Hyperbranched Polycarbosiloxanes: Synthesis by Piers-Rubinsztajn Reaction and Application as Precursors to Magnetoceramics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Synthesis of Si-H Containing Aromatic Monomers
2.3. Synthesis of Si-H Containing Aromatic Monomers
2.4. Synthesis of Magnetic Ceramics from Si-HBP-3a
3. Results and Discussion
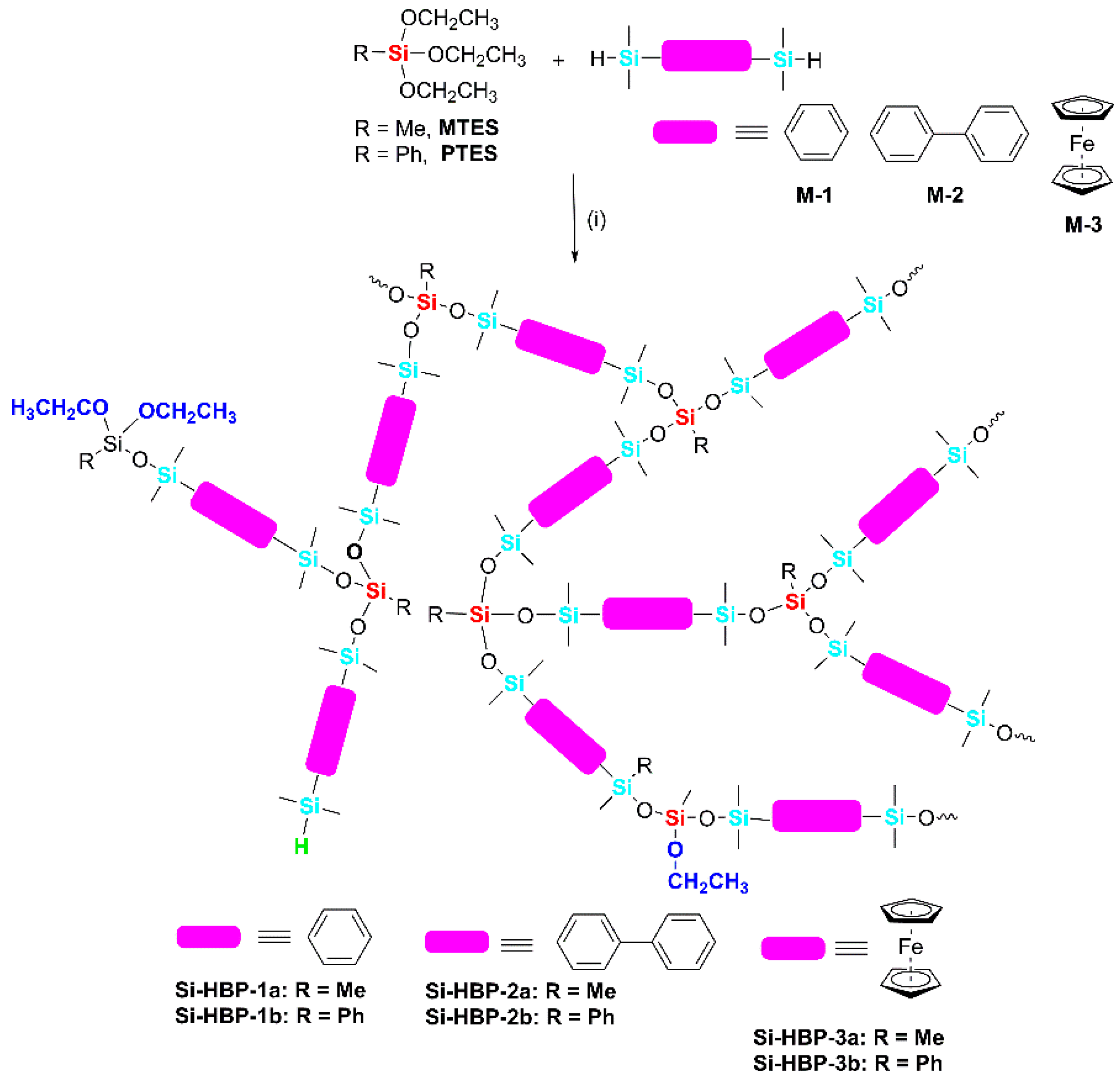
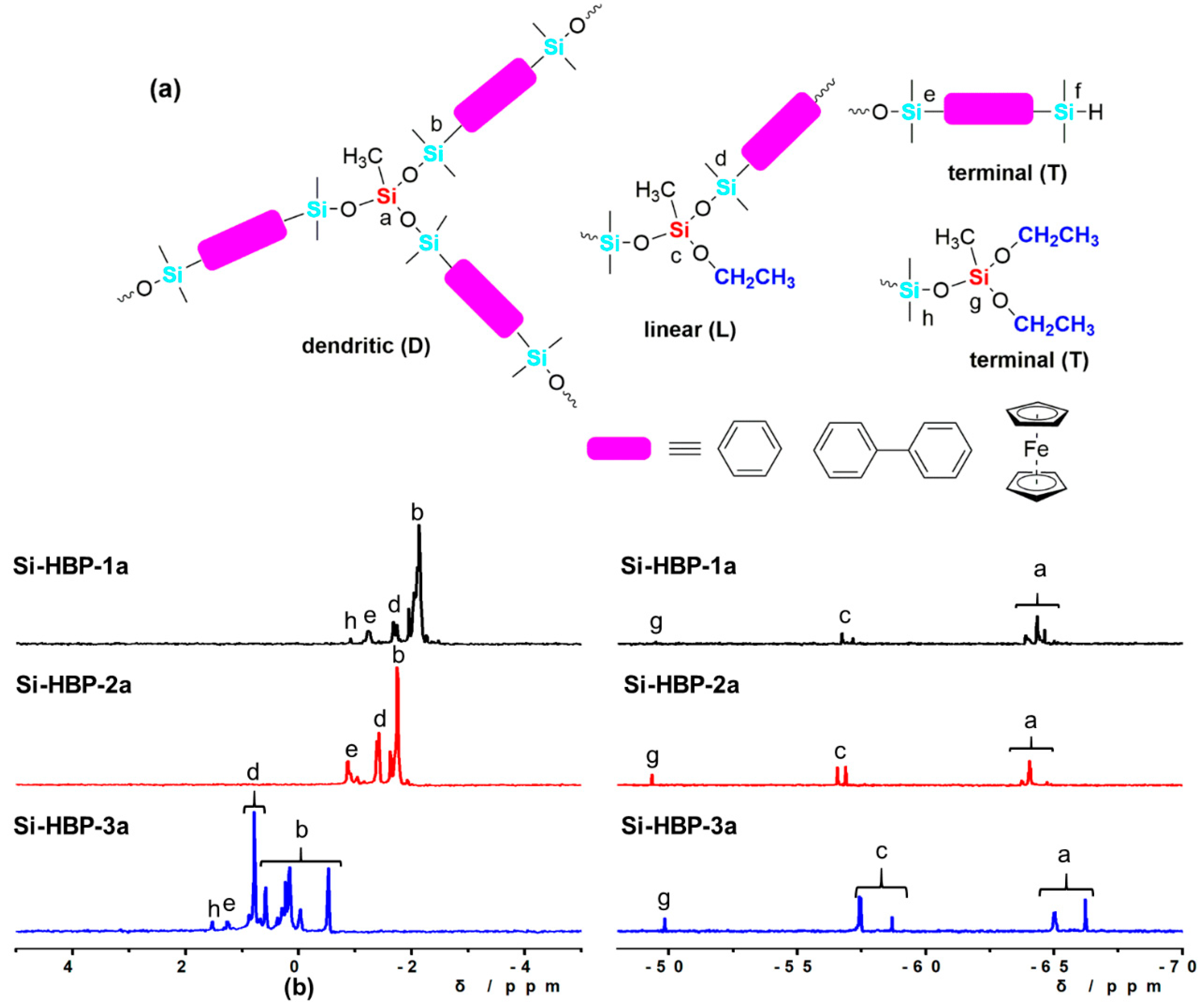
3.1. Synthesis
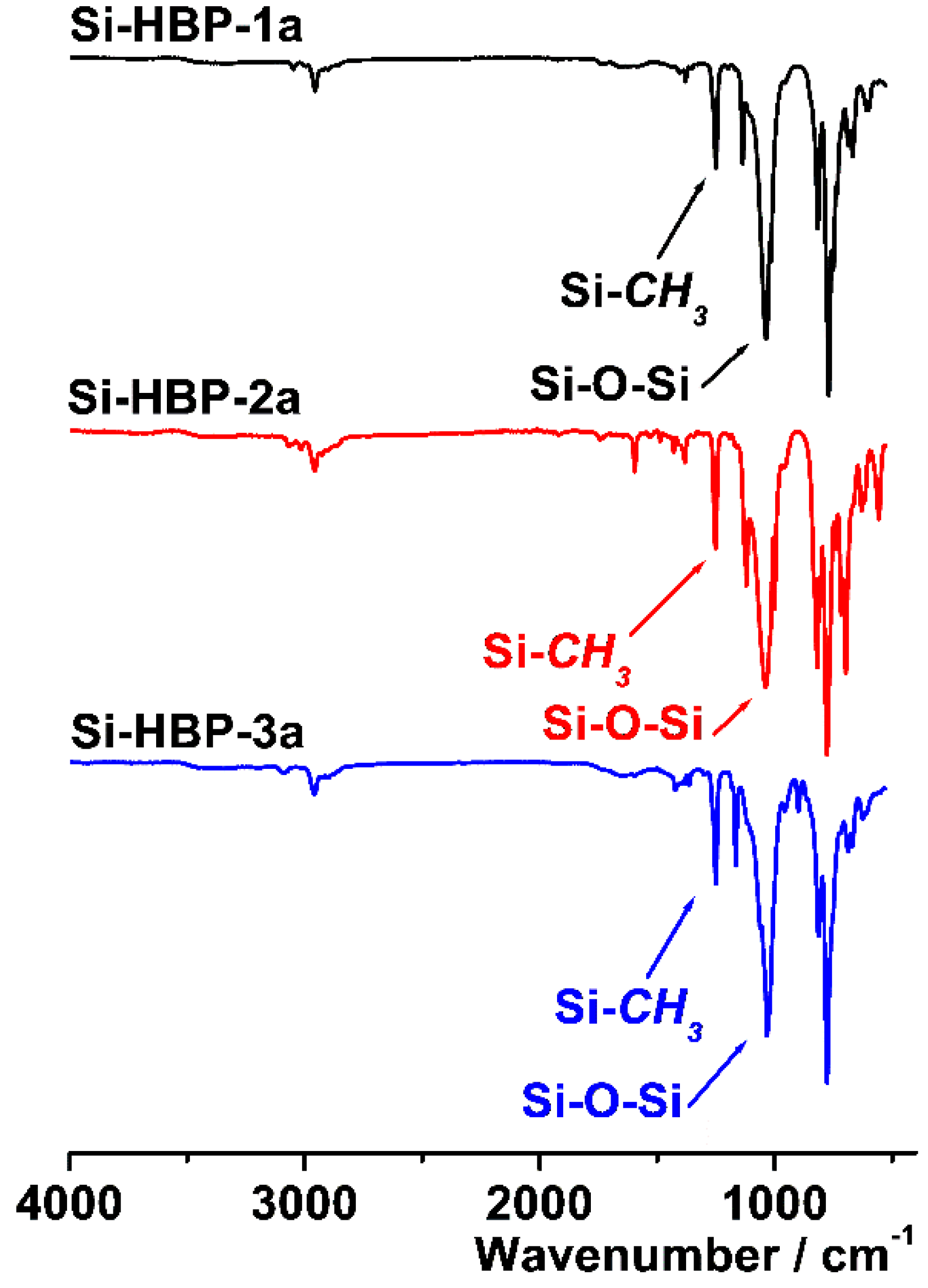
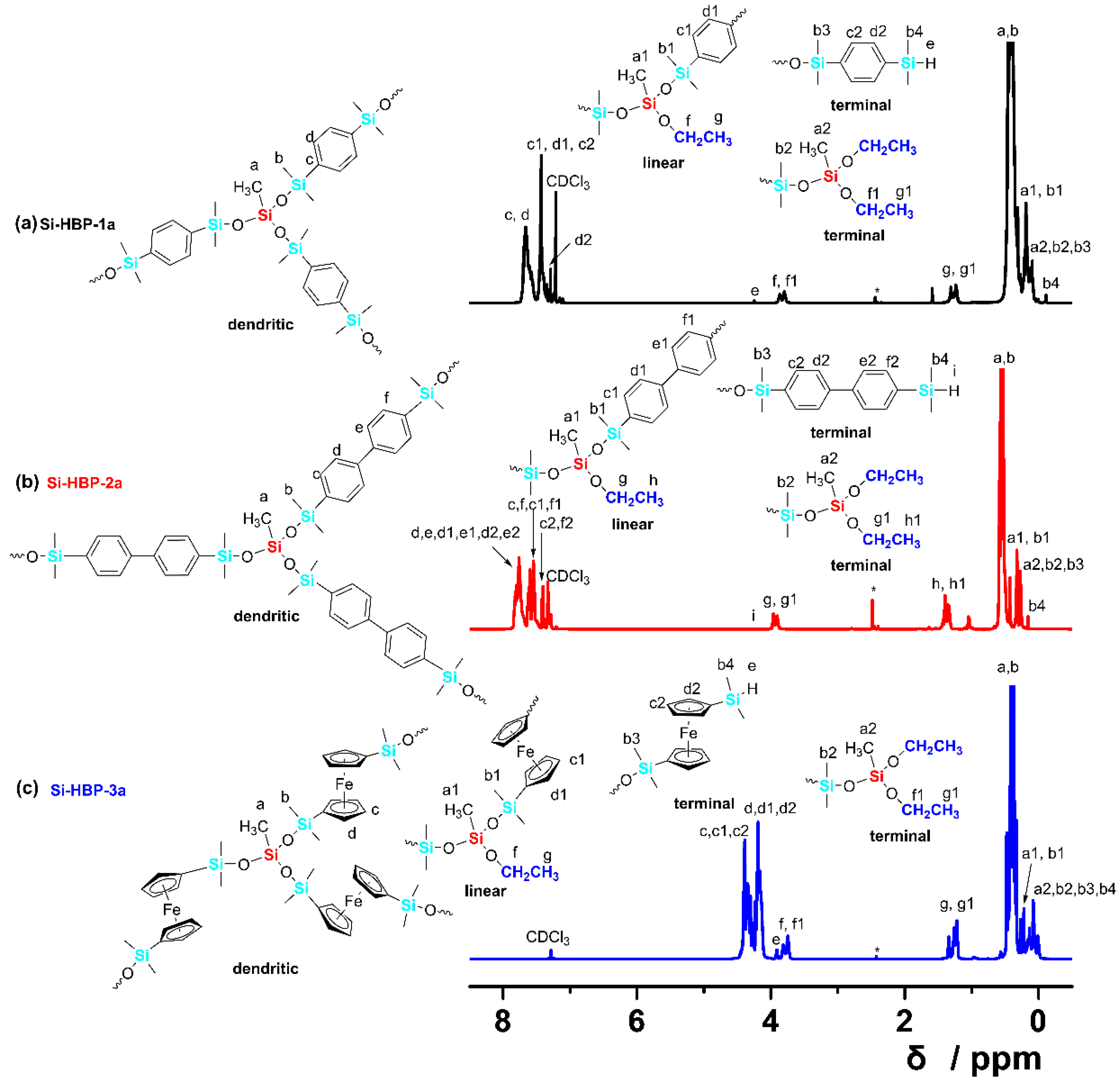
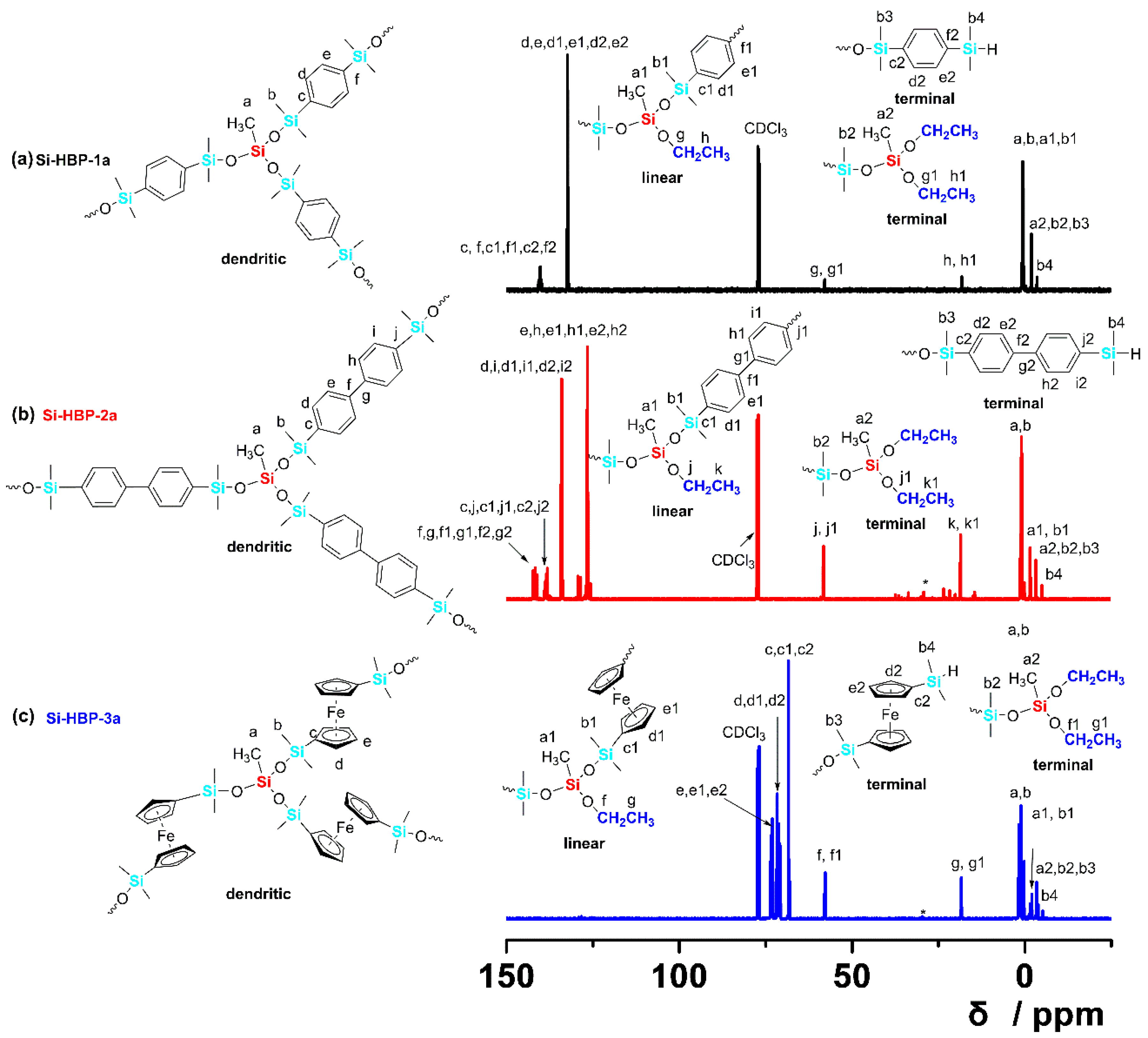
3.2. Characterization
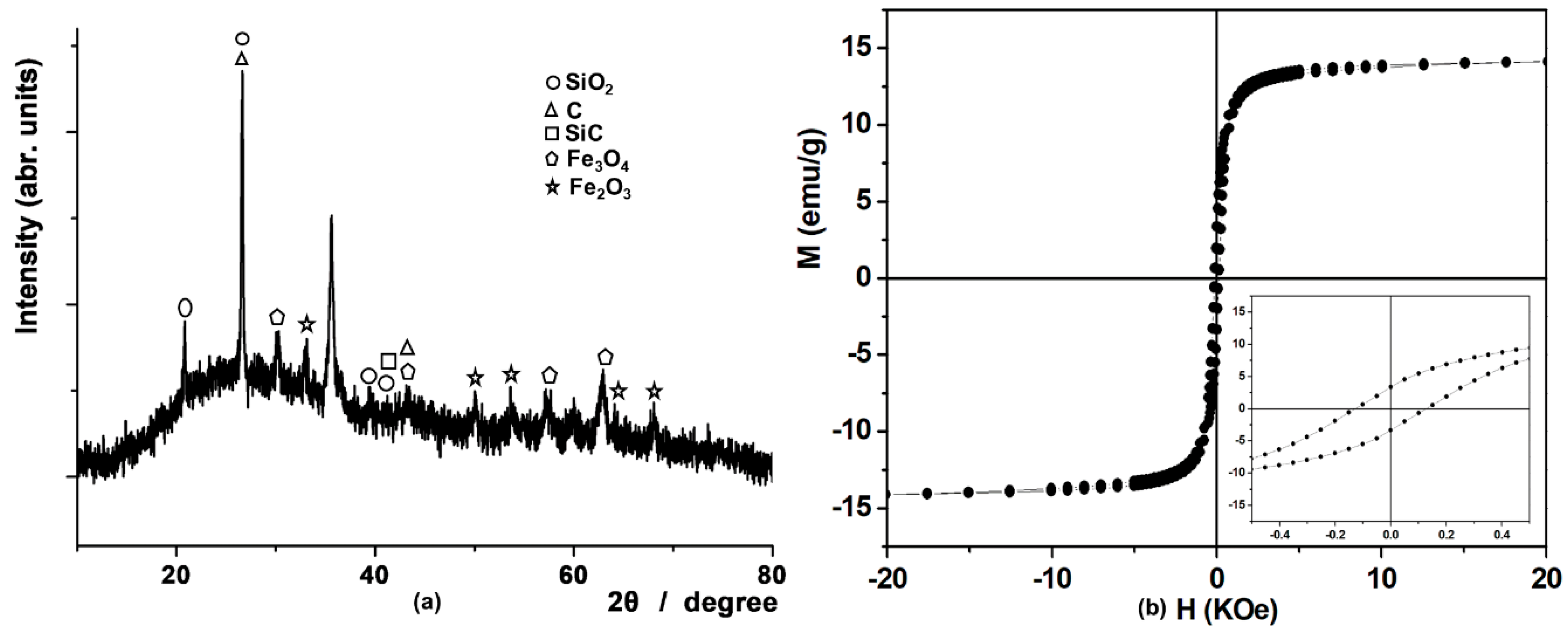
3.3. Magnetoceramic from Ferrocene-Based Hyperbranched Polymer Si-HBP-3a
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, Y.; Huang, W.; Liu, J.; Zhu, X.; Yan, D. Self-Assembly of Hyperbranched Polymers and Its Biomedical Applications. Adv. Mater. 2010, 22, 4567–4590. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, Y.; Yan, D. Hyperbranched polymer vesicles: From self-assembly, characterization, mechanisms, and properties to applications. Chem. Soc. Rev. 2015, 44, 3874–3889. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, T.; Zhu, X.; Yan, D.; Wang, W. Bioapplications of hyperbranched polymers. Chem. Soc. Rev. 2015, 44, 4023–4071. [Google Scholar] [PubMed]
- Wu, W.; Tang, R.; Li, Q.; Li, Z. Functional hyperbranched polymers with advanced optical, electrical and magnetic properties. Chem. Soc. Rev. 2015, 44, 3997–4022. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [PubMed]
- Zeng, H.; Wang, L.; Zhang, D.; Yan, P.; Nie, J.; Sharma, V.K.; Wang, C. Highly efficient and selective removal of mercury ions using hyperbranched polyethylenimine functionalized carboxymethyl chitosan composite adsorbent. Chem. Eng. J. 2019, 358, 253–263. [Google Scholar] [CrossRef]
- Chen, H.; Kong, J. Hyperbranched polymers from A2 + B3 strategy: Recent advances in description and control of fine topology. Polym. Chem. 2016, 7, 3643–3663. [Google Scholar] [CrossRef]
- Yao, J.Z.; Son, D.Y. Synthesis of an organosilicon hyperbranched oligomer containing alkenyl and silyl hydride groups. J. Polym. Sci.-Polym. Chem. 1999, 37, 3778–3784. [Google Scholar] [CrossRef]
- Yoon, K.; Son, D.Y. Syntheses of hyperbranched poly(carbosilarylenes). Macromolecules 1999, 32, 5210–5216. [Google Scholar] [CrossRef]
- Rim, C.; Son, D.Y. Hyperbranched poly(carbosilanes) from silyl-substituted furans and thiophenes. Macromolecules 2003, 36, 5580–5584. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Organosilicon dendritic networks in porous ceramics for water purification. Chem. Mater. 2005, 17, 3439–3444. [Google Scholar] [CrossRef]
- Li, D.; Niu, Y.; Yang, Y.; Wang, X.; Yang, F.; Shen, H.; Wu, D. Synthesis and self-assembly behavior of POSS-embedded hyperbranched polymers. Chem. Commun. 2015, 51, 8296–8299. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.W.; Li, W.J.; Zhang, C.; Zhou, C.J.; Feng, S.Y. Preparation of a novel hyperbranched carbosilane-silica hybrid coating for trace amount detection by solid phase microextraction/gas chromatography. J. Chromatog. A 2012, 1256, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Bai, T.; Yan, H.; Ding, F.; Bai, L.; Feng, W. High Fluorescence Quantum Yield Based on the Through-Space Conjugation of Hyperbranched Polysiloxane. Macromolecules 2019, 52, 3075–3082. [Google Scholar] [CrossRef]
- Bai, L.; Yan, H.; Bai, T.; Feng, Y.; Zhao, Y.; Ji, Y.; Feng, W.; Lu, T.; Nie, Y. High Fluorescent Hyperbranched Polysiloxane Containing beta-Cyclodextrin for Cell Imaging and Drug Delivery. Biomacromolecules 2019, 20, 4230–4240. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, Y.; Jiang, T.; Chang, Z.; Lam, J.W.Y.; Xu, L.; Qiu, H.; Tang, B.Z. A Fully Substituted 3-Silolene Functions as Promising Building Block for Hyperbranched Poly(Silylenevinylene). Macromol. Rapid Commun. 2012, 33, 1074–1079. [Google Scholar] [CrossRef]
- Kong, J.; Schmalz, T.; Motz, G.; Müller, A.H.E. Novel Hyperbranched Ferrocene-Containing Poly(boro)carbosilanes Synthesized via a Convenient “A2 + B3” Approach. Macromolecules 2011, 44, 1280–1291. [Google Scholar] [CrossRef]
- Liu, X.; Rathore, J.S.; Dubois, G.; Interrante, L.V. Grignard Condensation Routes to 1,3-Disilacyclobutane-Containing Cyclolinear Polycarbosilanes. J. Polym. Sci. Polym. Chem. 2017, 55, 1547–1557. [Google Scholar] [CrossRef]
- Migulin, D.; Milenin, S.; Cherkaev, G.; Svidchenko, E.; Surin, N.; Muzafarov, A. Sodiumoxy(aminopropyl) alkoxysilanes-AB2 type monomers for the synthesis of hyperbranched poly(aminopropyl) alkoxysiloxanes and their derivatives. J. Organomet. Chemistry 2018, 859, 24–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, Y.J.; Yang, T.X.; Gou, Z.M.; Lin, W.Y. Polysiloxane-based hyperbranched fluorescent materials prepared by thiolene “click” chemistry as potential cellular imaging polymers. Eur. Polym. J. 2019, 112, 515–523. [Google Scholar] [CrossRef]
- Xue, L.; Yang, Z.; Wang, D.; Wang, Y.; Zhang, J.; Feng, S. Synthesis and characterization of silicon-containing hyperbranched polymers via thiol-ene click reaction. J. Organomet. Chem. 2013, 732, 1–7. [Google Scholar] [CrossRef]
- Parks, D.J.; Piers, W.E. Tris(pentafluorophenyl)boron-Catalyzed Hydrosilation of Aromatic Aldehydes, Ketones, and Esters. J. Am. Chem. Soc. 1996, 118, 9440–9441. [Google Scholar] [CrossRef]
- Rubinsztajn, S.; Cella, J.A. A New Polycondensation Process for the Preparation of Polysiloxane Copolymers. Macromolecules 2005, 38, 1061–1063. [Google Scholar] [CrossRef]
- Brook, M.A. New Control Over Silicone Synthesis using SiH Chemistry: The Piers–Rubinsztajn Reaction. Chem. Eur. J. 2018, 24, 8458–8469. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yi, M.; Wu, S.; Tan, L.; Ge, X.; He, M.; Yin, G. Synthesis of Structurally Precise Polysiloxanes via the Piers-Rubinsztajn Reaction. Materials 2019, 12, 304. [Google Scholar] [CrossRef] [PubMed]
- Sample, C.S.; Lee, S.-H.; Bates, M.W.; Ren, J.M.; Lawrence, J.; Lensch, V.; Gerbec, J.A.; Bates, C.M.; Li, S.; Hawker, C.J. Metal-Free Synthesis of Poly(silyl ether)s under Ambient Conditions. Macromolecules 2019, 52, 1993–1999. [Google Scholar] [CrossRef]
- Schneider, A.F.; Brook, M.A. High-Throughput Synthesis and Characterization of Aryl Silicones by Using the Piers-Rubinsztajn Reaction. Chem. Eur. J. 2019, 25, 15367–15374. [Google Scholar] [CrossRef]
- Ai, L.; Chen, Y.; He, L.; Luo, Y.; Li, S.; Xu, C. Synthesis of structured polysiloxazanes via a Piers-Rubinsztajn reaction. Chem. Commun. 2019, 55, 14019–14022. [Google Scholar] [CrossRef]
- Yi, M.; Chen, X.; Wu, S.; Ge, J.; Zhou, X.; Yin, G. Fabrication of Reactive Poly(Phenyl-Substituted Siloxanes/Silsesquioxanes) with Si-H and Alkoxy Functional Groups via the Piers-Rubinsztajn Reaction. Polymers 2018, 10, 1006. [Google Scholar] [CrossRef]
- Macphail, B.; Brook, M.A. Controlling silicone-saccharide interfaces: Greening silicones. Green Chem. 2017, 19, 4373–4379. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Sewell, P.; Brook, M.A. Utilization of softwood lignin as both crosslinker and reinforcing agent in silicone elastomers. Green Chem. 2015, 17, 1811–1819. [Google Scholar] [CrossRef]
- Yu, J.Y.; Liu, Y.Z. Cyclic Polysiloxanes with Linked Cyclotetrasiloxane Subunits. Ang. Chem. Int. Ed. 2017, 56, 8706–8710. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Oba, Y.; Nakajima, Y.; Shimada, S.; Sato, K. One-Pot Sequence-Controlled Synthesis of Oligosiloxanes. Ang. Chem. Int. Ed. 2018, 57, 4637–4641. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yu, J.Y.; Meng, Y.Y.; Liu, Y.Z. Hyperbranched Polysiloxanes Based on Polyhedral Oligomeric Silsesquioxane Cages with Ultra-High Molecular Weight and Structural Tuneability. Polymers 2018, 10, 496. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hu, C.H.; Liu, Y.Z. Hyperbranched polysiloxane with highly constrained rings and the effect of the attached arms on the assembly behavior. Polym. Chem. 2017, 8, 6490–6495. [Google Scholar] [CrossRef]
- Chojnowski, J.; Rubinsztajn, S.; Fortuniak, W.; Kurjata, J. Synthesis of Highly Branched Alkoxysiloxane−Dimethylsiloxane Copolymers by Nonhydrolytic Dehydrocarbon Polycondensation Catalyzed by Tris(pentafluorophenyl)borane. Macromolecules 2008, 41, 7352–7358. [Google Scholar] [CrossRef]
- Grande, J.B.; Urlich, T.; Dickie, T.; Brook, M.A. Silicone dendrons and dendrimers from orthogonal SiH coupling reactions. Polym. Chem. 2014, 5, 6728–6739. [Google Scholar] [CrossRef]
- Chen, H.; Kong, J.; Tian, W.; Fan, X.-D. Intramolecular Cyclization in A2 + B3 Polymers via Step-Wise Polymerization Resulting in a Highly Branched Topology: Quantitative Determination of Cycles by Combined NMR and SEC Analytics. Macromolecules 2012, 45, 6185–6195. [Google Scholar] [CrossRef]
- Frey, H.; Hölter, D. Degree of branching in hyperbranched polymers. 3 Copolymerization of ABm-monomers with AB and ABn-monomers. Acta Polym. 1999, 50, 67–76. [Google Scholar] [CrossRef]
- Hawker, C.J.; Lee, R.; Frechet, J.M.J. One-step synthesis of hyperbranched dendritic polyesters. J. Am. Chem. Soc. 1991, 113, 4583–4588. [Google Scholar] [CrossRef]
- Li, H.; Chi, W.; Liu, Y.; Yuan, W.; Li, Y.; Li, Y.; Tang, B.Z. Ferrocene-Based Hyperbranched Polytriazoles: Synthesis by Click Polymerization and Application as Precursors to Nanostructured Magnetoceramics. Macromol. Rapid Commun. 2017, 38, 1700075. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Kong, M.; Zhang, X.; Chen, L.; An, L. Magnetoceramics from the Bulk Pyrolysis of Polysilazane Cross-Linked by Polyferrocenylcarbosilanes with Hyperbranched Topology. ACS Appl. Mater. Interface 2013, 5, 10367–10375. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Schmalz, T.; Motz, G.; Muller, A.H.E. Magnetoceramic nanocrystals from the bulk pyrolysis of novel hyperbranched polyferrocenyl(boro)carbosilanes. J. Mater. Chem. C 2013, 1, 1507–1514. [Google Scholar] [CrossRef]
- Häußler, M.; Sun, Q.; Xu, K.; Lam, J.W.Y.; Dong, H.; Tang, B.Z. Hyperbranched Poly(ferrocenylene)s Containing Groups 14 and 15 Elements: Syntheses, Optical and Thermal Properties, and Pyrolytic Transformations into Nanostructured Magnetoceramics. J. Inorg. Organomet. Polym. Mater. 2005, 15, 67–81. [Google Scholar] [CrossRef]
| Entry | The Mount of Catalyst (mol %) | Gel Time |
|---|---|---|
| 1 | 0.2 | ca. 5 h |
| 2 | 0.5 | 32 min |
| 3 | 0.8 | < 30 s |
| 4 | 1.1 | < 20 s |
| Samples | Mn (g mol−1) a | Mw (g mol−1) a | PDI a | DB b | DB c |
|---|---|---|---|---|---|
| Si-HBP-1a | 4300 | 18100 | 4.21 | 0.89 | 0.81 |
| Si-HBP-1b | 3600 | 14300 | 3.97 | 0.89 | 0.88 |
| Si-HBP-2a | 3000 | 11800 | 3.93 | 0.77 | 0.65 |
| Si-HBP-2b | 2800 | 10100 | 3.61 | 0.84 | 0.83 |
| Si-HBP-3a | 1950 | 4700 | 2.41 | 0.69 | 0.55 |
| Si-HBP-3b | 1850 | 4000 | 2.16 | 0.69 | 0.61 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xue, L.; Li, J.; Ma, Q. Hyperbranched Polycarbosiloxanes: Synthesis by Piers-Rubinsztajn Reaction and Application as Precursors to Magnetoceramics. Polymers 2020, 12, 672. https://doi.org/10.3390/polym12030672
Zhang H, Xue L, Li J, Ma Q. Hyperbranched Polycarbosiloxanes: Synthesis by Piers-Rubinsztajn Reaction and Application as Precursors to Magnetoceramics. Polymers. 2020; 12(3):672. https://doi.org/10.3390/polym12030672
Chicago/Turabian StyleZhang, Huayu, Lei Xue, Jianquan Li, and Qingyu Ma. 2020. "Hyperbranched Polycarbosiloxanes: Synthesis by Piers-Rubinsztajn Reaction and Application as Precursors to Magnetoceramics" Polymers 12, no. 3: 672. https://doi.org/10.3390/polym12030672
APA StyleZhang, H., Xue, L., Li, J., & Ma, Q. (2020). Hyperbranched Polycarbosiloxanes: Synthesis by Piers-Rubinsztajn Reaction and Application as Precursors to Magnetoceramics. Polymers, 12(3), 672. https://doi.org/10.3390/polym12030672






