Synergies between Surface Microstructuring and Molecular Nanopatterning for Controlling Cell Populations on Polymeric Biointerfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Computer-Aided Design of Microstructured Surfaces
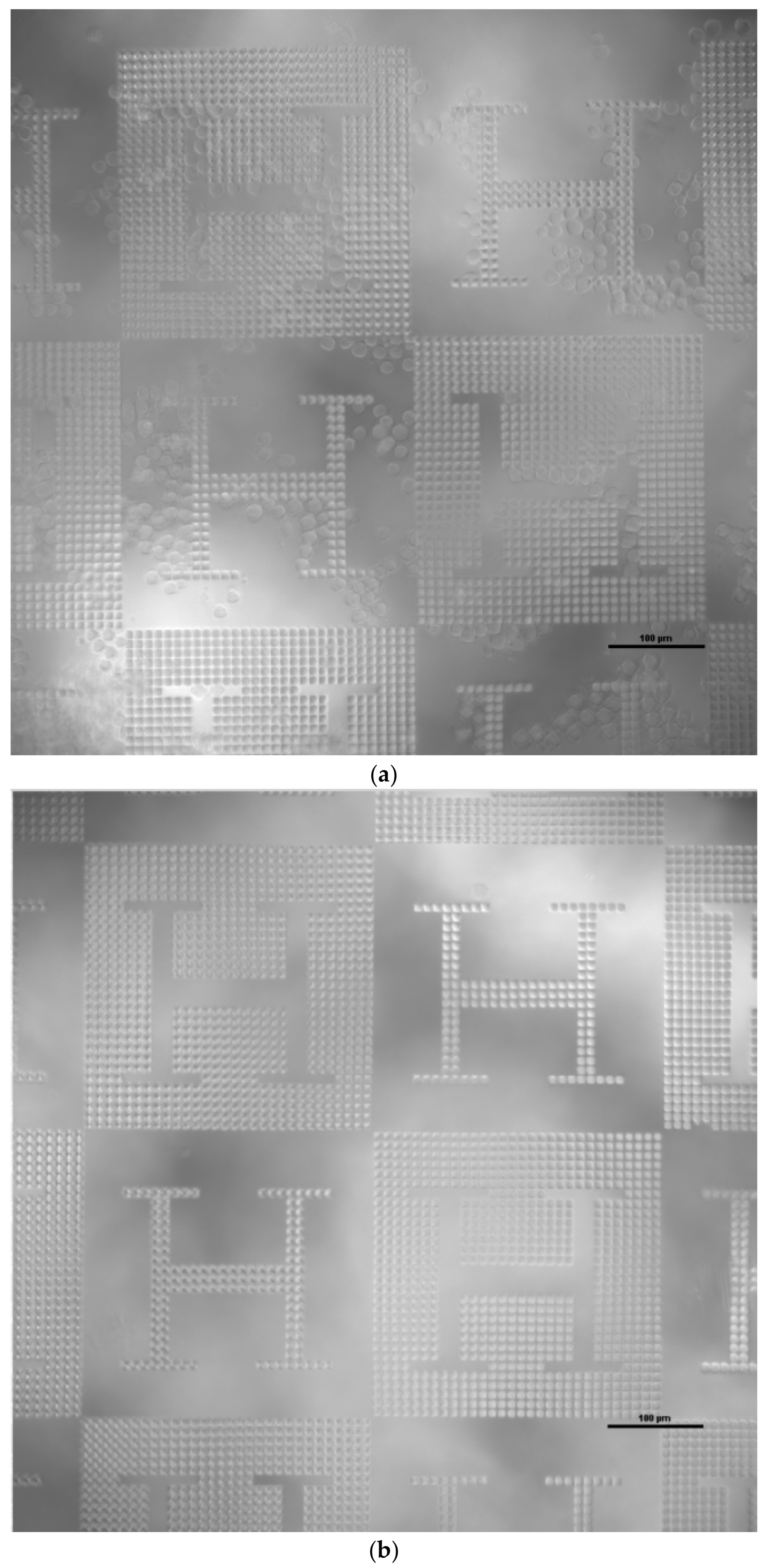
2.2. Production of Microstructured Surfaces
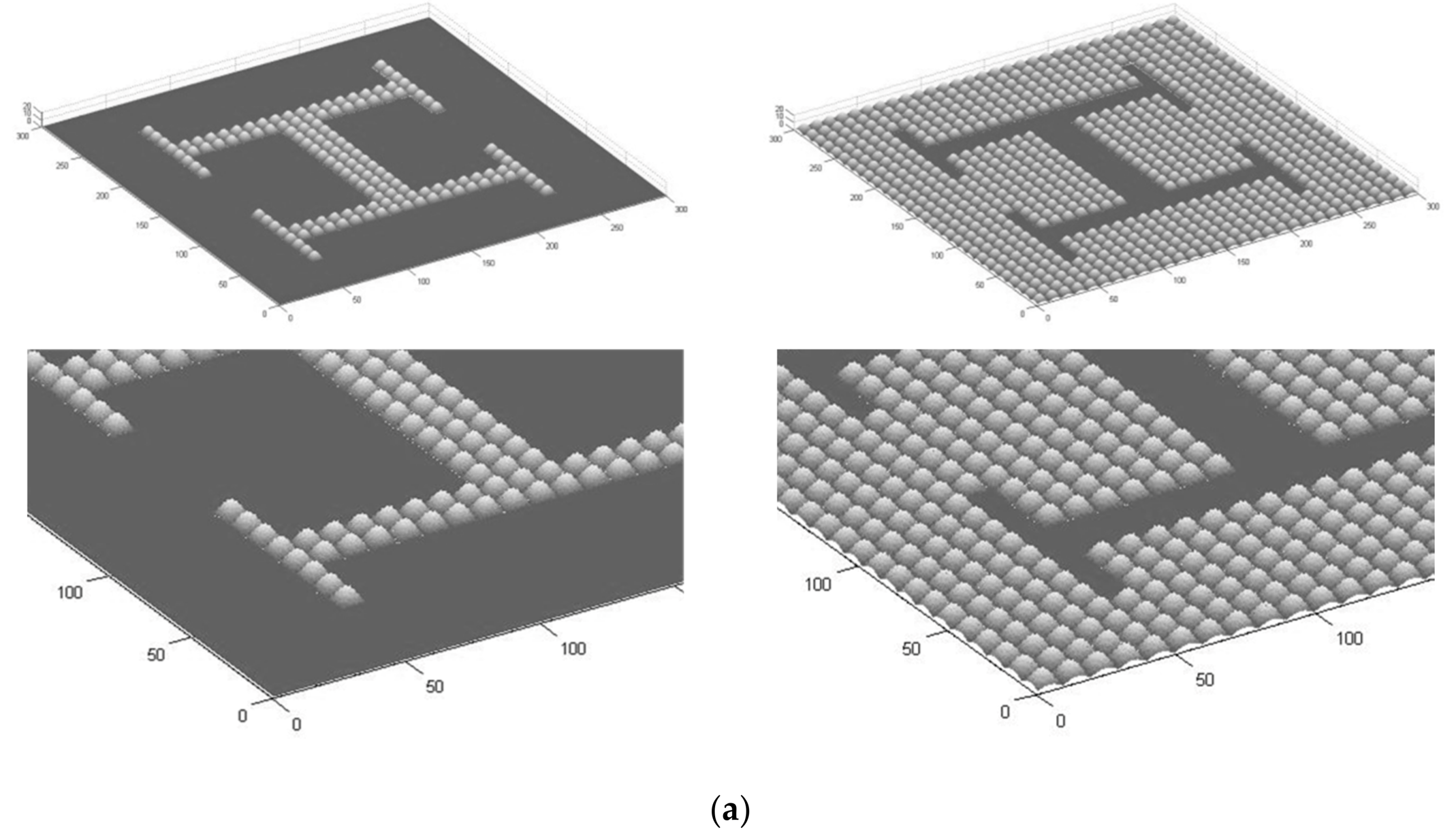
2.2.1. Three-Dimensional Direct Laser Writing of Master Models with Design-Controlled Features
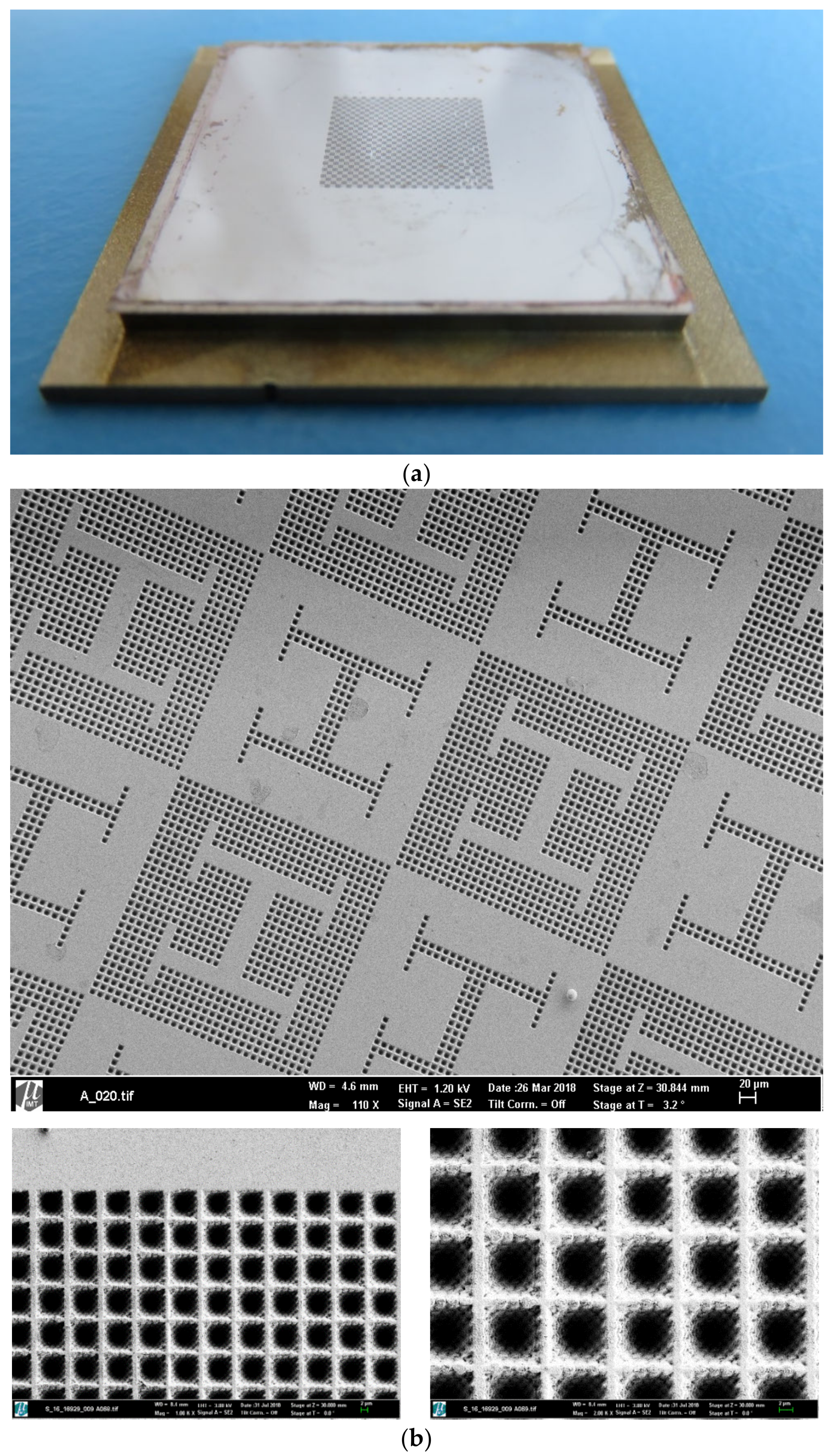
2.2.2. Electroplating of Master Models as Compression Molding Tools
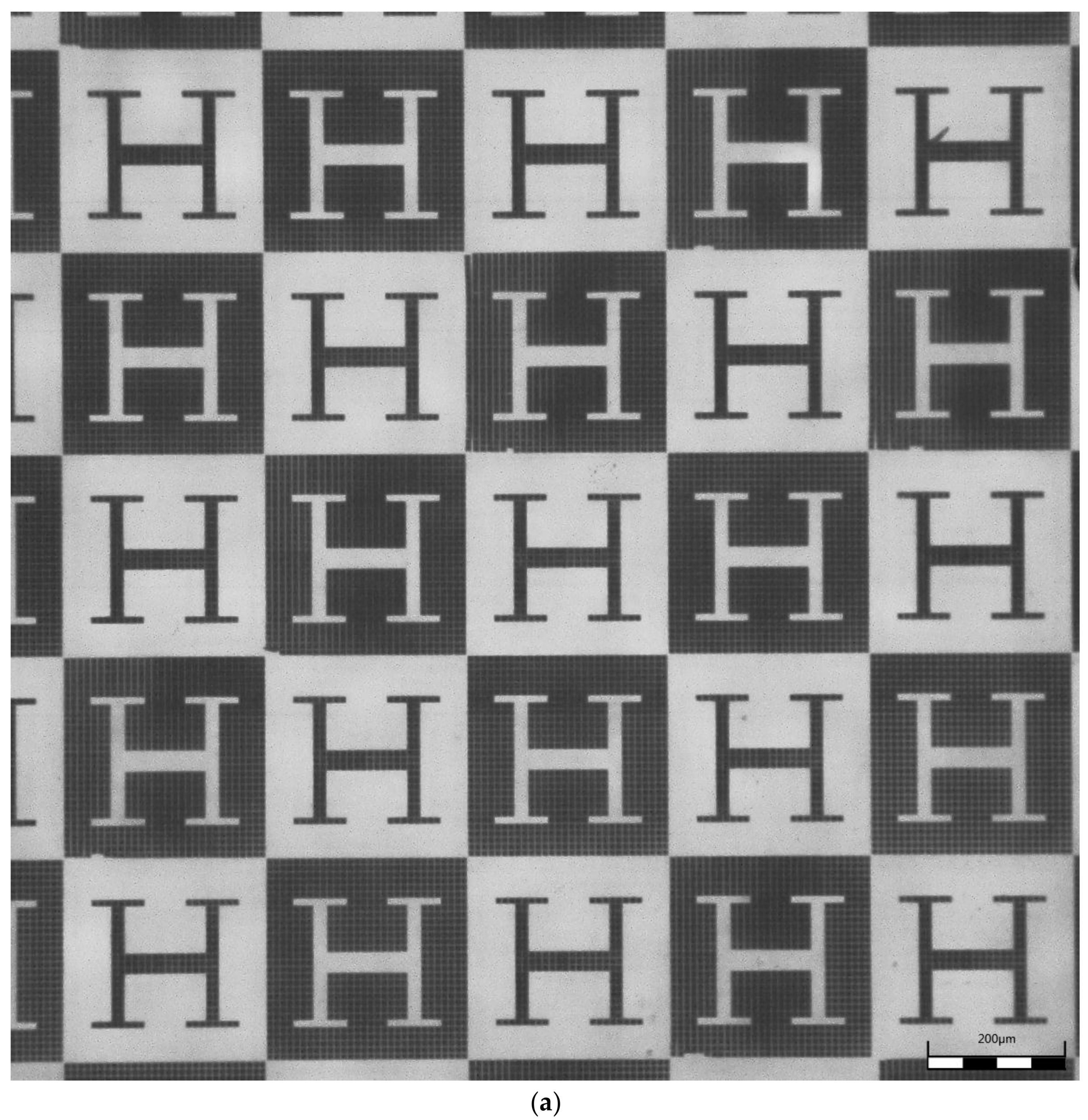
2.2.3. Hot Embossing of Microstructured Surfaces
| Embossing temperature: | 165 °C |
| Embossing force: | 18 kN |
| Demolding temperature: | 95 °C |
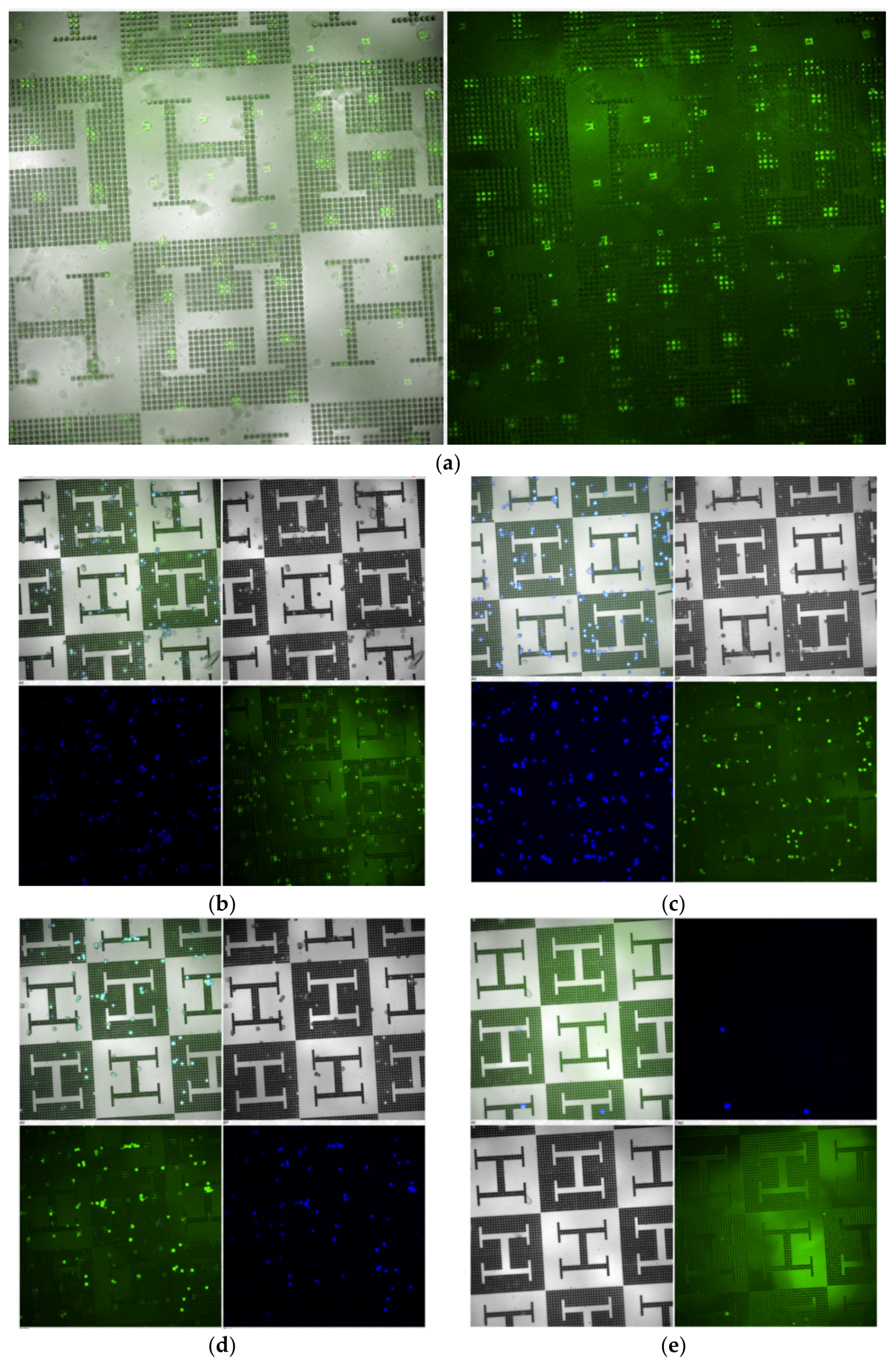
2.3. Cell Culture Experiments
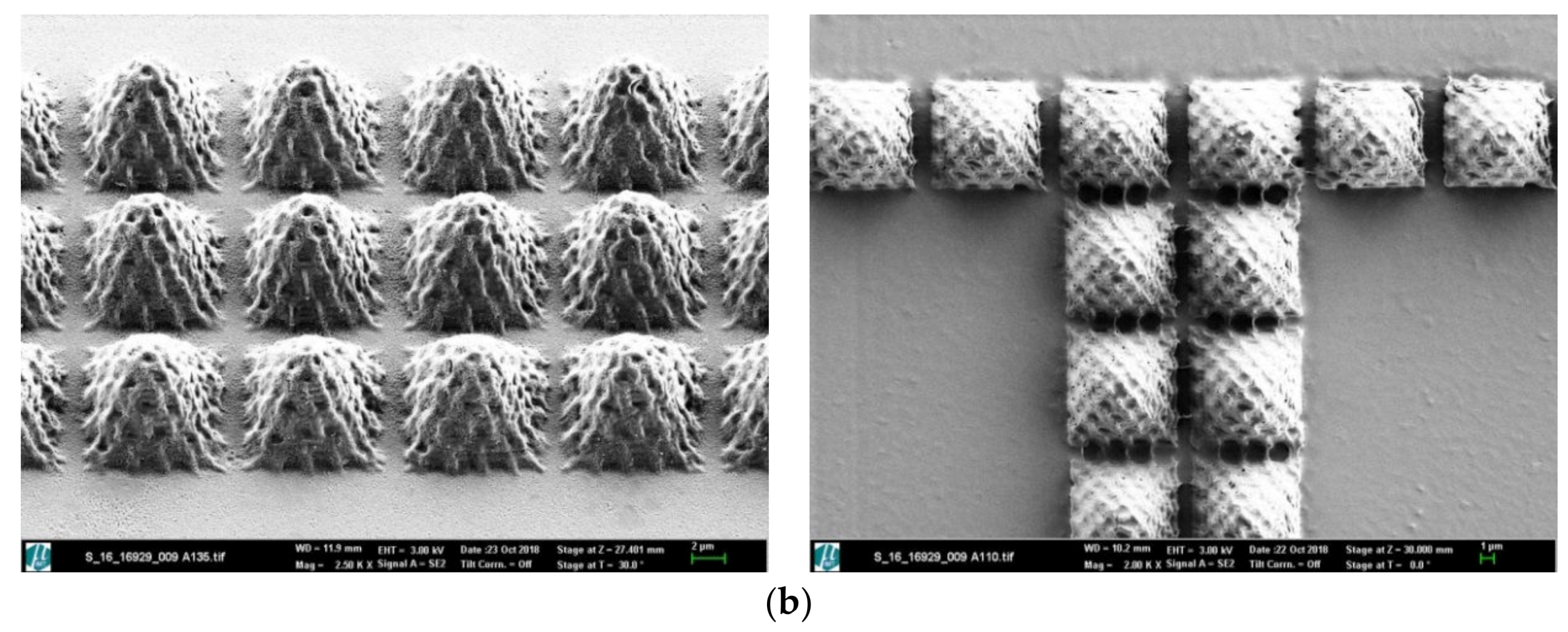
2.4. Molecular Nanopatterning of Microstructured Surfaces
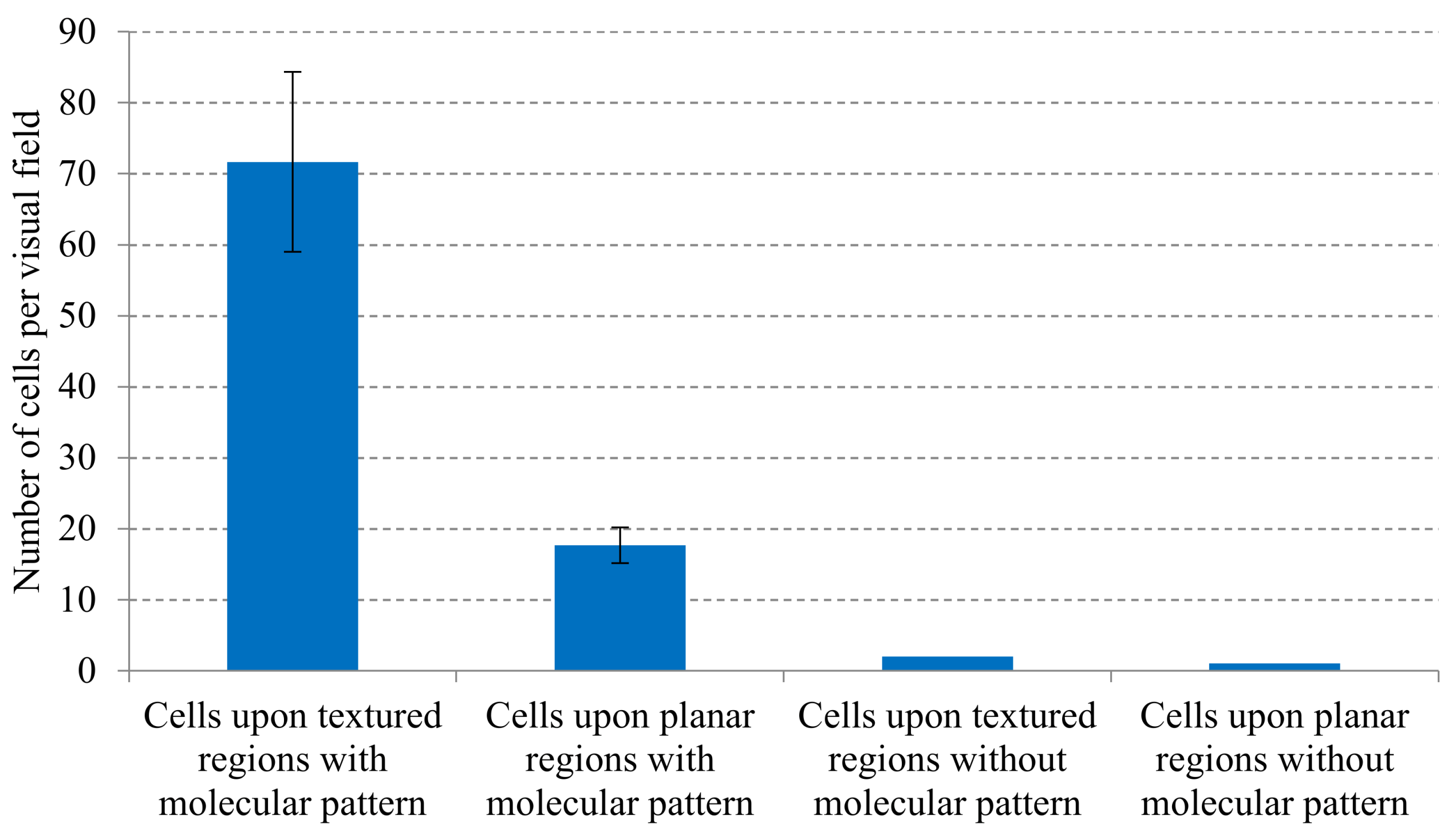
3. Results
4. Discussion: Potentials, Limitations and Continuation Proposals
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niemeyer, C.M.; Bastmeyer, M.; Bräse, S.; Lahann, J.; Woell, C. White Paper on the Biologization of Materials Research. Preprints 2018, 120, 329. [Google Scholar]
- Sun, A.; Lahann, J. Dynamically switchable biointerfaces. Soft Matter 2009, 5, 1555–1561. [Google Scholar] [CrossRef]
- Hutmacher, D.; Chrzanowski, W. Biointerfaces: Where Material Meets Biology; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Khademhosseini, A.; Langer, R.; Borenstein, J.; Vacanti, J.P. Microscale technologies for tissue engineering and biology. Proc. Natl. Acad. Sci. USA 2006, 103, 2480–2487. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Madou, M.; Wang, C. Photolithography. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 2051–2060. [Google Scholar]
- Díaz Lantada, A.; Piotter, V.; Plewa, K. Toward mass production of microtextured microdevices: Linking rapid prototyping with microinjection molding. Int. J. Adv. Manuf. Technol. 2015, 76, 1011–1020. [Google Scholar] [CrossRef]
- Worgull, M. Hot Embossing; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Duque-Sanchez, L.; Brack, N.; Postma, A.; Meagher, L.; Pigram, P.J. Engineering the biointerface of electrospun 3D scaffolds with functionalized polymer brushes for enhanced cell binding. Biomacromolecules 2019, 20, 813–825. [Google Scholar] [CrossRef]
- Aldana, A.A.; Malatto, L.; Rehman, M.A.U.; Boccaccini, A.R.; Abraham, G.A. Fabrication of Gelatin Methacrylate (GelMA) Scaffolds with Nano- and Micro-Topographical and Morphological Features. Nanomaterials 2019, 9, 120. [Google Scholar] [CrossRef]
- Liu, G.; Hirtz, M.; Fuchs, H.; Zheng, Z. Development of dip-pen nanolithography (DPN) and Its derivatives. Small 2019, 15, 1900564. [Google Scholar] [CrossRef]
- Sekula, S.; Jeanette, F.; Susanne, W.-R.; Peter, N.; Stefan, S. Multiplexed lipid dip-pen nanolithography on subcellular scales for the templating of functional proteins and cell culture. Small 2008, 4, 1785–1793. [Google Scholar] [CrossRef]
- Ueda, E.; Levkin, P.A. Emerging applications of superhydrophilic-superhydrophobic micropatterns. Adv. Mater. 2013, 25, 1234–1247. [Google Scholar] [CrossRef]
- Geyer, F.L.; Ueda, E.; Liebel, U.; Grau, N.; Levkin, P.A. Superhydrophobic–Superhydrophilic Micropatterning: Towards Genome-on-a-Chip Cell Microarrays. Angew. Chem. Int. Ed. 2011, 50, 8424–8427. [Google Scholar] [CrossRef] [PubMed]
- Ueda, E.; Geyer, F.L.; Nedashkivska, V.; Levkin, P.A. Droplet microarray: Facile formation of arrays of microdroplets and hydrogel micropads for cell screening applications. Lab Chip 2012, 12, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- Hengsbach, S.; Díaz Lantada, A. Rapid prototyping of multi-scale biomedical microdevices by combining additive manufacturing technologies. Biomed. Microdevices 2014, 16, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Hippler, M. 3D Scaffolds to Study Basic Cell Biology. Adv. Mater. 2019, 31, 1808110. [Google Scholar] [CrossRef] [PubMed]
- Gunnewiek, M.K.; Luca, A.D.; Bollemaat, H.Z.; van Blitterswijk, C.A.; Vancso, G.J.; Moroni, L. Creeping proteins in microporous structures: Polymer brush-assisted fabrication of 3D gradients for tissue engineering. Adv. Healthc. Mater. 2015, 4, 1169–1174. [Google Scholar] [CrossRef]
- DíazLantada, A.; Hengsbach, S.; Bade, K. Lotus-on-chip: Computer-aided design and 3D direct laser writing of bioinspired surfaces for controlling the wettability of materials and devices. Bioinspiration Biomim. 2017, 12, 066004. [Google Scholar]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Wissmann, M.; Guttmann, M.; Hartmann, M.; Hofmann, A.; Hummel, B. Alternative Mould Insert Fabrication Technology for Micromoulding by Galvanic Replication. In Proceedings of the Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS (DTIP 2010), Sevilla, Spain, 5–7 May 2010. [Google Scholar]
- Schanz, G.; Bade, K. Microelectroforming of Metal. In Advanced Micro and Nanosystems, Vol. 4, Microengineering of Metals and Ceramics; Baltes, H., Ed.; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2005; pp. 395–420. [Google Scholar]
- Guttmann, M.; Schulz, J.; Saile, V. Lithographic Fabrication of Mold Inserts. In Advanced Micro and Nanosystems, Vol. 3, Microengineering of Metals and Ceramics; Baltes, H., Ed.; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2005; pp. 187–219. [Google Scholar]
- Huo, F.; Zheng, Z.; Zheng, G.; Giam, L.R.; Zhang, H.; Mirkin, C.A. Polymer Pen Lithography. Science 2008, 321, 1658. [Google Scholar] [CrossRef]
- Ballester-Beltran, J.; Lebourg, M.; Salmeron-Sanchez, M. Dorsal and ventral stimuli in sandwich-like microenvironments. Effect on cell differentiation. Biotechnol. Bioeng. 2013, 110, 3048–3058. [Google Scholar] [CrossRef]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef]
- Wang, Z.; Lang, B.; Qu, Y.; Li, L.; Song, Z.; Wang, Z. Single-cell patterning technology for biological applications. Biomicrofluidics 2019, 13, 061502. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kuska, J.-P.; Zscharnack, M.; Loeffler, M.; Galle, J. Spatial organization of mesenchymal stem cells in vitro—Results from a new individual cell-based model with podia. PLoS ONE 2011, 6, e21960. [Google Scholar] [CrossRef] [PubMed]
- Jachetti, E.; Di Renzo, C.; Meucci, S.; Nocchi, F.; Beltram, F.; Cecchini, M. Wharton’s jelly human mesenchymal stem cell contact guidance by noisy nanotopographies. Nat. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Díaz Lantada, A.; Alarcón Iniesta, H.; García-Ruíz, J.P. Multi-channeled polymeric microsystem for studying the impact of surface topography on cell adhesion and motility. Polymers 2015, 7, 2371–2388. [Google Scholar] [CrossRef]
- Brinkmann, F.; Hirtz, M.; Greiner, A.M.; Weschenfelder, M.; Waterkotte, B.; Bastmeyer, M.; Fuchs, H. Interdigitated Multicolored Bioink Micropatterns by Multiplexed Polymer Pen Lithography. Small 2013, 9, 3266. [Google Scholar] [CrossRef]
- Arrabito, G.; Schroeder, H.; Schröder, K.; Filips, C.; Marggraf, U.; Dopp, C.; Venkatachalapathy, M.; Dehmelt, L.; Bastiaens, P.I.H.; Neyer, A.; et al. Configuarble Low-Cost Plotter Device for Fabrication of Multi-Color Sub-Cellular Scale Microarrays. Small 2014, 10, 2870. [Google Scholar] [CrossRef]
- Kumar, R.; Weigel, S.; Meyer, R.; Niemeyer, C.M.; Fuchs, H.; Hirtz, M. Multi-color polymer pen lithography for oligonucleotide arrays. Chem. Commun. 2016, 52, 12310. [Google Scholar] [CrossRef]
- Kumar, R.; Urtizberea, A.; Ghosh, S.; Bog, U.; Rainer, Q.; Lenhert, S.; Fuchs, H.; Hirtz, M. Polymer Pen Lithography with Lipids for Large-Area Gradient Patterns. Langmuir 2017, 33, 8739. [Google Scholar] [CrossRef]
- Bitterman, P.B.; Rennard, S.I.; Adelberg, S.; Crystal, R.G. Role of fibronectin as a growth factor for fibroblasts. J. Cell Biol. 1983, 97, 1925–1932. [Google Scholar] [CrossRef]
- Lehnert, D.; Wehrle-Haller, B.; David, C.; Weiland, U.; Ballestrem, C.; Imhof, B.A.; Bastmeyer, M. Cell behaviour on micropatterned substrata: Limits of extracellular matrix geometry for spreading and adhesion. J. Cell Sci. 2004, 117, 41–45. [Google Scholar] [CrossRef]
- Rodriguez-Emmenegger, C. Controlled cell adhesion on poly(dopamine) interfaces photopatterned with non-fouling brushes. Adv. Mater. 2013, 25, 6123–6127. [Google Scholar] [CrossRef] [PubMed]
- Parrillo, V.; de Los Santos Pereira, A.; Riedel, T.; Rodriguez-Emmenegger, C. Catalyst-free ‘click’ functionalization of polymer brushes preserves antifouling properties enabling detection in blood plasma. Anal. Chim. Acta 2017, 971, 78–87. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz Lantada, A.; Kumar, R.; Guttmann, M.; Wissmann, M.; Schneider, M.; Worgull, M.; Hengsbach, S.; Rupp, F.; Bade, K.; Hirtz, M.; et al. Synergies between Surface Microstructuring and Molecular Nanopatterning for Controlling Cell Populations on Polymeric Biointerfaces. Polymers 2020, 12, 655. https://doi.org/10.3390/polym12030655
Díaz Lantada A, Kumar R, Guttmann M, Wissmann M, Schneider M, Worgull M, Hengsbach S, Rupp F, Bade K, Hirtz M, et al. Synergies between Surface Microstructuring and Molecular Nanopatterning for Controlling Cell Populations on Polymeric Biointerfaces. Polymers. 2020; 12(3):655. https://doi.org/10.3390/polym12030655
Chicago/Turabian StyleDíaz Lantada, Andrés, Ravi Kumar, Markus Guttmann, Markus Wissmann, Marc Schneider, Matthias Worgull, Stefan Hengsbach, Florian Rupp, Klaus Bade, Michael Hirtz, and et al. 2020. "Synergies between Surface Microstructuring and Molecular Nanopatterning for Controlling Cell Populations on Polymeric Biointerfaces" Polymers 12, no. 3: 655. https://doi.org/10.3390/polym12030655
APA StyleDíaz Lantada, A., Kumar, R., Guttmann, M., Wissmann, M., Schneider, M., Worgull, M., Hengsbach, S., Rupp, F., Bade, K., Hirtz, M., & Sekula-Neuner, S. (2020). Synergies between Surface Microstructuring and Molecular Nanopatterning for Controlling Cell Populations on Polymeric Biointerfaces. Polymers, 12(3), 655. https://doi.org/10.3390/polym12030655






