Elaboration of Charged Poly(Lactic-co-Glycolic Acid) Microparticles for Effective Release of Tranexamic Acid
Abstract
1. Introduction
2. Materials and Methods
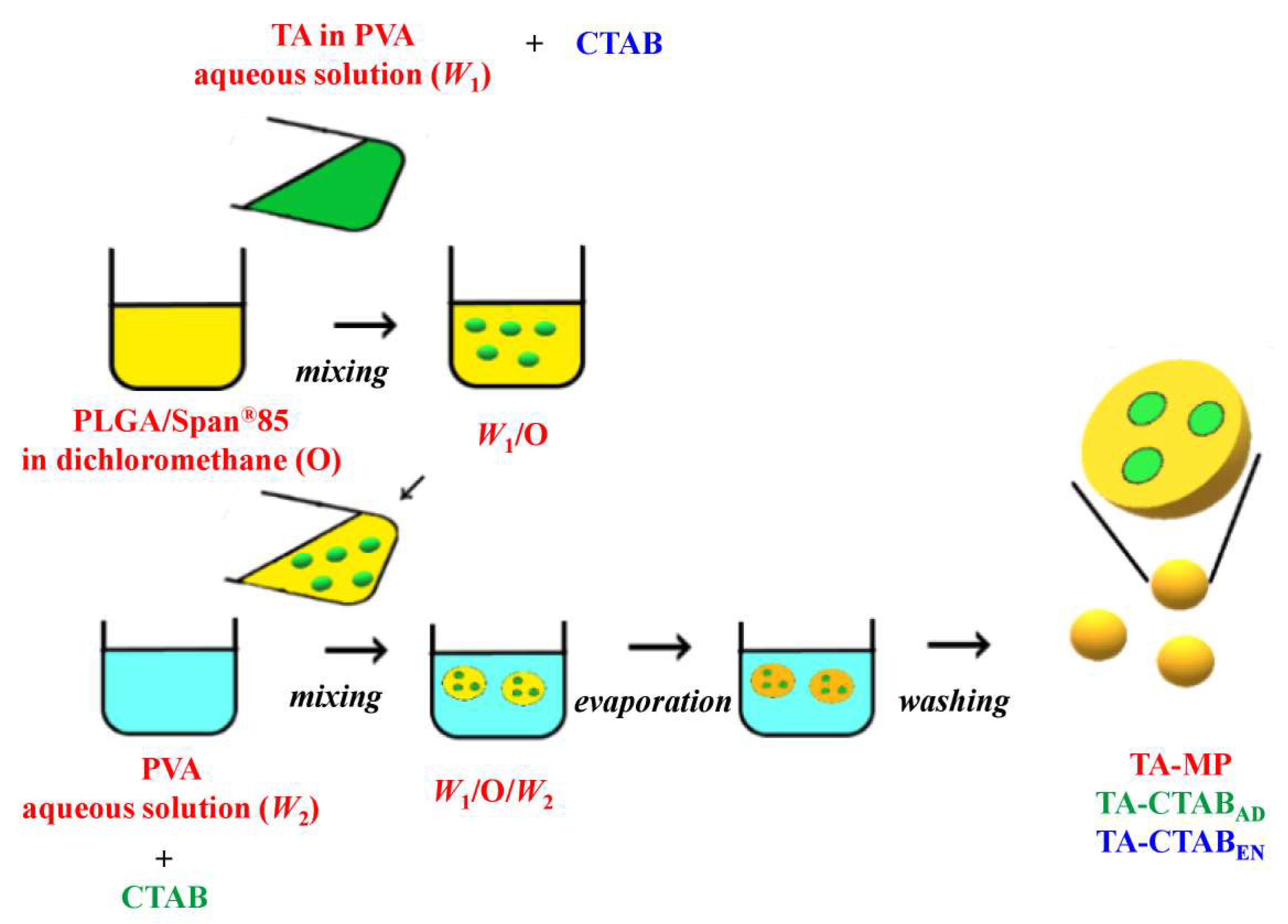
2.1. Preparation of PLGA MPs
2.2. Morphology and Particle Size Distribution
2.3. Zeta Potential
2.4. Loading Efficiency
2.5. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
2.6. In Vitro Release
3. Results and Discussion
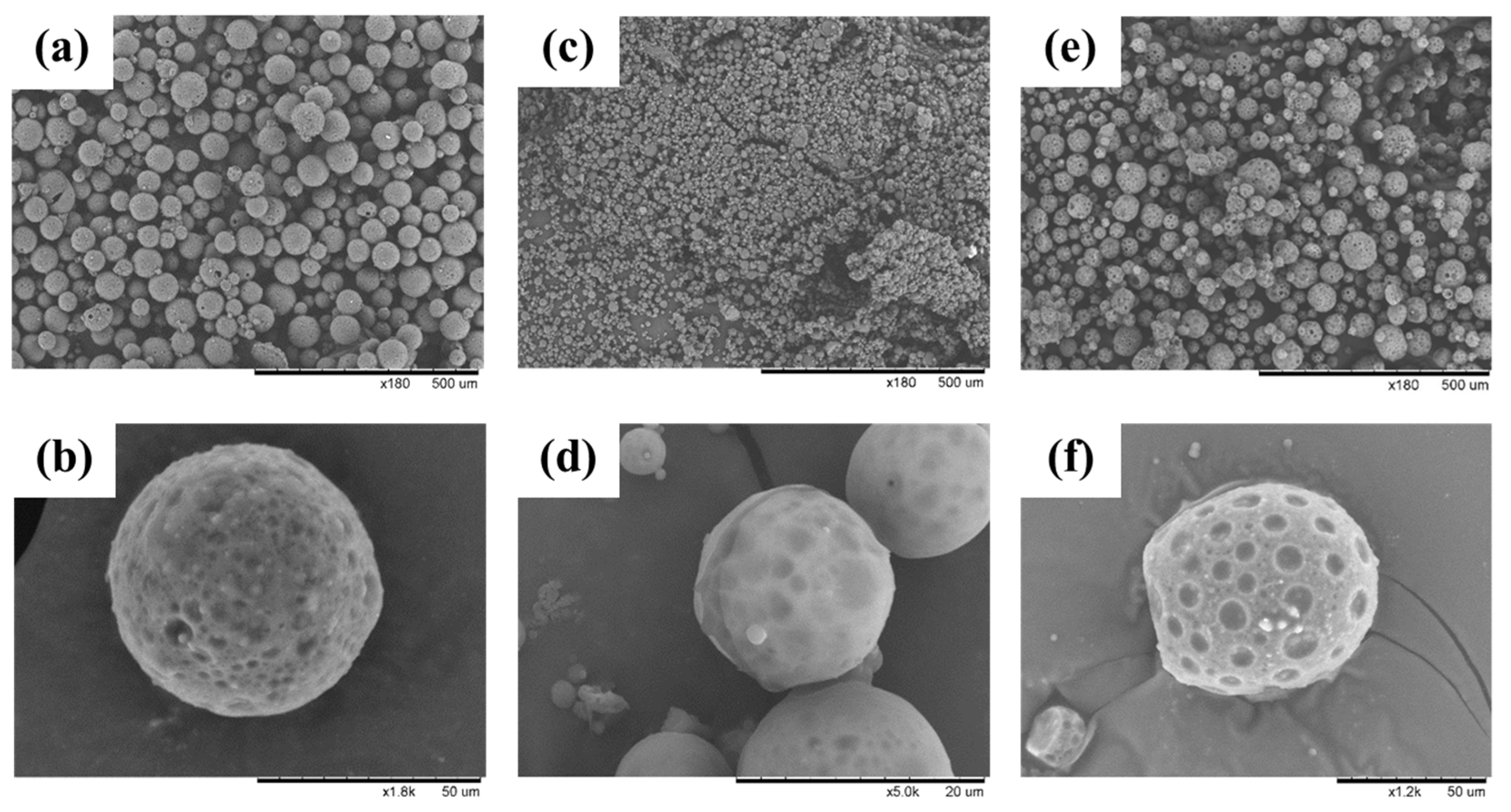
3.1. Morphology
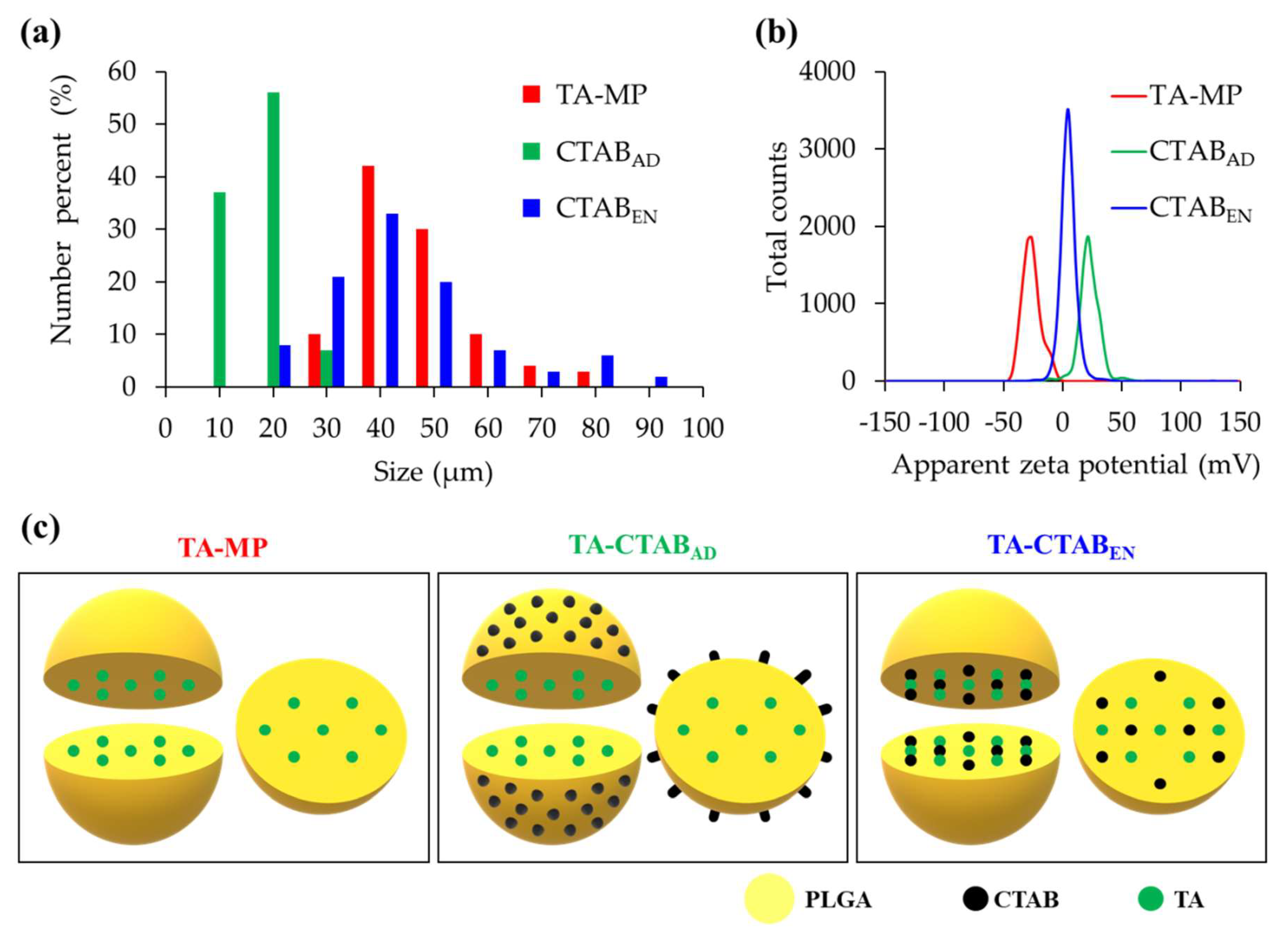
3.2. Particle Size and Zeta Potential
3.3. Loading Efficiency
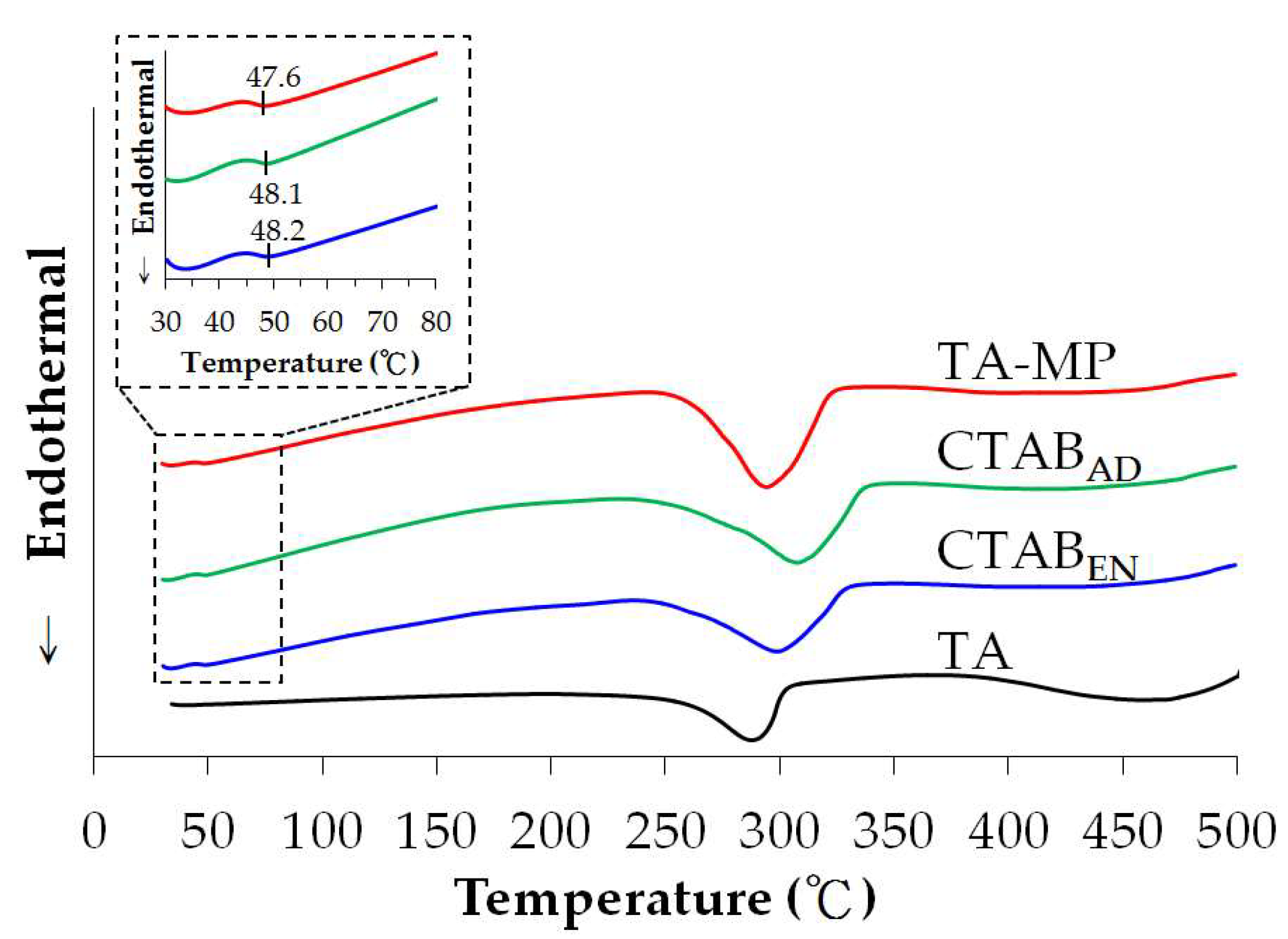
3.4. Differential Scanning Calorimetry
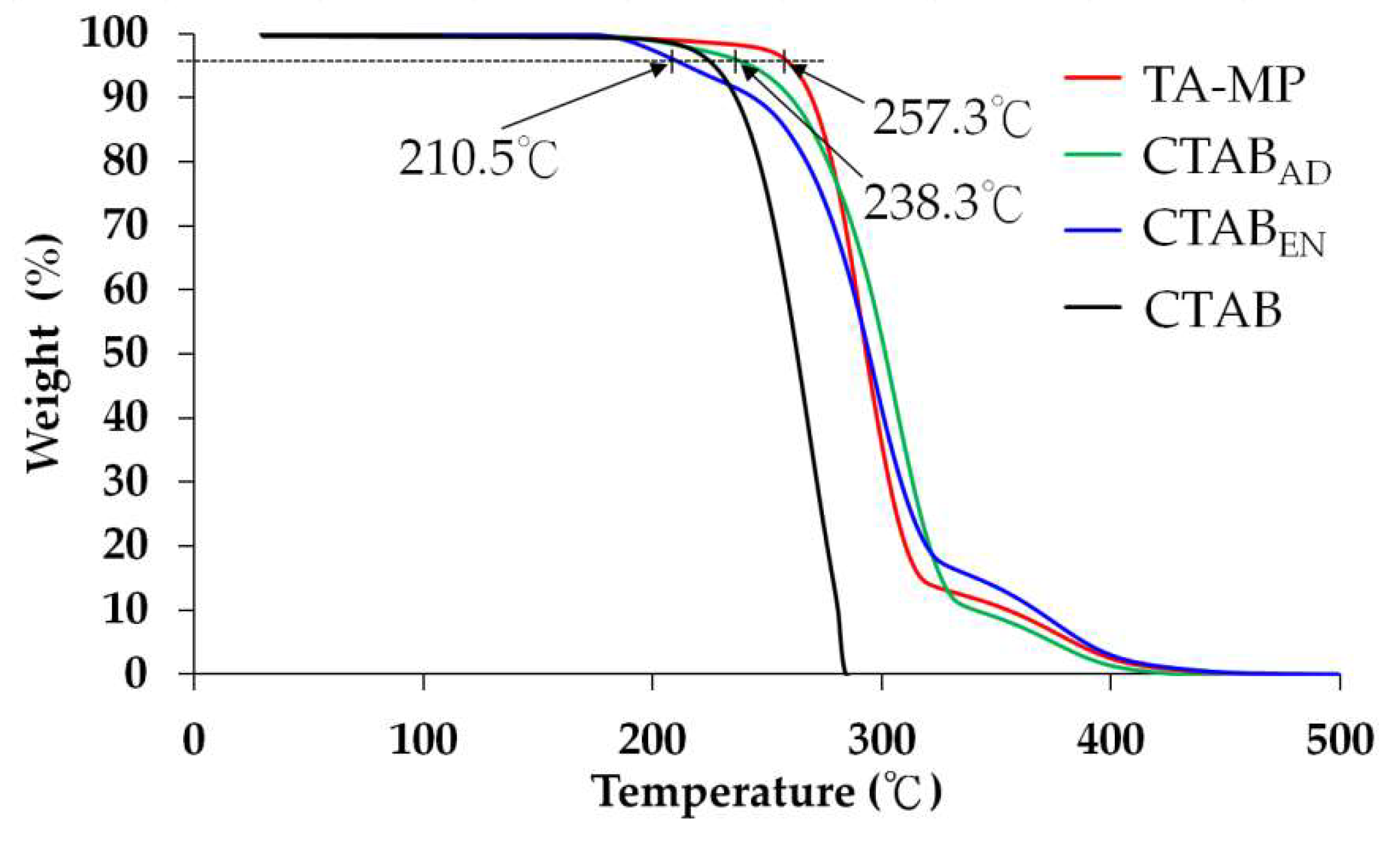
3.5. Thermogravimetric Analysis
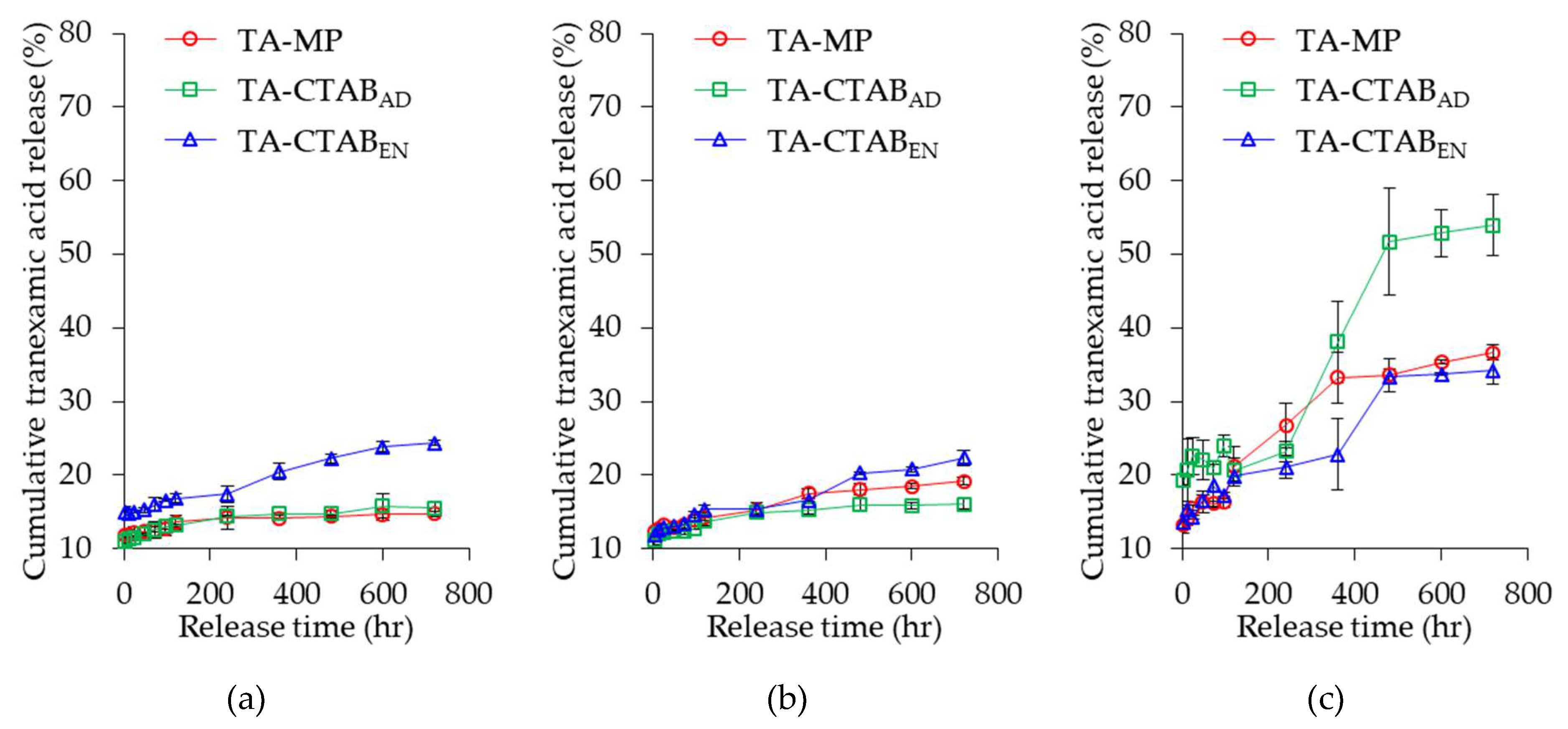
3.6. In Vitro Release
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Martin-Banderas, L.; Durán-Lobato, M.; Muñoz-Rubio, I.; Alvarez-Fuentes, J.; Fernández-Arevalo, M.; Holgado, M.A. Functional PLGA NPs for oral drug delivery: Recent strategies and developments. Mini Rev. Med. Chem. 2013, 13, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Shubhra, Q.T.H.; Tóth, J.; Gyenis, J.; Feczkó, T. Surface modification of HSA containing magnetic PLGA nanoparticles by poloxamer to decrease plasma protein adsorption. Colloids Surf. B Biointerfaces 2014, 122, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Ravi-Kumar, M.N.; Bakowsky, U.; Lehr, C.M. Preparation and characterization of cationic PLGA nanospheres as DNA carriers. Biomaterials 2004, 25, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Radovic-Moreno, A.F.; Lu, T.K.; Puscasu, V.A.; Yoon, C.J.; Langer, R.; Farokhzad, O.C. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano 2012, 6, 4279–4287. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, S.; Haddadi, A.; Hung, R.W.; Lavasanifar, A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv. Drug Deliv. Rev. 2011, 63, 943–955. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Haghi, M.; van den Oetelaar, W.; Moir, L.M.; Zhu, B.; Phillips, G.; Crapper, J.; Young, P.M.; Traini, D. Inhalable tranexamic acid for haemoptysis treatment. Eur. J. Pharm. Biopharm. 2015, 93, 311–319. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Baskaran, R.; Yoo, B.K. Skin permeation and retention of topical bead formulation containing tranexamic acid. J. Cosmet. Laser Ther. 2017, 19, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.R.; Corkill, A.G. Use of an anti-fibrinolytic agent (tranexamic acid) in the management of ruptured intracranial aneurysms. Postgrad. Med. J. 1971, 47, 199–200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanechorn Na Ayuthaya, P.; Niumphradit, N.; Manosroi, A.; Nakakes, A. Topical 5% tranexamic acid for the treatment of melasma in Asians: A double-blind randomized controlled clinical trial. J. Cosmet. Laser Ther. 2012, 14, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Tse, T.W.; Hui, E. Tranexamic acid: An important adjuvant in the treatment of melasma. J. Cosmet. Dermatol. 2013, 12, 57–66. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Valiante, N.M. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug Discov. 2003, 2, 727–735. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Singh, M.; Ulmer, J.B. Microparticles for the delivery of DNA vaccines. Immunol. Rev. 2004, 199, 191–200. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Babu, V.R.; Rangaswamy, V.; Patel, P.; Aminabhavi, T.M. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J. Control. Release 2008, 125, 193–209. [Google Scholar] [CrossRef]
- Cun, D.; Jensen, D.K.; Maltesen, M.J.; Bunker, M.; Whiteside, P.; Scurr, D.; Foged, C.; Nielsen, H.M. High loading efficiency and sustained release of siRNA encapsulated in PLGA nanoparticles: Quality by design optimization and characterization. Eur. J. Pharm. Biopharm. 2011, 77, 26–35. [Google Scholar] [CrossRef]
- El-Aroud, K.A.; Abushoffa, A.M.; Abdellatef, H.E. Spectrophotometric and spectrofluorimetric methods for the determination of tranexamic acid in pharmaceutical formulation. Chem. Pharm. Bull. 2007, 55, 364–367. [Google Scholar] [CrossRef][Green Version]
- Amoyav, B.; Benny, O. Microfluidic based fabrication and characterization of highly porous polymeric microspheres. Polymers 2019, 11, 419. [Google Scholar] [CrossRef]
- Icart, L.P.; Souza, F.G., Jr.; Lima, L.M.T.R. Sustained release and pharmacologic evaluation of human glucagon-like peptide-1 and liraglutide from polymeric microparticles. J. Microencapsul. 2019, 36, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, A.E.; Hu, W.; Nguyen, K.T.; Menon, J.U. Scaffold-based lung tumor culture on porous PLGA microparticle substrates. PLoS ONE 2019, 14, e0217640. [Google Scholar] [CrossRef] [PubMed]
- Biswal, A.K.; Hariprasad, P.; Saha, S. Efficient and prolonged antibacterial activity from porous PLGA microparticles and their application in food preservation. Mater. Sci. Eng. C 2020, 108, 110496. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.L.; Lowman, A.M. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Passerini, N.; Craig, D.Q. An investigation into the effects of residual water on the glass transition temperature of polylactide microspheres using modulated temperature DSC. J. Control. Release 2001, 73, 111–115. [Google Scholar] [CrossRef]
- Mu, L.; Feng, S.S. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin ETPGS. J. Control. Release 2003, 86, 33–48. [Google Scholar] [CrossRef]
- Attwood, D.; Elworthy, P.H.; Kayne, S.B. Membrane osmometry of aqueous micellar solutions of pure nonionic and ionic surfactants. J. Phys. Chem. 1970, 74, 3529–3534. [Google Scholar] [CrossRef]
- Huang, L.; Chen, X.; Li, Q. Synthesis of microporous molecular sieves by surfactant decomposition. J. Mater. Chem. 2001, 11, 610–615. [Google Scholar] [CrossRef]
- Li, J.; Jiang, G.; Ding, F. The effect of pH on the polymer degradation and drug release from PLGA-mPEG microparticles. J. Appl. Polym. Sci 2008, 109, 475–482. [Google Scholar] [CrossRef]
- Singh, V.; Singh, S.; Das, S.; Kumar, A.; Self, W.T.; Seal, S. A facile synthesis of PLGA encapsulated cerium oxide nanoparticles: Release kinetics and biological activity. Nanoscale 2012, 4, 2597–2605. [Google Scholar] [CrossRef]
- Sousa, F.; Cruz, A.; Fonte, P.; Pinto, I.M.; Neves-Petersen, M.T.; Sarmento, B. A new paradigm for antiangiogenic therapy through controlled release of bevacizumab from PLGA nanoparticles. Sci. Rep. 2017, 7, 3736. [Google Scholar] [CrossRef] [PubMed]
- Grassin Delyle, S.; Abe, E.; Batisse, A.; Tremey, B.; Fischler, M.; Devillier, P.; Alvarez, J.C. A validated assay for the quantitative analysis of tranexamic acid in human serum by liquid chromatography coupled with electrospray ionization mass spectrometry. Clin. Chim. Acta 2010, 411, 438–443. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.-H.; Huang, S.-Y.; Chen, Y.-X.; Chen, C.-Y.; Lin, Y.-S. Elaboration of Charged Poly(Lactic-co-Glycolic Acid) Microparticles for Effective Release of Tranexamic Acid. Polymers 2020, 12, 808. https://doi.org/10.3390/polym12040808
Huang M-H, Huang S-Y, Chen Y-X, Chen C-Y, Lin Y-S. Elaboration of Charged Poly(Lactic-co-Glycolic Acid) Microparticles for Effective Release of Tranexamic Acid. Polymers. 2020; 12(4):808. https://doi.org/10.3390/polym12040808
Chicago/Turabian StyleHuang, Ming-Hsi, Shun-Ying Huang, Yi-Xuan Chen, Cheng-You Chen, and Yung-Sheng Lin. 2020. "Elaboration of Charged Poly(Lactic-co-Glycolic Acid) Microparticles for Effective Release of Tranexamic Acid" Polymers 12, no. 4: 808. https://doi.org/10.3390/polym12040808
APA StyleHuang, M.-H., Huang, S.-Y., Chen, Y.-X., Chen, C.-Y., & Lin, Y.-S. (2020). Elaboration of Charged Poly(Lactic-co-Glycolic Acid) Microparticles for Effective Release of Tranexamic Acid. Polymers, 12(4), 808. https://doi.org/10.3390/polym12040808





