Recent Progress on Luminescent Metal-Organic Framework-Involved Hybrid Materials for Rapid Determination of Contaminants in Environment and Food
Abstract
1. Introduction
1.1. The Harmful Substance for Health
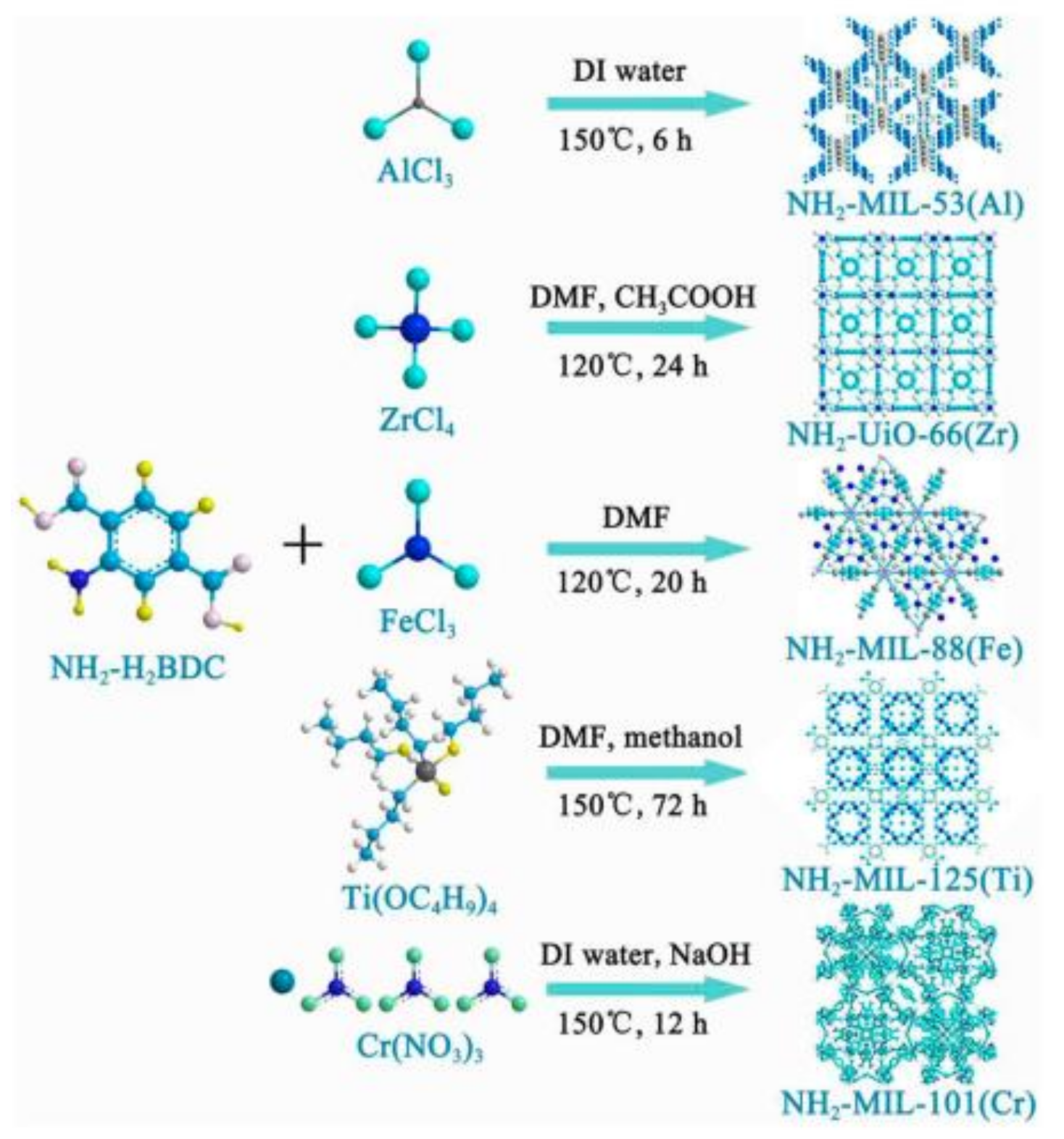
1.2. The Introduction of Metal-Organic Frameworks
1.2.1. The Solvothermal Method
1.2.2. The Mechanochemical Synthesis Method
1.2.3. The Sonochemical Method
1.2.4. The Electrochemical Synthesis
1.3. The Hybrid Materials Based on MOFs
1.4. The Harnessing of MOFs and Their Hybrid Materials
1.5. The Advantages of Metal-Organic Framework as Fluorescent Sensing Materials
- There are many ways to synthesize luminescent MOFs conveniently [108]. Diverse organic ligands and metal nodes of MOFs could recruit optical emission upon excitation with exposure to UV or visible light [109,110]. Additionally, the doping of the luminescent component expanded the application greatly.
- The organic-inorganic hybrid properties of metal-organic frameworks which containing organic ligands and inorganic metal ions enable coexistence of hydrophilic and hydrophobic channels [111]. The proper pretreatment and sample extraction procedures were indispensable to the traditional detection of food contaminants [112]. The detection sensors based on MOFs and its functional materials could overcome such constraints. The sensors possessed rapid response under practical conditions [113]. Meanwhile, they can realize intelligence, miniaturization, and both qualitative and quantitative analysis [114].
- MOFs with unlimited active sites can be prone to functionalize with some biological or chemical components [115,116,117,118,119]. The various fabrication strategies, along with the notable luminescence variation for target analytes, enable the luminescent MOFs to be broader applications in monitoring of quality and safety on environment and food.
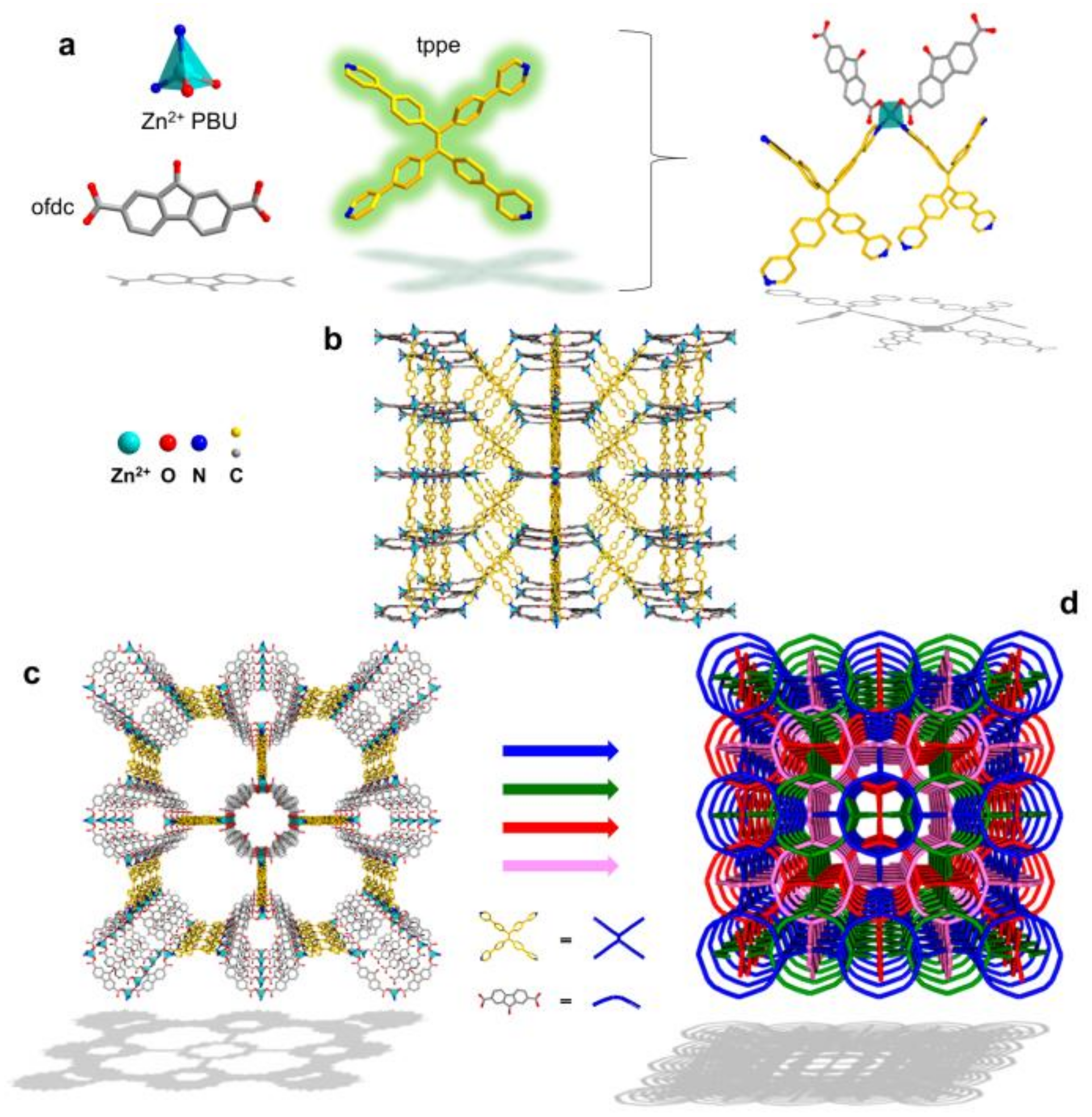
2. The Current States and Progress of MOFs Fluorescence Sensing Application
2.1. Heavy Metal Detection
2.2. Pharmaceuticals
2.2.1. Antibiotic Detection
2.2.2. Hormones
2.3. Pesticides
2.4. Persistent Organic Pollutants
2.5. Other Hazardous Substances
2.5.1. Mycotoxins
2.5.2. Amines
3. Future Perspectives and Concluding Remarks
- Raising the water stability and reusability of LMOFs. This can be achieved by introducing high valence metallic metals and multi-dental organic ligands into LMOFs.
- Enhance the fluorescence stability of LMOFs to improve the performance of targets detection, particularly.
- For improving adsorption efficiency for the range of quantitative phase analysis of targets: (i) the precise control for the channel of LMOFs, where the pore diameter is fitting the molecular dimension of targets; (ii) the improvement of pore affinity, which can be tuned by adjusting the organic ligands to increase the number of electrophilic groups of to bind the targets; (iii) increase hydrogen bond binding sites, which can be enhanced by introducing oxygen-containing functional groups on MOFs to bind targets.
Author Contributions
Funding
Conflicts of Interest
References
- Clarkson, T. Environmental contaminants in the food chain. Am. J. Clin. Nutr. 1995, 61, 682S–686S. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.; Mukherjee, S.; Rudd, N.; Desai, A.; Li, J.; Ghosh, S. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Song, S.; Wang, R.; Liu, Z.; Meng, J.; Sweetman, A.J.; Jenkins, A.; Ferrier, R.C.; Li, H.; Luo, W.; et al. Impacts of soil and water pollution on food safety and health risks in China. Environ. Int. 2015, 77, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, E.; Calamari, D.; Natangelo, M.; Fanelli, R. Presence of therapeutic drugs in the environment. Lancet 2000, 355, 1789–1790. [Google Scholar] [CrossRef]
- van Nuijs, A.; Castiglioni, S.; Tarcomnicu, I.; Postigo, C.; Lopez de Alda, M.; Neels, H.; Zuccato, E.; Barcelo, D.; Covaci, A. Illicit drug consumption estimations derived from wastewater analysis: A critical review. Sci. Total Environ. 2011, 409, 3564–3577. [Google Scholar] [CrossRef]
- Zuccato, E.; Chiabrando, C.; Castiglioni, S.; Bagnati, R.; Fanelli, R. Estimating Community Drug Abuse by Wastewater Analysis. Environ. Health Perspect. 2008, 116, 1027–1032. [Google Scholar] [CrossRef]
- Lam, H.-M.; Remais, J.; Fung, M.-C.; Xu, L.; Sun, S.S.-M. Food supply and food safety issues in China. Lancet 2013, 381, 2044–2053. [Google Scholar] [CrossRef]
- Guerinot, M. To improve nutrition for the world’s population—Response. Science 2000, 288, 1966–1967. [Google Scholar]
- Rojstaczer, S.; Sterling, S.; Moore, N. Human Appropriation of Photosynthesis Products. Science 2002, 294, 2549–2552. [Google Scholar] [CrossRef]
- Beeton, R.J.S. Sustainably managing food production resources to maximise human nutritional benefit. Asia Pac. J. Clin. Nutr. 2003, 12, S50. [Google Scholar]
- Imhoff, M.L.; Bounoua, L.; Ricketts, T.; Loucks, C.; Harriss, R.; Lawrence, W.T. Global patterns in human consumption of net primary production. Nature 2004, 429, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.; O’Keeffe, M.; Ockwig, N.; Chae, H.; Eddaoudi, M.; Kim, J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Chang, Z.; Yang, D.; Xu, J.; Hu, T.-L.; Bu, X.-H. Flexible Metal–Organic Frameworks: Recent Advances and Potential Applications. Adv. Mater. 2015, 27, 5432–5441. [Google Scholar] [CrossRef] [PubMed]
- Gangu, K.; Maddila, S.; Mukkamala, S.; Jonnalagadda, S. A Review on contemporary Metal-Organic Framework materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.-H.; Bansal, V.; Paul, A.K.; Deep, A. Practical utilization of nanocrystal metal organic framework biosensor for parathion specific recognition. Microchem. J. 2016, 128, 102–107. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Xu, Q. Porous metal–organic frameworks as platforms for functional applications. Chem. Commun. 2011, 47, 3351–3370. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Kustov, L. The application of metal-organic frameworks in catalysis (Review). Pet. Chem. 2010, 50, 167–180. [Google Scholar] [CrossRef]
- Wang, Y.; Rui, M.; Lu, G. Recent applications of metal-organic frameworks in sample pretreatment. J. Sep. Sci. 2017, 41, 180–194. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. Trac Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Salar-Amoli, J.; Ali-Esfahani, T. Determination of hazardous substances in food basket eggs in Tehran, Iran: A preliminary study. Vet. Res. Forum. Int. Q. J. 2015, 6, 155–159. [Google Scholar]
- Lee, Y.-R.; Kim, J.; Ahn, W.-S. Synthesis of metal-organic frameworks: A mini review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Zhang, J. Three two-dimensional coordination polymers constructed from transition metals and 2,3-norbornanedicarboxylic acid: Hydrothermal synthesis, crystal structures and photocatalytic properties. J. Mol. Struct. 2017, 1130, 223–230. [Google Scholar] [CrossRef]
- Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Férey, G. A New Photoactive Crystalline Highly Porous Titanium (IV) Dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Liu, X.; Shi, W.; Huang, Y. Metal–organic framework MIL-53(Fe): Facile microwave-assisted synthesis and use as a highly active peroxidase mimetic for glucose biosensing. RSC Adv. 2015, 5, 17451–17457. [Google Scholar] [CrossRef]
- Taddei, M.; Dau, P.; Cohen, S.; Ranocchiari, M.; Bokhoven, J.; Costantino, F.; Sabatini, S.; Vivani, R. Efficient Microwave Assisted Synthesis of Metal-Organic Framework UiO-66: Optimization and Scale Up. Dalton Trans. 2015, 44, 14019–14026. [Google Scholar] [CrossRef]
- Hu, S.-M.; Niu, H.-L.; Qiu, L.-G.; Yuan, Y.-P.; Jiang, X.; Xie, A.-J.; Shen, Y.-H.; Zhu, J.-F. Facile synthesis of highly luminescent nanowires of a terbium-based metal–organic framework by an ultrasonic-assisted method and their application as a luminescent probe for selective sensing of organoamines. Inorg. Chem. Commun. 2012, 17, 147–150. [Google Scholar] [CrossRef]
- Wang, G.-Y.; Song, C.; Kong, D.-M.; Ruan, W.-J.; Chang, Z.; Li, Y. Two luminescent metal–organic frameworks for the sensing of nitroaromatic explosives and DNA strands. J. Mater. Chem. A 2014, 2, 2213–2220. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, Y.; Liu, X.; Ye, W.; Hu, G.; Lei, B.; Yan, Y. Luminescence enhancement of CaMoO4:Eu3+ phosphor by charge compensation using microwave sintering method. J. Adv. Ceram. 2015, 4, 318–325. [Google Scholar] [CrossRef]
- Klimakow, M.; Klobes, P.; Thünemann, A.F.; Rademann, K.; Emmerling, F. Mechanochemical Synthesis of Metal−Organic Frameworks: A Fast and Facile Approach toward Quantitative Yields and High Specific Surface Areas. Chem. Mater. 2010, 22, 5216–5221. [Google Scholar] [CrossRef]
- Yuan, W.; Friscic, T.; Apperley, D.; James, S. High Reactivity of Metal-Organic Frameworks under Grinding Conditions: Parallels with Organic Molecular Materials. Angew. Chem. 2010, 49, 3916–3919. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Reid, D.G.; Halasz, I.; Stein, R.S.; Dinnebier, R.E.; Duer, M.J. Ion- and Liquid-Assisted Grinding: Improved Mechanochemical Synthesis of Metal–Organic Frameworks Reveals Salt Inclusion and Anion Templating. Angew. Chem. Int. Ed. 2010, 49, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Alammar, T.; Hlova, I.Z.; Gupta, S.; Balema, V.; Pecharsky, V.K.; Mudring, A.-V. Luminescence properties of mechanochemically synthesized lanthanide containing MIL-78 MOFs. Dalton Trans. 2018, 47, 7594–7601. [Google Scholar] [CrossRef] [PubMed]
- Son, W.-J.; Kim, J.; Kim, J.; Ahn, W.-S. Sonochemical synthesis of MOF-5. Chem. Commun. 2009, 47, 6336–6338. [Google Scholar] [CrossRef]
- Yang, D.-A.; Cho, H.-Y.; Kim, J.; Yang, S.-T.; Ahn, W.-S. CO2 capture and conversion using Mg-MOF-74 prepared by a sonochemical method. Energy Environ. Sci. 2012, 5, 6465–6467. [Google Scholar] [CrossRef]
- Jung, D.-W.; Yang, D.-A.; Kim, J.; Kim, J.; Ahn, W.-S. Facile synthesis of MOF-177 by a sonochemical method using 1-methyl-2-pyrrolidinone as a solvent. Dalton Trans. 2010, 39, 2883–2887. [Google Scholar] [CrossRef]
- Ni, Z.; Masel, R. Rapid Production of Metal-Organic Frameworks Via Microwave-Assisted Solvothermal Synthesis. J. Am. Chem. Soc. 2006, 128, 12394–12395. [Google Scholar] [CrossRef]
- Wu, X.; Bao, Z.; Yuan, B.; Wang, J.; Sun, Y.; Luo, H.; Deng, S. 308394 Microwave Synthesis and Characterization of MOF-74 (M = Ni, Mg) for Gas Separation. Microporous Mesoporous Mater. 2013, 180, 114–122. [Google Scholar] [CrossRef]
- Ren, J.; Langmi, H.W.; North, B.; Mathe, M.; Bessarabov, D. Modulated synthesis of zirconium-metal organic framework (Zr-MOF) for hydrogen storage applications. Int. J. Hydrogen Energy 2014, 39, 890–895. [Google Scholar] [CrossRef]
- Van Assche, T.R.C.; Desmet, G.; Ameloot, R.; De Vos, D.E.; Terryn, H.; Denayer, J.F.M. Electrochemical synthesis of thin HKUST-1 layers on copper mesh. Microporous Mesoporous Mater. 2012, 158, 209–213. [Google Scholar] [CrossRef]
- Ameloot, R.; Stappers, L.; Fransaer, J.; Alaerts, L.; Sels, B.; De Vos, D. Patterned Growth of Metal-Organic Framework Coatings by Electrochemical Synthesis. Chem. Mater. 2009, 21, 2580–2582. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Senthil Kumar, S.; Anbu Kulandainathan, M. Efficient electrosynthesis of highly active Cu3(BTC)2-MOF and its catalytic application to chemical reduction. Microporous Mesoporous Mater. 2013, 168, 57–64. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material. Science 1999, 283, 1148. [Google Scholar] [CrossRef] [PubMed]
- Campagnol, N.; Souza, E.R.; De Vos, D.E.; Binnemans, K.; Fransaer, J. Luminescent terbium-containing metal–organic framework films: New approaches for the electrochemical synthesis and application as detectors for explosives. Chem. Commun. 2014, 50, 12545–12547. [Google Scholar] [CrossRef]
- Gu, Z.-G.; Fu, H.; Neumann, T.; Xu, Z.-X.; Fu, W.-Q.; Wenzel, W.; Zhang, L.; Zhang, J.; Wöll, C. Chiral Porous Metacrystals: Employing Liquid-Phase Epitaxy to Assemble Enantiopure Metal-Organic Nanoclusters into Molecular Framework Pores. ACS Nano 2015, 10, 977–983. [Google Scholar] [CrossRef]
- Gu, Z.-G.; Zhang, J. Epitaxial growth and applications of oriented metal–organic framework thin films. Coord. Chem. Rev. 2019, 378, 513–532. [Google Scholar] [CrossRef]
- Gu, Z.-G.; Li, D.-J.; Zheng, C.; Kang, Y.; Wöll, C.; Zhang, J. MOF-Templated Synthesis of Ultrasmall Photoluminescent Carbon-Nanodot Arrays for Optical Applications. Angew. Chem. Int. Ed. 2017, 56, 6853–6858. [Google Scholar] [CrossRef]
- Rösler, C.; Fischer, R.A. Metal–organic frameworks as hosts for nanoparticles. CrystEngComm 2015, 17, 199–217. [Google Scholar] [CrossRef]
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal–Organic Frameworks as Platforms for Functional Materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, Y.; Liu, Q.; Xia, Z. Encapsulation of CH3NH3PbBr3 Perovskite Quantum Dots in MOF-5 Microcrystals as a Stable Platform for Temperature and Aqueous Heavy Metal Ion Detection. Inorg. Chem. 2018, 57, 4613–4619. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, G.; Wei, F.; Cen, Y.; Ma, Y.; Song, Y.; Xu, X.; Shi, M.; Muhammad, S.; Hu, Q. A dual-emissive fluorescent sensor fabricated by encapsulating quantum dots and carbon dots into metal–organic frameworks for the ratiometric detection of Cu2+ in tap water. J. Mater. Chem. C 2017, 5, 8566–8571. [Google Scholar] [CrossRef]
- Cao, F.; Ju, E.; Liu, C.; Li, W.; Zhang, Y.; Dong, K.; Liu, Z.; Ren, J.; Qu, X. Encapsulation of aggregated gold nanoclusters in a metal–organic framework for real-time monitoring of drug release. Nanoscale 2017, 9, 4128–4134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wang, Y.; Li, X.; Dong, X.; Wang, H.; Du, B.; Cao, W.; Wei, Q. Quenching Electrochemiluminescence Immunosensor Based on Resonance Energy Transfer between Ruthenium (II) Complex Incorporated in the UiO-67 Metal–Organic Framework and Gold Nanoparticles for Insulin Detection. ACS Appl. Mater. Interfaces 2018, 10, 22932–22938. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Gao, G.; Zheng, L.; Chi, Y.; Chen, G. Encapsulation of Strongly Fluorescent Carbon Quantum Dots in Metal–Organic Frameworks for Enhancing Chemical Sensing. Anal. Chem. 2014, 86, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, J.; Li, Z.; Wang, X.; Xu, Y.; Chen, S.; Wang, Z. Optimized synthesis of Zr (Ⅳ) metal organic frameworks (MOFs-808) for efficient hydrogen storage. New J. Chem. 2019, 43, 4092–4099. [Google Scholar] [CrossRef]
- Van de Voorde, B.; Bueken, B.; Denayer, J.; De Vos, D. Adsorptive separation on metal-organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef]
- Maya Alejandro, F.; Palomino Cabello, C.; Frizzarin, R.; Estela, J.; Turnes Palomino, G.; Cerdà, V. Magnetic solid-phase extraction using metal-organic frameworks (MOFs) and their derived carbons. TRAC Trends Anal. Chem. 2017, 90, 142–152. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Du, X.-M.; Zhao, B.; Li, Y.; Ruan, W.-J. White-light-emitting single MOF sensor based array for berberine homologues discrimination. J. Mater. Chem. C 2020, 8, 1433–1439. [Google Scholar] [CrossRef]
- Lin, C.; He, H.; Zhang, Y.; Xu, M.; Tian, F.; Li, L.; Wang, Y. Acetaldehyde-modified-cystine functionalized Zr-MOFs for pH/GSH dual-responsive drug delivery and selective visualization of GSH in living cells. RSC Adv. 2020, 10, 3084–3091. [Google Scholar] [CrossRef]
- Minguez, I.; Parvathikar, S.; Carpenter, M.; Bellamy, T.; Amato, K.; Carpenter, J.; Lail, M. MOF-derived nanostructured catalysts for low-temperature ammonia synthesis. Catal. Sci. Technol. 2019, 10, 105–112. [Google Scholar] [CrossRef]
- Wang, F.; Liang, C.; Tang, J.; Zhang, F.; Qu, F. Promotion of proton conductivity by Immobilizing molybdovanadophosphoric acids in metal–organic frameworks. New J. Chem. 2020, 44, 1912–1920. [Google Scholar] [CrossRef]
- Lin, J.-M.; He, C.-T.; Liu, Y.; Liao, P.-Q.; Zhou, D.-D.; Zhang, J.-P.; Chen, X.-M. A Metal–Organic Framework with a Pore Size/Shape Suitable for Strong Binding and Close Packing of Methane. Angew. Chem. Int. Ed. 2016, 55, 4674–4678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-B.; Furukawa, H.; Ko, N.; Nie, W.; Park, H.J.; Okajima, S.; Cordova, K.E.; Deng, H.; Kim, J.; Yaghi, O.M. Introduction of Functionality, Selection of Topology, and Enhancement of Gas Adsorption in Multivariate Metal–Organic Framework-177. J. Am. Chem. Soc. 2015, 137, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, X.; Huang, H.; Zhang, Z.; Yildirim, T.; Zhou, W.; Xiang, S.; Chen, B. A microporous aluminum-based metal-organic framework for high methane, hydrogen, and carbon dioxide storage. Nano Res. 2020. advance article. [Google Scholar] [CrossRef]
- Gutiérrez-Serpa, A.; Jiménez-Abizanda, A.I.; Jiménez-Moreno, F.; Pasán, J.; Pino, V. Core-shell microparticles formed by the metal-organic framework CIM-80(Al) (Silica@CIM-80(Al)) as sorbent material in miniaturized dispersive solid-phase extraction. Talanta 2020, 211, 120723. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, J.; Zhu, J.; Yu, Y.; Duan, G.; Zhou, L.; Li, Y. Synthesis of sandwich-structured magnetic graphene-Zn-MOFs composites for quantitative determination of acarbose in rat plasma. Talanta 2020, 209, 120514. [Google Scholar] [CrossRef]
- Tian, K.; Ma, Y.; Liu, Y.; Wang, M.; Guo, C.; He, L.; Song, Y.; Zhang, Z.; Du, M. Hierarchically structured hollow bimetallic ZnNi MOF microspheres as a sensing platform for adenosine detection. Sens. Actuators B Chem. 2020, 303, 127199. [Google Scholar] [CrossRef]
- Orellana-Tavra, C.; Köppen, M.; Li, A.; Stock, N.; Fairen-Jimenez, D. Biocompatible, Crystalline, and Amorphous Bismuth-Based Metal–Organic Frameworks for Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 5633–5641. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Huo, H.; Wang, Z.; Xu, X.; Yang, Y.; Lin, K.; Fan, R. One-pot synthesis of bimetallic metal–organic frameworks (MOFs) as acid–base bifunctional catalysts for tandem reaction. Catal. Sci. Technol. 2020, 10, 315–322. [Google Scholar] [CrossRef]
- Chueh, C.-C.; Chen, C.-I.; Su, Y.-A.; Konnerth, H.; Gu, Y.-J.; Kung, C.-W.; Wu, K. Harnessing of MOF Materials in Photovoltaic Devices: Recent Advance, Challenge, and Perspective. J. Mater. Chem. A 2019, 7, 17079–17095. [Google Scholar] [CrossRef]
- Janghouri, M.; Hosseini, H. Water-Soluble Metal–Organic Framework Hybrid Electron Injection Layer for Organic Light-Emitting Devices. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1–6. [Google Scholar] [CrossRef]
- Karim, N.A.; Mehmood, U.; Zahid, H.F.; Asif, T. Nanostructured photoanode and counter electrode materials for efficient Dye-Sensitized Solar Cells (DSSCs). Sol. Energy 2019, 185, 165–188. [Google Scholar] [CrossRef]
- Sun, Y.; Cao, Y.; Huang, W.; Zhu, Y.; Heng, L.; Tang, B. High-performance photoanode for dye sensitized solar cells with graphene modified two-layer construction. Mater. Lett. 2016, 165, 178–180. [Google Scholar] [CrossRef]
- Park, J.T.; Moon, J.; Choi, G.H.; Lim, S.M.; Kim, J.H. Facile graft copolymer template synthesis of mesoporous polymeric metal-organic frameworks to produce mesoporous TiO2: Promising platforms for photovoltaic and photocatalytic applications. J. Ind. Eng. Chem. 2020, 84, 384–392. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Z.; Wang, W.; Fu, L. Metal organic frameworks derived high-performance photoanodes for DSSCs. J. Alloy. Compd. 2020, 825, 154089. [Google Scholar] [CrossRef]
- Shen, D.; Pang, A.; Li, Y.; Dou, J.; Wei, M. Metal–organic frameworks at interfaces of hybrid perovskite solar cells for enhanced photovoltaic properties. Chem. Commun. 2018, 54, 1253–1256. [Google Scholar] [CrossRef]
- Nguyen, T.M.H.; Bark, C.W. Synthesis of Cobalt-Doped TiO2 Based on Metal–Organic Frameworks as an Effective Electron Transport Material in Perovskite Solar Cells. ACS Omega 2020, 5, 2280–2286. [Google Scholar] [CrossRef]
- Xing, W.; Ye, P.; Lu, J.; Wu, X.; Chen, Y.; Zhu, T.; Peng, A.; Huang, H. Tellurophene-based metal-organic framework nanosheets for high-performance organic solar cells. J. Power Sources 2018, 401, 13–19. [Google Scholar] [CrossRef]
- Sasitharan, K.; Bossanyi, D.G.; Vaenas, N.; Parnell, A.J.; Clark, J.; Iraqi, A.; Lidzey, D.G.; Foster, J.A. Metal–organic framework nanosheets for enhanced performance of organic photovoltaic cells. J. Mater. Chem. A 2020. advance article. [Google Scholar] [CrossRef]
- Thejo Kalyani, N.; Dhoble, S.J. Organic light emitting diodes: Energy saving lighting technology—A review. Renew. Sustain. Energy Rev. 2012, 16, 2696–2723. [Google Scholar] [CrossRef]
- Torsi, L.; Magliulo, M.; Manoli, K.; Palazzo, G. Organic field-effect transistor sensors: A tutorial review. Chem. Soc. Rev. 2013, 42, 8612–8628. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huo, F. Metal–organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Sundriyal, S.; Pachauri, V.; Ingebrandt, S.; Kim, K.-H.; Sharma, A.L.; Deep, A. Luminescent metal-organic frameworks and their composites: Potential future materials for organic light emitting displays. Coord. Chem. Rev. 2019, 401, 213077. [Google Scholar] [CrossRef]
- Liu, X.; Hu, H.; Liu, Y.; Huang, Z.; Lu, Y.; Zhou, X.; Wang, J. Experimental investigation on fluorescence polarization properties of isomerical MOF⊃RhB crystals. J. Solid State Chem. 2020, 284, 121179. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, J.; Wang, J.; Yang, X.; Zhang, X.; Li, S.; Sun, P.; Chen, J.; Li, B.; Lü, X. Color-tunable white-light of binary tris-β-diketonate-(Dy3+, Gd3+x) complexes’ blend under single wavelength excitation. Inorg. Chem. Commun. 2020, 113, 107814. [Google Scholar] [CrossRef]
- Manousiadis, P.P.; Yoshida, K.; Turnbull, G.A.; Samuel, I.D.W. Organic semiconductors for visible light communications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20190186. [Google Scholar] [CrossRef]
- McDonagh, C.; Burke, C.S.; MacCraith, B.D. Optical Chemical Sensors. Chem. Rev. 2008, 108, 400–422. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, Z.; Xin, X.; Sun, D. Metal–organic frameworks based luminescent materials for nitroaromatics sensing. CrystEngComm 2016, 18, 193–206. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Zhao, Y.; Wang, P.; Kang, Y.-S.; Azam, M.; Al-Resayes, S.I.; Liu, X.-H.; Lu, Q.-Y.; Sun, W.-Y. Fluorescent sensing and selective adsorption properties of metal–organic frameworks with mixed tricarboxylate and 1H-imidazol-4-yl-containing ligands. Dalton Trans. 2017, 46, 9022–9029. [Google Scholar] [CrossRef]
- Huo, L.; Zhang, J.; Gao, L.; Wang, X.; Fan, L.; Fang, K.; Hu, T. Two cadmium coordination polymers based on tris(p-carboxyphenyl) phosphane oxide with highly selective sensing of nitrobenzene derivatives and Hg2+ cations. CrystEngComm 2017, 19, 5285–5292. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, Y.; Yin, Y.; Li, H.; Wang, J.; Zhu, L. A novel “on-off-on” fluorescent sensor for 6-thioguanine and Hg2+ based on g-C3N4 nanosheets. Sens. Actuators B Chem. 2018, 257, 504–510. [Google Scholar] [CrossRef]
- Kumar, G.G.V.; Kesavan, M.P.; Sivaraman, G.; Rajesh, J. Colorimetric and NIR fluorescence receptors for F− ion detection in aqueous condition and its Live cell imaging. Sens. Actuators B Chem. 2018, 255, 3194–3206. [Google Scholar] [CrossRef]
- Anand, T.; Sivaraman, G.; Iniya, M.; Siva, A.; Chellappa, D. Aminobenzohydrazide based colorimetric and ‘turn-on’ fluorescence chemosensor for selective recognition of fluoride. Anal. Chim. Acta 2015, 876, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Zhang, J.-W.; Huang, G.; Du, Z.-Y.; Jiang, H.-L. An amine-functionalized metal–organic framework as a sensing platform for DNA detection. Chem. Commun. 2014, 50, 12069–12072. [Google Scholar] [CrossRef]
- Makwana, B.A.; Darjee, S.; Jain, V.K.; Kongor, A.; Sindhav, G.; Rao, M.V. A comparative study: Metal nanoparticles as fluorescent sensors for biomolecules and their biomedical application. Sens. Actuators B: Chem. 2017, 246, 686–695. [Google Scholar] [CrossRef]
- Liu, X.; Fu, W.; Bouwman, E. One-step growth of lanthanoid metal-organic framework (MOF) films under solvothermal conditions for temperature sensing. Chem. Commun. 2016, 52, 6926–6929. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Feng, D.; Wang, K.; Gu, Z.-Y.; Wei, Z.; Chen, Y.-P.; Zhou, H.-C. An Exceptionally Stable, Porphyrinic Zr Metal–Organic Framework Exhibiting pH-Dependent Fluorescence. J. Am. Chem. Soc. 2013, 135, 13934–13938. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Y.; Zapata, F.; Lin, G.; Qian, G.; Lobkovsky, E. Luminescent Open Metal Sites within a Metal–Organic Framework for Sensing Small Molecules. Adv. Mater. 2007, 19, 1693–1696. [Google Scholar] [CrossRef]
- Bajpai, A.; Mukhopadhyay, A.; Krishna, M.; Govardhan, S.; Moorthy, J. A fluorescent paramagnetic Mn metal-organic framework based on semi-rigid pyrene tetra-carboxylic acid: Sensing of solvent polarity and explosive nitroaromatics. IUCrJ 2015, 2, 552–562. [Google Scholar] [CrossRef]
- Xu, L.; Fang, G.; Liu, J.; Pan, M.; Wang, R.; Wang, S. One-pot synthesis of nanoscale carbon dots-embedded metal–organic frameworks at room temperature for enhanced chemical sensing. J. Mater. Chem. A 2016, 4, 15880–15887. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.-R.; Wang, K.; Han, T.; Tong, M.; Li, L.; Xie, Y.; Yang, Q.; Liu, D.; Zhong, C. An in situ self-assembly template strategy for the preparation of hierarchical-pore metal-organic frameworks. Nat. Commun. 2015, 6, 8847. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-H.; Zhang, L.; Yu, H.-Y. A new self-penetrating amine-decorated microporous metal–organic framework: Crystal structure, adsorption selectivity, and luminescence properties. Inorg. Chem. Commun. 2015, 54, 77–80. [Google Scholar] [CrossRef]
- Desai, A.V.; Samanta, P.; Manna, B.; Ghosh, S.K. Aqueous phase nitric oxide detection by an amine-decorated metal–organic framework. Chem. Commun. 2015, 51, 6111–6114. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Desai, A.V.; Sharma, S.; Chandra, P.; Ghosh, S.K. Selective Recognition of Hg2+ ion in Water by a Functionalized Metal–Organic Framework (MOF) Based Chemodosimeter. Inorg. Chem. 2018, 57, 2360–2364. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-L.; Xie, L.-H.; Joseph, E.A.; Li, J.-R.; Su, X.-O.; Zhou, H.-C. Metal–Organic Frameworks for Food Safety. Chem. Rev. 2019, 119, 10638–10690. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, Y.; Shao, Z.; Li, N.; Huang, C.; Hou, H. Water-stable Eu-MOF fluorescent sensors for trivalent metal ions and nitrobenzene. Dalton Trans. 2017, 46, 12201–12208. [Google Scholar] [CrossRef]
- Wang, X.; Qin, T.; Bao, S.-S.; Zhang, Y.-C.; Shen, X.; Zheng, L.-M.; Zhu, D. Facile synthesis of a water sTable 3D Eu-MOF showing high proton conductivity and its application as a sensitive luminescent sensor for Cu2+ ions. J. Mater. Chem. A 2016, 4, 16484–16489. [Google Scholar] [CrossRef]
- Huang, Y.-Q.; Ding, B.; Song, H.-B.; Zhao, B.; Ren, P.; Cheng, P.; Wang, H.-G.; Liao, D.-Z.; Yan, S.-P. A novel 3D porous metal–organic framework based on trinuclear cadmium clusters as a promising luminescent material exhibiting tunable emissions between UV and visible wavelengths. Chem. Commun. 2006, 47, 4906–4908. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y.; Zhou, X.; Brites, C.D.S.; Ananias, D.; Lin, Z.; Paz, F.A.A.; Rocha, J.; Huang, W.; Carlos, L.D. Visible-Light Excited Luminescent Thermometer Based on Single Lanthanide Organic Frameworks. Adv. Funct. Mater. 2016, 26, 8677–8684. [Google Scholar] [CrossRef]
- Zang, S.; Su, Y.; Duan, C.; Li, Y.; Zhu, H.; Meng, Q. Coexistence of chiral hydrophilic and achiral hydrophobic channels in one multi-helical-array metal-organic framework incorporating helical water cluster chains. Chem. Commun. 2007, 48, 4997–4999. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kim, K.-H.; Deep, A. Recent advancements in sensing techniques based on functional materials for organophosphate pesticides. Biosens. Bioelectron. 2015, 70, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Deng, Q.; Fang, G.; Wang, J.; Pan, M.; Wang, S.; Pu, Y. Metal–organic frameworks supported surface–imprinted nanoparticles for the sensitive detection of metolcarb. Biosens. Bioelectron. 2016, 79, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, E.; Yakimov, S.A.; Naidenko, E.V.; Kudrik, E.; Makarov, S.V. Application of metal–organic frameworks for purification of vegetable oils. Food Chem. 2016, 190, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; He, J.; Chen, J.; Niu, Y.; Zhao, Y.; Zhang, Y.; Yu, C. A sensitive sandwich-type immunosensor for the detection of galectin-3 based on N-GNRs-Fe-MOFs@AuNPs nanocomposites and a novel AuPt-methylene blue nanorod. Biosens. Bioelectron. 2018, 101, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zou, Y.; Huang, M.; Dong, S.; Wu, X.; Liang, G.; Han, Z.; Zhang, Z. A sensitive, colorimetric immunosensor based on Cu-MOFs and HRP for detection of dibutyl phthalate in environmental and food samples. Talanta 2018, 186, 104–109. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Ma, D.; Li, L.; Li, G.; Li, G.; Shi, Z.; Feng, S. A strategy toward constructing a bifunctionalized MOF catalyst: Post-synthetic modification of MOFs on organic ligands and coordinatively unsaturated metal sites. Chem. Commun. 2012, 48, 6151–6153. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, C.; Niu, Y.; Chen, J.; Zhao, Y.; Zhang, Y.; Gao, R.; He, J. Target triggered cleavage effect of DNAzyme: Relying on Pd-Pt alloys functionalized Fe-MOFs for amplified detection of Pb2+. Biosens. Bioelectron. 2018, 101, 297–303. [Google Scholar] [CrossRef]
- Lian, X.; Yan, B. Phosphonate MOFs Composite as Off–On Fluorescent Sensor for Detecting Purine Metabolite Uric Acid and Diagnosing Hyperuricuria. Inorg. Chem. 2017, 56, 6802–6808. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Yue, D.; Cui, Y.; Yang, Y.; Qian, G. A luminescent turn-up metal–organic framework sensor for tryptophan based on singlet–singlet Förster energy transfer. J. Mater. Chem. B 2018, 6, 5174–5180. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Q.; Xia, T.; Zhang, J.; Yang, Y.; Cui, Y.; Chen, B.; Qian, G. Turn-on and Ratiometric Luminescent Sensing of Hydrogen Sulfide Based on Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2016, 8, 32259–32265. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ghosh, S.K. Metal–Organic Framework-Based Selective Sensing of Biothiols via Chemidosimetric Approach in Water. ACS Omega 2018, 3, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Habi, S.; Daba, H. Plasmid Incidence, Antibiotic and Metal Resistance among Enterobacteriaceae Isolated from Algerian Streams. Pak. J. Biol. Sci. Pjbs 2009, 12, 1474–1482. [Google Scholar] [CrossRef]
- Tang, J.; He, J.; Liu, T.; Xin, X. Extraction and environmental risk assessment of heavy metal in the municipal dewatered sludge using rhamnolipid treatment. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 1522–1538. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Asgharinezhad, A.A.; Pooladi, M.; Barzin, M.; Abbaszadeh, A.; Tadjarodi, A. A novel magnetic metal organic framework nanocomposite for extraction and preconcentration of heavy metal ions, and its optimization via experimental design methodology. Microchim. Acta 2013, 180, 1073–1084. [Google Scholar] [CrossRef]
- Hassanpour, A.; Hosseinzadeh-Khanmiri, R.; Babazadeh, M.; Abolhasani, J.; Ghorbani-Kalhor, E. Determination of heavy metal ions in vegetable samples using a magnetic metal-organic framework nanocomposite sorbent. Food Addit. Contam. Part A Chem. Anal. ControlExpo. Risk Assess. 2015, 32, 725–736. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Abbaszadeh, A. A magnetic nanocomposite prepared from chelator-modified magnetite (Fe3O4) and HKUST-1 (MOF-199) for separation and preconcentration of mercury (II). Microchim. Acta 2016, 183, 1391–1399. [Google Scholar] [CrossRef]
- Diamantis, S.; Margariti, A.; Pournara, A.; Papaefstathiou, G.; Manos, M.; Lazarides, T. Luminescent metal-organic frameworks as chemical sensors: Common pitfalls and proposed best practices. Inorg. Chem. Front. 2018, 5, 1493–1511. [Google Scholar] [CrossRef]
- Wu, X.-J.; Kong, F.; Zhao, C.-Q.; Ding, S.-N. Ratiometric fluorescent nanosensors for ultra-sensitive detection of mercury ion based on AuNCs/ MOFs. Analyst 2019, 144, 2523–2530. 2019, 144, 2523–2530. [Google Scholar] [CrossRef]
- Xiao, Y.; Cui, Y.; Zheng, Q.; Xiang, S.; Qian, G.; Chen, B. A microporous luminescent metal-organic framework for highly selective and sensitive sensing of Cu2+ in aqueous solution. Chem. Commun. 2010, 46, 5503–5505. [Google Scholar] [CrossRef]
- Ji, G.; Liu, J.; Gao, X.; Sun, W.; Wang, J.; Zhao, S.; Liu, Z. A luminescent lanthanide MOF for selectively and ultra-high sensitively detecting Pb2+ ions in aqueous solution. J. Mater. Chem. A 2017, 5, 10200–10205. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Li, G.-P.; Liu, W.-C.; Li, Q.-L.; Li, B.-H.; Gable, R.W.; Hou, L.; Batten, S.R. Two Unusual Nanocage-Based Ln-MOFs with Triazole Sites: Highly Fluorescent Sensing for Fe3+ and Cr2O72−, and Selective CO2 Capture. Chem. Plus. Chem. 2016, 81, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, L.; Xiao, Y.; Fronczek, F.R.; Xue, M.; Cui, Y.; Qian, G. A Luminescent Metal–Organic Framework with Lewis Basic Pyridyl Sites for the Sensing of Metal Ions. Angew. Chem. Int. Ed. 2009, 48, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Rudd, N.D.; Wang, H.; Fuentes-Fernandez, E.M.A.; Teat, S.J.; Chen, F.; Hall, G.; Chabal, Y.J.; Li, J. Highly Efficient Luminescent Metal–Organic Framework for the Simultaneous Detection and Removal of Heavy Metals from Water. ACS Appl. Mater. Interfaces 2016, 8, 30294–30303. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Bai, Z.; Li, Y.; Wang, Y.; Chen, L.; Xu, L.; Diwu, J.; Chai, Z.; Wang, S. A Hydrolytically Stable Luminescent Cationic Metal-organic Framework for Highly Sensitive and Selective Sensing of Chromate Anion in Natural Water Systems. ACS Appl. Mater. Interfaces 2017, 9, 16448–16457. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.-X.; Yao, R.-X.; Zhang, F.-Q.; Zhang, X.-M. A Fluorescent Anionic MOF with Zn4(trz)2 Chain for Highly Selective Visual Sensing of Contaminants: Cr(III) Ion and TNP. Inorg. Chem. 2017, 56, 2690–2696. [Google Scholar] [CrossRef]
- Yu, H.; Fan, M.; Liu, Q.; Su, Z.; Li, X.; Pan, Q.; Hu, X. Two Highly Water-Stable Imidazole-Based Ln-MOFs for Sensing Fe3+,Cr2O72–/CrO42– in a Water Environment. Inorg. Chem. 2020, 59, 2005–2010. [Google Scholar] [CrossRef]
- Du, J.-L.; Gao, J.-P.; Li, C.-P.; Zhang, X.-Y.; Hou, J.-X.; Jing, X.; Mu, Y.-J.; Li, L.-J. A sTable 3D Cd( ii ) metal–organic framework for highly sensitive detection of Cu2+ ions and nitroaromatic explosives. RSC Adv. 2017, 7, 49618–49625. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Du, T.; Zhang, W.; Zhu, W.; Yang, C.; Yue, T.; Sun, J.; Li, T.; Wang, J. NH2-MIL-53 (Al) Metal–Organic Framework as the Smart Platform for Simultaneous High-Performance Detection and Removal of Hg2+. Inorg. Chem. 2019, 58, 12573–12581. [Google Scholar] [CrossRef]
- Lv, S.-W.; Liu, J.-M.; Li, C.-Y.; Zhao, N.; Wang, Z.-H.; Wang, S. A novel and universal metal-organic frameworks sensing platform for selective detection and efficient removal of heavy metal ions. Chem. Eng. J. 2019, 375, 122111. [Google Scholar] [CrossRef]
- Hao, J.-N.; Yan, B. A water-stable lanthanide-functionalized MOF as a highly selective and sensitive fluorescent probe for Cd2+. Chem. Commun. 2015, 51, 7737–7740. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, F.; Cui, Y.; Chen, B.; Qian, G. A luminescent nanoscale metal–organic framework for sensing of nitroaromatic explosives. Chem. Commun. 2011, 47, 3153–3155. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Joarder, B.; Chaudhari, A.K.; Mukherjee, S.; Ghosh, S.K. Highly Selective Detection of Nitro Explosives by a Luminescent Metal–Organic Framework. Angew. Chem. Int. Ed. 2013, 52, 2881–2885. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, K.C.; Heck, R.; Chong, S.Y.; Bacsa, J.; Jones, J.T.A.; Khimyak, Y.Z.; Bradshaw, D.; Rosseinsky, M.J. A Guest-Responsive Fluorescent 3D Microporous Metal−Organic Framework Derived from a Long-Lifetime Pyrene Core. J. Am. Chem. Soc. 2010, 132, 4119–4130. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, H.; Stil, H.; Kuipers, H.; Gascon, J.; Kapteijn, F. Shape and Transition State Selective Hydrogenations Using Egg-Shell Pt-MIL-101 (Cr) Catalyst. ACS Catal. 2013, 3, 2617–2626. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.; Lu, H.; Hong, X.; Jiang, H.-L.; Wu, Y.; Li, Y. Hollow Zn/Co ZIF Particles Derived from Core–Shell ZIF-67@ZIF-8 as Selective Catalyst for the Semi-Hydrogenation of Acetylene. Angew. Chem. Int. Ed. 2015, 54, 10889–10893. [Google Scholar] [CrossRef]
- Chao, H.; Zhao, G.; Ji, Y.; Niu, Z.; Wang, D.; Li, Y. Hydroformylation of alkenes over rhodium supported on the metal-organic framework ZIF-8. Nano Res. 2014, 7, 1364–1369. [Google Scholar] [CrossRef]
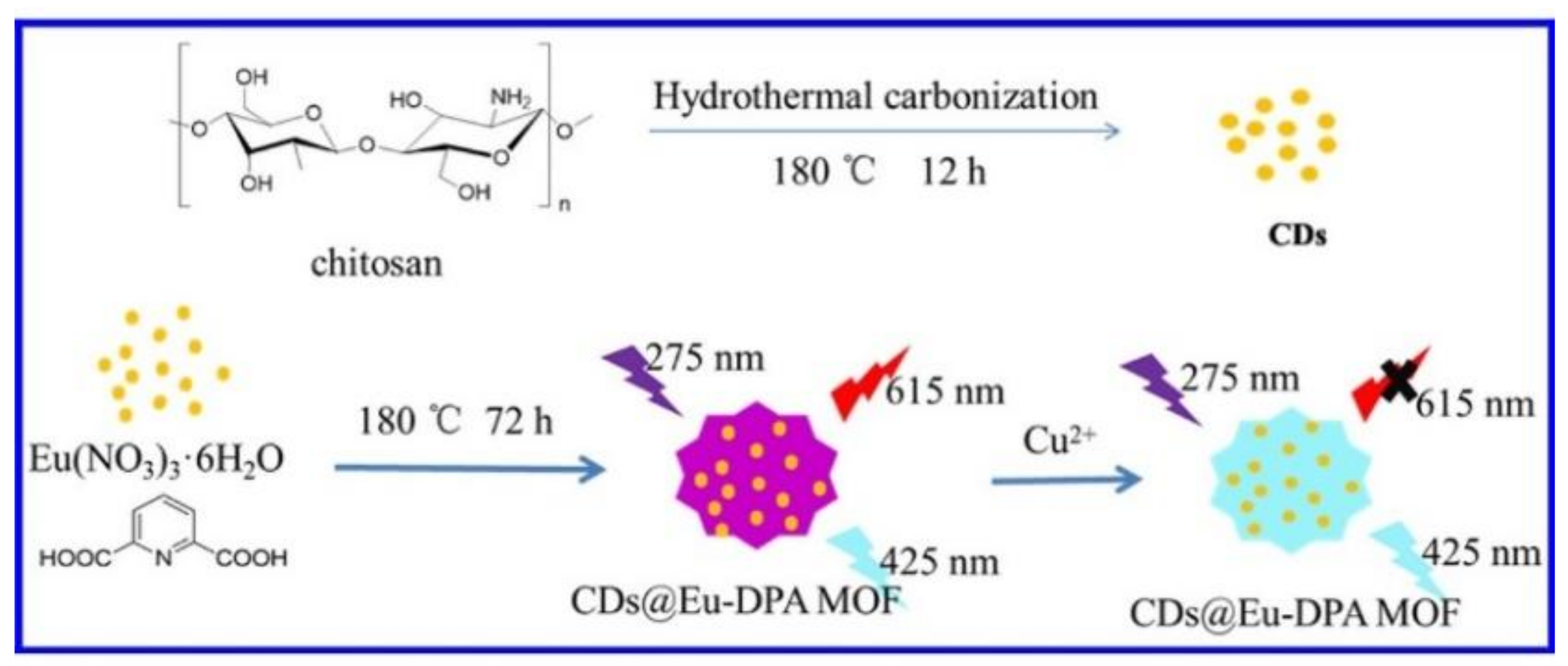
- Hao, J.; Liu, F.; Liu, N.; Zeng, M.; Song, Y.; Wang, L. Ratiometric fluorescent detection of Cu2+ with carbon dots chelated Eu-based metal-organic frameworks. Sens. Actuators B Chem. 2017, 245, 641–647. [Google Scholar] [CrossRef]
- Fan, C.; Lv, X.; Liu, F.; Feng, L.; Liu, M.; Cai, Y.; Liu, H.; Wang, J.; Yang, Y.; Wang, H. Silver Nanoclusters Encapsulated into Metal–Organic Frameworks with Enhanced Fluorescence and Specific Ion Accumulation toward the Microdot Array-Based Fluorimetric Analysis of Copper in Blood. ACS Sens. 2018, 3, 441–450. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: An overview. Radiat. Phys. Chem. 2016, 125, 56–64. [Google Scholar] [CrossRef]
- Jones, O.; Voulvoulis, N.; Lester, J. Human Pharmaceuticals in Wastewater Treatment Processes. Crit. Rev. Environ. Sci. Technol. 2005, 35, 401–427. [Google Scholar] [CrossRef]
- Gurke, R.; Roessler, M.; Marx, C.; Diamond, S.; Schubert, S.; Oertel, R.; Fauler, J. Occurrence and removal of frequently prescribed pharmaceuticals and corresponding metabolites in wastewater of a sewage treatment plant. Sci. Total Environ. 2015, 532, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhao, Y.; Yun, Y.-S. Highly Effective Removal of Nonsteroidal Anti-inflammatory Pharmaceuticals from Water by Zr (IV)-Based Metal–Organic Framework: Adsorption Performance and Mechanisms. ACS Appl. Mater. Interfaces 2018, 10, 28076–28085. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.; Bhadra, B.; Ahmed, I.; Khan, N.; Jhung, S. Adsorptive Removal of Pharmaceuticals and Personal Care Products from Water with Functionalized Metal-organic Frameworks: Remarkable Adsorbents with Hydrogen-bonding Abilities. Sci. Rep. 2016, 6, 34462. [Google Scholar] [CrossRef] [PubMed]
- DeFuria, M.; Zeller, M.; Genna, D. Removal of Pharmaceuticals from Water via π-π Stacking Interactions in Perfluorinated Metal Organic Frameworks. Cryst. Growth Des. 2016, 16, 3530–3534. [Google Scholar] [CrossRef]
- Hasan, Z.; Khan, N.; Jhung, S. Adsorptive removal of diclofenac sodium from water with Zr-based metal-organic frameworks. Chem. Eng. J. 2015, 284, 1406–1413. [Google Scholar] [CrossRef]
- Hasan, Z.; Choi, E.-J.; Jhung, S. Adsorption of naproxen and clofibric acid over a metal-organic framework MIL-101 functionalized with acidic and basic groups. Chem. Eng. J. 2013, 219, 537–544. [Google Scholar] [CrossRef]
- Hahn, T.; Schulz, R. Indirect Effects of Antibiotics in the Aquatic Environment: A Laboratory Study on Detritivore Food Selection Behavior. Hum Ecol Risk Assess. 2007, 13, 535–542. [Google Scholar] [CrossRef]
- Ji, X.N.; Wang, D.M.; Ren, Z.M.; Yang, H.; Xiao, Y.B.; Bian, D.J. Detection of the Antibiotic TMP in the Water with HPLC-DAD. Appl. Mech. Mater. 2015, 727–728, 790–793. [Google Scholar] [CrossRef]
- Lopez, M.I.; Feldlaufer, M.F.; Williams, A.D.; Chu, P.-S. Determination and confirmation of nitrofuran residues in honey using LC-MS/MS. J. Agric. Food Chem. 2007, 55, 1103–1108. [Google Scholar] [CrossRef]
- Petsch, M.; Mayer-Helm, B.X.; Sauermann, R.; Joukhadar, C.; Kenndler, E. Capillary electrophoresis analysis of fosfomycin in biological fluids for clinical pharmacokinetic studies. Electrophoresis 2004, 25, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Sakohara, S.; Kuriyama, Y.; Kobayashi, K.; Gotoh, T.; Iizawa, T. Adsorption and desorption of calcium ions by temperature swing with copolymer of thermosensitive and chelating components grafted on porous ethylene vinyl acetate disk. React. Funct. Polym. 2013, 73, 1632–1638. [Google Scholar] [CrossRef]
- Mehlhorn, A.; Rahimi, P.; Joseph, Y. Aptamer-Based Biosensors for Antibiotic Detection: A Review. Biosensors 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Lirio, S.; Chen, Y.-T.; Lin, C.-H.; Huang, H.-Y. A Novel Hybrid Metal-Organic Framework-Polymeric Monolith for Solid-Phase Microextraction. Chemistry 2014, 20, 3317–3321. [Google Scholar] [CrossRef]
- Jin, W.-G.; Chen, W.; Xu, P.-H.; Lin, X.-W.; Huang, X.-C.; Chen, G.; Lu, F.; Chen, X.-M. An Exceptionally Water Stable Metal-Organic Framework with Amide-Functionalized Cages: Selective CO2/CH4 Uptake, Removal of Antibiotics and Dyes from Water. Chemistry 2017, 23, 13058–13066. [Google Scholar] [CrossRef]
- Yang, X.-Q.; Yang, C.-X.; Yan, X.-P. Zeolite imidazolate framework-8 as sorbent for on-line solid-phase extraction coupled with high-performance liquid chromatography for the determination of tetracyclines in water and milk samples. J. Chromatogr. A 2013, 1304, 28–33. [Google Scholar] [CrossRef]
- Wu, M.; Ai, Y.; Zeng, B.; Zhao, F. In situ solvothermal growth of metal-organic framework-ionic liquid functionalized graphene nanocomposite for highly efficient enrichment of chloramphenicol and thiamphenicol. J. Chromatogr. A 2016, 1427, 1–7. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.-L.; Feng, D.; Xie, L.-H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.-R.; Zhou, H.-C. Highly Stable Zr (IV)-Based Metal–Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef]
- Senturk, S.; Temizel, E. Clinical efficacy of rifamycin SV combined with oxytetracycline in the treatment of caseous lymphadenitis in sheep. Vet. Rec. 2006, 159, 216–217. [Google Scholar] [CrossRef]
- Molina, A.; Molina, M.P.; Althaus, R.; Gallego, L. Residue Persistence in Sheep Milk Following Antibiotic Therapy. Vet. J. 2003, 165, 84–89. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Q.; Zhang, D.; Gan, N.; Li, Q.; Cuan, J. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sens. Actuators B Chem. 2018, 262, 137–143. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Wang, J.; Fan, Y.; Zhang, S.; Dai, W. A Novel Multifunctional p-Type Semiconductor@MOFs Nanoporous Platform for Simultaneous Sensing and Photodegradation of Tetracycline. ACS Appl. Mater. Interfaces 2020, 12, 11036–11044. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ning, D.; Li, W.-J.; Du, X.-M.; Wang, Q.; Li, Y.; Ruan, W.-J. Metal–organic framework-based fluorescent sensing of tetracycline-type antibiotics applicable to environmental and food analysis. Analyst 2019, 144, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-M.; Li, R.; Li, X. Construction of metal–organic frameworks (MOFs) and highly luminescent Eu (iii)-MOF for the detection of inorganic ions and antibiotics in aqueous medium. CrystEngComm 2018, 20, 4962–4972. [Google Scholar] [CrossRef]
- Rogge, S.M.J.; Waroquier, M.; Van Speybroeck, V. Reliably Modeling the Mechanical Stability of Rigid and Flexible Metal–Organic Frameworks. Acc. Chem. Res. 2018, 51, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wang, Y.; Han, J.; Ni, L.; Zhang, H.; Li, C.; Li, J.; Qiu, Y. A novel fluorescent probe based on biphenyl and rhodamine for multi-metal ion recognition and its application. Dalton Trans. 2018, 47, 3378–3387. [Google Scholar] [CrossRef]
- Momidi, B.K.; Tekuri, V.; Trivedi, D.R. Multi-signaling thiocarbohydrazide based colorimetric sensors for the selective recognition of heavy metal ions in an aqueous medium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 180, 175–182. [Google Scholar] [CrossRef]
- Yu, M.; Yao, X.; Wang, X.; Li, Y.; Li, G. White-Light-Emitting Decoding Sensing for Eight Frequently-Used Antibiotics Based on a Lanthanide Metal-Organic Framework. Polymers 2019, 11, 99. [Google Scholar] [CrossRef]
- Witte, W.; Klare, I.; Werner, G. The use of antibiotics as growth promoters in animal husbandry and antibiotic resistance in bacterial pathogens of humans. Fleischwirtsch. Frankf. 1999, 79, 90–94. [Google Scholar]
- Veach, B.; Baker, C.; Kibbey, J.; Fong, A.; Broadaway, B.; Drake, C. Quantitation of Chloramphenicol and Nitrofuran Metabolites in Aquaculture Products Using Microwave-Assisted Derivatization, Automated SPE, and LC-MS/MS. J. AOAC Int. 2015, 98, 588–594. [Google Scholar] [CrossRef]
- Wickramanayake, P.U.; Tran, T.C.; Hughes, J.G.; Macka, M.; Simpson, N.; Marriott, P.J. Simultaneous separation of nitrofuran antibiotics and their metabolites by using micellar electrokinetic capillary chromatography. Electrophoresis 2006, 27, 4069–4077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yao, H.; Chu, T.; Zhang, G.; Wang, Y.; Yang, Y. A Lanthanide MOF Thin-Film Fixed with Co3O4 Nano-Anchors as a Highly Efficient Luminescent Sensor for Nitrofuran Antibiotics. Chem. A Eur. J. 2017, 23, 10293–10300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yao, H.; Zhao, Y.; Li, X.; Zhang, G.; Yang, Y. Mixed matrix membranes incorporated with Ln-MOF for selective and sensitive detection of nitrofuran antibiotics based on inner filter effect. Talanta 2017, 174, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-Q.; He, H.; Yan, Y.; Yuan, J.; Lu, D.-Q.; Zhang, D.-Y.; Sun, F.; Zhu, G. An Exceptionally Stable TbIII-Based Metal–Organic Framework for Selectively and Sensitively Detecting Antibiotics in Aqueous Solution. Inorg. Chem. 2019, 58, 7746–7753. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-Q.; Zhou, Q.-S.; Zhang, H.-W.; Zhang, W.-W.; Lu, D.-Q.; Guo, M.-T.; Yuan, Y.; Sun, F.; He, H. Design and Construction of a Metal–Organic Framework as an Efficient Luminescent Sensor for Detecting Antibiotics. Inorg. Chem. 2020, 59, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Kuller, L.H.; Matthews, K.A.; Meilahn, E.N. Estrogens and women’s health: Interrelation of coronary heart disease, breast cancer and osteoporosis. J. Steroid Biochem. Mol. Biol. 2000, 74, 297–309. [Google Scholar] [CrossRef]
- Zsarnovszky, A.; Kiss, D.; Gergely, J.; Nemeth, G.; Toth, I.; Horvath, T. Thyroid hormone- and estrogen receptor interactions with natural ligands and endocrine disruptors in the cerebellum. Front. Neuroendocrinol. 2017, 48, 23–36. [Google Scholar] [CrossRef]
- Smy, L.; Straseski, J.A. Measuring estrogens in women, men, and children: Recent advances 2012–2017. Clin. Biochem. 2018, 62, 11–23. [Google Scholar] [CrossRef]
- Manson, J.E.; Bassuk, S.S. Menopausal Estrogen-Alone Therapy and Health Outcomes in Women With and Without Bilateral Oophorectomy. Ann. Intern. Med. 2020, 172, 227. [Google Scholar] [CrossRef]
- Jarque, S.; Quiros Pesudo, L.; Grimalt, J.; Gallego, E.; Catalan, J.; Lackner, R.; Piña, B. Background fish feminization effects in European remote sites. Sci. Rep. 2015, 5, 11292. [Google Scholar] [CrossRef]
- Ma, L.; Yates, S. Dissolved organic matter and estrogen interactions regulate estrogen removal in the aqueous environment: A review. Sci. Total Environ. 2018, 640–641, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Robles, J.; Marcos, J.; Renau, N.; Garrostas, L.; Segura, J.; Ventura, R.; Barceló, B.; Barceló, A.; Pozo, O.J. Quantifying endogenous androgens, estrogens, pregnenolone and progesterone metabolites in human urine by gas chromatography tandem mass spectrometry. Talanta 2017, 169, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Nogueira, J.M.F. Determination of steroid sex hormones in real matrices by bar adsorptive microextraction (BAμE). Talanta 2015, 136, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xu, X.; Liu, Z.; Xue, S.; Zhang, L. Novel functionalized magnetic ionic liquid green separation technology coupled with high performance liquid chromatography: A rapid approach for determination of estrogens in milk and cosmetics. Talanta 2020, 209, 120542. [Google Scholar] [CrossRef]
- Häkkinen, M.R.; Heinosalo, T.; Saarinen, N.; Linnanen, T.; Voutilainen, R.; Lakka, T.; Jääskeläinen, J.; Poutanen, M.; Auriola, S. Analysis by LC–MS/MS of endogenous steroids from human serum, plasma, endometrium and endometriotic tissue. J. Pharm. Biomed. Anal. 2018, 152, 165–172. [Google Scholar] [CrossRef]
- Abdel-Khalik, J.; Björklund, E.; Hansen, M. Simultaneous determination of endogenous steroid hormones in human and animal plasma and serum by liquid or gas chromatography coupled to tandem mass spectrometry. J. Chromatogr. B 2013, 928, 58–77. [Google Scholar] [CrossRef]
- Merib, J.; Spudeit, D.A.; Corazza, G.; Carasek, E.; Anderson, J.L. Magnetic ionic liquids as versatile extraction phases for the rapid determination of estrogens in human urine by dispersive liquid-liquid microextraction coupled with high-performance liquid chromatography-diode array detection. Anal. Bioanal. Chem. 2018, 410, 4689–4699. [Google Scholar] [CrossRef]
- Kislyuk, S.; Bosch, W.; Adams, E.; de Witte, P.; Cabooter, D. Development of a sensitive and quantitative capillary LC-UV method to study the uptake of pharmaceuticals in zebrafish brain. Anal. Bioanal. Chem. 2018, 410. [Google Scholar] [CrossRef]
- Ragab, D.; Gomaa, H.; Sabouni, R.; Salem, M.; Ren, M.; Zhu, J. Micropollutants Removal from Water using Microfiltration Membrane Modified with ZIF-8 Metal Organic Frameworks (MOFs). Chem. Eng. J. 2016, 300, 273–279. [Google Scholar] [CrossRef]
- Gao, G.; Li, S.; Li, S.; Zhao, L.; Wang, T.; Hou, X. Development and application of vortex-assisted membrane extraction based on metal–organic framework mixed-matrix membrane for the analysis of estrogens in human urine. Anal. Chim. Acta 2018, 1023, 35–43. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Liu, M.; Wang, Y.-Q.; Li, Y.; Zhang, J.-M.; Huo, S.-H. Hydrothermal synthesis of functionalized magnetic MIL-101 for magnetic enrichment of estrogens in environmental water samples. RSC Adv. 2016, 6, 15362–15369. [Google Scholar] [CrossRef]
- Chen, M.-L.; Chen, J.-H.; Ding, L.; Xu, Z.; Wen, L.; Wang, L.-B.; Cheng, Y.-H. Study of the detection of bisphenol A based on a nano-sized metal–organic framework crystal and an aptamer. Anal. Methods 2017, 9, 906–909. [Google Scholar] [CrossRef]
- Khalilian, F.; Rezaee, M. Ultrasound-Assisted Extraction Followed by Solid-Phase Extraction Followed by Dispersive Liquid–Liquid Microextraction for the Sensitive Determination of Diazinon and Chlorpyrifos in Rice. Food Anal. Methods 2017, 10, 885–891. [Google Scholar] [CrossRef]
- Kumar, P.; Paul, A.K.; Deep, A. Sensitive chemosensing of nitro group containing organophosphate pesticides with MOF-5. Microporous Mesoporous Mater. 2014, 195, 60–66. [Google Scholar] [CrossRef]
- Zhu, X.-D.; Zhang, K.; Wang, Y.; Long, W.-W.; Sa, R.-J.; Liu, T.-F.; Lü, J. Fluorescent Metal–Organic Framework (MOF) as a Highly Sensitive and Quickly Responsive Chemical Sensor for the Detection of Antibiotics in Simulated Wastewater. Inorg. Chem. 2018, 57, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Xie, Y.; Wang, X.; Li, Y. Highly Water-Stable Dye@Ln-MOFs for Sensitive and Selective Detection toward Antibiotics in Water. Acs Appl. Mater. Interfaces 2019, 11, 21201–21210. [Google Scholar] [CrossRef]
- Lohmann, R.; Breivik, K.; Dachs, J.; Muir, D. Global fate of POPs: Current and future research directions. Environ. Pollut. 2007, 150, 150–165. [Google Scholar] [CrossRef]
- Baughman, G.; Weber, E. Transformation of Dyes and Related Compounds in Anoxic Sediment: Kinetics and Products. Environ. Sci. Technol. 1994, 28, 267–276. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef]
- Lin, S.; Gan, N.; Qiao, L.; Zhang, J.; Cao, Y.; Chen, Y. Magnetic metal-organic frameworks coated stir bar sorptive extraction coupled with GC–MS for determination of polychlorinated biphenyls in fish samples. Talanta 2015, 144, 1139–1145. [Google Scholar] [CrossRef]
- Huo, S.-H.; Yu, J.; Fu, Y.-Y.; Zhou, P.-X. In situ hydrothermal growth of a dual-ligand metal–organic framework film on a stainless steel fiber for solid-phase microextraction of polycyclic aromatic hydrocarbons in environmental water samples. RSC Adv. 2016, 6, 14042–14048. [Google Scholar] [CrossRef]
- Pi, Y.; Li, X.; Xia, Q.; Wu, J.; Li, Y.; Xiao, J.; Li, Z. Adsorptive and photocatalytic removal of Persistent Organic Pollutants (POPs) in water by metal-organic frameworks (MOFs). Chem. Eng. J. 2018, 337, 351–371. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244–245, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Xiao, Y.; Tong, M.; Huang, H.; Liu, D.; Wang, L.; Zhong, C. Synthesis of CNT@MIL-68(Al) composites with improved adsorption capacity for phenol in aqueous solution. Chem. Eng. J. 2015, 275, 134–141. [Google Scholar] [CrossRef]
- Park, E.Y.; Hasan, Z.; Khan, N.; Jhung, S. Adsorptive Removal of Bisphenol-A from Water with a Metal-Organic Framework, a Porous Chromium-Benzenedicarboxylate. J. Nanosci. Nanotechnol. 2013, 13, 2789–2794. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Li, p.; Sui, Q.; Chen, J.; Xu, J.; Gao, E.-Q. Stable electron-deficient metal-organic framework for colorimetric and luminescent sensing of phenols and anilines. J. Mater. Chem. A 2018, 6, 2939–2952. [Google Scholar] [CrossRef]
- Ning, D.; Liu, Q.; Wang, Q.; Du, X.-M.; Li, Y.; Ruan, W.-J. Pyrene based MOFs as fluorescent sensors for PAHs: An energetic pathway of backbone structure effect on response. Dalton Trans. 2019, 48, 5705–5712. [Google Scholar] [CrossRef]
- Lucille, G.; Valence, F.; Mounier, J. Diversity and Control of Spoilage Fungi in Dairy Products: An Update. Microorganisms 2017, 5, 42. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. In Encyclopedia of Microbiology; Schaechter, M., Ed.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A Producing Fungi, Biosynthetic Pathway and Regulatory Mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef]
- Ryu, D.-J.; Bianchini, A.; Bullerman, B.L. Effects of processing on mycotoxins. Stewart Postharvest Rev. 2008, 4, 1–7. [Google Scholar] [CrossRef]
- Wei, Y.-K.; Zhao, X.-M.; Li, M.-M.; Yu, J.-X.; Selvaraj, G.; Hu, Y.-F.; Ji, G.-F.; Wei, D.-Q. Detoxification of aflatoxins on prospective approach: Effect on structural, mechanical, and optical properties under pressures. Interdiscip. Sci. Comput. Life Sci. 2017, 10, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Devi, Y.; Thirumdas, R.; Sarangapani, C.; Deshmukh, R.; Annapure, U. Influence of cold plasma on fungal growth and aflatoxins production on groundnuts. Food Control 2017, 77, 187–191. [Google Scholar] [CrossRef]
- Mehrzad, J.; Devriendt, B.; Baert, K.; Cox, E. Aflatoxin B1 interferes with the antigen-presenting capacity of porcine dendritic cells. Toxicol. Vitr. 2014, 28, 531–537. [Google Scholar] [CrossRef] [PubMed]
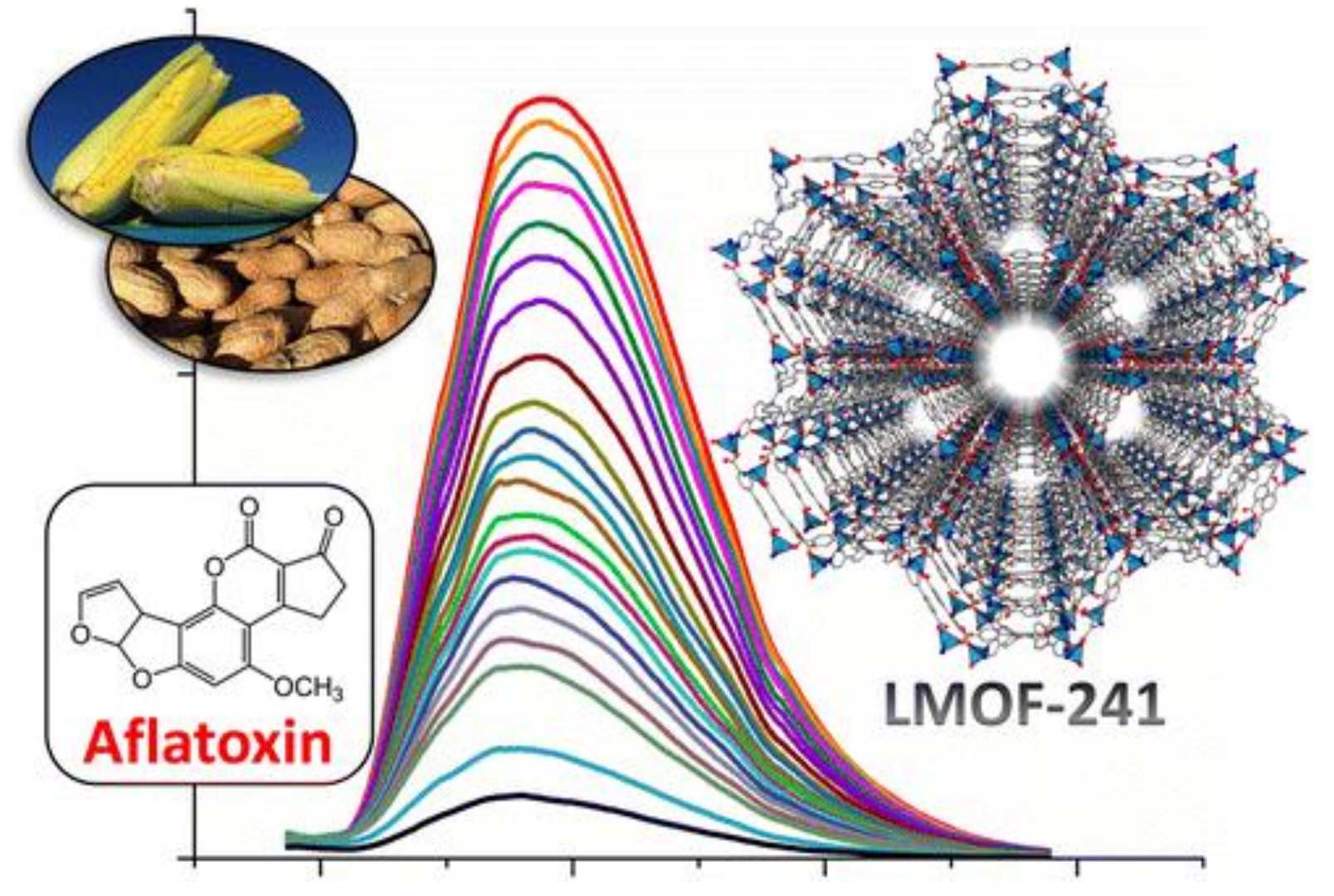
- Hu, Z.; Lustig, W.; Zhang, J.; Zheng, C.; Wang, H.; Teat, S.; Gong, Q.; Rudd, N.; Li, J. Effective Detection of Mycotoxins by a Highly Luminescent Metal-Organic Framework. J. Am. Chem. Soc. 2015, 137, 16209–16215. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Fu, Y.; Guo, Y.; Zhang, Q.; Zhang, Q.; Yang, H.; Li, Y. A water-stable luminescent metal–organic framework for effective detection of aflatoxin B1 in walnut and almond beverages. RSC Adv. 2019, 9, 620–625. [Google Scholar] [CrossRef]
- Hu, S.; Ouyang, W.; Guo, L.; Lin, Z.; Jiang, X.; Qiu, B.; Chen, G. Facile synthesis of Fe3O4/g-C3N4/HKUST-1 composites as a novel biosensor platform for ochratoxin A. Biosens. Bioelectron. 2017, 92, 718–723. [Google Scholar] [CrossRef]
- Bao, B.; Yuwen, L.; Zheng, X.; Weng, L.; Zhu, X.; Zhan, X.; Wang, L. A fluorescent conjugated polymer for trace detection of diamines and biogenic polyamines. J. Mater. Chem. 2010, 20, 9628–9634. [Google Scholar] [CrossRef]
- Vineis, P.; Pirastu, R. Aromatic amines and cancer. Cancer Causes Control 1997, 8, 346–355. [Google Scholar] [CrossRef]
- Medina, M.; Urdiales, J.; Rodríguez-Caso, C.; Ramírez, F.; Sánchez-Jiménez, F. Biogenic Amines and Polyamines: Similar Biochemistry for Different Physiological Missions and Biomedical Applications. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 23–59. [Google Scholar] [CrossRef]
- Mallick, A.; El-Zohry, A.M.; Shekhah, O.; Yin, J.; Jia, J.; Aggarwal, H.; Emwas, A.-H.; Mohammed, O.F.; Eddaoudi, M. Unprecedented Ultralow Detection Limit of Amines using a Thiadiazole-Functionalized Zr (IV)-Based Metal–Organic Framework. J. Am. Chem. Soc. 2019, 141, 7245–7249. [Google Scholar] [CrossRef]
- Pinheiro, H.; Touraud, E.; Thomas, O. Aromatic amines from azo dye reduction: Status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dye. Pigment. 2004, 61, 121–139. [Google Scholar] [CrossRef]
- Wang, F.; Dong, C.; Wang, C.; Yu, Z.; Guo, S.; Wang, Z.; Zhao, Y.; Li, G. Fluorescence detection of aromatic amines and photocatalytic degradation of rhodamine B under UV light irradiation by luminescent metal–organic frameworks. New J. Chem. 2015, 39, 4437–4444. [Google Scholar] [CrossRef]
- Benigni, R.; Passerini, L. Carcinogenicity of the aromatic amines: From structure–activity relationships to mechanisms of action and risk assessment. Mutat. Res. Rev. Mutat. Res. 2002, 511, 191–206. [Google Scholar] [CrossRef]
- Guo, Z.; Song, X.; Lei, H.; Wang, H.; Su, S.; Xu, H.; Qian, G.; Zhang, H.; Chen, B. A ketone functionalized luminescent terbium metal–organic framework for sensing of small molecules. Chem. Commun. 2015, 51, 376–379. [Google Scholar] [CrossRef] [PubMed]

| Material | Target | Strategy | Response Range | Detection Limit/Stern- Volmer Constant (Ksv) M-1 | Ref |
|---|---|---|---|---|---|
| MIL-101-NH2 | Cu2+, Pb2+ | turn-off | 10–1000 μM | ~1.8 μM | [140] |
| CQDs/ZIF-8 | Cu2+ | turn-off | 2–1000 nM | 80 pM | [54] |
| [Cd3(L)2(H2O)5] · (H2O)4 | Cu2+ | turn-off | 0–100 μM | 3.65 × 104 M−1 | [138] |
| Eu2(FMA)2(OX) (H2O)4·4H2O | Cu2+ | turn-off | - | 528.7 M−1 | [130] |
| CDs@Eu-DPAMOFs | Cu2+ | turn-off | 50 nM–10 μM | 26.3 nM) | [148] |
| AgNCs-BSA@ZIF-8 | Cu2+ | turn-off | 2.0 × 10-4–80.0 μM | 0.05 nM | [149] |
| NH2-MIL-53(Al) | Hg(Ⅱ) | turn-off | 1–17.3 μM | 0.15 μM | [139] |
| LMOF-263 | Hg2+ | turn-off | - | ~3.3 ppb | [129] |
| Tb-MOF | Pb2+ | turn-off | 8 × 10−4–3.4 × 10−7 M | 10-7 M | [131] |
| [Eu7(mtb)5 (H2O)16]·NO3·8DMA·18H2O | Cr(VI) | turn-off | 1 ppb–300 ppm | 0.56–1.75 | [132] |
| [Me2NH2]4[Zn6 (qptc)3(trz)4]· 6H2O | Cr(III) | turn-off | 0–40 μM | 4.39 × 104 M−1 | [136] |
| Ln-MOFs | Cr2O72−, CrO42− | turn-off | - | 1.915 × 104 M−1, 1.141 × 104 M−1 | [137] |
| UiO-66(Zr)–(COOH)2 | Cd2+ | turn-on | 0–500 μM | 0.06 μM | [141] |
| PCN-128Y | TCs | turn-off | 0–0.9 μM | 30 nM | [171] |
| CuBi2O4@ZIF-8 | TC | turn-on | 0–45 μM | 26 nM | [172] |
| In-sbdc | CTC, OTC | turn-off | - | 0.28–0.30 μM | [173] |
| [Eu2(2,3΄-oba)3(phen)2]n | MDZ | turn-off | 0.06–0.17 mM | 2.75 μM | [174] |
| Eu/Gd/Tb-dcpcpt | TC NZF NFT SDZ CBZ MDZ DTZ ODZ | turn-on; turn-off | 0–100 μM | 0.887 ppm 2.770 ppm 0.189 ppm 1.890 ppm 0.373 ppm 0.217 ppm 0.219 ppm 0.142 ppm | [178] |
| Eu-BCA thin-film | NFAs | turn-off | 0–40 μM | ~0.16 μM | [182] |
| Tb-AIP MMMs | NFAs | turn-off | 0–60 μM | ~ 0.30 μM | [183] |
| {[Tb-(TATMA) (H2O) · 2H2O}n | NFAs | turn-off | 0–5 μM | up to 3.35 × 104 M−1 | [184] |
| {[Cd3(TDCPB)·2DMAc]·DMAc·4H2O}n | NFAs | turn-off | 0–100 μM | up to 7.46 × 104M−1 | [185] |
| Fe-MIL-88B–NH2 | BPA | turn-on | 5.0 × 10−14 –2.0 × 10−9 mol L−1 | 4.1 × 10−14 mol L−1 | [202] |
| MOF-5 | nitro OPs | turn-off | 5–600 ppb | 5 ppb | [204] |
| FCS-1 | sulfonamide antibiotics | turn-off | - | 1.87 × 103- 3.7 × 104 M−1 | [205] |
| RhB@Tb-dcpcpt | NZF; NFT NFX; CPFX | turn-off | 0–0.1 mM | 99 ppb; 107 ppb; 69 ppb; 53 ppb | [206] |
| LVMOF-1 | Phenol; aniline; benzenediols; aminophenols | turn-off | 0–4 μM | 1–9 μg L−1 | [216] |
| NU – 1000 | acenaphthylene; pyrene; fluoranthene | turn-on | 0–16 μg L–1; 0–8 μg L–1; 0–10 μg L–1 | 183 ng L–1; 40 ng L–1; 35 ng L–1 | [217] |
| LMOF-241 | aflatoxin B1 | turn-off | 0–5.0 × 10−6 M | 46 ppb | [225] |
| Zr-CAU-24 | aflatoxin B1 | turn-off | 0.075–25 mM | 19.97 ppb | [226] |
| Fe3O4/g-C3N4/HKUST-1 | ochratoxin A | turn-on | 5.0–160.0 ng mL−1 | 2.57 ng/mL | [227] |
| Zr-BTDB-fcu-MOF | methylamine; aniline | turn-on; turn-off | (0.5–5) × 10−6 M (3–18) × 10−6 M | 66.2 nM 160 nM | [231] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, C.-X.; Zhao, N.; Liu, J.-C.; Chen, L.-J.; Liu, J.-M.; Fang, G.-Z.; Wang, S. Recent Progress on Luminescent Metal-Organic Framework-Involved Hybrid Materials for Rapid Determination of Contaminants in Environment and Food. Polymers 2020, 12, 691. https://doi.org/10.3390/polym12030691
Yao C-X, Zhao N, Liu J-C, Chen L-J, Liu J-M, Fang G-Z, Wang S. Recent Progress on Luminescent Metal-Organic Framework-Involved Hybrid Materials for Rapid Determination of Contaminants in Environment and Food. Polymers. 2020; 12(3):691. https://doi.org/10.3390/polym12030691
Chicago/Turabian StyleYao, Chi-Xuan, Ning Zhao, Ji-Chao Liu, Li-Jun Chen, Jing-Min Liu, Guo-Zhen Fang, and Shuo Wang. 2020. "Recent Progress on Luminescent Metal-Organic Framework-Involved Hybrid Materials for Rapid Determination of Contaminants in Environment and Food" Polymers 12, no. 3: 691. https://doi.org/10.3390/polym12030691
APA StyleYao, C.-X., Zhao, N., Liu, J.-C., Chen, L.-J., Liu, J.-M., Fang, G.-Z., & Wang, S. (2020). Recent Progress on Luminescent Metal-Organic Framework-Involved Hybrid Materials for Rapid Determination of Contaminants in Environment and Food. Polymers, 12(3), 691. https://doi.org/10.3390/polym12030691





