Effects of Graphene Nanosheets with Different Lateral Sizes as Conductive Additives on the Electrochemical Performance of LiNi0.5Co0.2Mn0.3O2 Cathode Materials for Li Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Electrode Composition and Characterizations
2.2. Slurry Preparation
2.3. Electrochemical Measurements
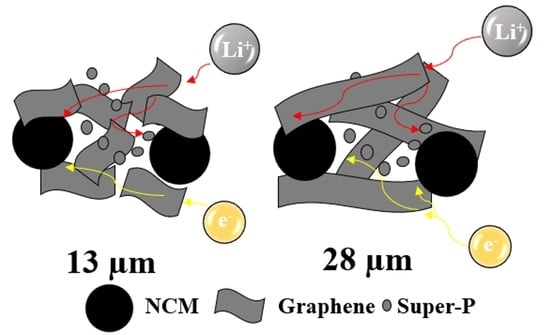
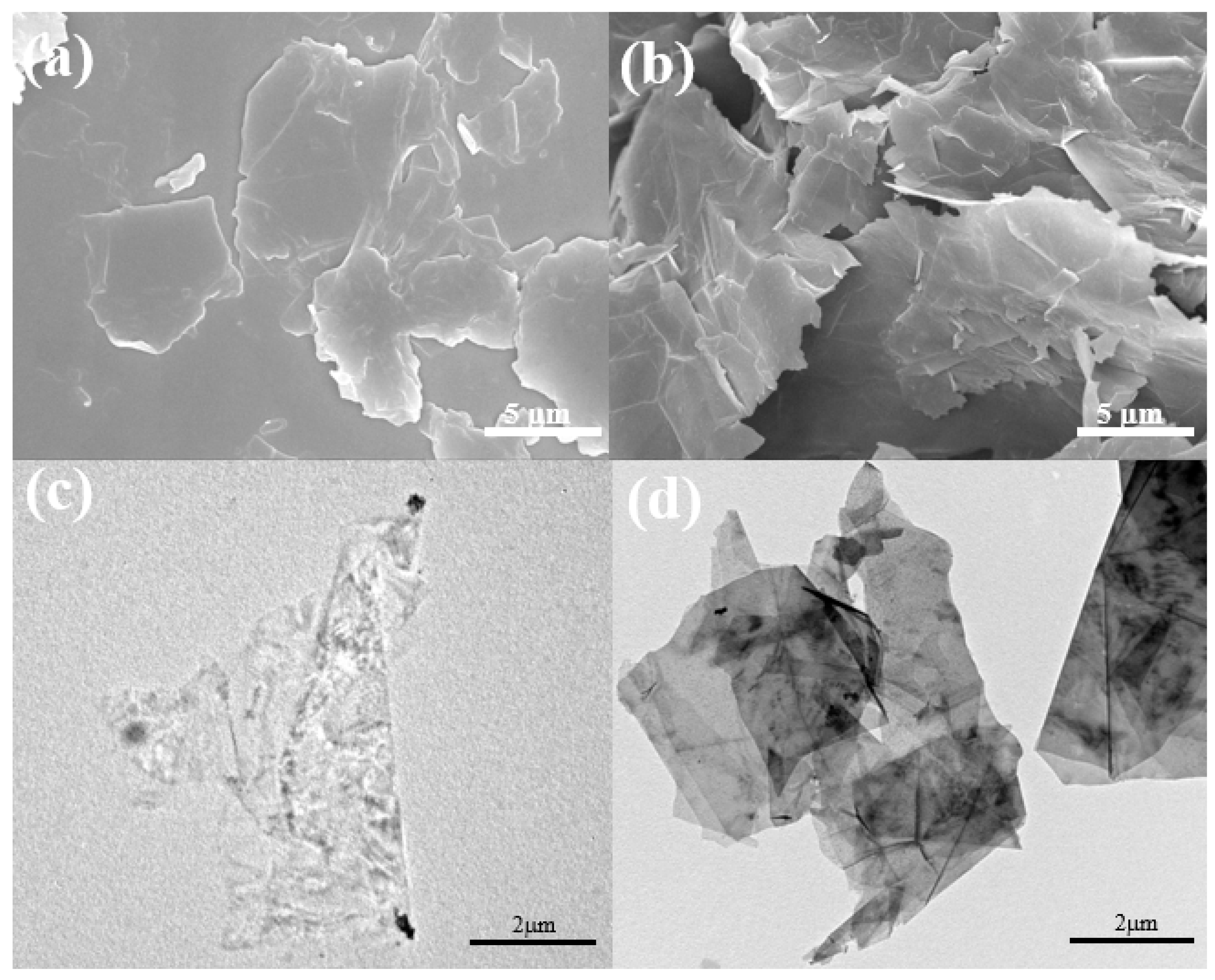
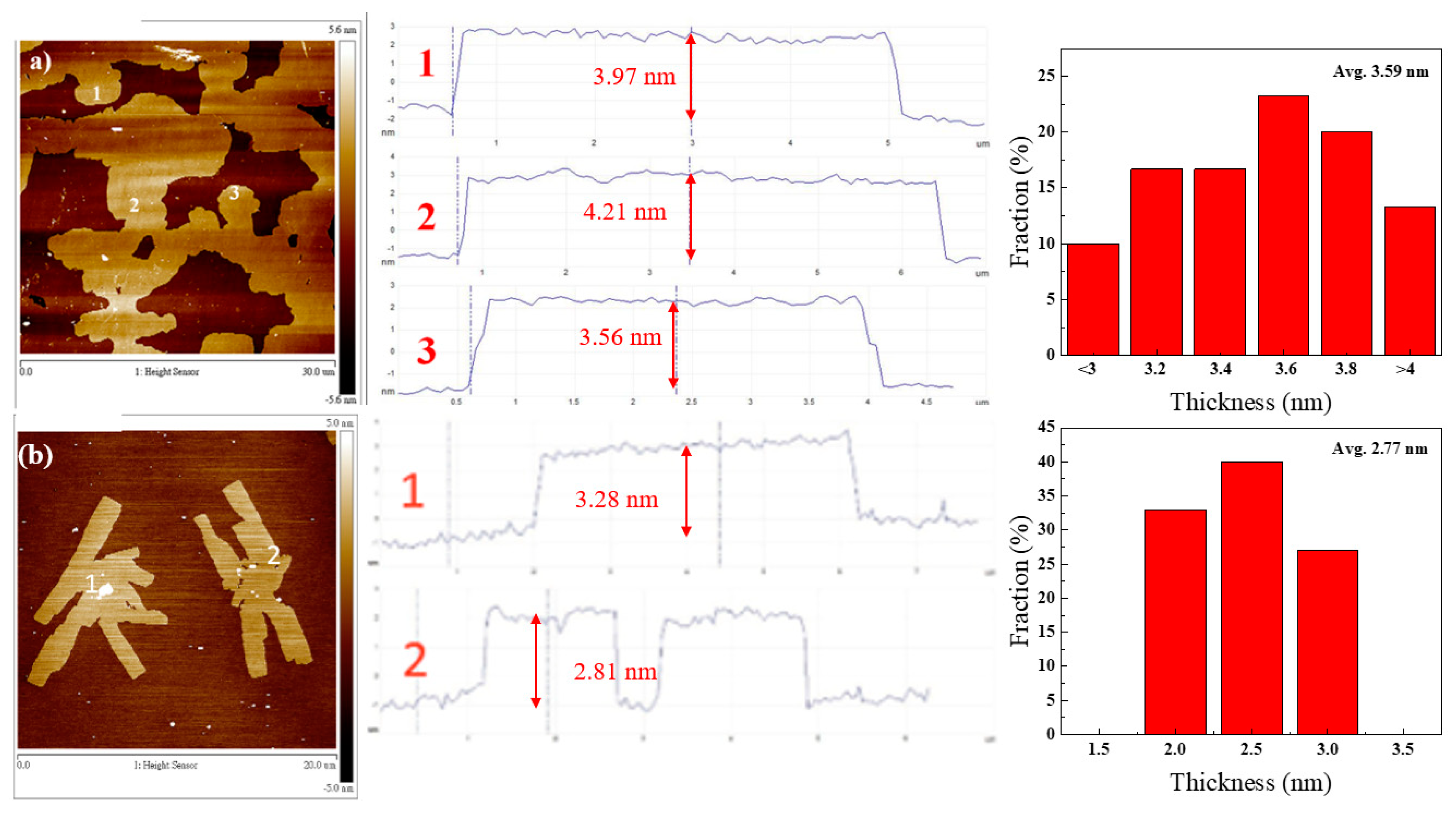
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chou, S.-L.; Wang, J.-Z.; Wexler, D.; Li, H.-J.; Liu, H.-K.; Wu, Y. Graphene wrapped LiFePO4/C composites as cathode materials for Li-ion batteries with enhanced rate capability. J. Mater. Chem. 2012, 22, 16465–16470. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.-L.; Hsieh, C.-T.; Li, J.; Gandomi, Y.A. Enabling high rate charge and discharge capability, low internal resistance, and excellent cycleability for Li-ion batteries utilizing graphene additives. Electrochim. Acta 2018, 273, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cao, G. Developments in nanostructured cathode materials for high-performance lithium-ion batteries. Adv. Mater. 2008, 20, 2251–2269. [Google Scholar] [CrossRef]
- Krüger, S.; Hanisch, C.; Kwade, A.; Winter, M.; Nowak, S. Effect of impurities caused by a recycling process on the electrochemical performance of Li[Ni0.33Co0.33Mn0.33]O2. J. Electroanal. Chem. 2014, 726, 91–96. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Li, W.; Liu, X.; Zhang, J. Enhancing the high voltage interface compatibility of LiNi0.5Co0.2Mn0.3O2 in the succinonitrile-based electrolyte. Electrochim. Acta 2019, 298, 818–826. [Google Scholar] [CrossRef]
- Streich, D.; Guéguen, A.; Mendez, M.; Chesneau, F.; Novák, P.; Berg, E. Online electrochemical mass spectrometry of high energy lithium nickel cobalt manganese oxide/graphite half-and full-cells with ethylene carbonate and fluoroethylene carbonate based electrolytes. J. Electrochem. Soc. 2016, 163, A964–A970. [Google Scholar] [CrossRef] [Green Version]
- Kitada, K.; Murayama, H.; Fukuda, K.; Arai, H.; Uchimoto, Y.; Ogumi, Z.; Matsubara, E. Factors determining the packing-limitation of active materials in the composite electrode of lithium-ion batteries. J. Power Sources 2016, 301, 11–17. [Google Scholar] [CrossRef]
- Bockholt, H.; Indrikova, M.; Netz, A.; Golks, F.; Kwade, A. The interaction of consecutive process steps in the manufacturing of lithium-ion battery electrodes with regard to structural and electrochemical properties. J. Power Sources 2016, 325, 140–151. [Google Scholar] [CrossRef]
- Forouzan, M.M.; Chao, C.-W.; Bustamante, D.; Mazzeo, B.A.; Wheeler, D.R. Experiment and simulation of the fabrication process of lithium-ion battery cathodes for determining microstructure and mechanical properties. J. Power Sources 2016, 312, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Cui, P.; Gan, B.; Ma, Y.; Liang, Y. Graphene Oxide Modified LiNi1/3Co1/3Mn1/3O2 as Cathode Material for Lithium Ion Batteries and Its Electrochemical Performances. Int. J. Electrochem. Sci. 2018, 13, 10440–10453. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Wang, M.-S.; Zhang, K.-J.; Huang, Y.; Qu, M.-Z.; Yu, Z.-L.; Geng, D.-S.; Zhao, W.-G.; Zheng, J.-M. Improved rate capability of a LiNi1/3Co1/3Mn1/3O2/CNT/graphene hybrid material for Li-ion batteries. RSC Adv. 2017, 7, 24359–24367. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Zhang, H.; Yue, X.; Yu, L.; Fan, W. Triallyl phosphite as an electrolyte additive to improve performance at elevated temperature of LiNi0.6Co0.2Mn0.2O2/graphite cells. J. Electroanal. Chem. 2019, 832, 408–416. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, L.; Lu, J. Improve safety of high energy density LiNi1/3Co1/3Mn1/3O2/graphite battery using organosilicon electrolyte. Electrochim. Acta 2019, 296, 149–154. [Google Scholar] [CrossRef]
- Zhu, L.; Yan, T.-F.; Jia, D.; Wang, Y.; Wu, Q.; Gu, H.-T.; Wu, Y.-M.; Tang, W.-P. LiFePO4-Coated LiNi0.5Co0.2Mn0.3O2 Cathode Materials with Improved High Voltage Electrochemical Performance and Enhanced Safety for Lithium Ion Pouch Cells. J. Electrochem. Soc. 2019, 166, A5437–A5444. [Google Scholar] [CrossRef]
- Dong, S.; Zhou, Y.; Hai, C.; Zeng, J.; Sun, Y.; Shen, Y.; Li, X.; Ren, X.; Qi, G.; Zhang, X. Ultrathin CeO2 coating for improved cycling and rate performance of Ni-rich layered LiNi0.7Co0.2Mn0.1O2 cathode materials. Ceram. Int. 2019, 45, 144–152. [Google Scholar] [CrossRef]
- Ding, Y.; Deng, B.; Wang, H.; Li, X.; Chen, T.; Yan, X.; Wan, Q.; Qu, M.; Peng, G. Improved electrochemical performances of LiNi0.6Co0.2Mn0.2O2 cathode material by reducing lithium residues with the coating of Prussian blue. J. Alloys Compd. 2019, 774, 451–460. [Google Scholar] [CrossRef]
- Dannehl, N.; Steinmuüller, S.O.; Szabó, D.V.; Pein, M.; Sigel, F.; Esmezjan, L.; Hasenkox, U.; Schwarz, B.R.; Indris, S.; Ehrenberg, H. High-Resolution Surface Analysis on Aluminum Oxide-Coated Li1.2Mn0.55Ni0.15Co0.1O2 with Improved Capacity Retention. ACS Appl. Mater. Interfaces 2018, 10, 43131–43143. [Google Scholar]
- Huang, Y.; Wang, X.; Guo, Y.; Jia, D.; Tang, X. Improved electrochemical performance of lithium iron phosphate in situ coated with hierarchical porous nitrogen-doped graphene-like membrane. J. Power Sources 2016, 305, 122–127. [Google Scholar]
- Lv, C.; Yang, J.; Peng, Y.; Duan, X.; Ma, J.; Li, Q.; Wang, T. 1D Nb-doped LiNi1/3Co1/3Mn1/3O2 nanostructures as excellent cathodes for Li-ion battery. Electrochim. Acta 2019, 297, 258–266. [Google Scholar] [CrossRef]
- Chen, Z.; Gong, X.; Zhu, H.; Cao, K.; Liu, Q.; Liu, J.; Li, L.; Duan, J. High performance and structural stability of K and Cl co-doped LiNi0.5Co0.2Mn0.3O2 cathode materials in 4.6 voltage. Front. Chem. 2018, 6, 643. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Z.; Li, H.; Yang, T.; Zhang, H.; Shi, X.; Song, D.; Zhang, L. Influence of Ni/Mn distributions on the structure and electrochemical properties of Ni-rich cathode materials. Dalton Trans. 2018, 47, 16651–16659. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Wang, C.-W.; Liu, G.; Song, X.-Y.; Battaglia, V.; Sastry, A.M. Selection of conductive additives in li-ion battery cathodes a numerical study. J. Electrochem. Soc. 2007, 154, A978–A986. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Li, J.; Wood, M.; Mao, C.; Daniel, C.; Wood, D.L., III. Three-dimensional conductive network formed by carbon nanotubes in aqueous processed NMC electrode. Electrochim. Acta 2018, 270, 54–61. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Li, X.-X.; Huang, J.-R.; Li, J.-J.; Zhou, P.; Liu, J.-H.; Huang, X.-J. Three-dimensional graphene-based nanocomposites for high energy density Li-ion batteries. J. Mater. Chem. A 2017, 5, 5977–5994. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Nemes-Incze, P.; Osváth, Z.; Kamarás, K.; Biró, L. Anomalies in thickness measurements of graphene and few layer graphite crystals by tapping mode atomic force microscopy. Carbon 2008, 46, 1435–1442. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Zhu, N.; Liu, W.; Xue, M.; Xie, Z.; Zhao, D.; Zhang, M.; Chen, J.; Cao, T. Graphene as a conductive additive to enhance the high-rate capabilities of electrospun Li4Ti5O12 for lithium-ion batteries. Electrochim. Acta 2010, 55, 5813–5818. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of large-area graphene films for high-performance transparent conductive electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Sim, D.; Wong, K.; Zhu, J.; Liu, W.; Xu, C.; Tan, H.; Xiao, N.; Hng, H.H.; Lim, T.M. Li3V2(PO4)3 nanocrystals embedded in a nanoporous carbon matrix supported on reduced graphene oxide sheets: Binder-free and high rate cathode material for lithium-ion batteries. J. Power Sources 2012, 214, 171–177. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, B.; Wang, Y.; Zheng, L.; Duan, X.; Wang, L.; Yang, J.; Qian, Y. Improved electrochemical performance of LiMn2O4/graphene composite as cathode material for lithium ion battery. Int. J. Electrochem. Sci. 2012, 7, 10627–10632. [Google Scholar]
- He, J.-R.; Chen, Y.-F.; Li, P.-J.; Wang, Z.-G.; Qi, F.; Liu, J.-B. Synthesis and electrochemical properties of graphene-modified LiCo1/3 Ni1/3Mn1/3O2 cathodes for lithium ion batteries. RSC Adv. 2014, 4, 2568–2572. [Google Scholar] [CrossRef]
- Choi, M.; Yoon, I.-H.; Jo, I.-H.; Kim, H.-S.; Jin, B.-S.; Lee, Y.M.; Choi, W.-K. The effect of SiO2 nanoparticles in Li3V2(PO4)3/graphene as a cathode material for Li-ion batteries. Mater. Lett. 2015, 160, 206–209. [Google Scholar] [CrossRef]
- Yang, J.; Kang, X.; He, D.; Zheng, A.; Pan, M.; Mu, S. Graphene activated 3D-hierarchical flower-like Li2FeSiO4 for high-performance lithium-ion batteries. J. Mater. Chem. A 2015, 3, 16567–16573. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-wrapped sulfur particles as a rechargeable lithium–sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 2011, 11, 2644–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seh, Z.W.; Wang, H.; Liu, N.; Zheng, G.; Li, W.; Yao, H.; Cui, Y. High-capacity Li2S–graphene oxide composite cathodes with stable cycling performance. Chem. Sci. 2014, 5, 1396–1400. [Google Scholar] [CrossRef]
- Wang, L.; Sofer, Z.; Pumera, M. Will Any Crap We Put into Graphene Increase Its Electrocatalytic Effect? ACS Nano 2020, 14, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Shen, Z.; Yi, M. Liquid-exfoliated graphene as highly efficient conductive additives for cathodes in lithium ion batteries. Carbon 2019, 153, 156–163. [Google Scholar] [CrossRef]
- Chen, X.; Lu, W.; Chen, C.; Xue, M. Improved electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode with different carbon additives for lithium-ion batteries. Int. J. Electrochem. Sci. 2018, 13, 296–304. [Google Scholar] [CrossRef]
- Liu, T.; Sun, S.; Zang, Z.; Li, X.; Sun, X.; Cao, F.; Wu, J. Effects of graphene with different sizes as conductive additives on the electrochemical performance of a LiFePO4 cathode. RSC Adv. 2017, 7, 20882–20887. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.-C.; Wu, J.-Y.; Liu, W.-R. Liu. Green and facile synthesis of few-layer graphene via liquid exfoliation process for Lithium-ion batteries. Sci. Rep. 2018, 8, 9766. [Google Scholar] [CrossRef]



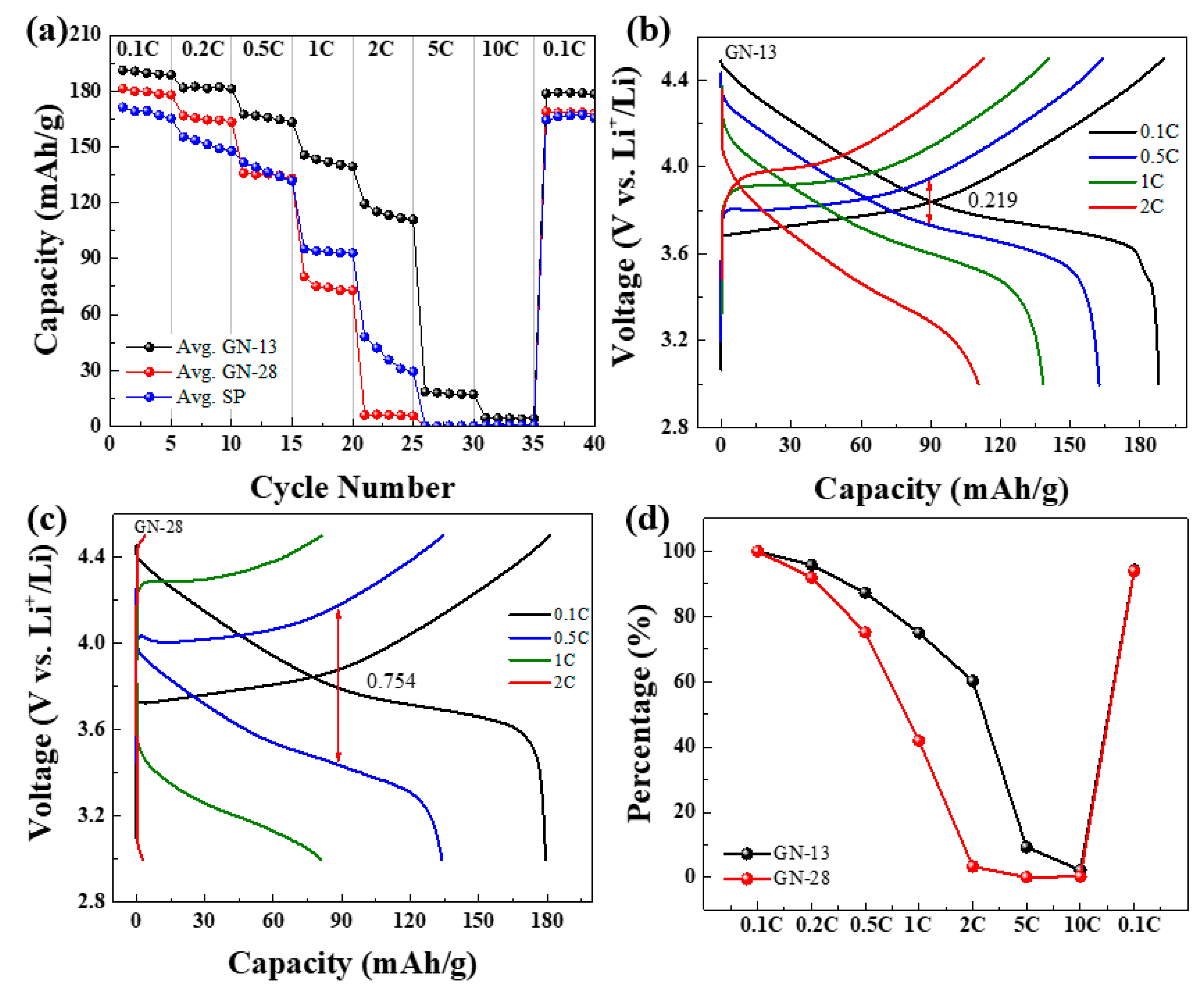

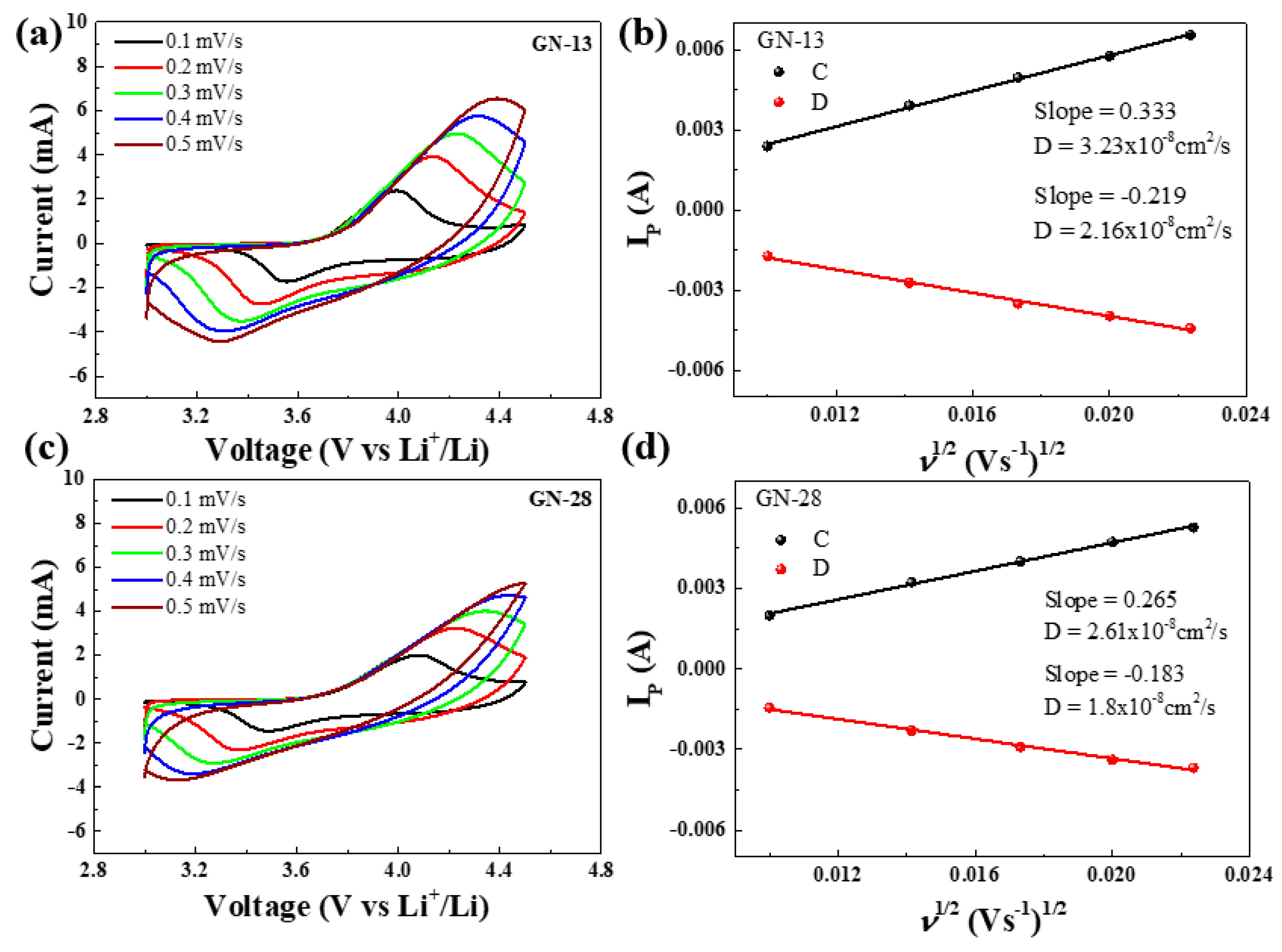
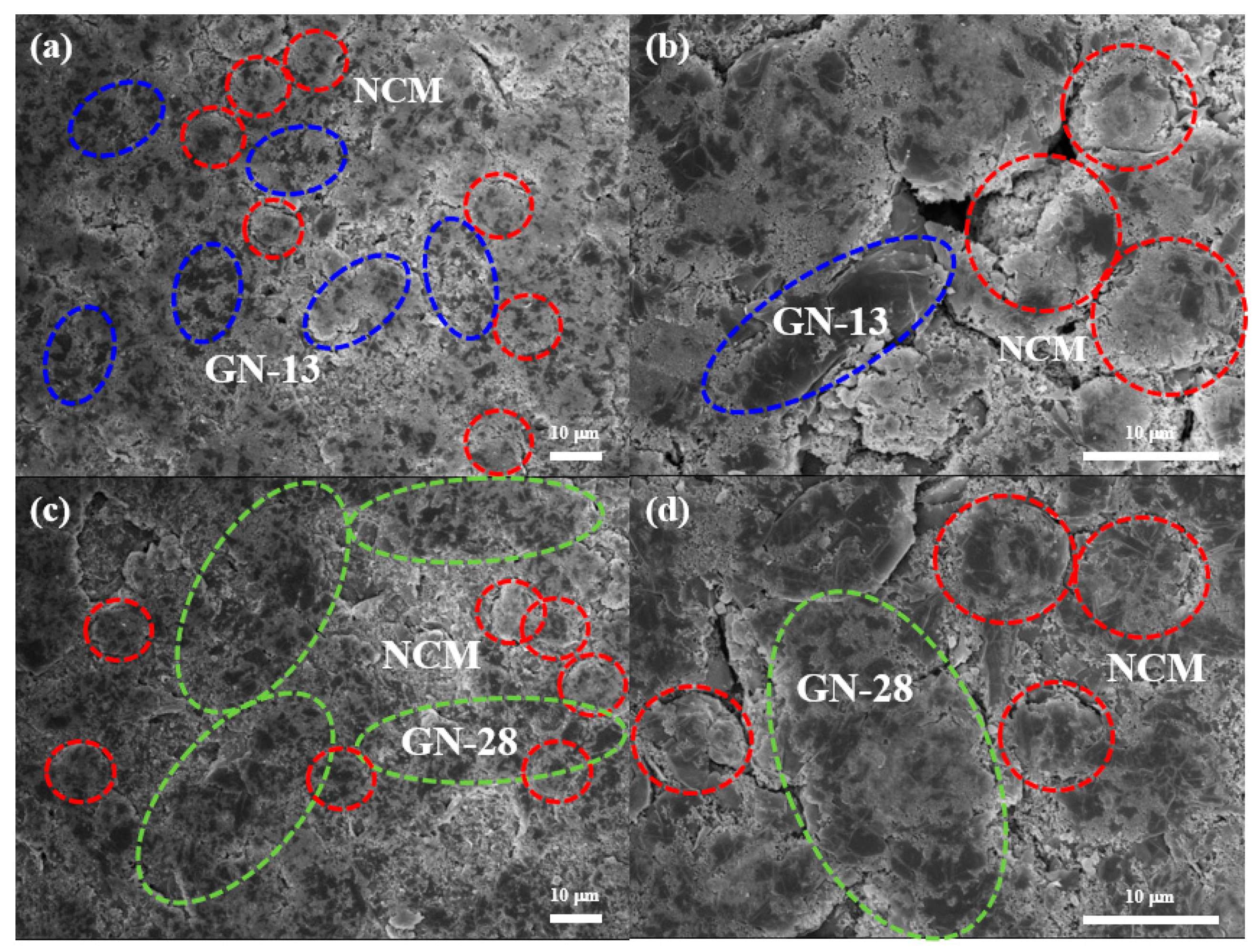
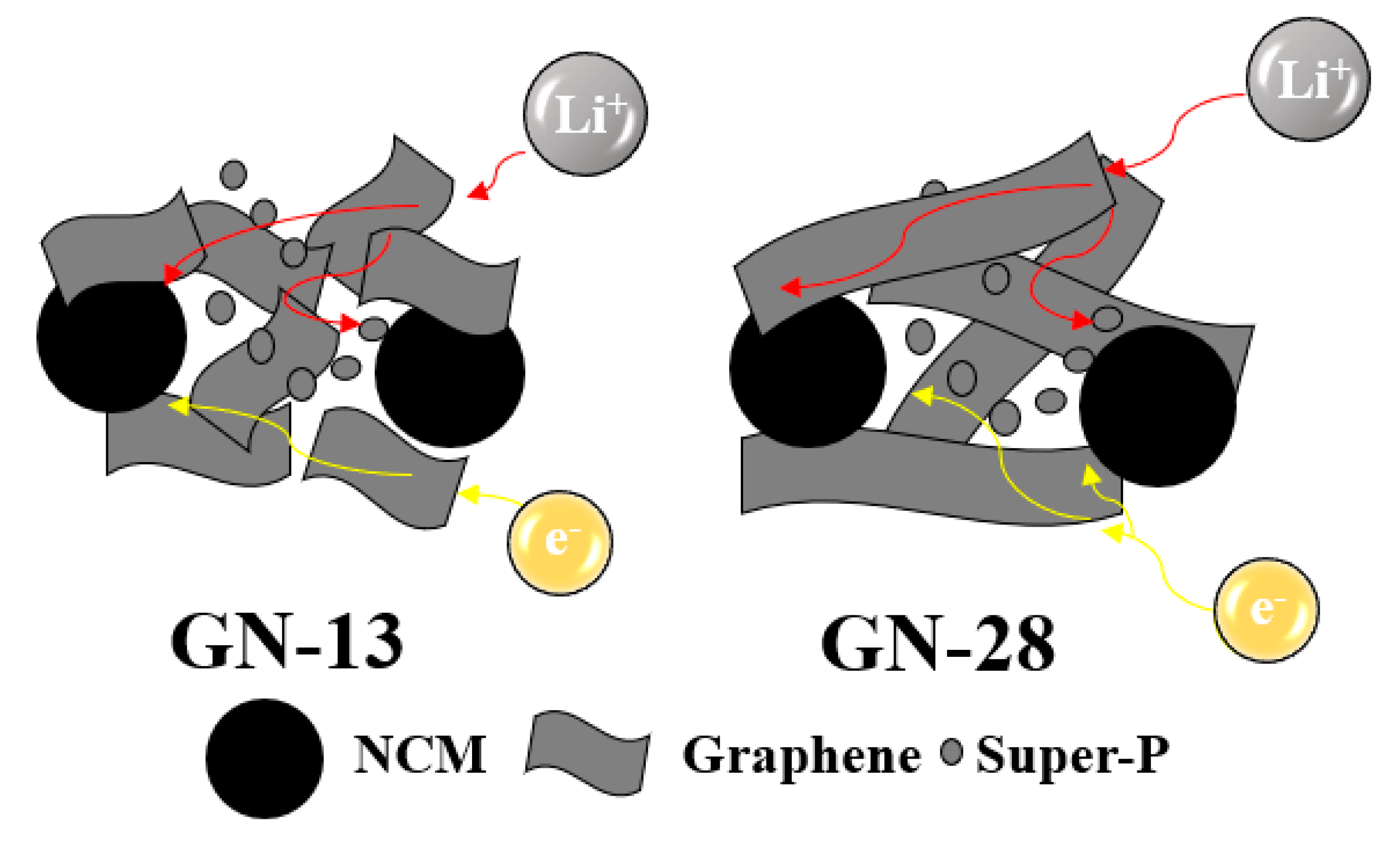
| Samples (Scan Rate) | Anodic Peak (V) | Cathodic Peak (V) | Peak Separation (V) |
|---|---|---|---|
| GN-13 (0.1 mV/s) | 3.999 | 3.555 | 0.444 |
| GN-28 (0.1 mV/s) | 4.079 | 3.485 | 0.594 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, T.-H.; Liu, W.-R. Effects of Graphene Nanosheets with Different Lateral Sizes as Conductive Additives on the Electrochemical Performance of LiNi0.5Co0.2Mn0.3O2 Cathode Materials for Li Ion Batteries. Polymers 2020, 12, 1162. https://doi.org/10.3390/polym12051162
Hsu T-H, Liu W-R. Effects of Graphene Nanosheets with Different Lateral Sizes as Conductive Additives on the Electrochemical Performance of LiNi0.5Co0.2Mn0.3O2 Cathode Materials for Li Ion Batteries. Polymers. 2020; 12(5):1162. https://doi.org/10.3390/polym12051162
Chicago/Turabian StyleHsu, Ting-Hao, and Wei-Ren Liu. 2020. "Effects of Graphene Nanosheets with Different Lateral Sizes as Conductive Additives on the Electrochemical Performance of LiNi0.5Co0.2Mn0.3O2 Cathode Materials for Li Ion Batteries" Polymers 12, no. 5: 1162. https://doi.org/10.3390/polym12051162
APA StyleHsu, T.-H., & Liu, W.-R. (2020). Effects of Graphene Nanosheets with Different Lateral Sizes as Conductive Additives on the Electrochemical Performance of LiNi0.5Co0.2Mn0.3O2 Cathode Materials for Li Ion Batteries. Polymers, 12(5), 1162. https://doi.org/10.3390/polym12051162