Tungsten-Doped Zinc Oxide and Indium–Zinc Oxide Films as High-Performance Electron-Transport Layers in N–I–P Perovskite Solar Cells
Abstract
1. Introduction
2. Experimental Section
2.1. Fabrication Process of the ZnON and WIZO Films
2.2. Device Fabrication
2.3. Characterization
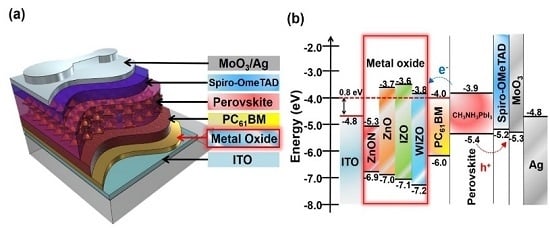
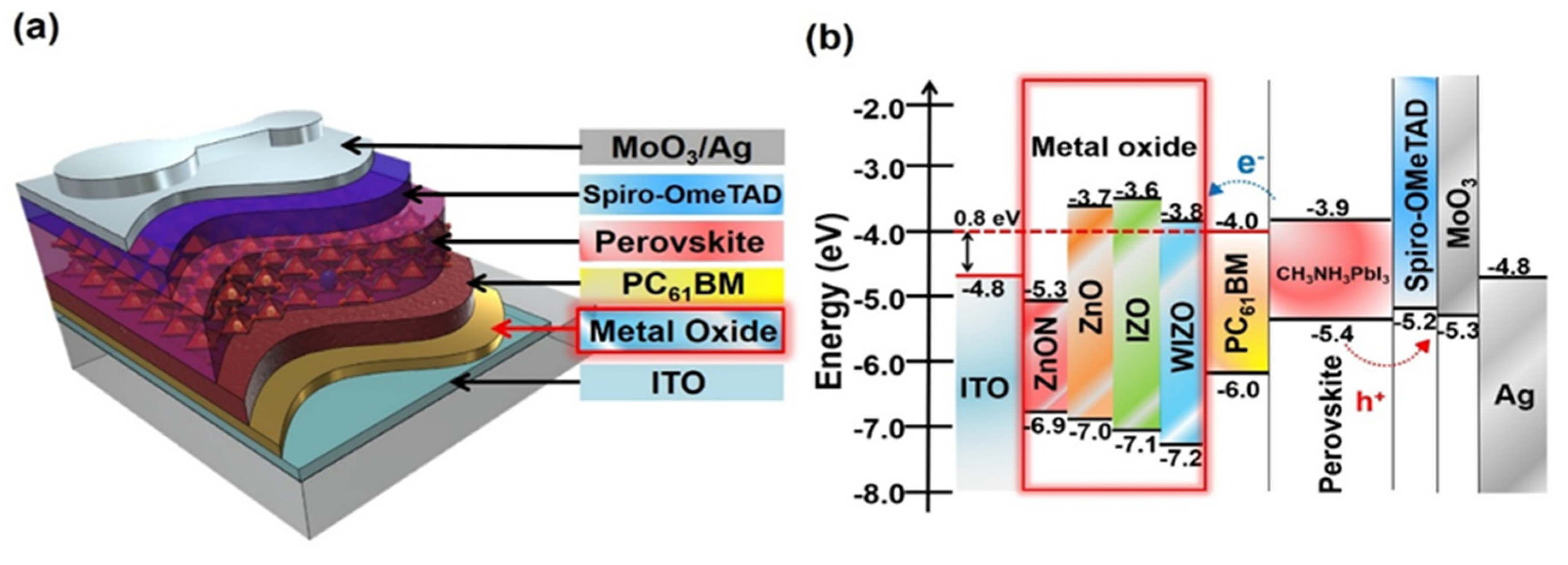
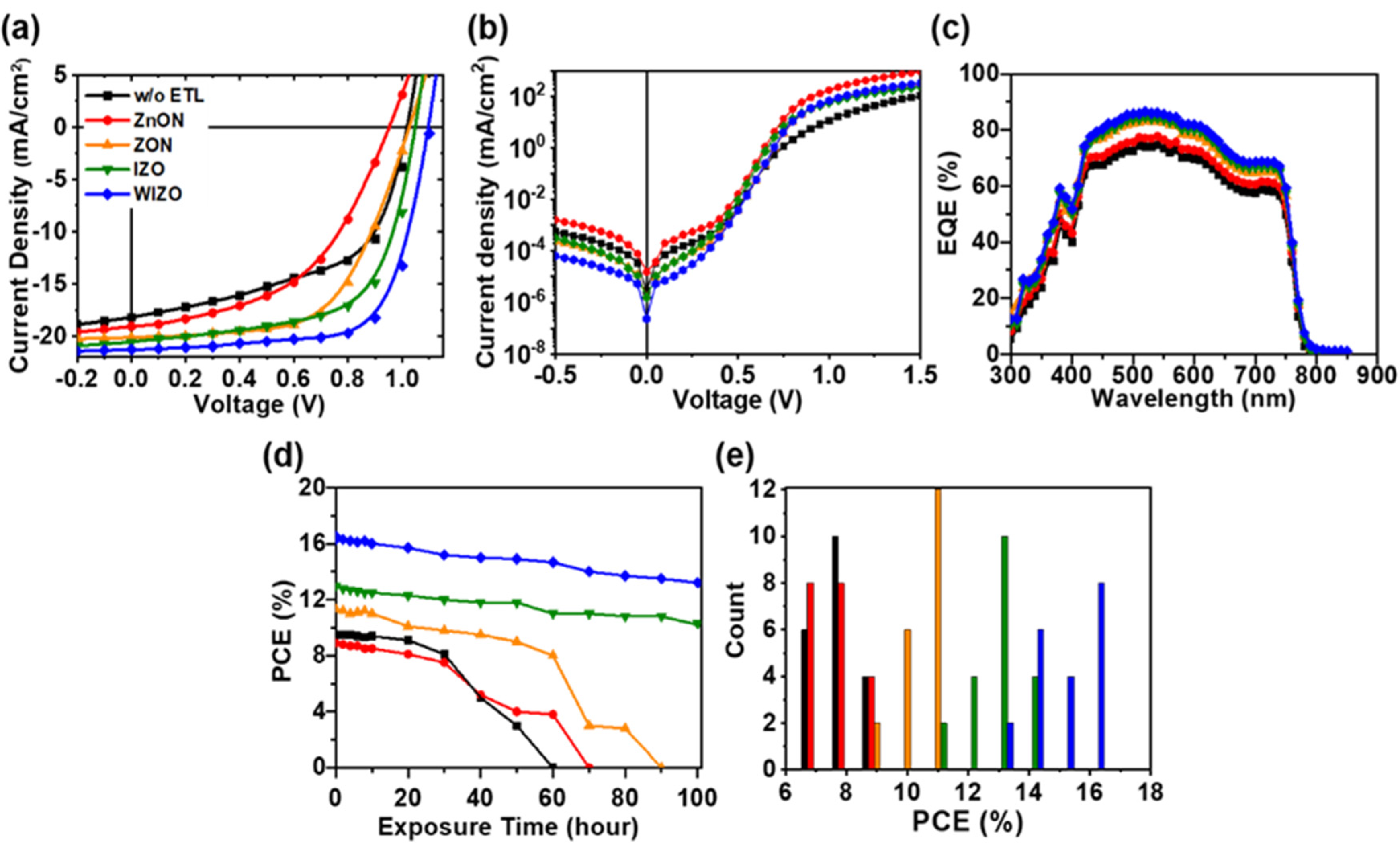
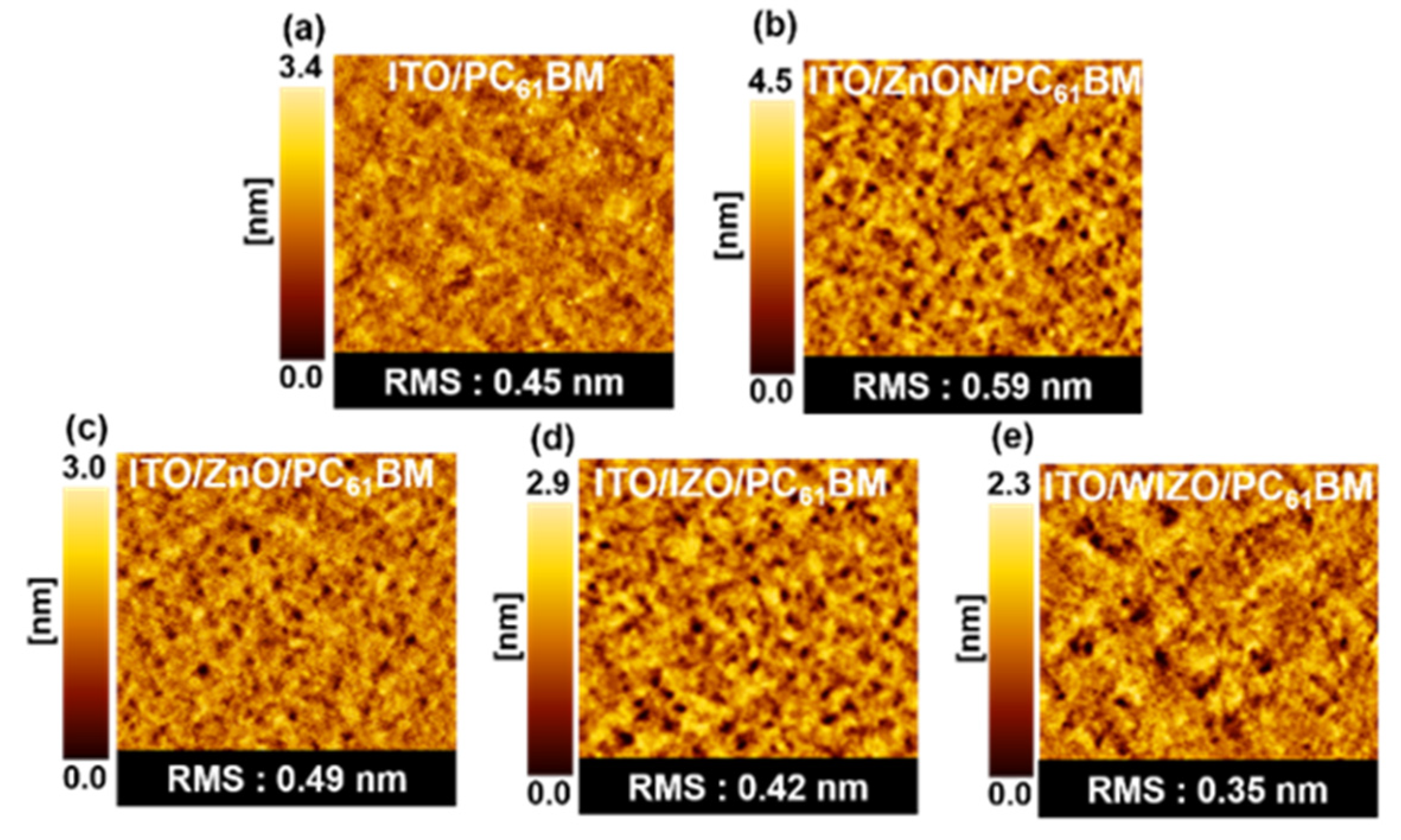
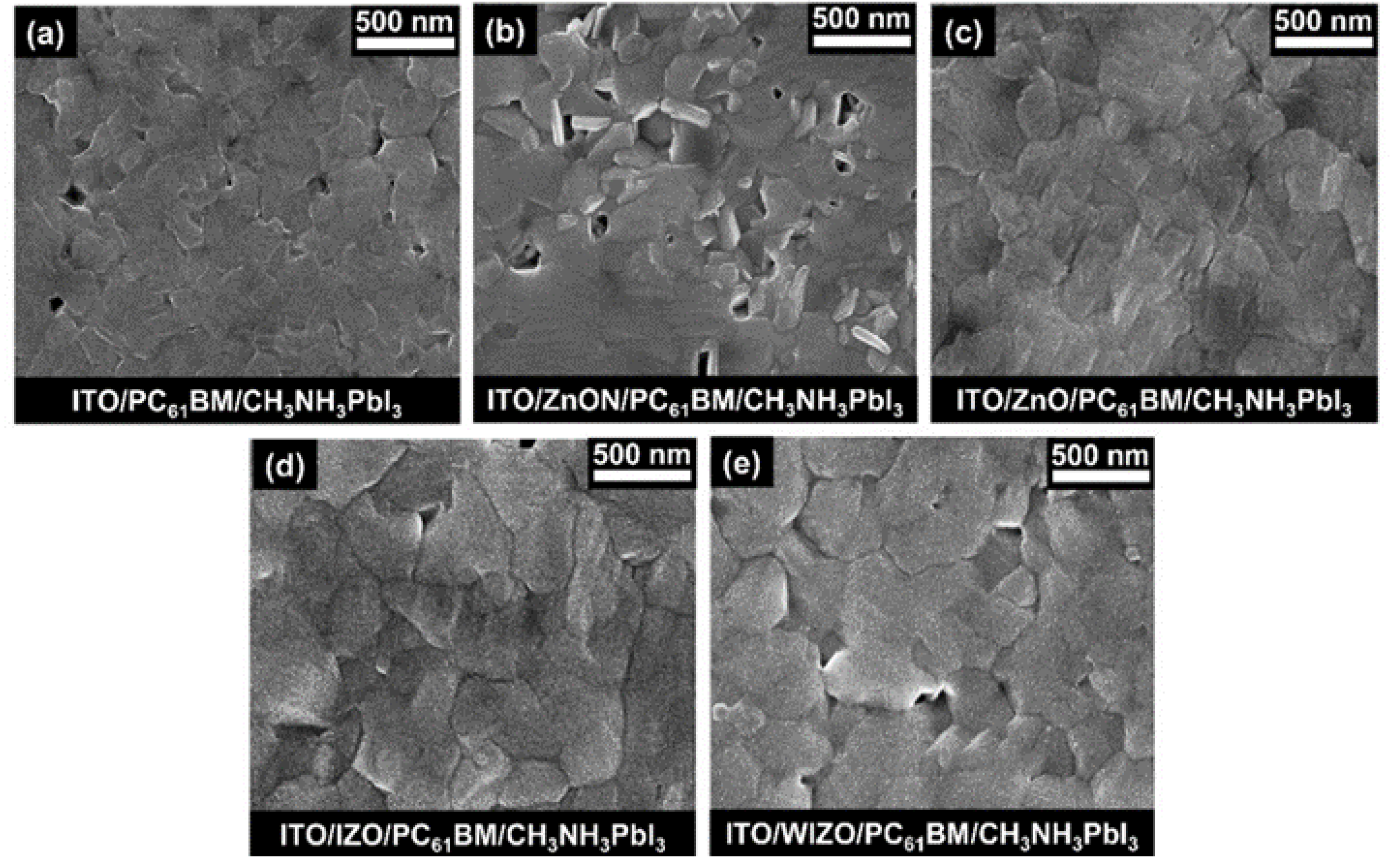
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.-C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef]
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; De Arquer, F.P.G.; Fan, J.Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y. Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths> 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.X.; Zhang, D.; Su, H.; Ren, X.; Wong, K.S.; Gratzel, M.; Choy, W.C. Vacuum-assisted thermal annealing of CH3NH3PbI3 for highly stable and efficient perovskite solar cells. ACS Nano 2015, 9, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Filip, M.R.; Eperon, G.E.; Snaith, H.J.; Giustino, F. Steric engineering of metal-halide perovskites with tunable optical band gaps. Nat. Commun. 2014, 5, 5757. [Google Scholar] [CrossRef]
- Wang, F.; Bai, S.; Tress, W.; Hagfeldt, A.; Gao, F. Defects engineering for high-performance perovskite solar cells. NPJ Flex. Electron. 2018, 2, 22. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- X Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Grätzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838. [Google Scholar] [CrossRef]
- Edri, E.; Kirmayer, S.; Mukhopadhyay, S.; Gartsman, K.; Hodes, G.; Cahen, D. Elucidating the charge carrier separation and working mechanism of CH3NH3PbI3−xClx perovskite solar cells. Nat. Commun. 2014, 5, 3461. [Google Scholar] [CrossRef]
- Shih, Y.-C.; Wang, L.; Hsieh, H.-C.; Lin, K.-F. Enhancing the photocurrent of perovskite solar cells via modification of the TiO2/CH3NH3PbI3 heterojunction interface with amino acid. J. Mater. Chem. A 2015, 3, 9133–9136. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, J.; Wang, Z.; Mu, C.; Fan, Z.; Du, L.; Bai, Y.; Fan, L.; Yan, H.; Phillips, D.L. Efficiency enhancement of perovskite solar cells through fast electron extraction: The role of graphene quantum dots. J. Am. Chem. Soc. 2014, 136, 3760–3763. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, K.; Saliba, M.; Leijtens, T.; Abate, A.; Snaith, H.J. Sub-150 °C processed meso-superstructured perovskite solar cells with enhanced efficiency. Energy Environ. Sci. 2014, 7, 1142–1147. [Google Scholar] [CrossRef]
- Liu, D.; Kelly, T.L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat. Photonics 2014, 8, 133. [Google Scholar] [CrossRef]
- Ouyang, D.; Huang, Z.; Choy, W.C. Solution-processed metal oxide nanocrystals as carrier transport layers in organic and perovskite solar cells. Adv. Funct. Mater. 2019, 29, 1804660. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.; Ban, H.; Sun, Q.; Shen, Y.; Wang, M. Efficient CsSnI3-based inorganic perovskite solar cells based on a mesoscopic metal oxide framework via incorporating a donor element. J. Mater. Chem. A 2020, 8, 4118–4124. [Google Scholar] [CrossRef]
- Fernandes, S.L.; Véron, A.C.; Neto, N.F.; Nüesch, F.A.; da Silva, J.H.D.; Zaghete, M.A.; Carlos, F.d.O. Nb2O5 hole blocking layer for hysteresis-free perovskite solar cells. Mater. Lett. 2016, 181, 103–107. [Google Scholar] [CrossRef]
- Anaraki, E.H.; Kermanpur, A.; Steier, L.; Domanski, K.; Matsui, T.; Tress, W.; Saliba, M.; Abate, A.; Grätzel, M.; Hagfeldt, A. Highly efficient and stable planar perovskite solar cells by solution-processed tin. Energy Environ. Sci. 2016, 9, 3128–3134. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J.J.N.E. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Ozaki, M.; Ishikura, Y.; Truong, M.A.; Liu, J.; Okada, I.; Tanabe, T.; Sekimoto, S.; Ohtsuki, T.; Murata, Y.; Murdey, R. Iodine-rich mixed composition perovskites optimised for tin (iv) oxide transport layers: The influence of halide ion ratio, annealing time, and ambient air aging on solar cell performance. J. Mater. Chem. A 2019, 7, 16947–16953. [Google Scholar] [CrossRef]
- Zhang, W.; Wan, L.; Li, X.; Wu, Y.; Fu, S.; Fang, J. A dopant-free polyelectrolyte hole-transport layer for high efficiency and stable planar perovskite solar cells. J. Mater. Chem. A 2019, 7, 18898–18905. [Google Scholar] [CrossRef]
- Zhao, J.; Tavakoli, R.; Tavakoli, M.M. Synergistic interface and compositional engineering of inverted perovskite solar cells enables highly efficient and stable photovoltaic devices. Chem. Commun. 2019, 55, 9196–9199. [Google Scholar] [CrossRef] [PubMed]
- Tsarev, S.; Yakushchenko, I.K.; Luchkin, S.Y.; Kuznetsov, P.M.; Timerbulatov, R.S.; Dremova, N.N.; Frolova, L.A.; Stevenson, K.J.; Troshin, P.A. A new polytriarylamine derivative for dopant-free high-efficiency perovskite solar cells. Sustain. Energy Fuels 2019, 3, 2627–2632. [Google Scholar] [CrossRef]
- Mali, S.S.; Kim, H.; Kim, H.H.; Shim, S.E.; Hong, C.K. Nanoporous p-type NiOx electrode for pin inverted perovskite solar cell toward air stability. Mater. Today 2018, 21, 483–500. [Google Scholar] [CrossRef]
- Cao, J.; Wu, B.; Chen, R.; Wu, Y.; Hui, Y.; Mao, B.W.; Zheng, N. Efficient, Hysteresis-Free, and Stable Perovskite Solar Cells with ZnO as Electron-Transport Layer: Effect of Surface Passivation. Adv. Mater. 2018, 30, 1705596. [Google Scholar] [CrossRef]
- Yu, H.; Yeom, H.I.; Lee, J.W.; Lee, K.; Hwang, D.; Yun, J.; Ryu, J.; Lee, J.; Bae, S.; Kim, S.K. Superfast Room-Temperature Activation of SnO2 Thin Films via Atmospheric Plasma Oxidation and their Application in Planar Perovskite Photovoltaics. Adv. Mater. 2018, 30, 1704825. [Google Scholar] [CrossRef]
- Scheideler, W.J.; Rolston, N.; Zhao, O.; Zhang, J.; Dauskardt, R.H. Rapid Aqueous Spray Fabrication of Robust NiOx: A Simple and Scalable Platform for Efficient Perovskite Solar Cells. Adv. Energy. 2019, 9, 1803600. [Google Scholar] [CrossRef]
- Abzieher, T.; Moghadamzadeh, S.; Schackmar, F.; Eggers, H.; Sutterlüti, F.; Farooq, A.; Kojda, D.; Habicht, K.; Schmager, R.; Mertens, A. Electron-Beam-Evaporated Nickel Oxide Hole Transport Layers for Perovskite-Based Photovoltaics. Adv. Energy Mater. 2019, 9, 1802995. [Google Scholar] [CrossRef]
- Lu, G.; Wang, X.; Du, J.; Zhang, M.; Gao, Y.; Liu, Y.; Ma, J.; Lin, Z. Enhancing Perovskite Solar Cell Performance through Surface Engineering of Metal Oxide Electron-Transporting Layer. Coatings 2020, 10, 46. [Google Scholar] [CrossRef]
- Hossain, M.I.; Yumnam, N.; Qarony, W.; Salleo, A.; Wagner, V.; Knipp, D.; Tsang, Y.H. Non-resonant metal-oxide metasurfaces for efficient perovskite solar cells. Sol. Energy 2020, 198, 570–577. [Google Scholar] [CrossRef]
- Chiu, C.; Pei, Z.; Chang, S.; Chang, S.; Chang, S.-J. Effect of oxygen partial pressure on electrical characteristics of amorphous indium gallium zinc oxide thin-film transistors fabricated by thermal annealing. Vacuum 2011, 86, 246–249. [Google Scholar] [CrossRef]
- Chen, W.-T.; Lo, S.-Y.; Kao, S.-C.; Zan, H.-W.; Tsai, C.-C.; Lin, J.-H.; Fang, C.-H.; Lee, C.-C. Oxygen-dependent instability and annealing/passivation effects in amorphous In–Ga–Zn–O thin-film transistors. IEEE Electron. Device Lett. 2011, 32, 1552–1554. [Google Scholar] [CrossRef]
- Park, K.; Choi, J.-Y.; Lee, H.-J.; Kwon, J.-Y.; Kim, H. Thin film transistor using amorphous InGaZnO films as both channel and source/drain electrodes. Jpn. J. Appl. Phys. 2011, 50, 096504. [Google Scholar] [CrossRef]
- Park, Y.J.; Song, A.; Walker, B.; Seo, J.H.; Chung, K.B. Hybrid ZnON–Organic Light Emitting Transistors with Low Threshold Voltage< 5 V. Adv. Opt. Mater. 2019, 7, 1801290. [Google Scholar]
- Park, J.; Kim, Y.S.; Ok, K.-C.; Park, Y.C.; Kim, H.Y.; Park, J.-S.; Kim, H.-S. A study on the electron transport properties of ZnON semiconductors with respect to the relative anion content. Sci. Rep. 2016, 6, 24787. [Google Scholar] [CrossRef]
- Tseng, Z.-L.; Chiang, C.-H.; Wu, C.-G. Surface engineering of ZnO thin film for high efficiency planar perovskite solar cells. Sci. Rep. 2015, 5, 13211. [Google Scholar] [CrossRef]
- Kim, J.; Kim, G.; Kim, T.K.; Kwon, S.; Back, H.; Lee, J.; Lee, S.H.; Kang, H.; Lee, K. Efficient planar-heterojunction perovskite solar cells achieved via interfacial modification of a sol–gel ZnO electron. J. Mater. Chem. A 2014, 2, 17291–17296. [Google Scholar] [CrossRef]
- Paine, D.C.; Yaglioglu, B.; Beiley, Z.; Lee, S. Amorphous IZO-based transparent thin film transistors. Thin Solid Films 2008, 516, 5894–5898. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Cai, X.; Ma, T.; Jiang, C. Indium Zinc Oxide Electron Transport Layer for High-Performance Planar Perovskite Solar Cells. J. Phy. Chem. C 2018, 122, 28491–28496. [Google Scholar] [CrossRef]
- Park, H.-W.; Song, A.; Kwon, S.; Choi, D.; Kim, Y.; Jun, B.-H.; Kim, H.-K.; Chung, K.-B. Enhancing the performance of tungsten doped InZnO thin film transistors via sequential ambient annealing. Appl. Phys. Lett. 2018, 112, 123501. [Google Scholar] [CrossRef]
- Park, H.-W.; Song, A.; Kwon, S.; Du Ahn, B.; Chung, K.-B. Improvement of device performance and instability of tungsten-doped InZnO thin-film transistor with respect to doping concentration. Appl. Phys. Express 2016, 9, 111101. [Google Scholar] [CrossRef]
- Park, H.-W.; Song, A.; Choi, D.; Kim, H.-J.; Kwon, J.-Y.; Chung, K.-B. Enhancement of the device performance and the stability with a homojunction-structured tungsten indium zinc oxide thin film transistor. Sci. Rep. 2017, 7, 11634. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, J.; Xiao, L.; Chen, J.; Tan, Z.a.; Yao, J.; Dai, S. Efficient planar perovskite solar cells prepared via a low-pressure vapor-assisted solution process with fullerene/TiO2 as an electron collection bilayer. RSC Adv. 2016, 6, 78585–78594. [Google Scholar] [CrossRef]
- Ke, W.; Zhao, D.; Grice, C.R.; Cimaroli, A.J.; Ge, J.; Tao, H.; Lei, H.; Fang, G.; Yan, Y. Efficient planar perovskite solar cells using room-temperature vacuum-processed C60 electron selective layers. J. Mater. Chem. A 2015, 3, 17971–17976. [Google Scholar] [CrossRef]
- Ke, W.; Zhao, D.; Xiao, C.; Wang, C.; Cimaroli, A.J.; Grice, C.R.; Yang, M.; Li, Z.; Jiang, C.-S.; Al-Jassim, M. Cooperative tin oxide fullerene electron selective layers for high-performance planar perovskite solar cells. J. Mater. Chem. A 2016, 4, 14276–14283. [Google Scholar] [CrossRef]
- Mori, T.; Kato, K. Photovoltaic properties of organic thin-film solar cell using various exciton-diffusion blocking materials. J. Photopolym. Sci. Technol. 2007, 20, 61–66. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific: Singapore, 2011; pp. 80–84. [Google Scholar]
- Yang, W.; Zhong, D.; Shi, M.; Qu, S.; Chen, H. Toward Highly Thermal Stable Perovskite Solar Cells by Rational Design of Interfacial Layer. iScience 2019, 22, 534–543. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, F.; Wei, Q.; Li, H.; Shang, Y.; Zhou, W.; Wang, C.; Cheng, P.; Chen, Q.; Chen, L. Ultra-high open-circuit voltage of tin perovskite solar cells via an electron transporting layer design. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Mahmood, K.; Sarwar, S.; Mehran, M.T. Current status of electron transport layers in perovskite solar cells: Materials and properties. RSC Adv. 2017, 7, 17044–17062. [Google Scholar] [CrossRef]
- Shao, Y.; Xiao, Z.; Bi, C.; Yuan, Y.; Huang, J. Origin and elimination of photocurrent hysteresis by fullerene passivation in CH3NH3PbI3 planar heterojunction solar cells. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shao, Y.; Dong, Q.; Xiao, Z.; Yuan, Y.; Huang, J. Large fill-factor bilayer iodine perovskite solar cells fabricated by a low-temperature solution-process. Energy Environ. Sci. 2014, 7, 2359–2365. [Google Scholar] [CrossRef]
- Xu, J.; Buin, A.; Ip, A.H.; Li, W.; Voznyy, O.; Comin, R.; Yuan, M.; Jeon, S.; Ning, Z.; McDowell, J.J. Perovskite–fullerene hybrid materials suppress hysteresis in planar diodes. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, Y.S.; Kim, J.H.; Park, K.; Park, Y.C.; Kim, H.-S. The effects of active layer thickness and annealing conditions on the electrical performance of ZnON thin-film transistors. J. Alloys Compd. 2016, 688, 666–671. [Google Scholar] [CrossRef]
- Park, H.-W.; Park, K.; Kwon, J.-Y.; Choi, D.; Chung, K.-B. Effect of active layer thickness on device performance of tungsten-doped InZnO thin-film transistor. IEEE Trans. Electron Devices 2016, 64, 159–163. [Google Scholar] [CrossRef]
- Shao, Y.; Yuan, Y.; Huang, J. Correlation of energy disorder and open-circuit voltage in hybrid perovskite solar cells. Nat. Energy 2016, 1, 15001. [Google Scholar] [CrossRef]
- Zhang, M.-D.; Zhao, D.-X.; Chen, L.; Pan, N.; Huang, C.-Y.; Cao, H.; Chen, M.-D. Structure-performance relationship on the asymmetric methoxy substituents of spiro-OMeTAD for perovskite solar cells. Solar Energy Mater. Solar Cells 2018, 176, 318–323. [Google Scholar] [CrossRef]
- Zhu, Q.; Bao, X.; Yu, J.; Zhu, D.; Qiu, M.; Yang, R.; Dong, L. Compact layer free perovskite solar cells with a high-mobility hole-transporting layer. ACS Appl. Mater. Interfaces 2016, 8, 2652–2657. [Google Scholar] [CrossRef]
- Futsuhara, M.; Yoshioka, K.; Takai, O. Optical properties of zinc oxynitride thin films. Thin Solid Films 1998, 317, 322–325. [Google Scholar] [CrossRef]
- Tvingstedt, K.; Malinkiewicz, O.; Baumann, A.; Deibel, C.; Snaith, H.J.; Dyakonov, V.; Bolink, H.J. Radiative efficiency of lead iodide based perovskite solar cells. Sci. Rep. 2014, 4, 6071. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Wu, T.; Ahmadi, M.; Liu, L.; Haacke, S.; Guo, T.-F.; Hu, B. Simultaneously enhancing dissociation and suppressing recombination in perovskite solar cells. Nano Energy 2017, 36, 95–101. [Google Scholar] [CrossRef]
- Gelmetti, I.; Montcada, N.F.; Pérez-Rodríguez, A.; Barrena, E.; Ocal, C.; García-Benito, I.; Molina-Ontoria, A.; Martín, N.; Vidal-Ferran, A.; Palomares, E. Energy alignment and recombination in perovskite solar cells: Weighted influence on the open circuit voltage. Energy Environ. Sci. 2019, 12, 1309–1316. [Google Scholar] [CrossRef]
- Sherkar, T.S.; Momblona, C.; Gil-Escrig, L.N.; Ávila, J.; Sessolo, M.; Bolink, H.J.; Koster, L.J.A. Recombination in perovskite solar cells: Significance of grain boundaries, interface traps, and defect ions. ACS Energy Lett. 2017, 2, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Q.; Hong, Z.; Zhou, H.; Xu, X.; De Marco, N.; Sun, P.; Zhao, Z.; Cheng, Y.-B.; Yang, Y. Low-temperature TiO x compact layer for planar heterojunction perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 11076–11083. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.S.; Chen, B.X.; Li, W.G.; Xu, Y.F.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. Improving the extraction of photogenerated electrons with SnO2 nanocolloids for efficient planar perovskite solar cells. Adv. Funct. Mater. 2015, 25, 7200–7207. [Google Scholar] [CrossRef]
- Shi, J.; Xu, X.; Li, D.; Meng, Q. Interfaces in perovskite solar cells. Small 2015, 11, 2472–2486. [Google Scholar] [CrossRef] [PubMed]
- Berhe, T.A.; Su, W.-N.; Chen, C.-H.; Pan, C.-J.; Cheng, J.-H.; Chen, H.-M.; Tsai, M.-C.; Chen, L.-Y.; Dubale, A.A.; Hwang, B.-J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Jung, E.H.; Jeon, N.J.; Park, E.Y.; Moon, C.S.; Shin, T.J.; Yang, T.-Y.; Noh, J.H.; Seo, J. Efficient, stable and scalable perovskite solar cells using poly (3-hexylthiophene). Nature 2019, 567, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, H.; Li, G.; Fang, G. Stabilizer-assisted growth of formamdinium-based perovskites for highly efficient and stable planar solar cells with over 22% efficiency. Nano Energy 2019, 63, 103835. [Google Scholar] [CrossRef]
- Hu, M.; Bi, C.; Yuan, Y.; Bai, Y.; Huang, J. Stabilized wide bandgap MAPbBrxI3–x perovskite by enhanced grain size and improved crystallinity. Adv. Sci. 2016, 3, 1500301. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, A.K.K.; Wang, D.H.; Gupta, V.; Zhang, J.; Chand, S.; Bazan, G.C.; Heeger, A.J. Efficient solution-processed small-molecule solar cells with inverted structure. Adv. Mater. 2013, 25, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.J.; Choi, H.; Hoang, Q.V.; Song, C.E.; Morin, P.O.; Heo, J.; Leclerc, M.; Yoon, S.C.; Woo, H.Y.; Shin, W.S. Modeling and implementation of tandem polymer solar cells using wide-bandgap front cells. Carbon Energy 2020, 2, 131–142. [Google Scholar] [CrossRef]
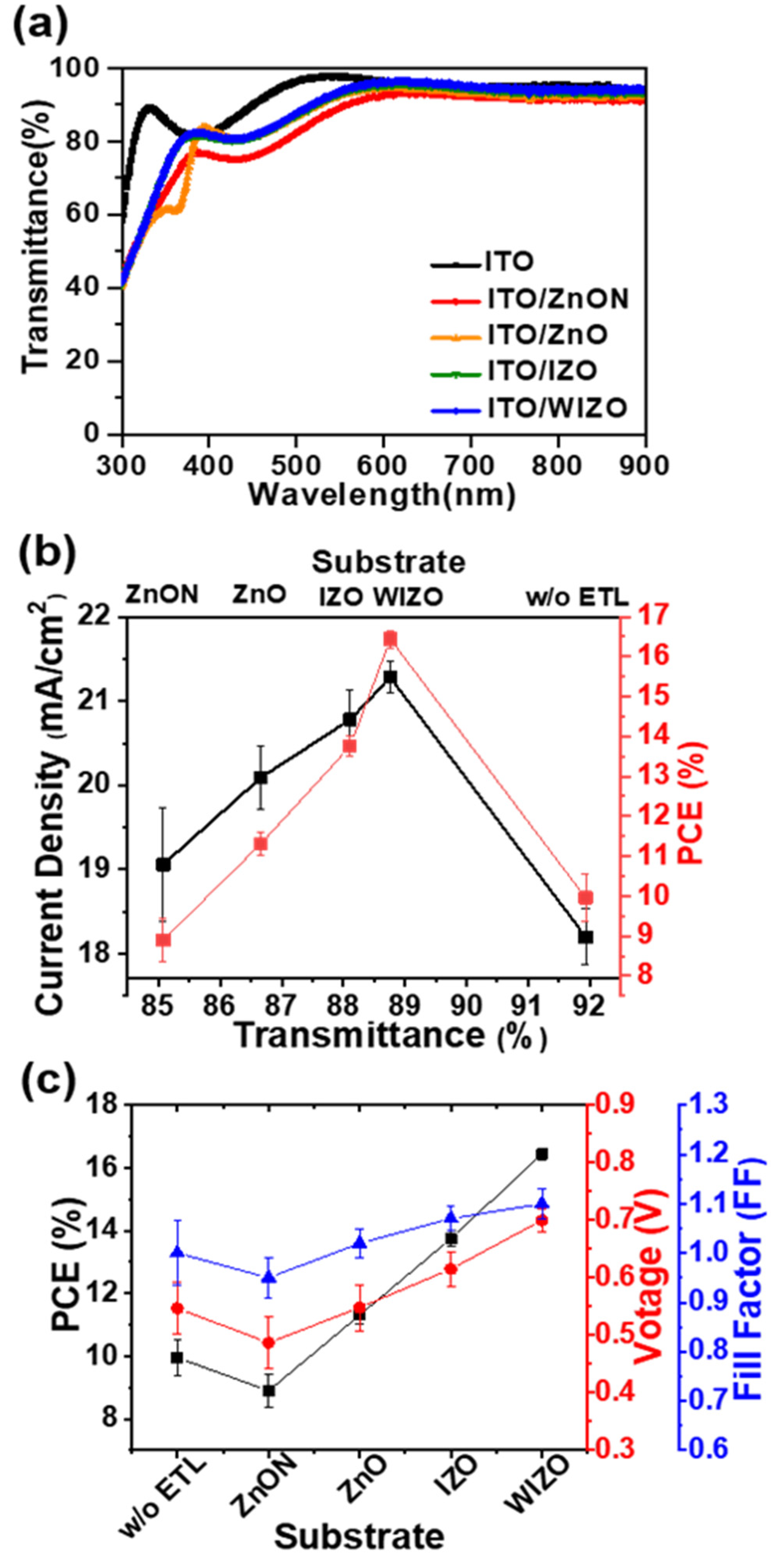
| Substrates | VOC (V) | JSC (mA cm-2) | FF | RS (Ω cm2) | RSH (Ω cm2) | PCE (%) | |
|---|---|---|---|---|---|---|---|
| Champion | Average | ||||||
| No ETL | 1.00 ± 0.13 | 18.20 ± 0.33 | 0.547 ± 0.09 | 14.4 | 173 | 9.955 | 8.98 ± 0.59 |
| ZnON | 0.95 ± 0.08 | 19.06 ± 0.53 | 0.487 ± 0.09 | 18.4 | 244 | 8.90 | 8.23 ± 0.67 |
| ZnO | 1.02 ± 0.06 | 20.09 ± 0.29 | 0.548 ± 0.08 | 15.8 | 354 | 11.31 | 10.94 ± 0.38 |
| IZO | 1.07 ± 0.05 | 20.78 ± 0.25 | 0.615 ± 0.06 | 12.4 | 383 | 13.75 | 13.32 ± 0.35 |
| WIZO | 1.10 ± 0.06 | 21.28 ± 0.19 | 0.700 ± 0.04 | 7.89 | 602 | 16.44 | 16.05 ± 0.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.H.; Song, A.; Park, Y.J.; Seo, J.H.; Walker, B.; Chung, K.-B. Tungsten-Doped Zinc Oxide and Indium–Zinc Oxide Films as High-Performance Electron-Transport Layers in N–I–P Perovskite Solar Cells. Polymers 2020, 12, 737. https://doi.org/10.3390/polym12040737
Kang JH, Song A, Park YJ, Seo JH, Walker B, Chung K-B. Tungsten-Doped Zinc Oxide and Indium–Zinc Oxide Films as High-Performance Electron-Transport Layers in N–I–P Perovskite Solar Cells. Polymers. 2020; 12(4):737. https://doi.org/10.3390/polym12040737
Chicago/Turabian StyleKang, Ju Hwan, Aeran Song, Yu Jung Park, Jung Hwa Seo, Bright Walker, and Kwun-Bum Chung. 2020. "Tungsten-Doped Zinc Oxide and Indium–Zinc Oxide Films as High-Performance Electron-Transport Layers in N–I–P Perovskite Solar Cells" Polymers 12, no. 4: 737. https://doi.org/10.3390/polym12040737
APA StyleKang, J. H., Song, A., Park, Y. J., Seo, J. H., Walker, B., & Chung, K.-B. (2020). Tungsten-Doped Zinc Oxide and Indium–Zinc Oxide Films as High-Performance Electron-Transport Layers in N–I–P Perovskite Solar Cells. Polymers, 12(4), 737. https://doi.org/10.3390/polym12040737