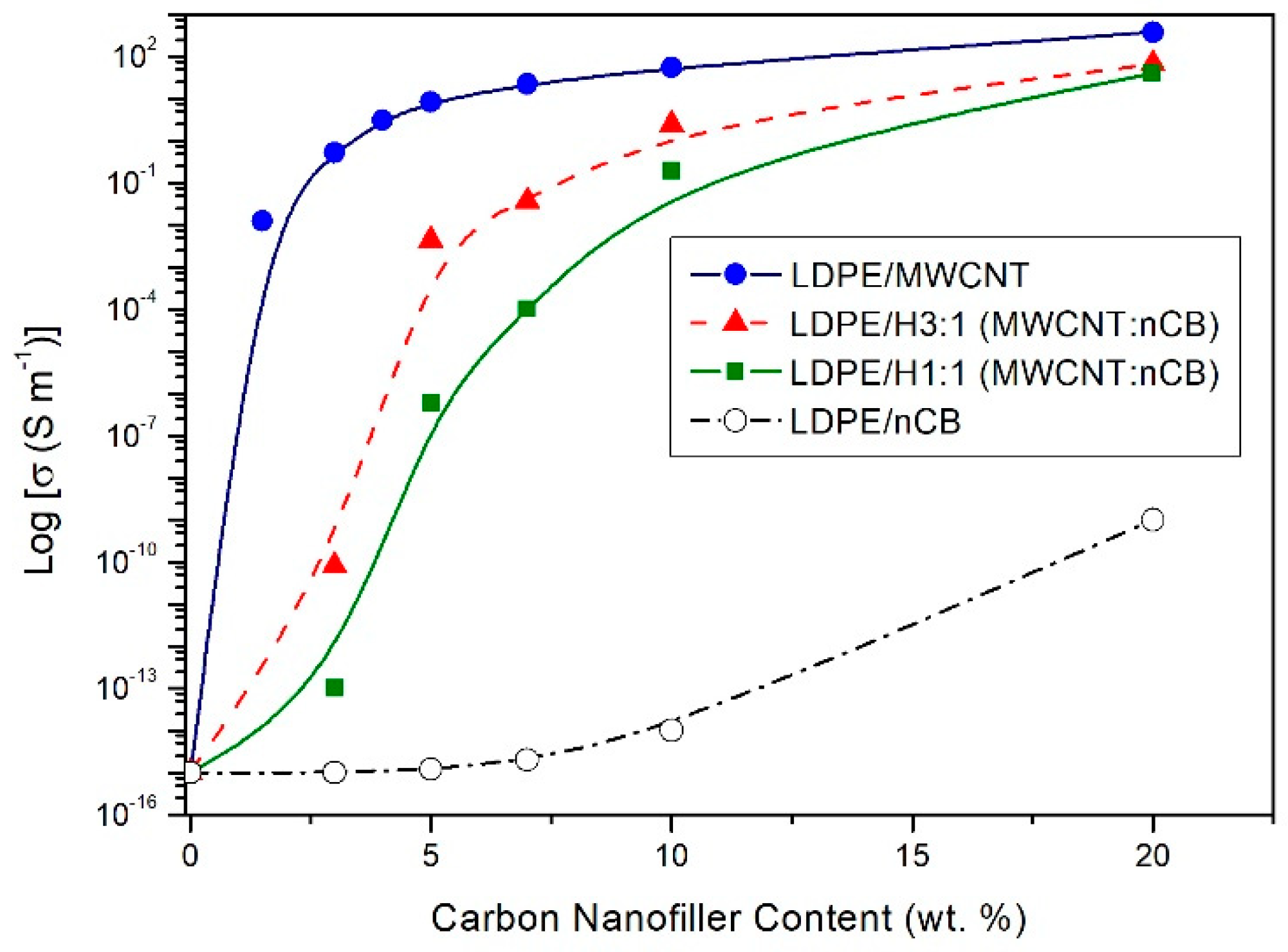
3.3. Electrical Properties
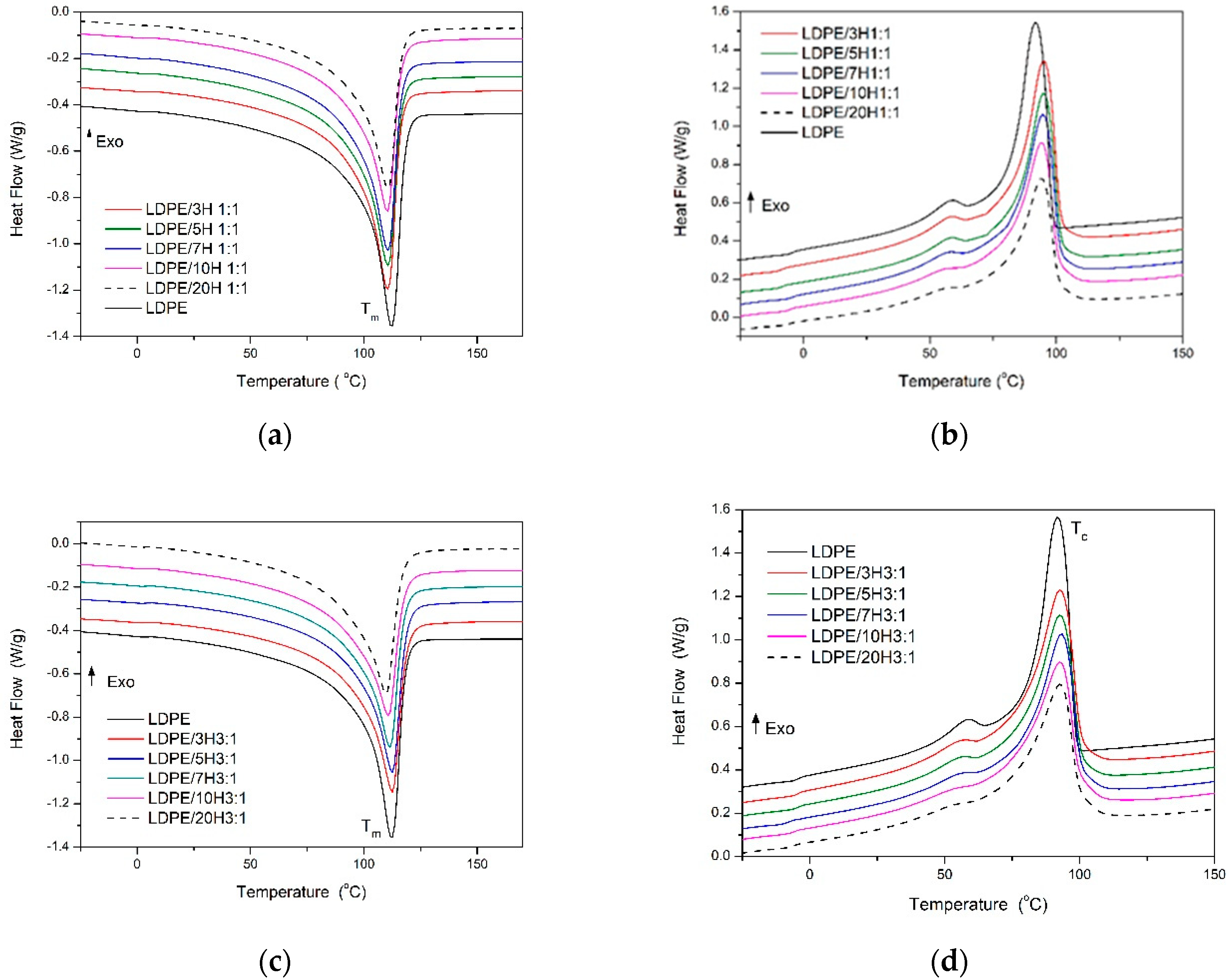
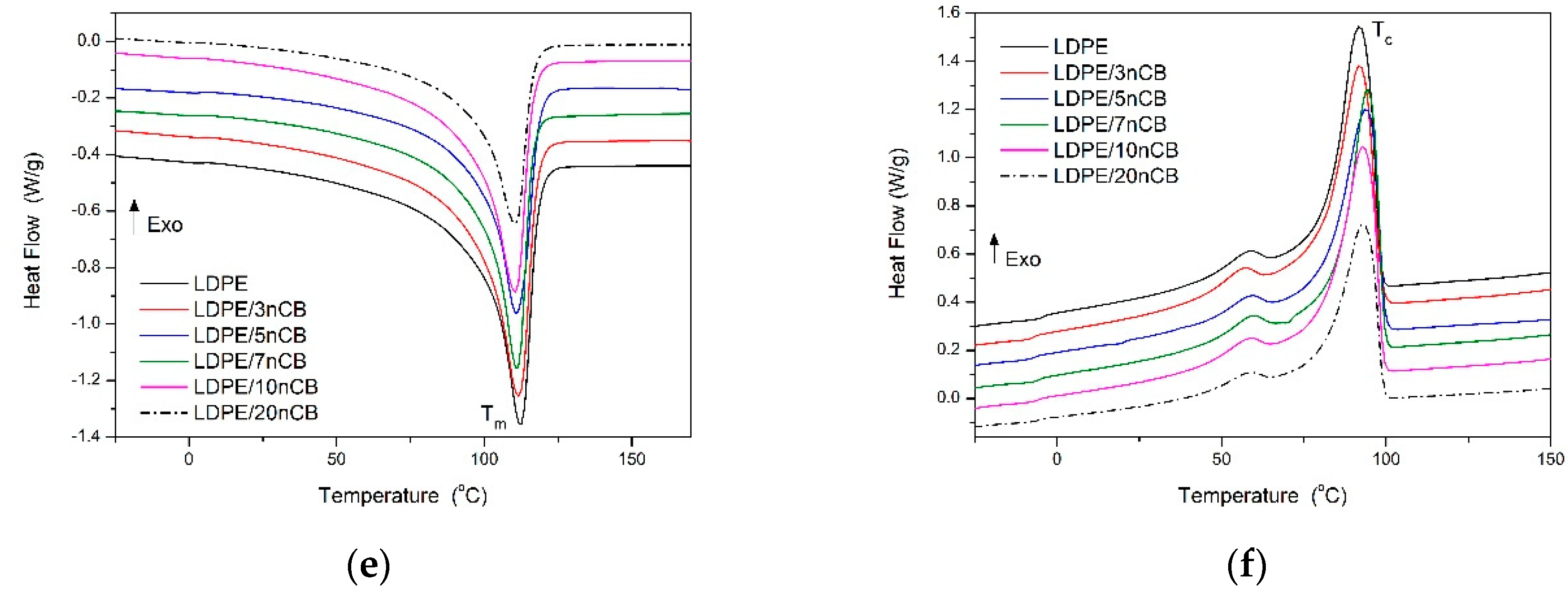
3.4. Thermal Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pötschke, P.; Arnaldo, M.H.; Radusch, H.J. Percolation behavior and mechanical properties of polycarbonate composites filled with carbon black/carbon nanotube systems. Polim. Polym. 2012, 57, 204–211. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction in Percolation Theory; Taylor & Francis: London, UK, 1994. [Google Scholar]
- Farrukh, M.A. Functionalized Nanomaterials; InTech: London, UK, 2016; ISBN 9789535128557. [Google Scholar]
- Krishnamoorti, R.; Vaia, R.A. Polymer Nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 3252–3256. [Google Scholar] [CrossRef]
- Bhattacharya, M. Polymer nanocomposites—A comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef]
- Huang, J.C. Carbon black filled conducting polymers and polymer blends. Adv. Polym. Technol. 2002, 21, 299–313. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Janowska, I.; Jedrzejewski, R.; Linares, A.; Ezquerra, T.A.; Wagner, H.D.; Tenne, R.; Rosłaniec, Z. Comparative study on the properties of poly(trimethylene terephthalate)-based nanocomposites containing multi-walled carbon (MWCNT) and tungsten disulfide (INT-WS2) nanotubes. Polym. Adv. Technol. 2017, 28, 645–657. [Google Scholar] [CrossRef]
- Rahmat, M.; Hubert, P. Carbon nanotube-polymer interactions in nanocomposites: A review. Compos. Sci. Technol. 2011, 72, 72–84. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Paszkiewicz, S. Polymer hybrid nanocomposites containing carbon nanoparticles. In Situ Synthesis and Physical Properties; West Pomeranian University of Technology in Szczecin: Szczecin, Poland, 2014. [Google Scholar]
- Sun, Y.; Bao, H.D.; Guo, Z.X.; Yu, J. Modeling of the electrical percolation of mixed carbon fillers in polymer-based composites. Macromolecules 2009, 42, 459–463. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Pawlikowska, D.; Subocz, J.; Zenker, M.; Masztak, R. Electrically and thermally conductive low-density polyethylene-based nanocomposites reinforced by MWCNT or hybrid MWCNT/graphene nanoplatelets with improved thermo-oxidative stability. Nanomaterials 2018, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hrymak, A.; Kamal, M. Effect of hybrid carbon fillers on the electrical and morphological properties of polystyrene nanocomposites in microinjection molding. Nanomaterials 2018, 8, 779. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, M.; Ruan, W. Studies on synergistic effect of CNT and CB nanoparticles on PVDF. Polym. Compos. 2015, 36, 2248–2254. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Sui, X.M.; Wagner, H.D.; Linares, A.; Ezquerra, T.A.; Rosłaniec, Z. Synergetic effect of single-walled carbon nanotubes (SWCNT) and graphene nanoplatelets (GNP) in electrically conductive PTT-block-PTMO hybrid nanocomposites prepared by in situ polymerization. Compos. Sci. Technol. 2015, 118, 72–77. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Pilawka, R.; Przybyszewski, B.; Czulak, A.; RosŁaniec, Z. Improved thermal conductivity of poly(trimethylene terephthalate-block-poly(tetramethylene oxide) based nanocomposites containing hybrid single-walled carbon nanotubes/graphene nanoplatelets fillers. Adv. Polym. Technol. 2017, 36, 236–242. [Google Scholar] [CrossRef]
- Chen, J.; Du, X.-C.; Zhang, W.-B.; Yang, J.-H.; Zhang, N.; Huang, T.; Wang, Y. Synergistic effect of carbon nanotubes and carbon black on electrical conductivity of PA6/ABS blend. Compos. Sci. Technol. 2013, 81, 1–8. [Google Scholar] [CrossRef]
- Baltá Calleja, F.J.; Bayer, R.K.; Ezquerra, T.A. Electrical conductivity of polyethylene-carbon-fibre composites mixed with carbon black. J. Mater. Sci. 1988, 23, 1411–1415. [Google Scholar] [CrossRef]
- Kim, S.H.; Mulholland, G.W.; Zachariah, M.R. Density measurement of size selected multiwalled carbon nanotubes by mobility-mass characterization. Carbon 2009, 47, 1297–1302. [Google Scholar] [CrossRef]
- Wunderlich, B.; Doyle, M. Specific heat of synthetic high polymers. VIII. Low pressure polyethylene. J. Polym. Sci. 1957, 24, 201–213. [Google Scholar] [CrossRef]
- Szymczyk, A. Poly(trimethylene terephthalate-block-tetramethylene oxide) elastomer/single-walled carbon nanocompsites: Syntheis, structure, and properies. J. Appl. Polym. Sci. 2012, 126, 796–807. [Google Scholar] [CrossRef]
- Li, H.; Zare, Y.; Rhee, K.Y. The percolation threshold for tensile strength of polymer/CNT nanocomposites assuming filler network and interphase regions. Mater. Chem. Phys. 2018, 207, 76–83. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, F.; Guo, Z.; Liu, B.; Zhang, J. Stiffness threshold of randomly distributed carbon nanotube networks. J. Mech. Phys. Solids 2015, 84, 395–423. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Analysis of the connecting effectiveness of the interphase zone on the tensile properties of carbon nanotubes (CNT) reinforced nanocomposite. Polymers 2020, 12, 896. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Mohseni, M.; Ranjbar, Z. An attempt to quantitatively predict the interfacial adhesion of differently surface treated nanosilicas in a polyurethane coating matrix using tensile strength and DMTA analysis. Int. J. Adhes. Adhes. 2012, 34, 24–31. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Li, C.; Chou, T.W. Nanocomposites in context. Compos. Sci. Technol. 2005, 65, 491–516. [Google Scholar] [CrossRef]
- Zhang, Q.; Rastogi, S.; Chen, D.; Lippits, D.; Lemstra, P.J. Low percolation threshold in single-walled carbon nanotube/high density polyethylene composites prepared by melt processing technique. Carbon 2006, 44, 778–785. [Google Scholar] [CrossRef]
- Ciselli, P.; Zhang, R.; Wang, Z.; Reynolds, C.T.; Baxendale, M.; Peijs, T. Oriented UHMW-PE/CNT composite tapes by a solution casting-drawing process using mixed-solvents. Eur. Polym. J. 2009, 45, 2741–2748. [Google Scholar] [CrossRef]
- Du, J.; Zhao, L.; Zeng, Y.; Zhang, L.; Li, F.; Liu, P.; Liu, C. Comparison of electrical properties between multi-walled carbon nanotube and graphene nanosheet/high density polyethylene composites with a segregated network structure. Carbon 2011, 49, 1094–1100. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, S.K.; Kim, N.H. Effects of the addition of multi-walled carbon nanotubes on the positive temperature coefficient characteristics of carbon-black-filled high-density polyethylene nanocomposites. Scr. Mater. 2006, 55, 1119–1122. [Google Scholar] [CrossRef]
- Wang, L.; Hong, J.; Chen, G. Comparison study of graphite nanosheets and carbon black as fillers for high density polyethylene. Polym. Eng. Sci. 2010, 50, 2176–2181. [Google Scholar] [CrossRef]
- Dufresne, A.; Paillet, M.; Putaux, J.L.; Canet, R.; Carmona, F.; Delhaes, P.; Cui, S. Processing and characterization of carbon nanotube/poly(styrene-co-butyl acrylate) nanocomposites. J. Mater. Sci. 2002, 37, 3915–3923. [Google Scholar] [CrossRef]
- Balberg, I.; Azulay, D.; Toker, D.; Millo, O. Precolation and Tunneling in composite Materials. Int. J. Mod. Phys. B 2004, 18, 2091–2121. [Google Scholar] [CrossRef]
- Kirkpatrick, S. Percolation and Conduction. Rev. Mod. Phys. 1973, 45, 574. [Google Scholar] [CrossRef]
- Trujillo, M.; Arnal, M.L.; Müller, A.J.; Bredeau, S.; Bonduel, D.; Dubois, P.; Hamley, I.W.; Castelletto, V. Thermal fractionation and isothermal crystallization of polyethylene nanocomposites prepared by in situ polymerization. Macromolecules 2008, 41, 2087–2095. [Google Scholar] [CrossRef]
- Su, Z.; Li, Q.; Liu, Y.; Guo, W.; Wu, C. The nucleation effect of modified carbon black on crystallization of poly(lactic acid). Polym. Eng. Sci. 2010, 50, 1658–1666. [Google Scholar] [CrossRef]
- Szymczyk, A.A.; Roslaniec, Z.; Zenker, M.; García-Gutiérrez, M.C.; Hernández, J.J.; Rueda, D.R.; Nogales, A.; Ezquerra, T.A. Preparation and characterization of nanocomposites based on COOH functionalized multi-walled carbon nanotubes and on poly(trimethylene terephthalate). Express Polym. Lett. 2011, 5, 977–995. [Google Scholar] [CrossRef]
- Perez, R.A.; Lopez, J.V.; Hoskins, J.N.; Zhang, B.; Grayson, S.M.; Casas, M.T.; Puiggali, J.; Müller, A.J. Nucleation and antinucleation effects of functionalized carbon nanotubes on cyclic and linear poly(ε-caprolactones). Macromolecules 2014, 47, 3553–3566. [Google Scholar] [CrossRef]
- Müller, A.J.; Arnal, M.L.; Trujillo, M.; Lorenzo, A.T. Super-nucleation in nanocomposites and confinement effects on the crystallizable components within block copolymers, miktoarm star copolymers and nanocomposites. Eur. Polym. J. 2011, 47, 614–629. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, P.; Du, J.; Zhao, L.; Ajayan, P.M.; Cheng, H.-M. Increasing the electrical conductivity of carbon nanotube/polymer composites by using weak nanotube–polymer interactions. Carbon 2010, 48, 3551–3558. [Google Scholar] [CrossRef]
- Hong, J.; Park, D.W.; Shim, S.E. Electrical, thermal, and rheological properties of carbon black and carbon nanotube dual filler-incorporated poly(dimethylsiloxane) nanocomposites. Macromol. Res. 2012, 20, 465–472. [Google Scholar] [CrossRef]
- Wen, X.; Tian, N.; Gong, J.; Chen, Q.; Qi, Y.; Liu, Z.; Liu, J.; Jiang, Z.; Chen, X.; Tang, T. Effect of nanosized carbon black on thermal stability and flame retardancy of polypropylene/carbon nanotubes nanocomposites. Polym. Adv. Technol. 2013, 24, 971–977. [Google Scholar] [CrossRef]
- Lorenz, H.; Fritzsche, J.; Das, A.; Stöckelhuber, K.W.; Jurk, R.; Heinrich, G.; Klüppel, M. Advanced elastomer nanocomposites based on CNT-hybrid filler systems. Compos. Sci. Technol. 2009, 69, 2135–2143. [Google Scholar] [CrossRef]
- Zweifel, H. Stabilization of Polymeric Materials; Springer: Berlin, Germany, 1998. [Google Scholar]
- Gugumus, F. Re-examination of the thermal oxidation reactions of polymers 1. New views of an old reaction. Polym. Degrad. Stab. 2001, 74, 327–339. [Google Scholar] [CrossRef]
- Gugumus, F. Re-examination of the thermal oxidation reactions of polymers 2. Thermal oxidation of polyethylene. Polym. Degrad. Stab. 2002, 76, 329–340. [Google Scholar] [CrossRef]
- Bravo, A.; Hotchkiss, J.H. Identification of volatile compound resulting from the thermal oxidation of polyethylene. J. Appl. Polym. Sci. 1993, 47, 1741–1748. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Y.; Gong, J.; Liu, J.; Tian, N.; Wang, Y.; Jiang, Z.; Qiu, J.; Tang, T. Thermal and flammability properties of polypropylene/carbon black nanocomposites. Polym. Degrad. Stab. 2012, 97, 793–801. [Google Scholar] [CrossRef]
- Galano, A. Carbon nanotubes as free-radical scavengers. J. Phys. Chem. C 2008, 112, 8922–8927. [Google Scholar] [CrossRef]
- Azizi, S.; David, E.; Fréchette, M.F.; Nguyen-Tri, P.; Ouellet-Plamondon, C.M. Electrical and thermal conductivity of ethylene vinyl acetate composite with graphene and carbon black filler. Polym. Test. 2018, 72, 24–31. [Google Scholar] [CrossRef]
- Azizi, S.; David, E.; Fréchette, M.F.; Nguyen-Tri, P.; Ouellet-Plamondon, C.M. Electrical and thermal phenomena in low-density polyethylene/carbon black composites near the percolation threshold. J. Appl. Polym. Sci. 2019, 136, 1–13. [Google Scholar] [CrossRef]
- Song, J.; Li, X.; Ma, L.; Li, W. YaoShichune Thermal conductivity of natural rubber nanocomposites with hybrid fillers. Chin. J. Chem. Eng. 2019, 27, 928–934. [Google Scholar] [CrossRef]
| Sample | Carbon Nanofiller Content | dt | d | MFI (190 °C/5 kg) | E | σm | εb | |
|---|---|---|---|---|---|---|---|---|
| MWCT | nCB | |||||||
| wt.% | wt.% | g/cm3 | g/cm3 | g/10 min | MPa | MPa | % | |
| LDPE | - | - | 0.924 | 0.925 | 1.44 | 182.17 ± 10.12 | 22.93 ± 0.54 | 58.54 ± 2.74 |
| LDPE/3H 1:1 | 1.5 | 1.5 | 0.937 | 0.931 | 1.41 | 256.90 ± 17.87 | 20.71 ± 0.16 | 64.23 ± 10.74 |
| LDPE/5H 1:1 | 2.5 | 2.5 | 0.947 | 0.955 | 1.39 | 244.32 ± 20.98 | 19.39 ± 0.38 | 54.90 ± 9.93 |
| LDPE/7H 1:1 | 3.5 | 3.5 | 0.956 | 0.961 | 1.06 | 279.75 ± 23.06 | 18.63 ± 0.17 | 56.59 ± 4.77 |
| LDPE/10H 1:1 | 5 | 5 | 0.969 | 0.976 | 0.71 | 297.99 ± 21.17 | 17.60 ± 0.64 | 49.97 ± 4.04 |
| LDPE/20H 1:1 | 10 | 10 | 1.014 | 0.998 | NF | 595.22 ± 47.43 | 17.89 ± 0.07 | 43.09 ± 1.66 |
| LDPE/3H 3:1 | 2.25 | 0.75 | 0.938 | 0.932 | 0.781 | 298.42 ± 26.60 | 20.63 ± 0.14 | 61.33 ± 6.41 |
| LDPE/5H 3:1 | 3.75 | 1.25 | 0.948 | 0.955 | 0.55 | 267.10 ± 16.21 | 18.94 ± 0.51 | 54.39 ± 5.60 |
| LDPE/7H 3:1 | 5.25 | 1.75 | 0.959 | 0.965 | 0.29 | 266.23 ± 35.47 | 18.34 ± 0.15 | 60.63 ± 10.24 |
| LDPE/10H 3:1 | 7.5 | 2.5 | 0.972 | 0.979 | 0.45 | 301.32 ± 28.71 | 16.88 ± 0.47 | 48.56 ± 7.48 |
| LDPE/20H 3:1 | 15 | 5 | 1.021 | 1.007 | NF | 576.62 ± 58.23 | 19.75 ± 0.11 | 20.12 ± 4.19 |
| LDPE/3nCB | - | 3 | 0.907 | 0.942 | 1.48 | 191.48 ± 12.32 | 19.58 ± 0.50 | 64.74 ± 4.08 |
| LDPE/5nCB | - | 5 | 0.897 | 0.950 | 1.53 | 192.32 ± 19.68 | 19.33 ± 0.50 | 61.12 ± 9.10 |
| LDPE/7nCB | - | 7 | 0.886 | 0.956 | 1.50 | 197.31 ± 16.68 | 19.25 ± 0.56 | 55.87 ± 5.52 |
| LDPE/10nCB | - | 10 | 0.870 | 0.966 | 1.37 | 230.78 ± 13.98 | 21.85 ± 0.85 | 58.74 ± 8.41 |
| LDPE/20nCB | - | 20 | 0.825 | 0.876 | NF | 412.52 ± 14.21 | 19.92 ± 0.51 | 44.92 ± 3.81 |
| Sample | Tm °C | ΔHm J/g | Tc °C | ΔHc J/g | Xc % | T10% °C | T50% °C |
|---|---|---|---|---|---|---|---|
| LDPE | 112 | 131.1 | 92 | 130.5 | 45.4 | 351 | 418 |
| LDPE/3H 1:1 | 112 | 128.5 | 94 | 127.3 | 45.8 | 436 | 461 |
| LDPE/5H 1:1 | 111 | 121.4 | 95 | 120.4 | 44.2 | 429 | 463 |
| LDPE/7H 1:1 | 111 | 120.4 | 95 | 120.5 | 44.8 | 432 | 469 |
| LDPE/10H 1:1 | 110 | 112.5 | 94 | 112.9 | 43.2 | 428 | 471 |
| LDPE/20H 1:1 | 110 | 100.2 | 94 | 101.0 | 43.4 | 419 | 472 |
| LDPE/3H 3:1 | 111 | 126.4 | 95 | 127.1 | 45.1 | 434 | 462 |
| LDPE/5H 3:1 | 112 | 118.8 | 93 | 117.8 | 44.2 | 424 | 469 |
| LDPE/7H 3:1 | 111 | 114.8 | 93 | 114.0 | 44.1 | 415 | 456 |
| LDPE/10H 3:1 | 110 | 99.6 | 93 | 98.9 | 43.0 | 426 | 472 |
| LDPE/20H 3:1 | 112 | 129.8 | 94 | 128.9 | 44.9 | 441 | 476 |
| LDPE/3nCB | 111 | 120.7 | 94 | 120.5 | 41.7 | 370 | 430 |
| LDPE/5nCB | 111 | 122.4 | 94 | 122.0 | 42.3 | 368 | 422 |
| LDPE/7nCB | 111 | 121.7 | 93 | 120.5 | 42.1 | 382 | 445 |
| LDPE/10nCB | 110 | 101.5 | 90 | 99.8 | 43.9 | 409 | 436 |
| LDPE/20nCB | 112 | 131.1 | 92 | 130.5 | 45.4 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paszkiewicz, S.; Szymczyk, A.; Zubkiewicz, A.; Subocz, J.; Stanik, R.; Szczepaniak, J. Enhanced Functional Properties of Low-Density Polyethylene Nanocomposites Containing Hybrid Fillers of Multi-Walled Carbon Nanotubes and Nano Carbon Black. Polymers 2020, 12, 1356. https://doi.org/10.3390/polym12061356
Paszkiewicz S, Szymczyk A, Zubkiewicz A, Subocz J, Stanik R, Szczepaniak J. Enhanced Functional Properties of Low-Density Polyethylene Nanocomposites Containing Hybrid Fillers of Multi-Walled Carbon Nanotubes and Nano Carbon Black. Polymers. 2020; 12(6):1356. https://doi.org/10.3390/polym12061356
Chicago/Turabian StylePaszkiewicz, Sandra, Anna Szymczyk, Agata Zubkiewicz, Jan Subocz, Rafal Stanik, and Jedrzej Szczepaniak. 2020. "Enhanced Functional Properties of Low-Density Polyethylene Nanocomposites Containing Hybrid Fillers of Multi-Walled Carbon Nanotubes and Nano Carbon Black" Polymers 12, no. 6: 1356. https://doi.org/10.3390/polym12061356
APA StylePaszkiewicz, S., Szymczyk, A., Zubkiewicz, A., Subocz, J., Stanik, R., & Szczepaniak, J. (2020). Enhanced Functional Properties of Low-Density Polyethylene Nanocomposites Containing Hybrid Fillers of Multi-Walled Carbon Nanotubes and Nano Carbon Black. Polymers, 12(6), 1356. https://doi.org/10.3390/polym12061356