High Capacity Prismatic Type Layered Electrode with Anionic Redox Activity as an Efficient Cathode Material and PVdF/SiO2 Composite Membrane for a Sodium Ion Battery
Abstract
1. Introduction
2. Materials and Methods
2.1. Solid State Method (SSR)
Sol–Gel Method (SGR)
2.2. Characterization Techniques
2.3. Electrochemical Testing—Half-Cell Preparation
2.3.1. Half-Cell Preparation
2.3.2. Pouch Cell Preparation
3. Results and Discussion
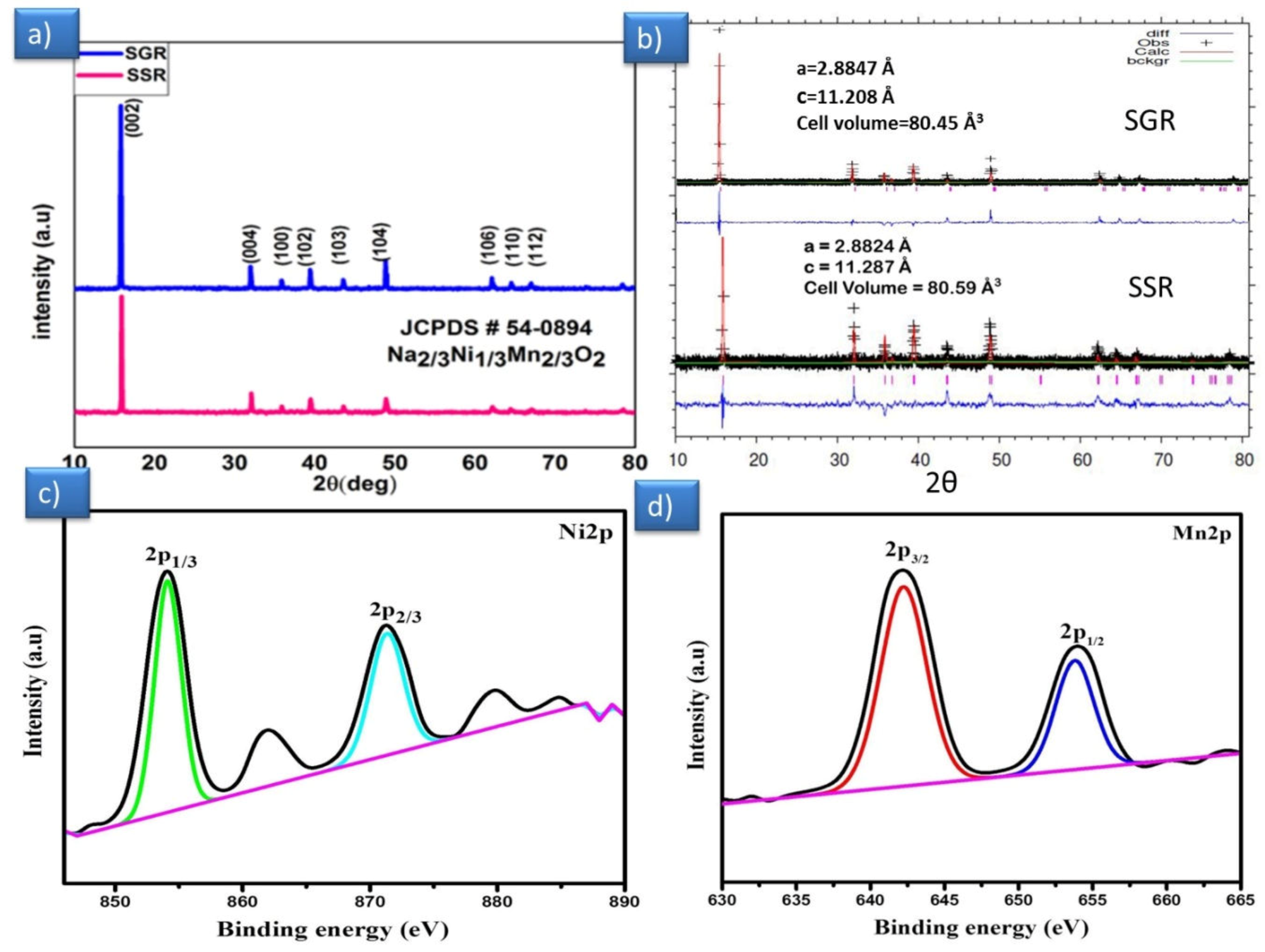
3.1. Structural Analysis
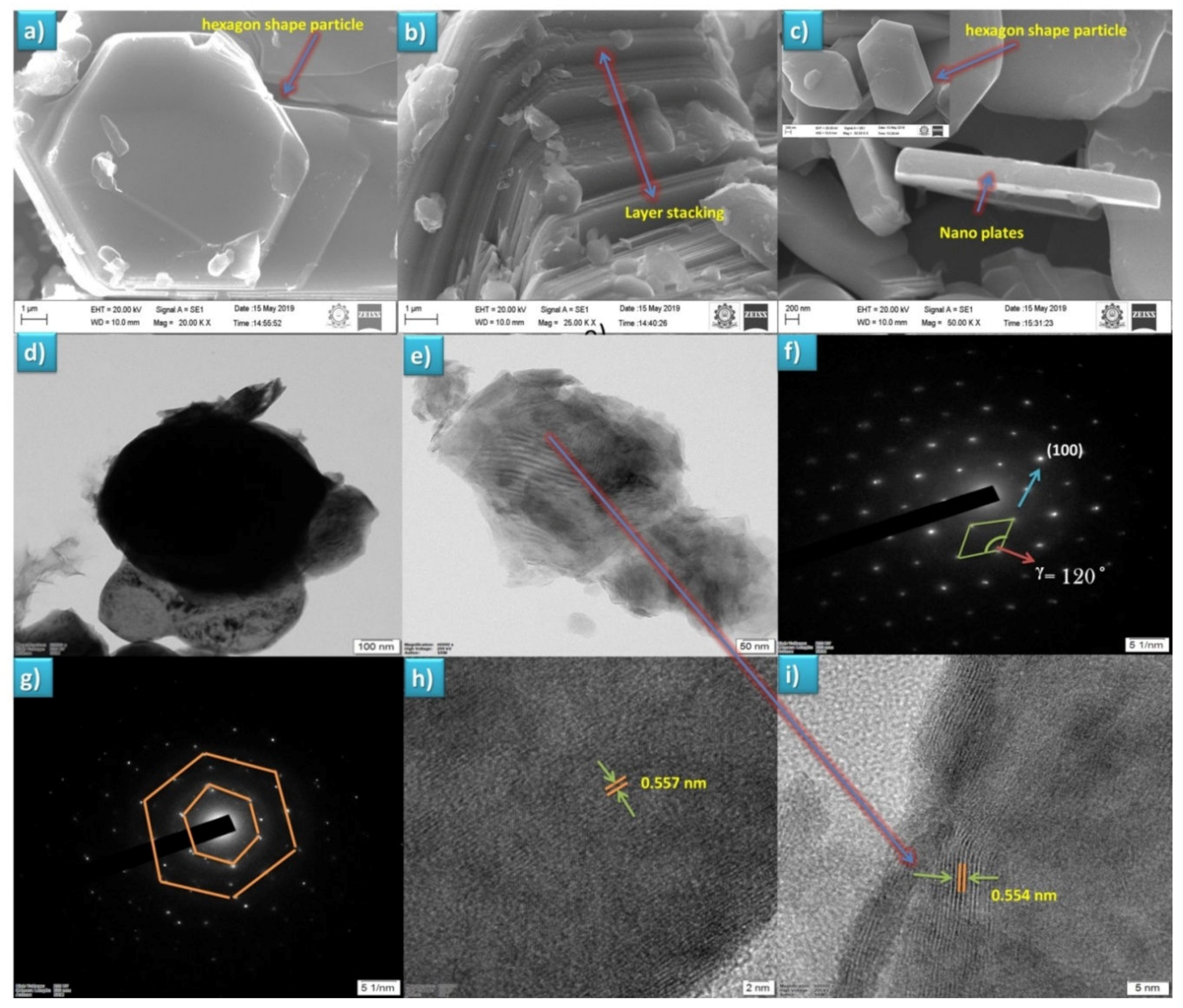
3.2. Surface Morphological Analysis
HR-TEM Analysis
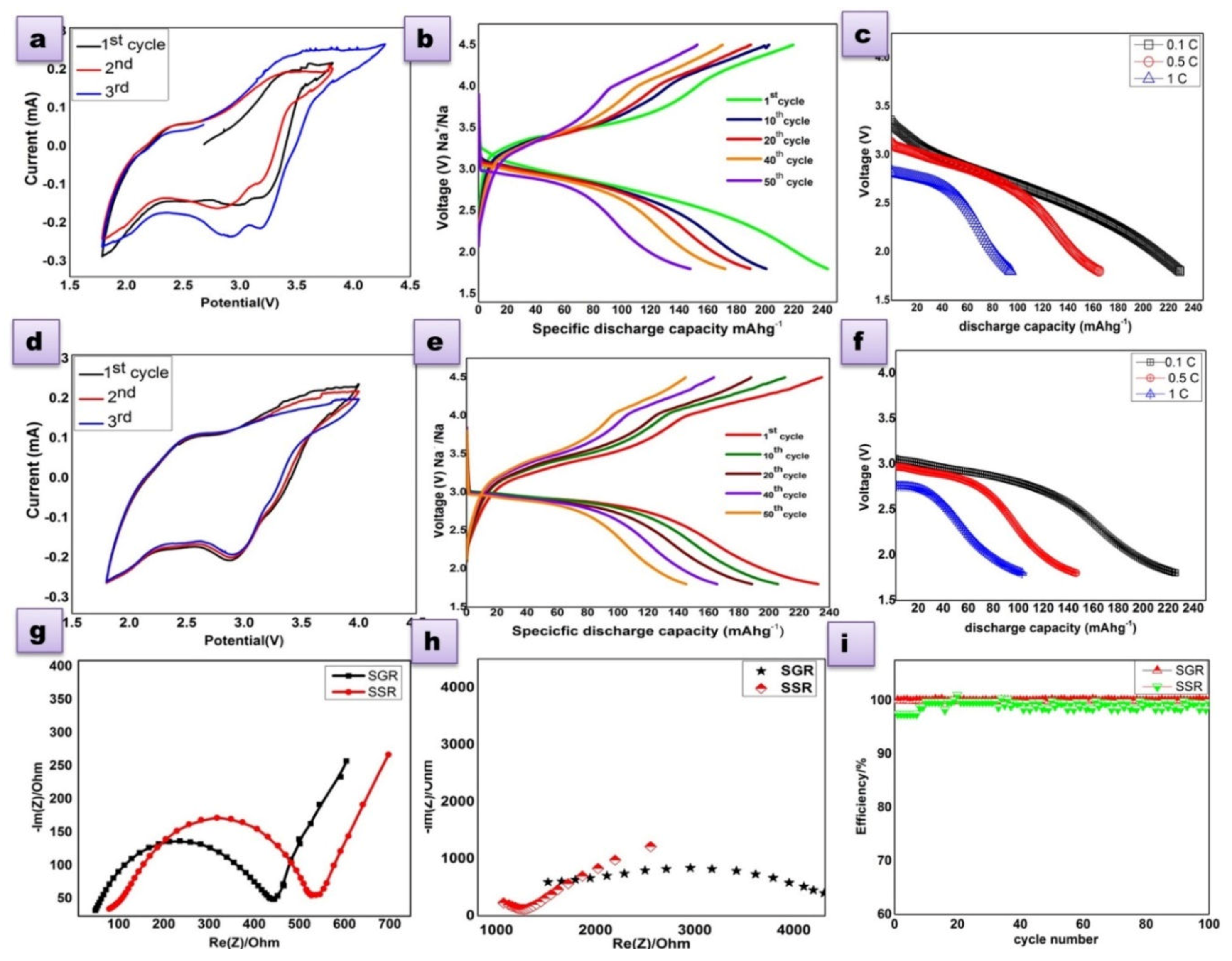
3.3. Electrochemical Analysis
3.3.1. Full-Cell
3.3.2. Charge compensation mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffert, M.I.; Caldeira, K.; Benford, G.; Criswell, D.R.; Green, C.; Herzog, H.; Jain, A.K.; Kheshgi, H.S.; Lackner, K.S.; Lewis, J.S.; et al. Advanced Technology Paths to Global Climate Stability: Energy for a Greenhouse Planet. Science 2002, 298, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.-M.; Armand, M.; Dusastre, V. Issues and challenges facing rechargeable lithium batteries. Mater. Sustain. Energy 2010, 171–179. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.; González, J.C.; Lu, J. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Qian, J.; Zhou, M.; Cao, Y.; Ai, X.; Yang, H. Nanosized Na4Fe(CN)6/C Composite as a Low-Cost and High-Rate Cathode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2012, 2, 410–414. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Sun, C.; Alonso, J.; Fernández-Díaz, M.T.; Chen, L. Experimental visualization of the diffusion pathway of sodium ions in the Na3[Ti2P2O10F] anode for sodium-ion battery. Sci. Rep. 2014, 4, 7231. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.D.; Kim, N.; Lee, E.; Johnson, C. Correction: Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 3255. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338. [Google Scholar] [CrossRef]
- Delmas, C.; Fouassier, C.; Hagenmuller, P. Structural classification and properties of the layered oxides. Phys. B+C 1980, 99, 81–85. [Google Scholar] [CrossRef]
- Buchholz, D.; Chagas, L.G.; Winter, M.; Passerini, S. P2-type layered Na0.45Ni0.22Co0.11Mn0.66O2 as intercalation host material for lithium and sodium batteries. Electrochim. Acta 2013, 110, 208–213. [Google Scholar] [CrossRef]
- Komaba, S.; Takei, C.; Nakayama, T.; Ogata, A.; Yabuuchi, N. Electrochemical intercalation activity of layered NaCrO2 vs. LiCrO2. Electrochem. Commun. 2010, 12, 355–358. [Google Scholar] [CrossRef]
- Komaba, S.; Murata, W.; Ishikawa, T.; Yabuuchi, N.; Ozeki, T.; Nakayama, T.; Ogata, A.; Gotoh, K.; Fujiwara, K. Electrochemical Na Insertion and Solid Electrolyte Interphase for Hard-Carbon Electrodes and Application to Na-Ion Batteries. Adv. Funct. Mater. 2011, 21, 3859–3867. [Google Scholar] [CrossRef]
- Delmas, C.; Braconnier, J.-J.; Fouassier, C.; Hagenmuller, P. Electrochemical intercalation of sodium in NaxCoO2 bronzes. Solid State Ion. 1981, 3, 165–169. [Google Scholar] [CrossRef]
- Berthelot, R.; Carlier, D.; Delmas, C. Electrochemical investigation of the P2–NaxCoO2 phase diagram. Nat. Mater. 2010, 10, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Dahn, J.R. In Situ X-Ray Diffraction Study of P 2 Na2/3. J. Electrochem. Soc. 2001, 148, A1225–A1229. [Google Scholar] [CrossRef]
- Koga, H.; Croguennec, L.; Ménétrier, M.; Mannessiez, P.; Weill, F.; Delmas, C. Different oxygen redox participation for bulk and surface: A possible global explanation for the cycling mechanism of Li1.20Mn0.54Co0.13Ni0.13O2. J. Power Sources 2013, 236, 250–258. [Google Scholar] [CrossRef]
- Koga, H.; Croguennec, L.; Mannessiez, P.; Ménétrier, M.; Weill, F.; Bourgeois, L.; Duttine, M.; Suard, E.; Delmas, C. Li1.20Mn0.54Co0.13Ni0.13O2 with Different Particle Sizes as Attractive Positive Electrode Materials for Lithium-Ion Batteries: Insights into Their Structure. J. Phys. Chem. C 2012, 116, 13497–13506. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Yoshii, K.; Myung, S.-T.; Nakai, I.; Komaba, S. Detailed Studies of a High-Capacity Electrode Material for Rechargeable Batteries, Li2MnO3−LiCo1/3Ni1/3Mn1/3O2. J. Am. Chem. Soc. 2011, 133, 4404–4419. [Google Scholar] [CrossRef]
- Sathiya, M.; Rousse, G.; Ramesha, K.; Laisa, C.P.; Vezin, H.; Sougrati, M.T.; Doublet, M.-L.; Foix, D.; Gonbeau, D.; Walker, W.; et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 2013, 12, 827–835. [Google Scholar] [CrossRef]
- Sathiya, M.; Abakumov, A.M.; Foix, D.; Rousse, G.; Ramesha, K.; Saubanère, M.; Doublet, M.-L.; Vezin, H.; Laisa, C.P.; Prakash, A.S.; et al. Origin of voltage decay in high-capacity layered oxide electrodes. Nat. Mater. 2014, 14, 230–238. [Google Scholar] [CrossRef]
- Sathiya, M.; Ramesha, K.; Rousse, G.; Foix, D.; Gonbeau, D.; Prakash, A.; Doublet, M.-L.; Hemalatha, K.; Tarascon, J.-M. High Performance Li2Ru1–yMnyO3 (0.2 ≤ y ≤ 0.8) Cathode Materials for Rechargeable Lithium-Ion Batteries: Their Understanding. Chem. Mater. 2013, 25, 1121–1131. [Google Scholar] [CrossRef]
- Risthaus, T.; Zhou, D.; Cao, X.; He, X.; Qiu, B.; Wang, J.; Winter, M. A high-capacity P2 Na2/3Ni1/3Mn2/3O2 cathode material for sodium ion batteries with oxygen activity. J. Power Sources 2018, 395, 16–24. [Google Scholar] [CrossRef]
- Ma, C.; Alvarado, J.; Xu, J.; Clément, R.J.; Kodur, M.; Tong, W.; Grey, C.P.; Meng, Y.S. Exploring Oxygen Activity in the High Energy P2-Type Na0.78Ni0.23Mn0.69O2 Cathode Material for Na-Ion Batteries. J. Am. Chem. Soc. 2017, 139, 4835–4845. [Google Scholar] [CrossRef] [PubMed]
- Hasa, I.; Buchholz, D.; Passerini, S.; Scrosati, B.; Hassoun, J. High Performance Na 0.5 [Ni 0.23 Fe 0.13 Mn 0.63 ]O 2 Cathode for Sodium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1400083. [Google Scholar] [CrossRef]
- Arjunan, P.; Kouthaman, M.; Subadevi, R.; Diwakar, K.; Liu, W.-R.; Huang, C.-H.; Sivakumar, M. Superior Ionic Transferring Polymer with Silicon Dioxide Composite Membrane via Phase Inversion Method Designed for High Performance Sodium-Ion Battery. Polymers 2020, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Donaberger, R.A.; Dahn, J.R. Superlattice Ordering of Mn, Ni, and Co in Layered Alkali Transition Metal Oxides with P2, P3, and O3 Structures. Chem. Mater. 2000, 12, 3583–3590. [Google Scholar] [CrossRef]
- Henrich, V.E.; Cox, P.A.; Diebold, U. The Surface Science of Metal Oxides. Phys. Today 1995, 48, 58. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, K.J. Structural and electrochemical evolution with post-annealing temperature of solution-based LiNi0.5Mn1.5O4 thin-film cathodes for microbatteries with cyclic stability. Electrochim. Acta 2014, 137, 169–174. [Google Scholar] [CrossRef]
- Yan, G.; Li, X.; Wang, Z.; Guo, H.; Xiong, X. Beneficial effects of 1-propylphosphonic acid cyclic anhydride as an electrolyte additive on the electrochemical properties of LiNi0.5Mn1.5O4 cathode material. J. Power Sources 2014, 263, 231–238. [Google Scholar] [CrossRef]
- Sathiya, M.; Hemalatha, K.; Ramesha, K.; Tarascon, J.M.; Prakash, A.S. Synthesis, Structure, and Electrochemical Properties of the Layered Sodium Insertion Cathode Material: NaNi1/3Mn1/3Co1/3O2. Chem. Mater. 2012, 24, 1846–1853. [Google Scholar] [CrossRef]
- De Boisse, B.M.; Carlier, D.; Guignard, M.; Delmas, C. Structural and Electrochemical Characterizations of P2 and New O3-NaxMn1-yFeyO2Phases Prepared by Auto-Combustion Synthesis for Na-Ion Batteries. J. Electrochem. Soc. 2013, 160, A569–A574. [Google Scholar] [CrossRef]
- Luo, L.B.; Zhao, Y.; Zhang, G.M.; Guo, S.M.; Li, Z.; Luo, J.L. Spin-glass behavior in hexagonalNa0.70MnO2. Phys. Rev. B 2007, 75, 125115. [Google Scholar] [CrossRef]
- Lee, D.H.; Xu, J.; Meng, Y.S. An advanced cathode for Na-ion batteries with high rate and excellent structural stability. Phys. Chem. Chem. Phys. 2013, 15, 3304. [Google Scholar] [CrossRef] [PubMed]
- Carlier, D.; Cheng, J.-H.; Berthelot, R.; Guignard, M.; Yoncheva, M.; Stoyanova, R.; Hwang, B.J.; Delmas, C. The P2-Na2/3Co2/3Mn1/3O2 phase: Structure, physical properties and electrochemical behavior as positive electrode in sodium battery. Dalton Trans. 2011, 40, 9306–9312. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Qiu, X.; Chen, H.; He, Z.; Zhu, W.; Chen, L. Analysis of high rate performance of nanoparticled lithium cobalt oxides prepared in molten KNO3 for rechargeable lithium-ion batteries. Electrochem. Commun. 2004, 6, 789–794. [Google Scholar] [CrossRef]
- Kang, E.; Jung, Y.S.; Kim, G.-H.; Chun, J.; Wiesner, U.; Dillon, A.C.; Kim, J.K.; Lee, J. Highly Improved Rate Capability for a Lithium-Ion Battery Nano-Li4Ti5O12 Negative Electrode via Carbon-Coated Mesoporous Uniform Pores with a Simple Self-Assembly Method. Adv. Funct. Mater. 2011, 21, 4349–4357. [Google Scholar] [CrossRef]
- Narayanan, S.R.; Shen, D.H.; Surampudi, S.; Attia, A.I.; Halpert, G. Stochastic reconstruction and electrical transport studies of porous cathode of Li-ion batteries. J. Electrochem. Soc. 1993, 140, 1854–1861. [Google Scholar] [CrossRef]
- Song, X.; Meng, T.; Deng, Y.; Gao, A.; Nan, J.; Shu, D.; Yi, F. The effects of the functional electrolyte additive on the cathode material Na0.76Ni0.3Fe0.4Mn0.3O2 for sodium-ion batteries. Electrochimica Acta 2018, 281, 370–377. [Google Scholar] [CrossRef]
- Hy, S.; Liu, H.; Zhang, M.; Qian, D.; Hwang, B.-J.; Meng, Y.S. Performance and design considerations for lithium excess layered oxide positive electrode materials for lithium ion batteries. Energy Environ. Sci. 2016, 9, 1931–1954. [Google Scholar] [CrossRef]
- Oxelius, V.-A. Immunoglobulin constant heavy g chain (ighg) (fcγ) (gm) genes identifying new innate igg subclasses and innate b cells. Morressier 2016, 8, 684. [Google Scholar]
- Seo, D.-H.; Lee, J.; Urban, A.; Malik, R.; Kang, S.; Ceder, G. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 2016, 8, 692–697. [Google Scholar] [CrossRef]
- Yuan, D.; Hu, X.; Qian, J.; Pei, F.; Wu, F.; Mao, R.; Cao, Y. P2-type Na0. 67Mn0. 65Fe0. 2Ni0. 15O2 cathode material with high-capacity for sodium-ion battery. Electrochim. Acta 2014, 116, 300–305. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, J.X.; Chen, Z. f-Orthomorphisms and f-Linear Operators on the Order Dual of an f-Algebra. Electrochim. Acta 2013, 113, 200–204. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.-Y.; Gao, R.; Sun, L.; Hu, Z. Designing an advanced P2-Na 0.67 Mn 0.65 Ni 0.2 Co 0.15 O 2 layered cathode material for Na-ion batteries. J. Mater. Chem. A 2015, 3, 16272–16278. [Google Scholar] [CrossRef]
- Wu, X.; Guo, J.; Wang, D.; Zhong, G.; McDonald, M.J.; Yang, Y. P2-type Na0. 66Ni0. 33–xZnxMn0. 67O2 as new high-voltage cathode materials for sodium-ion batteries. J. Power Sources 2015, 281, 18–26. [Google Scholar] [CrossRef]
- Pang, W.L.; Zhang, X.H.; Guo, J.Z.; Li, J.Y.; Yan, X.; Hou, B.H.; Wu, X.L. P2-type Na2/3Mn1-xAlxO2 cathode material for sodium-ion batteries: Al-doped enhanced electrochemical properties and studies on the electrode kinetics. J. Power Sources 2017, 356, 80–88. [Google Scholar] [CrossRef]
- Xu, X.; Ji, S.; Gao, R.; Liu, J. Facile synthesis of P2-type Na0.4Mn0.54Co0.46O2as a high capacity cathode material for sodium-ion batteries. RSC Adv. 2015, 5, 51454–51460. [Google Scholar] [CrossRef]
- Guo, S.; Yu, H.; Liu, P.; Ren, Y.; Zhang, T.; Chen, M.; Ishida, M.; Zhou, H. High-performance symmetric sodium-ion batteries using a new, bipolar O3-type material, Na0.8Ni0.4Ti0.6O2. Energy Environ. Sci. 2015, 8, 1237–1244. [Google Scholar] [CrossRef]
- Hasa, I.; Dou, X.; Buchholz, D.; Shao-Horn, Y.; Hassoun, J.; Passerini, S.; Scrosati, B. A sodium-ion battery exploiting layered oxide cathode, graphite anode and glyme-based electrolyte. J. Power Sources 2016, 310, 26–31. [Google Scholar] [CrossRef]
- Komaba, S.; Ishikawa, T.; Yabuuchi, N.; Murata, W.; Ito, A.; Ohsawa, Y. Fluorinated Ethylene Carbonate as Electrolyte Additive for Rechargeable Na Batteries. ACS Appl. Mater. Interfaces 2011, 3, 4165–4168. [Google Scholar] [CrossRef]


| Half-Cell Comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| S. No. | Cathode Materials | Capacity (mAhg−1) | Voltage (V) | Electrolyte Used | Ref. | ||||
| 1 | P2-Na0.67Mn0.65Fe0.2Ni0.15O2 | 208 | 2.0–4.0 | 1.0 M NaPF6 (EC/DEC, 50: 50 vol%) | [41] | ||||
| 2 | P2-Na2/3 [Ni1/3Mn2/3] O2 | 134 | 2.0–4.0 | 1.0 M NaClO4 (PC) + (FEC) (95:5, v/v) | [42] | ||||
| 3 | P2-Na0.67Mn0.65Ni0.1Co0.15O2 | 155 | 1.5–4.2 | 1.0 M NaClO4 in propylene carbonate (PC) | [43] | ||||
| 4 | P2- Na0.66Ni0.33-xZnxMn0.67O2 | 132 | 2.0–4.2 | 1 M NaClO4 dissolved in PC with 2 vol.% FEC | [44] | ||||
| 5 | P2- Na2/3Mn1-xAlxO2 | 162 | 2.0–4.0 | 1 mol NaClO4 in EC and PC was 1:1 in volume | [45] | ||||
| 6 | P2-Na0.4Mn0.54Co0.46O2 | 194 | 1.5–4.0 | 1 mol NaClO4 in (EC/DEC = 4:6 in volume) | [46] | ||||
| 7 | P2- Na2/3Ni1/3Mn2/3O2 | 228 | 1.5–4.5 | 1M NaPF6 in 1/1 weight ratio (EC/DMC) | [21] | ||||
| 8 | P2- Na2/3Ni1/3Mn2/3O2 | 244 | 1.8–4.5 | 1M NaClO4 in 1/1 weight ratio in EC/PC | This Work | ||||
| Full-Cell Comparison | |||||||||
| S. No. | Cathode | Anode | Capacity (mAhg−1) | Voltage (V) | Type of Cell | Ref. | |||
| 1 | Na0.83Ni0.40Ti0.60O2 | Na0.83Ni0.40Ti0.60O2 | 100 | 0.01–2.0 | Coin-2032 | [47] | |||
| 2 | Na0.7CoO2 | Graphite | 80 | 0.5–3.7 | Coin-2032 | [48] | |||
| 3 | NaNi1/2Mn1/2O2 | Hard carbon | 250 | 0.7–2.0 | Coin-2032 | [49] | |||
| 4 | Na0.76Ni0.3Fe0.4Mn0.3O2 | Hard carbon | 650 | 1.5–3.8 | Pouch cell | [37] | |||
| 5 | P2-Na2/3Ni1/3Mn2/3O2 | Tin-Carbon | 750 | 1.0–2.2 | Pouch cell | This work | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponnaiah, A.; Rengapillai, S.; Karuppiah, D.; Marimuthu, S.; Liu, W.-R.; Huang, C.-H. High Capacity Prismatic Type Layered Electrode with Anionic Redox Activity as an Efficient Cathode Material and PVdF/SiO2 Composite Membrane for a Sodium Ion Battery. Polymers 2020, 12, 662. https://doi.org/10.3390/polym12030662
Ponnaiah A, Rengapillai S, Karuppiah D, Marimuthu S, Liu W-R, Huang C-H. High Capacity Prismatic Type Layered Electrode with Anionic Redox Activity as an Efficient Cathode Material and PVdF/SiO2 Composite Membrane for a Sodium Ion Battery. Polymers. 2020; 12(3):662. https://doi.org/10.3390/polym12030662
Chicago/Turabian StylePonnaiah, Arjunan, Subadevi Rengapillai, Diwakar Karuppiah, Sivakumar Marimuthu, Wei-Ren Liu, and Chia-Hung Huang. 2020. "High Capacity Prismatic Type Layered Electrode with Anionic Redox Activity as an Efficient Cathode Material and PVdF/SiO2 Composite Membrane for a Sodium Ion Battery" Polymers 12, no. 3: 662. https://doi.org/10.3390/polym12030662
APA StylePonnaiah, A., Rengapillai, S., Karuppiah, D., Marimuthu, S., Liu, W.-R., & Huang, C.-H. (2020). High Capacity Prismatic Type Layered Electrode with Anionic Redox Activity as an Efficient Cathode Material and PVdF/SiO2 Composite Membrane for a Sodium Ion Battery. Polymers, 12(3), 662. https://doi.org/10.3390/polym12030662