3.1. Synthesis and Identification of Metal Containing Mono(hydroxyethoxyethyl) Phthalate (M(HEEP)2)
In our pre-experiment, the metal containing mono(hydroxyethoxyethyl) phthalate (M(HEEP)
2) was synthesized from diethyl glycol (DEG), phthalate (PA), and various of metal acetates including Zn, Mn, Pb, and Ca acetates at 60–70 °C as described in Matsuda [
20] and Jayakurmar et al. [
17], however, the yields of M(HEEP)
2 were only 4–5%. In our modified process, the mixture of PA and DEG was stirred and temperature was rose up to 135 °C, which was higher than the melt point of PA of 131 °C, and maintained for 1.5 h to obtain mono diglycolic phthalate. Then the divalent metal acetates were added respectively, and the reaction temperature of the mixtures was adjusted to a specific temperature (as listed in
Table 1) according to the insoluble precipitates produced. When the specific temperature reached, the mixture was continued stirred at the temperature for another 3 h. The results showed that the yields of the M(HEEP)
2 were increased to 43.4–55.1%. The appearance of the M(HEEP)
2 powder was a milk white color except the Mn(HEEP)
2 powder was a light pink color as shown in
Figure 1.
The FTIR spectra of M(HEEP)
2 are shown in
Figure 2. A broad absorption peak at 3400–3500 cm
−1, which indicates the presence of OH groups and the peak that represents the carbonyl (C=O) group stretching vibration was observed at 1700–1730 cm
−1. The peaks at 1400–1430 and 1545–1570 cm
−1 indicate the ion bonding vibrations between carbonyl group and metal ions. In addition, peaks at 1080–1130 cm
−1 (C–O–C stretching vibration), 1045–1060 cm
−1 (primary alcohol of C–O stretching vibration), and 725–735 cm
−1 (C–H out of plane bending vibration of the benzene ring) [
11,
13,
17,
18,
19,
20] indicates that M(HEEP)
2 was readily synthesized.
The structures of M(HEEP)
2 were also investigated by NMR spectrometry. Taking Ca(HEEP)
2 as an example, the
1H-NMR and
13C-NMR spectra are displayed by
Figure 3 and
Figure 4, respectively. In the
1H-NMR spectrum, the signals were observed in the 7.41–7.48 ppm (H1), which corresponded to the aromatic proton. The signals with the chemical shift at 4.21–4.25 ppm (H2) could be assigned to the proton of –COOC
H2–, and the proton of –O
H shows signals at 3.86–3.88 ppm. (H3) The peaks at the 3.71–3.76 ppm (H4) region could be attributed to the methylene group proton of R–COOAr, and the protons of –C
H2–O–C
H2– show signals at 3.61–3.64 ppm (H5) [
11,
13,
17,
18,
19,
20].
The
13C-NMR spectrum of Ca(HEEP)
2 (
Figure 4) shows the carbon of COO– bonding with Ca (C12) and COO– adjacent to CH
2 (C5) at 176.7–177.1 ppm and 169.3 ppm, respectively. The signal at 136.2 ppm (C11) and 132.5 ppm (C6) assigned to the carbon atoms of the aromatic group attached to COO–. The aromatic carbons C7, C8, C9, and C10 appeared in the 126.6–129.3 ppm regions. The carbon of the methylene shows a peak at 71.5 ppm (C1) for –
CH
2–OH, at 71.7 ppm (C4) for –
CH
2OOC–Ar, at 60.3 ppm (C2) for –
CH
2–CH
2OH, and 68.2 ppm (C3) for –
CH
2–CH
2OOC–Ar [
11,
13,
17,
18,
19,
20]. These results indicated clearly that the Ca(HEEP)
2 was synthesized successfully from DEG, PA, and calcium acetate.
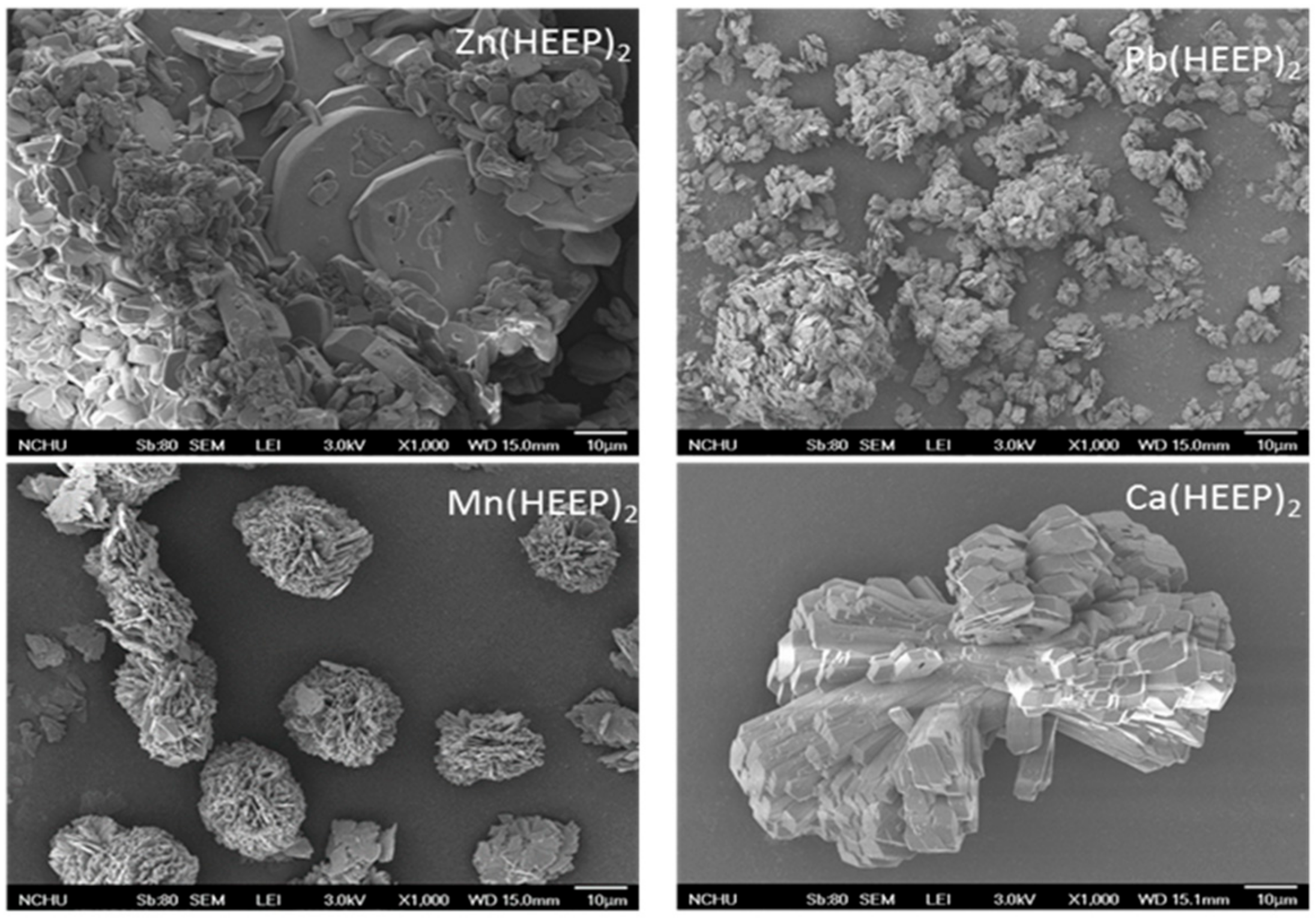
The morphologic characterization of M(HEEP)
2 was determined by the FE-SEM analysis as listed in
Figure 5. The Zn(HEEP)
2 was piled as a sheet-like shape and had the largest size; the Ca(HEEP)
2 was piled as a columnar shape; the Mn(HEEP)
2 was piled as a spherical shape; and the Pb(HEEP)
2 was piled as a lumpy shape and had the smallest size. The different morphology and size of M(HEEP)
2 crystals may be due to the different nucleation during the synthesis process [
29]. The melt points of zinc acetate (200 °C) and calcium acetate (160°C) were higher than or equal to the synthesis temperature (as shown in
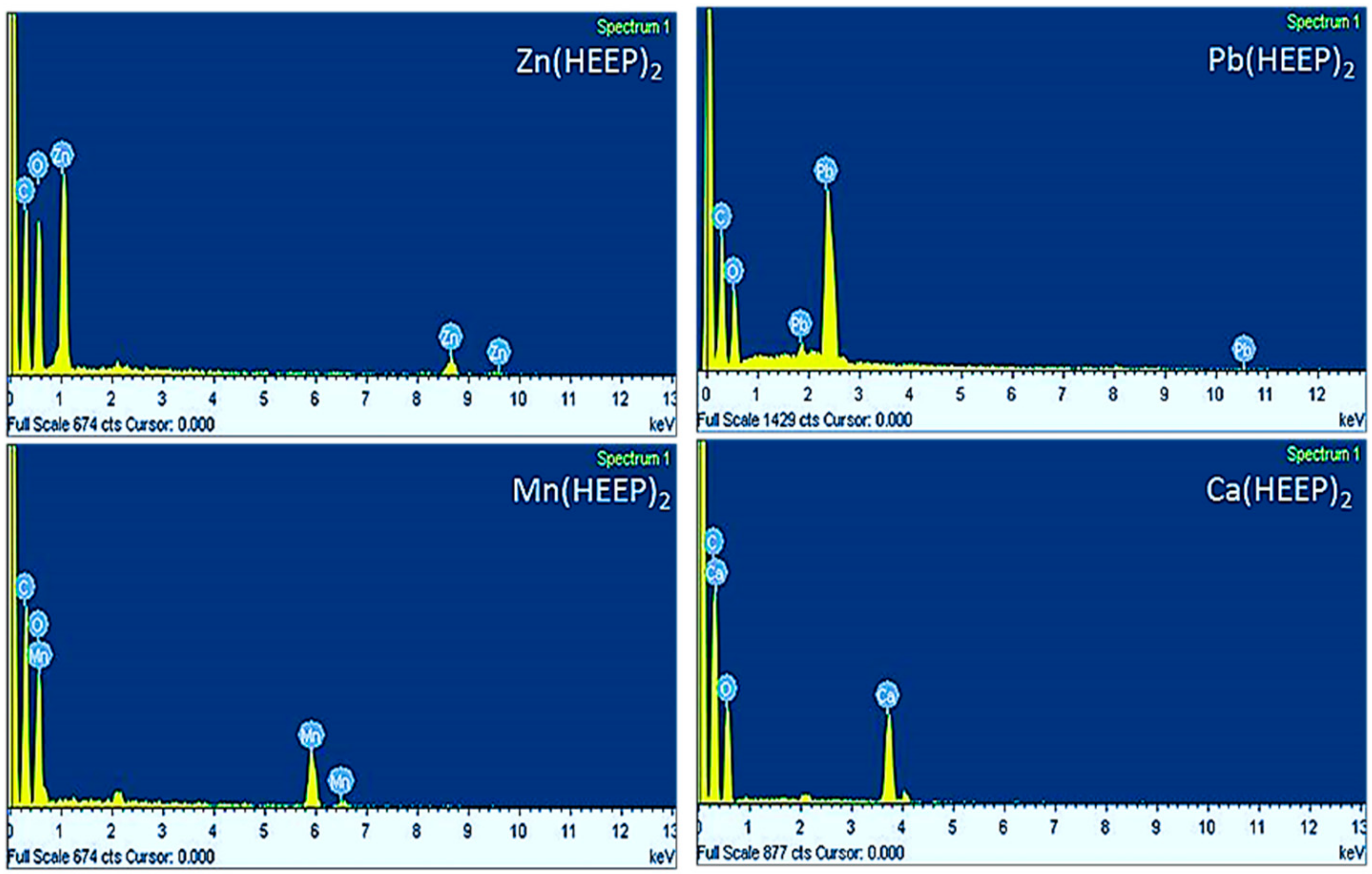
Table 1), resulting in an uneven nucleation and obtaining a larger size crystal. On the contrary, the manganese acetate and lead acetate had a lower melt point of 80 and 75 °C, which was also lower than the synthesis temperature, attributing to an even nucleation and a smaller size crystal obtained. In addition, the EDS was used for confirming the metal ions were introduced into the mono(hydroxyethoxyethyl) phthalate as displayed by
Figure 6. The results further revealed that the M(HEEP)
2 (M = Zn, Mn, Pb, and Ca) were synthesized successfully as shown in
Scheme 1.
3.2. Antibacterial Activity of M(HEEP)2-Containing WUO Coatings
In this study, the WUO coatings with hexamethylene diisocyanate (HDI) and isophorone diisocyanate (IPDI), and dimethylolpropionic acid at NCO/OH molars of 0.9 were used, which were, respectively, named the HDI-0.9 coating and IPDI-0.9 coating. The bacteria including
E. coli (Gram-negative bacterium) and
S. aureus (Gram-positive bacterium) were used and the agar disk diffusion method was applied for determining the antibacterial activity. The antibacterial activity of various M(HEEP)
2 (M = Zn, Mn, Pb, and Ca) and commercial nanoAg dosages containing WUO films synthesizing with HDI and IPDI for
E. coli are listed in
Table 2 and
Table 3, respectively, and the inhibitory zone of Zn(HEEP)
2 for
E. coli that was used as an example is presented in
Figure 7. The results showed that the inhibitory zone of the blank group (WUO films containing 0 phr Zn(HEEP)
2)) was 8 mm (
Figure 7a), meaning inactive on antibacterial activity, while the inhibitory zone increased to 15 mm (as shown in
Figure 7b) with increasing Zn(HEEP)
2 dosage of 2.0 phr, assigning mildly active antibacterial activity. However, the inhibitory zone decreased to 11 mm with increasing the Zn(HEEP)
2 content to 4.0 phr (
Figure 7c). The
Table 2 showed that in the HDI-0.9 coating, except the Zn(HEEP)
2 must add to 0.6 phr, the other M(HEEP)
2 ((M = Mn, Pb, and Ca) and commercial nanoAg had only the dosage of 0.2 phr had promptly a mildly active antibacterial activity for
E. coli.
Table 3 for IPDI-0.9 coatings, the inhibitory zone of without M(HEEP)
2 was 8 mm, indicating inactive antibacterial activity for
E. coli. The Zn(HEEP)
2 containing WUO film had a superior antibacterial activity, its inhibitory zones increased from 11 to 14 mm with an increasing dosage of 0.2–1.0 phr. The inhibitory zones of Mn(HEEP)
2 containing WUO films were 10–11 mm, also assigning a mildly active, but they show less efficiency on antibacterial activity than the Zn(HEEP)
2 containing WUO films. In addition, the Pb(HEEP)
2 and Ca(HEEP)
2 containing WUO films (IPDI-Pb and IPDI-Ca) had inactive antibacterial activity for
E. coli. However, the control group of commercial nanoAg containing WUO films (IPDI-Ag) possessed inhibitory zones of 19–23 mm and had the highest antibacterial activity for
E. coli. Even though by only adding 0.2 phr of nanoAg, it shows a highly active antibacterial activity.
The antibacterial activity of various M(HEEP)
2 (M = Zn, Mn, Pb, and Ca) and commercial nanoAg dosage containing WUO films synthesizing with HDI and IPDI for
S. aureus are listed in
Table 4 and
Table 5, respectively. The results showed that the inhibitory zone of WUO films without M(HEEP)
2 and commercial nanoAg was 9 mm, meaning inactive antibacterial activity for
S. aureus. The inhibitory zone of HDI-Zn, HDI-Mn, and HDI-Ca films were 11–14 mm, 12–14 mm, and 13–15 mm, respectively, assigning mildly active antibacterial activity. However, the HDI-Pb films had a larger inhibitory zone of 15–16 mm, especially the ones of 0.4–1.0 phr possessed a superior antibacterial activity, which was compared with the control group of HDI-Ag films, which the inhibitory zone was 18–19 mm and revealed a moderately active antibacterial activity for
S. aureus.
Table 5 shows the IPDI-0.9 coatings on antibacterial activity for
S. aureus. The inhibitory zone of IPDI-Mn, IPDI-Pb, and IPDI-Ca were 12–14 mm, 12–14mm, and 11–13 mm, respectively, indicating a mildly active on antibacterial activity for
S. aureus. However, the Zn(HEEP)
2 containing WUO films had a larger inhibitory zone of 15–19 mm, meaning a superior moderately active antibacterial activity, which was slightly less or equal to that of commercial nanoAg containing WUO films, which possessed a highly active antibacterial activity with a dosage of 0.8, 1.0, and 4.0 phr.
Integrating the results mentioned above, it could be concluded that except the IPDI-Pb and IPDI-Ca for
E. coli, all of the WUO films with M(HEEP)
2 had antibacterial activity for
E. coli and
S. aureus. Furthermore, the antibacterial efficiency for
S. aureus was higher than that for
E. coli. This might be due to the
S. aureus belonging to Gram-positive bacterium, which the cell wall is composed of thickening peptidoglycan and takes a negative charge on the surface, which is more sensitivity to the metal ion with a positive charge than the Gram-negative bacterium of
E. coli. In addition, the antibacterial efficiency of IPDI-Ag films was superior to that of HDI-Ag, which might be attributed to the IPDI-0.9 coating having a larger particle size of the z-average diameter of 995 nm than the HDI-0.9 coating of 719 nm [
21], and the nanoAg was easy and well dispersed in the waterborne IPDI-0.9 coating, on the contrary it was easy agglutinated in the HDI-0.9 coating, resulting in a less free Ag ion and leading to inferior antibacterial activity.
3.3. Antifungal Activity of M(HEEP)2-Containing WUO Coatings
In this study, the fungi including brown-rot fungus of
G. trabeum and white-rot fungus of
L. betulina were used and the agar disk diffusion method was applied for determining the antifungal activity. The antifungal activity of various M(HEEP)
2 (M = Zn, Mn, Pb, and Ca) and commercial nanoAg dosages containing WUO films synthesizing with HDI and IPDI for
G. trabeum are listed in
Table 6 and
Table 7, respectively. The results showed that the inhibitory zone of blank group (0 phr) was 8 mm, meaning inactive antifungal activity for
G. trabeum. The WUO films with HDI and containing M(HEEP)
2 including HDI-Zn, HDI-Mn, HDI-Pb, HDI-Ca, and commercial nanoAg (HDI-Ag) had the inhibitory zone of 11–13 mm, 9–12 mm, 11–13 mm, 9–11 mm, and 10–11 mm, respectively, revealing only a dosage of 0.2 phr had mildly active antifungal activity for
G. trabeum. Furthermore, even though the HDI-0.9 films with antimicrobial agents attributed to mildly active antifungal activity, the HDI-Zn and HDI-Pb exhibiting a superior antifungal efficiency compared to HDI-Ag.
The IPDI-0.9 films with antimicrobial agents (as shown in
Table 7) had similar results to the HDI-0.9 films, especially the IPDI-Zn, IPDI-Mn, and IPDI-Ca with a dosage of 0.2 phr had a higher antifungal activity for
G. trabeum than IPDI-Ag and that of HDI-0.9 films. In particular the inhibitory zone of IPDI-Mn was 16 mm, assigning a moderately active grade.
The antifungal activity of various M(HEEP)
2 (M = Zn, Mn, Pb, and Ca) and commercial nanoAg dosages containing WUO films synthesizing with HDI and IPDI for white-rot fungus of
L. betulina are listed in
Table 8 and
Table 9, respectively. The results indicated that among all of the WUO films, the HDI-Zn had the best antifungal activity for
L. betulina, but the dosage of Zn(HEEP)
2 must exceed 1.0 phr. In addition to this, the HDI-Mn, HDI-Pb, HDI-Ca, and control HDI-Ag had almost inactive antifungal activity for
L. betulina. The IPDI-0.9 films had similar results to HDI-0.9 films, only the IPDI-Zn with a dosage of 4.0 phr had an inhibitory zone of 13 mm, assigning a mildly active antifungal activity.
From the results of antimicrobial activity, it can be seen that the metal-containing WUO films had more efficiency on antibacterial activity for bacterial than on antifungal activity for fungi. This may be attributed to the metal ions that can directly and easily damage the bacterial cell wall, by release of ions followed by an increased membrane permeability, loss of the proton motive force, and efflux of intracellular components [
30]. The mechanism of metal ions on inhibiting microbial growth is an interesting topic and will be studied in the future.
3.4. Film Properties of WUO Coatings
In the present study, the M(HEEP)
2 (M = Zn, Mn, Pb, and Ca) were additives for antimicrobial agents. Even though the results revealed that the WUO film synthesizing with HDI films containing Zn(HEEP)
2 of 2.0 phr and Pb(HEEP)
2 of 0.4 phr had the best antibacterial activity for
E. coli and
S. aureus, respectively. The IPDI films containing Zn(HEEP)
2 of 1.0 phr had the best antibacterial activity for both
E. coli and
S. aureus. For antifungal activity, the WUO film synthesizing with HDI films containing Pb(HEEP)
2 of 0.8 phr and Zn(HEEP)
2 of 2.0 phr as well as IPDI films containing Mn(HEEP)
2 of 0.2 phr and Zn(HEEP)
2 of 4.0 phr had the best performances against
G. trabeum and
L. betulina, respectively. From the results mentioned above it also shows that the WUO films of HDI-Pb and IPDI-Zn, which were synthesized with HDI and IPDI, respectively, had the best antimicrobial efficiency for bacteria and fungi used in the study. According to the antimicrobial activity grades, the dosage of Zn(HEEP)
2 and Pb(HEEP)
2 was only 0.2 phr, which exhibited superiority of antimicrobial activity. Therefore, in this section, the films properties of HDI-0.9 (0.0 phr), HDI-Pb (0.2 phr), HDI-Ag (0.2 phr as control group), IPDI-0.9, IPDI-Zn, and IPDI-Ag were compared. The results are listed in
Table 10.
The WUO film of IPDI-0.9 had a higher hardness of 87 sec than HDI-0.9 of 13 sec, while the hardness of metal-containing WUO films decreased slightly by adding the Zn(HEEP)
2, Pb(HEEP)
2, and nanoAg, which was from 13 to 11 sec for HDI films and from 87 to 72–76 sec for IPDI films. The IPDI film had a higher hardness than the HDI film. The results were also confirmed by the studies of Chang et al. [
22]. The impact resistance of all HDI films was 25 cm, but of all IPDI films was lower than 5 cm. The bending resistance of all HDI films was lower than 2 mm-diameters and was superior to all IPDI films of 10 mm-diameter steel shaft. The results demonstrated that there had no difference between with or without metal-containing and kinds of metal ions on the impact and bending resistance of the WUO films. In addition, due to having a long alkyl structure for HDI films, it had a lower hardness, higher impact, and bending resistance than IPDI films with a hard and brittle alicyclic structure. However, all of the films showed an excellent adhesion of 10 grades on wood, exhibiting that the WUO coating was suitable for wood finishing. The mass retention of HDI-0.9 was 75.0%, which increased slightly for HDI-Zn and HDI-Ag of 77.4% and 77.1%, respectively. The similar result could also be found in IPDI-films. While, the HDI films had a higher mass retention than IPDI films, it may be due to the molecular weight of HDI (Mw = 10,734) being higher than that of IPDI (Mw = 6126) [
21], and the steric effect of IPDI hindering the oxidative polymerization of unsaturated fatty acids in linseed oil [
31]. The 60° gloss of HDI-0.9 had a value of 78%, whereas the HDI-Pb showed a decrease to 62% and an HDI-Ag increase to 81%. The results may be attributed to the Zn(HEEP)
2 and Pb(HEEP)
2 having a larger particle diameter than nanoAg. Similar results were also shown in IPDI films, but were exhibited at lower gloss values.
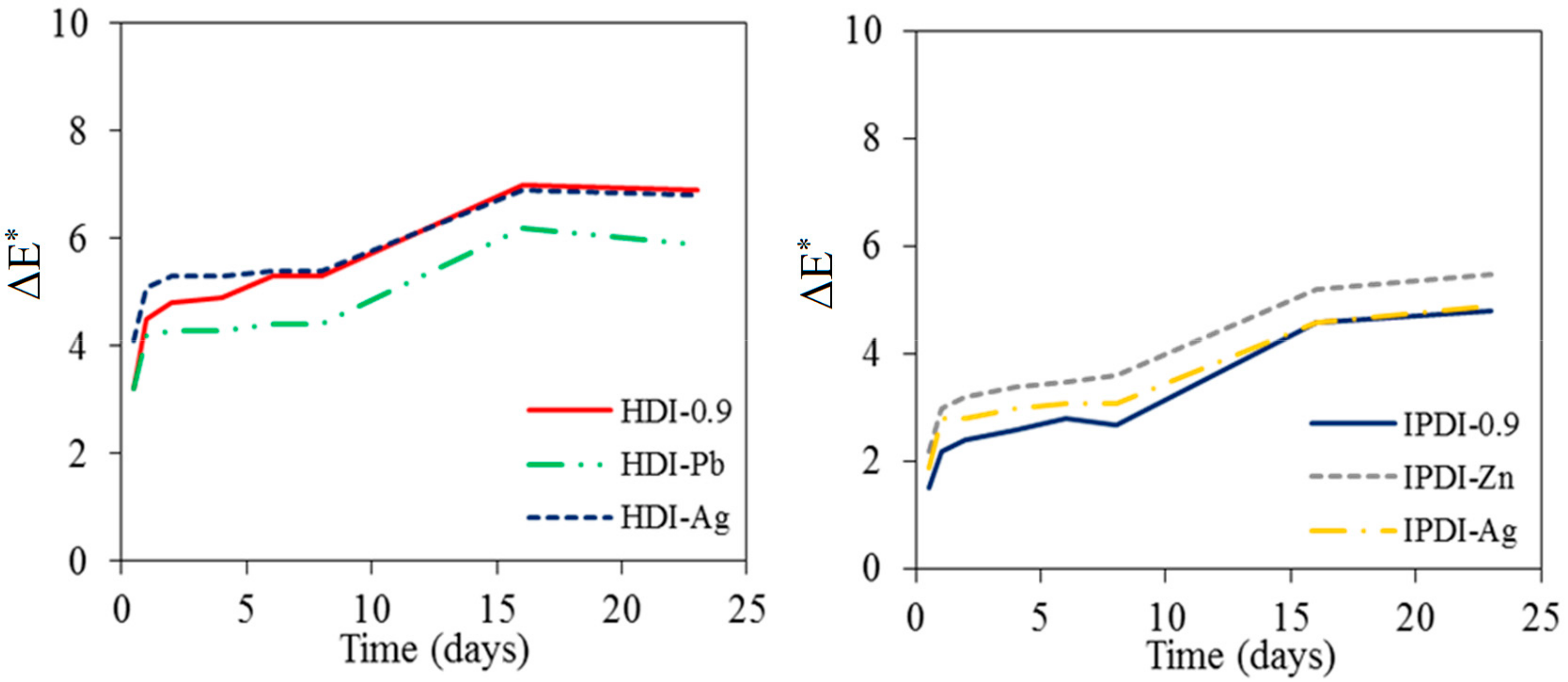
The lightfastness of antimicrobial WUO films synthesizing with HDI and IPDI before and after the color fading test are given in
Figure 8. The color difference (ΔE*) of WUO films increased with prolonged exposure under ultraviolet light irradiation, accounting for the color changes of the films, but the ΔE* values were found to stabilize at 16 days. The ΔE* values were 6.9, 5.9, and 6.8 for HDI-0.9, HDI-Pb, and HDI-Ag, and were 4.8, 5.5, and 4.9 for IPDI-0.9, IPDI-Zn, and IPDI-Ag, respectively, after ultraviolet light irradiation of 23 days. Generally speaking, it has no significant effect on the lightfastness of WUO films with adding antimicrobial agents. However, the IPDI films had a slight superiority in lightfastness over the HDI films, which all belonged to aliphatic isocyanates.
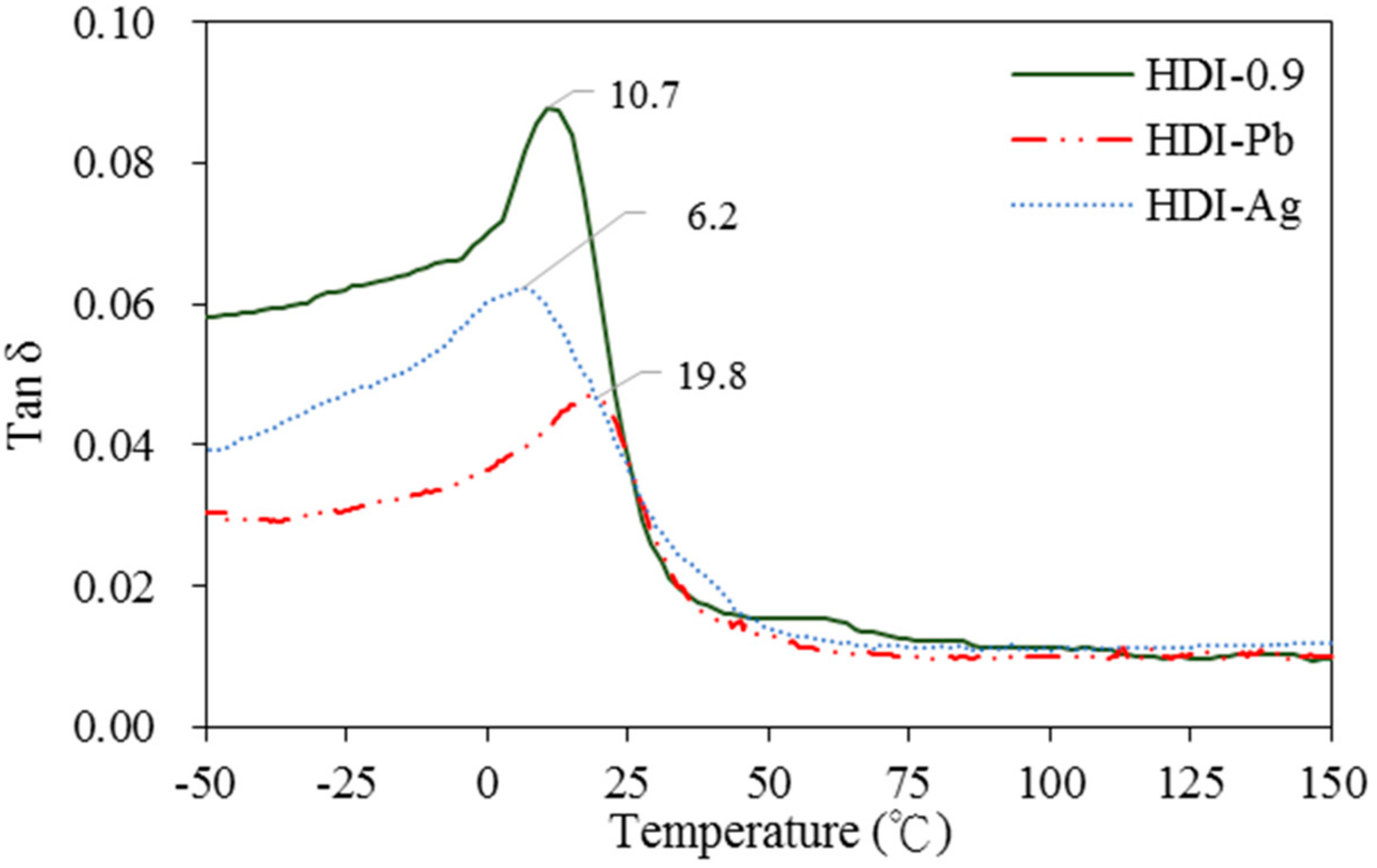
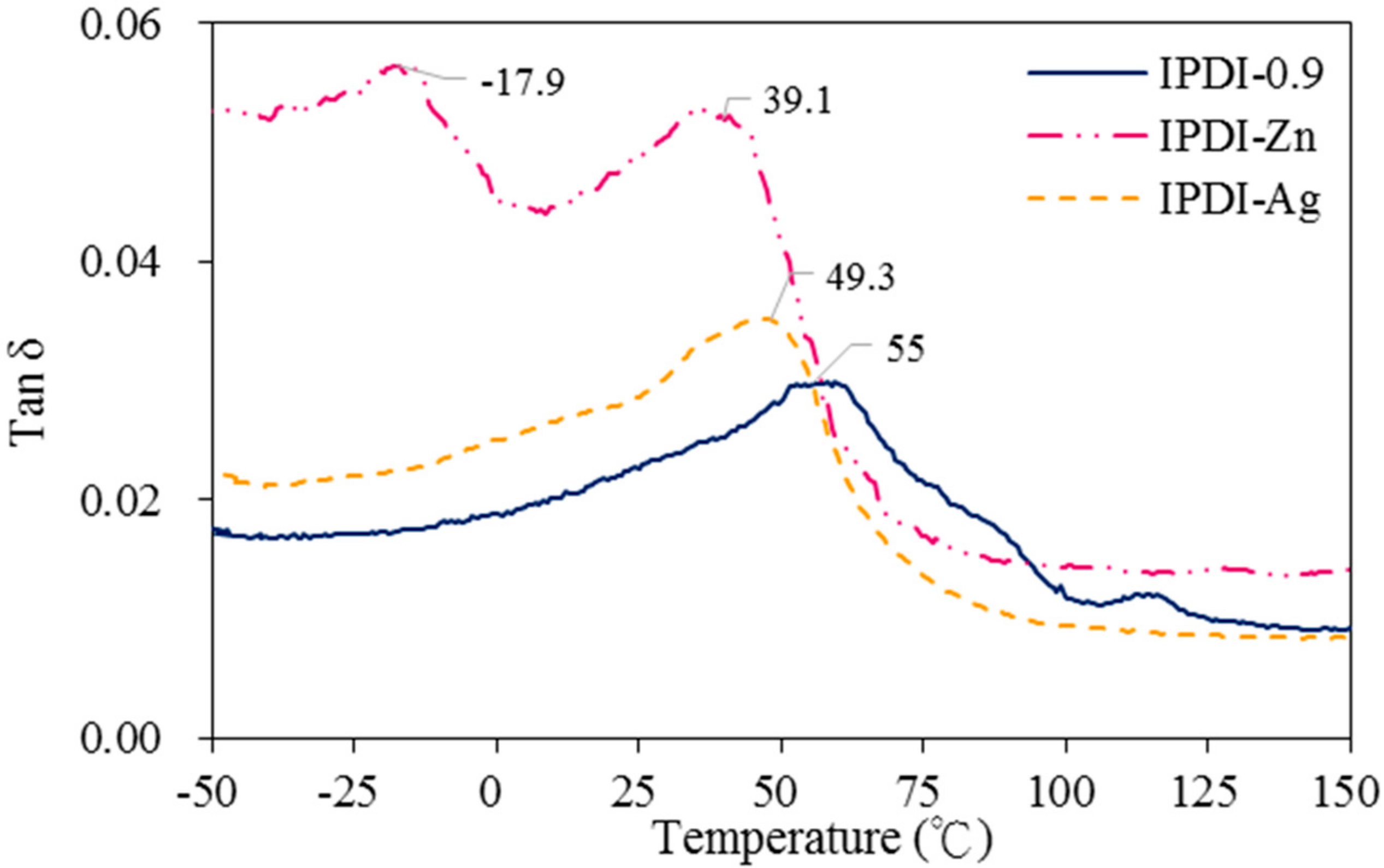
The Tan δ curves of antimicrobial WUO films synthesizing with HDI and IPDI by a dynamic mechanical analysis (DMA) are given in
Figure 9 and
Figure 10, respectively. The glass transition temperature (
Tg) of HDI-0.9 film, which possessed a softer long alkyl chain, was 10.7 °C and the IPDI-0.9 film, which possessed a harder alicyclic structure, was 55 °C. When adding the nanograde filler of the Ag ion, the
Tg was shifted to a lower temperature, i.e., HDI-Ag was 6.2 °C and IPDI-Ag was 49.3 °C. This was attributed to an interfacial layer of polymer molecules whose chain relaxation dynamics were altered by the interaction with the filler surface. This is accompanied by a shift of the
Tg itself to a lower temperature when the filler surface is organophilic [
32]. However, for the micron-grade filler of M(HEEP)
2, when adding the filler could change the
Tg of the softer polymer such as the HDI film to a higher temperature, i.e., HDI-Pb of 19.8 °C, whereas for the harder polymer such as the IPDI film to a lower temperature, i.e., IPDI-Zn of 39.1 °C [
32]. In addition, the IPDI-Zn had a phase separation, this may be due to the Zn(HEEP)
2 agglutinated mainly in the hard segment of IPDI film located at the main α relaxation, the glass transition
Tg of 39.1 °C, and a second relaxation occurred at −17.9 °C in the unfilled soft segment of the IPDI film.
The TGA curves of antimicrobial WUO films synthesizing with HDI and IPDI are displayed by
Figure 11 and
Figure 12, respectively. The WUO films with or without antimicrobial agents showed similar behavior of thermal degradation. They consisted of three dominant steps at around 250–350 °C, 350–420 °C, and 420–500 °C, corresponding to the decomposition of the urethane linkage, the degradation of an aliphatic long chain of fatty acid from the linseed oil glyceride component, and the dehydrogenation and depolycondensation of alkyl groups, respectively [
33,
34,
35,
36]. The results showed that adding metal-containing antimicrobial agents can slightly enhance the thermal stability of the WUO films, e.g., the weight loss at 450 °C for HDI films were 73%, 69%, and 71% of HDI-0.9, HDI-Pb, and HDI-Ag, respectively, and for IPDI films were 83%, 80%, and 80% of IPDI-0.9, IPDI-Zn, and IPDI-Ag, respectively. The result also confirmed by the studies of Zafar et al. [
11] and Chang and Chang [
37]. In addition, the M(HEEP)
2 and nanoAg containing WUO films had no significant difference in thermal stability.