Effect of Chemical Structure and Degree of Branching on the Stability of Proton Exchange Membranes Based on Sulfonated Polynaphthylimides
Abstract
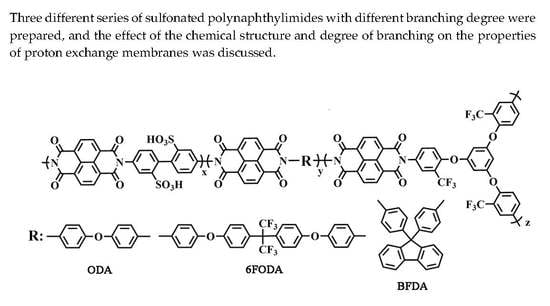
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of B3 Monomer (TFAPOB)
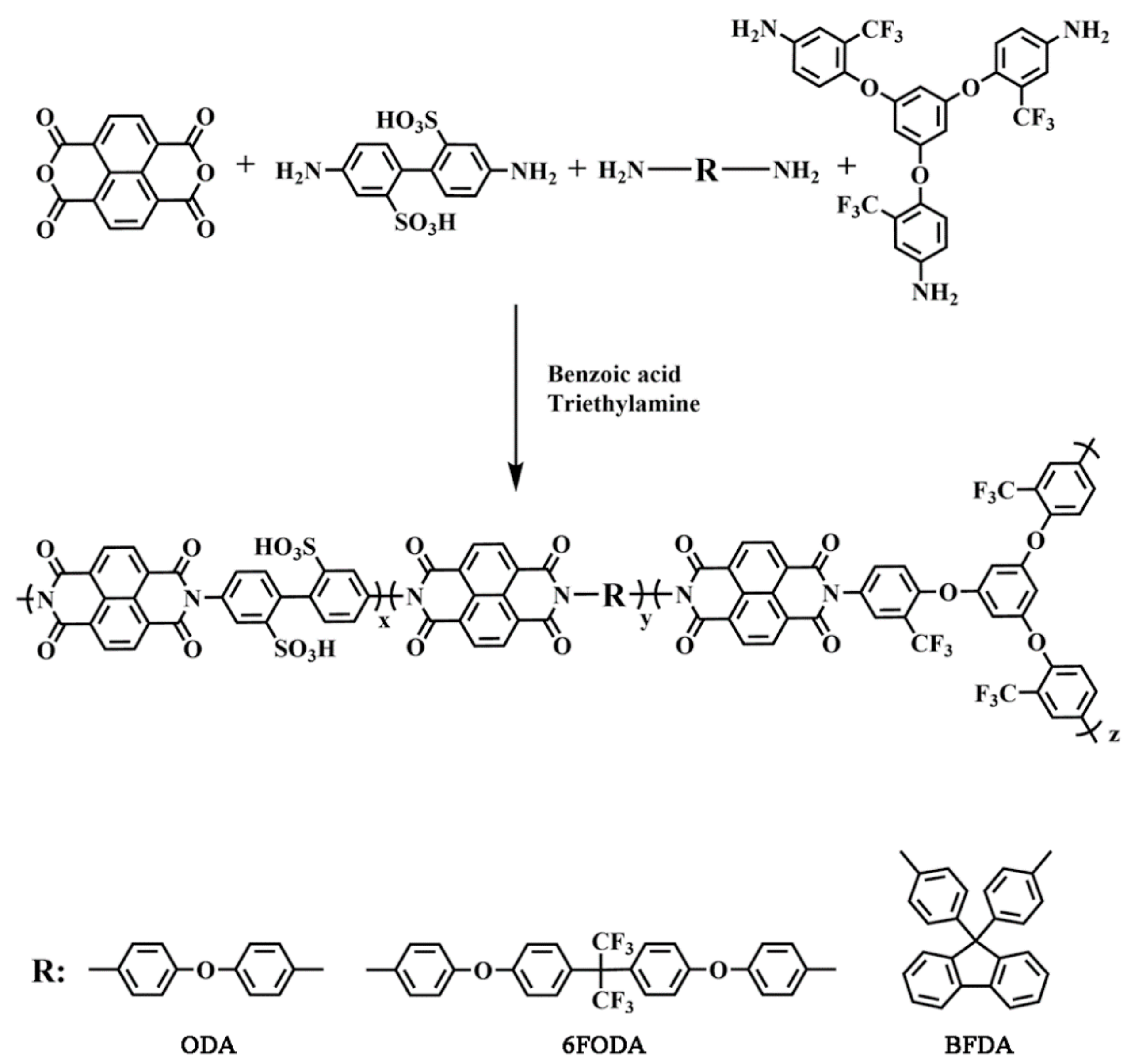
2.3. Synthesis of Sulfonated Polyimide (SPI)
2.3.1. Preparation of Linear SPI-ODA
2.3.2. Preparation of Branched SPI
2.4. Preparation of Membranes
2.5. Measurements
2.6. Water Uptake and Ion Exchange Capacity
2.7. Proton Conductivity
2.8. Chemical Stability
3. Results and Discussion
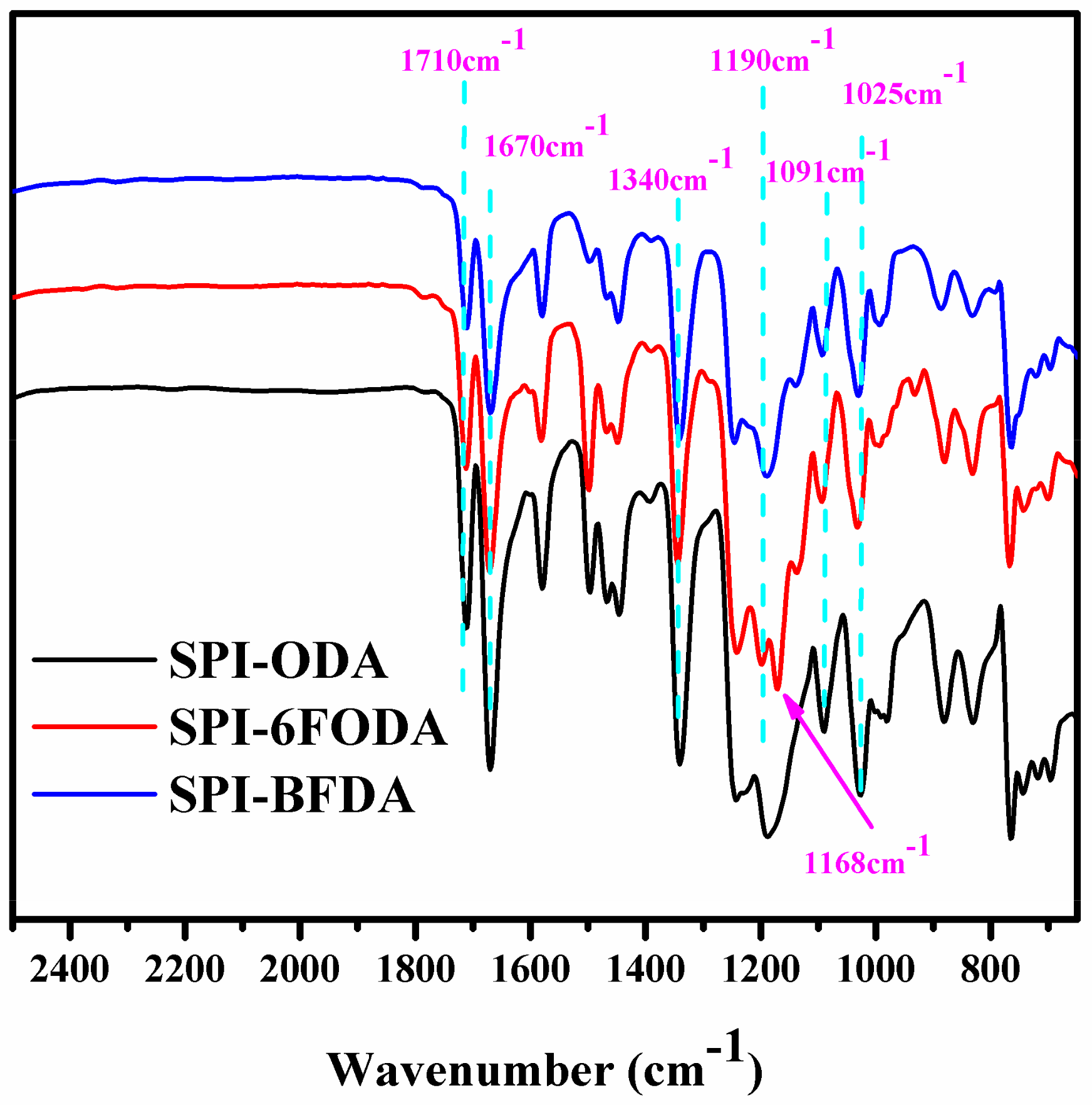
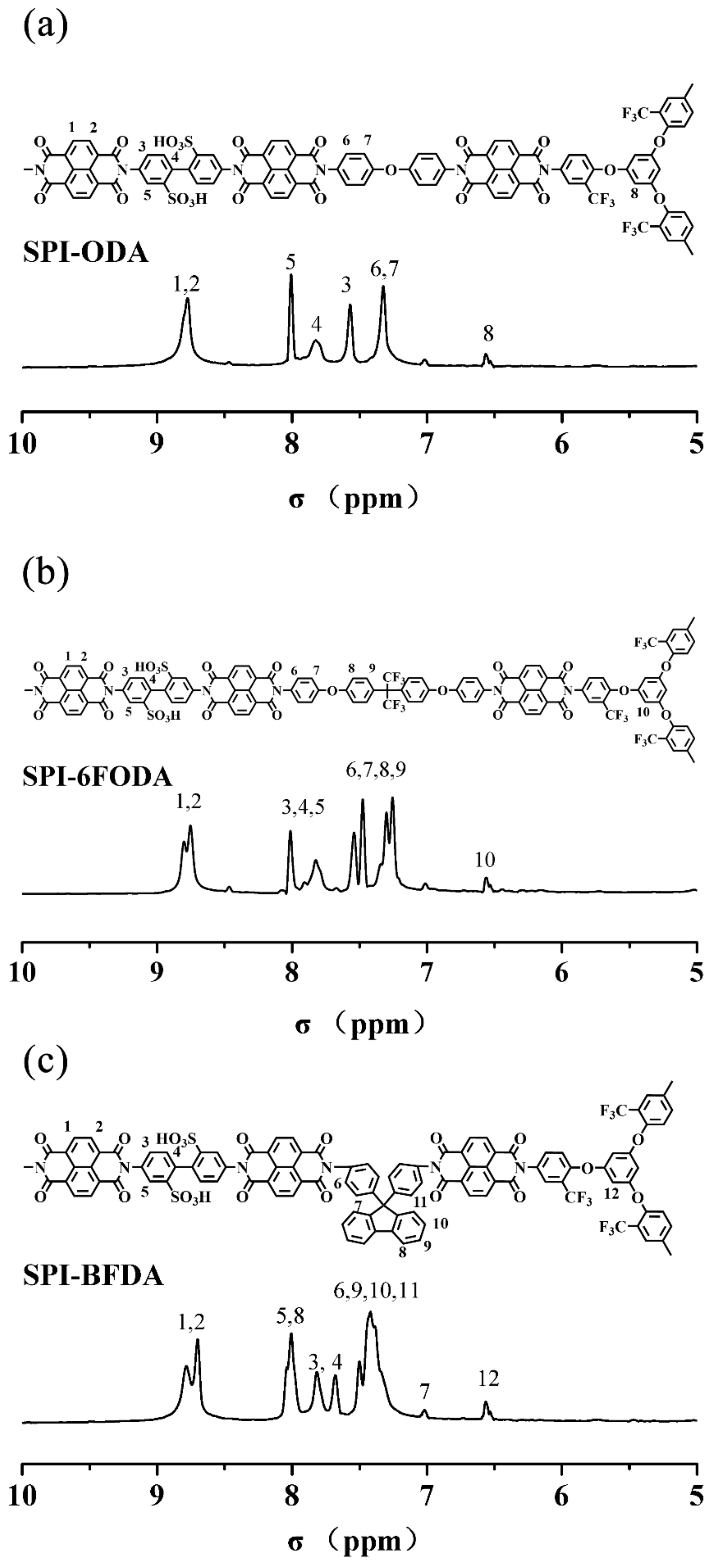
3.1. SPIs Synthesis And Characterization
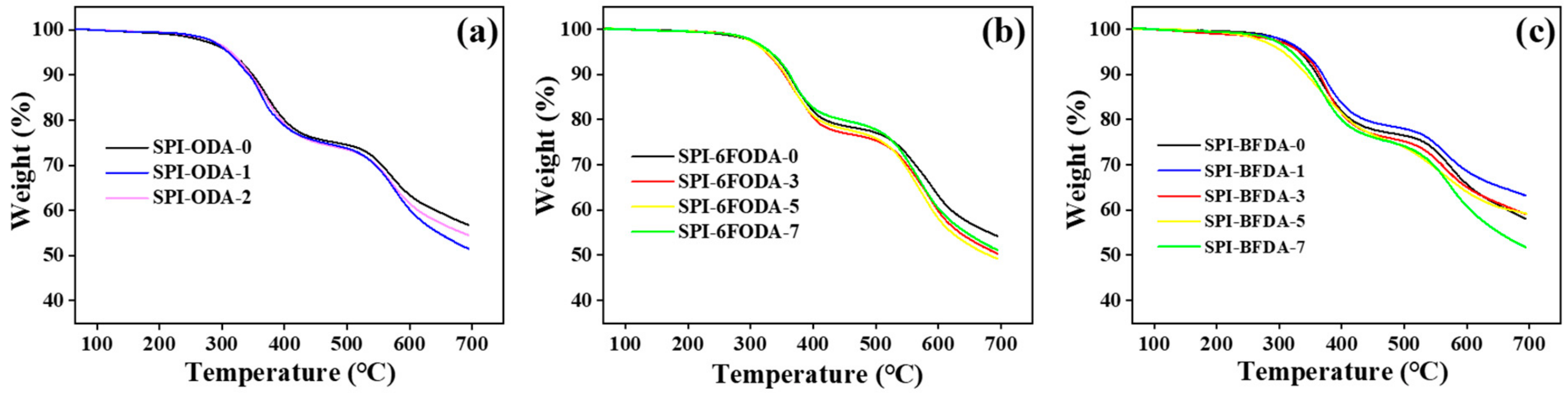
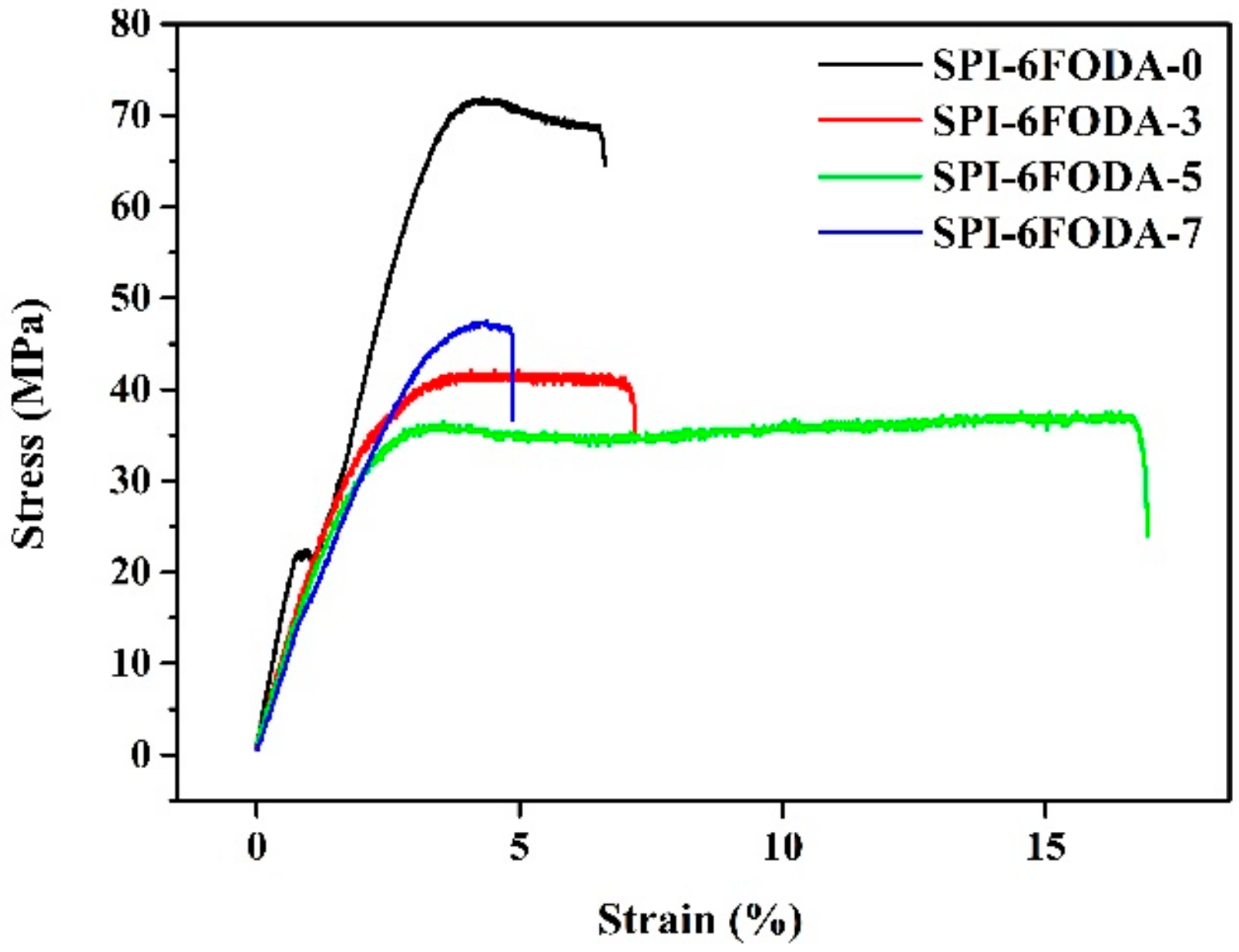
3.2. Thermal and Mechanical Properties
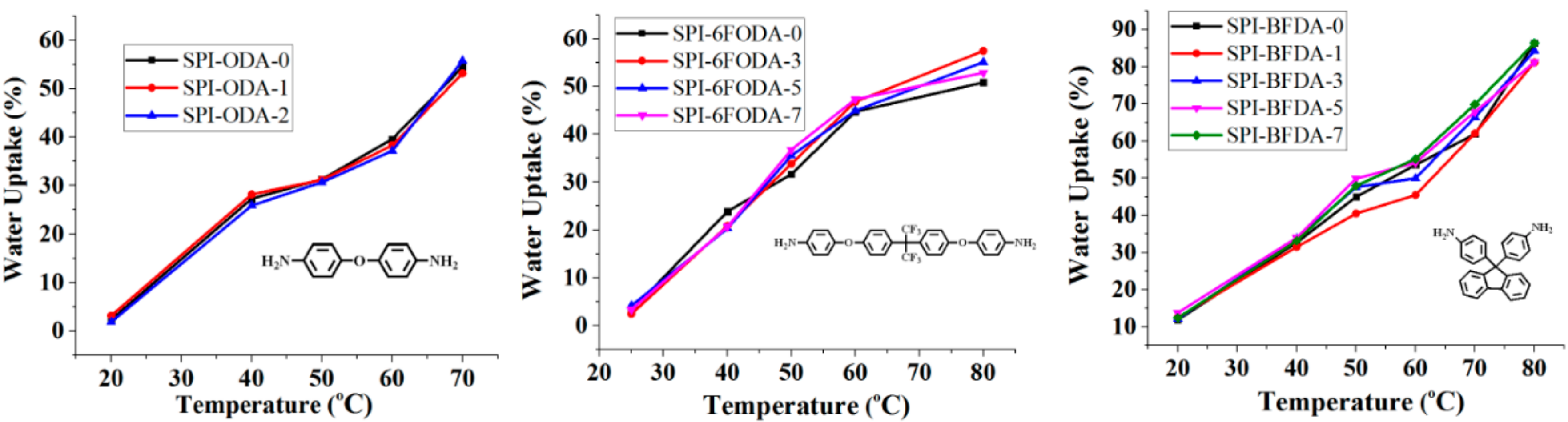
3.3. Water Uptake (WU) Values and Ion-Exchange Capacity (IEC)
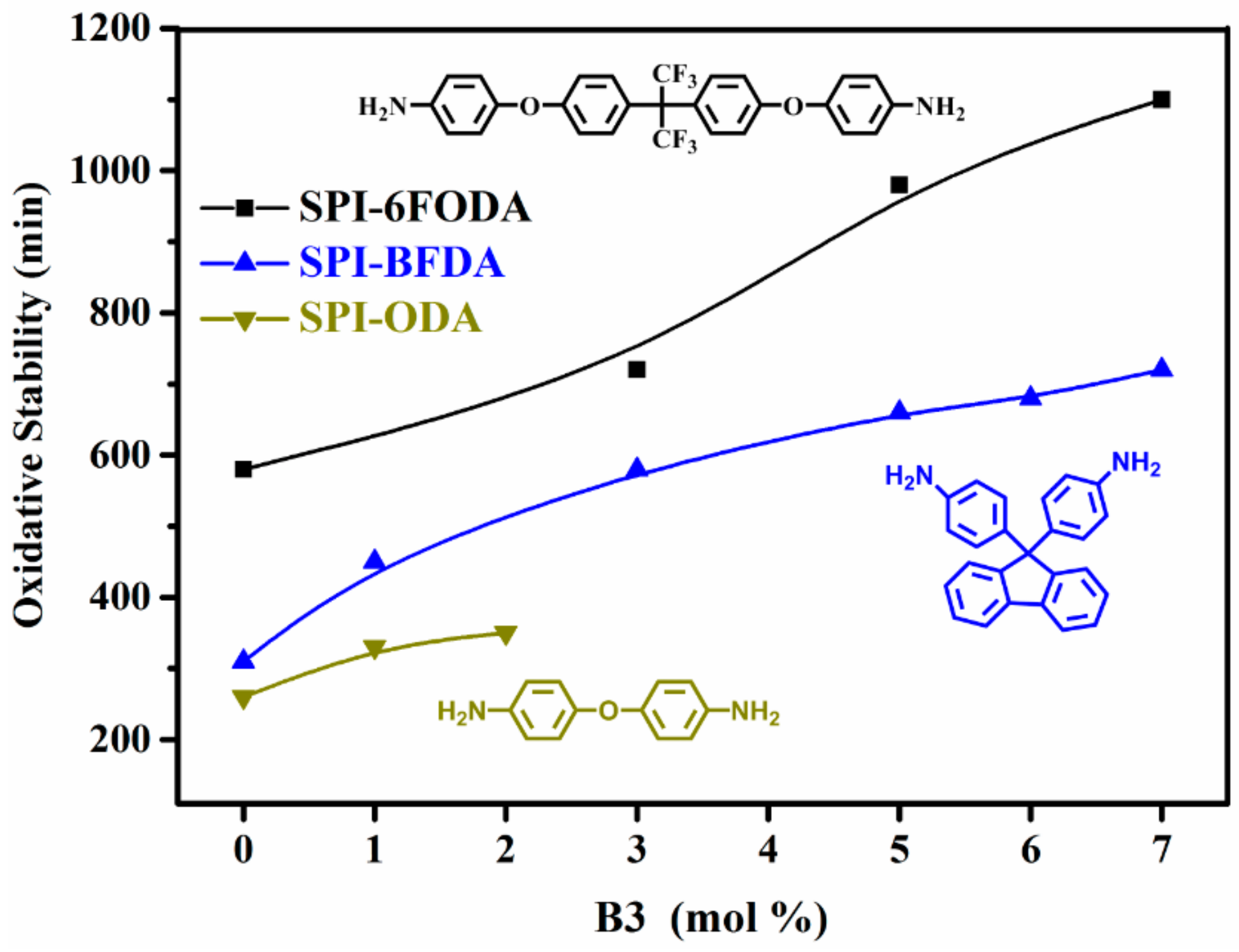
3.4. Chemical Stabilities
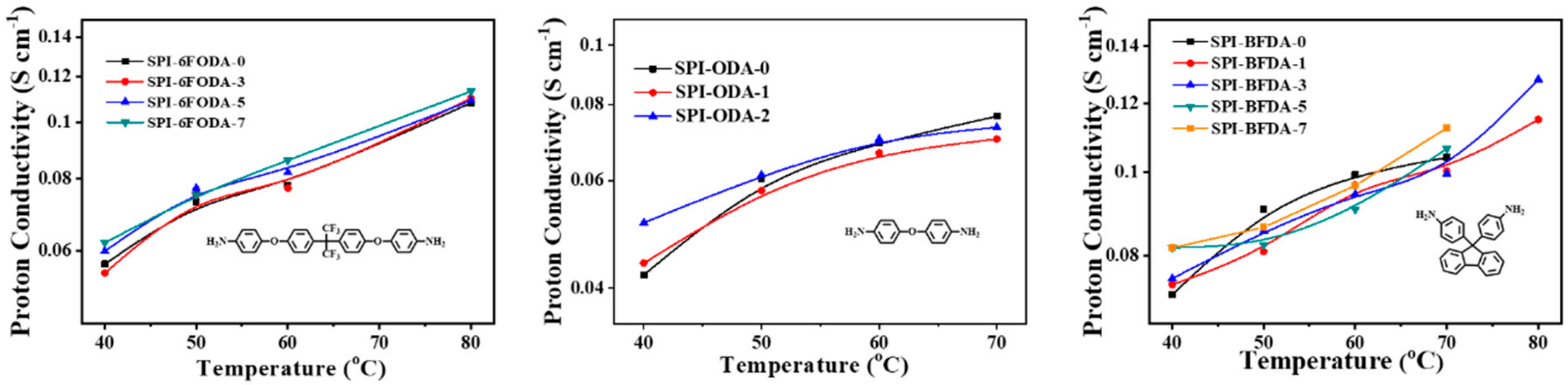
3.5. Proton Conductivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts-A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Wee, J.H. Applications of proton exchange membrane fuel cell systems. Renew. Sustain. Energy Rev. 2007, 11, 1720–1738. [Google Scholar] [CrossRef]
- Higashihara, T.; Matsumoto, K.; Ueda, M. Sulfonated aromatic hydrocarbon polymers as proton exchange membranes for fuel cells. Polymer 2009, 50, 5341–5357. [Google Scholar] [CrossRef] [Green Version]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.H.; Kim, A.R.; Yoo, D.J. Profile of extended chemical stability and mechanical integrity and high hydroxide ion conductivity of poly(ether imide) based membranes for anion exchange membrane fuel cells. Int. J. Hydrogen Energy 2019, 44, 4281–4292. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4611. [Google Scholar] [CrossRef]
- Alberti, G.; Narducci, R.; Di Vona, M.L.; Giancola, S. Annealing of Nafion 1100 in the Presence of an Annealing Agent: APowerful Method for Increasing Ionomer Working Temperature in PEMFCs. Fuel Cells 2013, 13, 42–47. [Google Scholar] [CrossRef]
- Alberti, G.; Narducci, R.; Di Vona, M.L.; Giancola, S. Preparation and Nc/T plots of un-crystallized Nafion 1100 and semi-crystalline Nafion 1000. Int. J. Hydrogen Energy 2017, 42, 15908–15912. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Kulkarni, S.; Mauritz, K.A. In Situ Grown Titania Composition for Optimal Performance and Durability of Nafion (R) Fuel Cell Membranes. J. Appl. Polym. Sci. 2011, 121, 2344–2353. [Google Scholar] [CrossRef]
- Shoesmith, J.P.; Collins, R.D.; Oakley, M.J.; Stevenson, D.K. Status of Solid Polymer Fuel-Cell System-Development. J. Power Sources 1994, 49, 129–142. [Google Scholar] [CrossRef]
- Chuang, S.W.; Hsu, S.L.C.; Yang, M.L. Preparation and characterization of fluorine-containing polybenzimidazole/imidazole hybrid membranes for proton exchange membrane fuel cells. Eur. Polym. J. 2008, 44, 2202–2206. [Google Scholar] [CrossRef]
- Feng, M.N.; Huang, Y.M.; Cheng, Y.; Liu, J.C.; Liu, X.B. Rational design of sulfonated poly(ether ether ketone) grafted graphene oxide-based composites for proton exchange membranes with enhanced performance. Polymer 2018, 144, 7–17. [Google Scholar] [CrossRef]
- Li, C.C.; Yang, Z.H.; Liu, X.P.; Zhang, Y.F.; Dong, J.M.; Zhang, Q.; Cheng, H.S. Enhanced performance of sulfonated poly (ether ether ketone) membranes by blending fully aromatic polyamide for practical application in direct methanol fuel cells (DMFCs). Int. J. Hydrogen Energy 2017, 42, 28567–28577. [Google Scholar] [CrossRef]
- Li, X.F.; Zhao, C.J.; Lu, H.; Wang, Z.; Na, H. Direct synthesis of sulfonated poly(ether ether ketone ketone)s (SPEEKKs) proton exchange membranes for fuel cell application. Polymer 2005, 46, 5820–5827. [Google Scholar] [CrossRef]
- Lee, C.; Jo, S.M.; Choi, J.; Baek, K.Y.; Truong, Y.B.; Kyratzis, I.L.; Shul, Y.G. SiO2/sulfonated poly ether ether ketone (SPEEK) composite nanofiber mat supported proton exchange membranes for fuel cells. J. Mater. Sci. 2013, 48, 3665–3671. [Google Scholar] [CrossRef]
- Anderson, L.J.; Yuan, X.; Fahs, G.B.; Moore, R.B. Blocky lonomers via Sulfonation of Poly(ether ether ketone) in the Semicrystalline Gel State. Macromolecules 2018, 51, 6226–6237. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Park, C.J.; Yoo, D.J. Alleviating the Mechanical and Thermal Degradations of Highly Sulfonated Poly(Ether Ether Ketone) Blocks via Copolymerization with Hydrophobic Unit for Intermediate Humidity Fuel Cells. Polymers 2018, 10, 1346. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Facile Fabrication and Characterization of Improved Proton Conducting Sulfonated Poly(Arylene Biphenylether Sulfone) Blocks Containing Fluorinated Hydrophobic Units for Proton Exchange Membrane Fuel Cell Applications. Polymers 2018, 10, 1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A.R.; Yoo, D.J. A Comparative Study on Physiochemical, Thermomechanical, and Electrochemical Properties of Sulfonated Poly(Ether Ether Ketone) Block Copolymer Membranes with and without Fe3O4 Nanoparticles. Polymers 2019, 11, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasikala, S.; Meenakshi, S.; Bhat, S.D.; Sahu, A.K. Functionalized Bentonite clay-sPEEK based composite membranes for direct methanol fuel cells. Electrochim. Acta 2014, 135, 232–241. [Google Scholar] [CrossRef]
- Gil, S.C.; Kim, J.C.; Ahn, D.; Jang, J.S.; Kim, H.; Jung, J.C.; Lim, S.; Jung, D.H.; Lee, W. Thermally crosslinked sulfonated polyethersulfone proton exchange membranes for direct methanol fuel cells. J. Membr. Sci. 2012, 417, 2–9. [Google Scholar]
- Di Vona, M.L.; Sgreccia, E.; Tamilvanan, M.; Khadhraoui, M.; Chassigneux, C.; Knauth, P. High ionic exchange capacity polyphenylsulfone (SPPSU) and polyethersulfone (SPES) cross-linked by annealing treatment: Thermal stability, hydration level and mechanical properties. J. Membr. Sci. 2010, 354, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.; Mohanty, S.; Nayak, S.K. Polymer electrolyte membranes based on sulfonated polysulfone and functionalized layered silicate for direct methanol fuel cell applications. High Perform. Polym. 2015, 27, 714–723. [Google Scholar] [CrossRef]
- Yu, J.J.; Dong, C.; Liu, J.H.; Li, C.H.; Fang, J.H.; Guan, R. Crosslinked sulfonated poly (bis-A)-sulfones as proton exchange membrane for PEM fuel cell application. J. Mater. Sci. 2010, 45, 1017–1024. [Google Scholar] [CrossRef]
- Zhang, B.P.; Ni, J.P.; Xiang, X.Z.; Wang, L.; Chen, Y.M. Synthesis and properties of reprocessable sulfonated polyimides cross-linked via acid stimulation for use as proton exchange membranes. J. Power Sources 2017, 337, 110–117. [Google Scholar] [CrossRef]
- Mistri, E.A.; Banerjee, S.; Komber, H.; Voit, B. Structure-property correlation of semifluorinated 6-membered co-SPIs for proton exchange membrane. Eur. Polym. J. 2015, 73, 466–479. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Dong, J.Y. Artificially designed, low humidifying organic–inorganic (SFBC-50/FSiO2 ) composite membrane for electrolyte applications of fuel cells. Compos. Part B Eng. 2017, 130, 103–118. [Google Scholar] [CrossRef]
- Kim, A.R.; Park, C.J.; Vinothkannan, M.; Dong, J.Y. Sulfonated poly ether sulfone/heteropoly acid composite membranes as electrolytes for the improved power generation of proton exchange membrane fuel cells. Compos. Part B Eng. 2018, 155, 272–281. [Google Scholar] [CrossRef]
- Date, B.; Han, J.; Park, S.; Park, E.J.; Shin, D.; Ryu, C.Y.; Bae, C. Synthesis and Morphology Study of SEBS Triblock Copolymers Functionalized with Sulfonate and Phosphonate Groups for Proton Exchange Membrane Fuel Cells. Macromolecules 2018, 51, 1020–1030. [Google Scholar] [CrossRef]
- Marestin, C.; Gebel, G.; Diat, O.; Mercier, R. Sulfonated Polyimides. Adv. Polym. Sci. 2008, 216, 185–258. [Google Scholar]
- Arico, A.S.; Srinivasan, S.; Antonucci, V. DMFCs: From Fundamental Aspects to Technology Development. Fuel Cells 2001, 1, 133–161. [Google Scholar] [CrossRef]
- Rusanov, A.L.; Komarova, L.G.; Bulycheva, E.G.; Bugaenko, M.G.; Belomoina, N.M. New Sulfonated Polyethers and Polynaphthylimides based on TNT Derivatives. High Perform. Polym. 2009, 21, 508–521. [Google Scholar] [CrossRef]
- Rusanov, A.L.; Bulycheva, E.G.; Bugaenko, M.G.; Leikin, A.Y.; Shevelev, S.A.; Dutov, M.D.; Serushkina, O.V.; Voitekunas, V.Y.; Abadi, M.J.P.S. New sulfonated polynaphthylimides: Synthesis and investigation. Polym. Sci. 2009, 51, 3–7. [Google Scholar] [CrossRef]
- Zhang, H.W.; Shen, P.K. Recent Development of Polymer Electrolyte Membranes for Fuel Cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef]
- Akbarian-Feizi, L.; Mehdipour-Ataei, S.; Yeganeh, H. Survey of sulfonated polyimide membrane as a good candidate for nafion substitution in fuel cell. Int. J. Hydrogen Energy 2010, 35, 9385–9397. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Li, Q.B.; Liu, B.; Du, F.F.; Tian, J.; Wang, H.; Wang, Y.X.; Bai, R.K. Conjugated polymers containing a 2,2 -biimidazole moiety-a novel fluorescent sensing platform. Chem. Commun. 2012, 48, 118–120. [Google Scholar] [CrossRef]
- Perrot, C.; Gonon, L.; Marestin, C.; Gebel, G. Hydrolytic degradation of sulfonated polyimide membranes for fuel cells. J. Membr. Sci. 2011, 379, 207–214. [Google Scholar] [CrossRef]
- Fang, J.H.; Zhai, F.X.; Guo, X.X.; Xu, H.J.; Okamoto, K. A facile approach for the preparation of cross-linked sulfonated polyimide membranes for fuel cell application. J. Mater. Chem. 2007, 17, 1102–1108. [Google Scholar] [CrossRef]
- Sutou, Y.; Yin, Y.; Hu, Z.; Chen, S.; Kita, H.; Okamoto, K.; Wang, H.; Kawasato, H. Synthesis and Properties of Sulfonated Polyimides Derived from Bis(sulfophenoxy) Benzidines. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1463–1477. [Google Scholar] [CrossRef]
- Miyatake, K.; Watanabe, M. Emerging membrane materials for high temperature polymer electrolyte fuel cells: Durable hydrocarbon ionomers. J. Mater. Chem. 2006, 16, 4465–4467. [Google Scholar] [CrossRef]
- Zhai, F.X.; Guo, X.X.; Fang, J.H.; Xu, H.J. Synthesis and properties of novel sulfonated polyimide membranes for direct methanol fuel cell application. J. Membr. Sci. 2007, 296, 102–109. [Google Scholar] [CrossRef]
- Li, N.W.; Cui, Z.M.; Zhang, S.B.; Li, S.H.; Zhang, F. Preparation and evaluation of a proton exchange membrane based on oxidation and water stable sulfonated polyimides. J. Power Sources 2007, 172, 511–519. [Google Scholar] [CrossRef]
- Xie, H.X.; Tao, D.; Xiang, X.Z.; Ou, Y.X.; Bai, X.J.; Wang, L. Synthesis and properties of highly branched star-shaped sulfonated block poly(arylene ether)s as proton exchange membranes. J. Membr. Sci. 2015, 473, 226–236. [Google Scholar] [CrossRef]
- Xie, H.X.; Wang, D.; Tao, D.; Wang, L. Synthesis of highly branched sulfonated polymers and the effects of degree of branching on properties of branched sulfonated polymers as proton exchange membranes. J. Power Sources 2014, 262, 328–337. [Google Scholar] [CrossRef]
- Xie, H.X.; Liu, D.; Xiang, X.Z.; Zhu, C.Z.; Wang, L. Preparation and properties of highly branched sulfonated poly(arylene ether)/polyacrylonitrile composite materials as proton exchange membranes. J. Mater. Sci. 2016, 51, 7119–7129. [Google Scholar] [CrossRef]
- Liu, D.; Xu, M.; Fang, M.; Chen, J.; Wang, L. Branched comb-shaped poly(arylene ether sulfone)s containing flexible alkyl imidazolium side chains as anion exchange membranes. J. Mater. Chem. A 2018, 6, 10879–10890. [Google Scholar] [CrossRef]
- Neelakandan, S.; Liu, D.; Wang, L.; Hu, M.S.; Wang, L. Highly branched poly(arylene ether)/surface functionalized fullerene-based composite membrane electrolyte for DMFC applications. Int. J. Energy Res. 2019, 43, 3756–3767. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.R.; Liu, Y.; Wang, L. Crosslinked polybenzimidazole containing branching structure with no sacrifice of effective N-H sites: Towards high-performance high-temperature proton exchange membranes for fuel cells. J. Membr. Sci. 2019, 583, 110–117. [Google Scholar] [CrossRef]
- Hu, M.; Li, T.; Neelakandan, S.; Wang, L.; Chen, Y. Cross-linked polybenzimidazoles containing hyperbranched cross-linkers and quaternary ammoniums as high-temperature proton exchange membranes: Enhanced stability and conductivity. J. Membr. Sci. 2020, 593, 117435. [Google Scholar] [CrossRef]
- Gao, C.; Liu, Y.; Gao, Y.; Zhou, Y.; Zhou, X.; Yin, X.; Pan, C.; Yang, C.; Wang, H.; Chen, G.; et al. High-performance n-type thermoelectric composites of acridones with tethered tertiary amines and carbon nanotubes. J. Mater. Chem. A 2018, 6, 20161–20169. [Google Scholar] [CrossRef]
- Guo, W.; Li, X.; Wang, H.; Pang, J.; Wang, G.; Jiang, Z.; Zhang, S. Synthesis of branched sulfonated poly(aryl ether ketone) copolymers and their proton exchange membrane properties. J. Membr. Sci. 2013, 444, 259–267. [Google Scholar] [CrossRef]


| SPIs | Molar Ratio | DB | |||||
|---|---|---|---|---|---|---|---|
| NDTA | Non-Sulfonated Diamine Monomers | DAPS | B3 | ||||
| ODA | 6FODA | BFDA | |||||
| SPI-ODA-0 | 3 | 1 | - | - | 2 | 0 | 0 |
| SPI-ODA-1 | 3 | 0.97 | - | - | 2 | 0.02 | 1 |
| SPI-ODA-2 | 3 | 0.94 | - | - | 2 | 0.04 | 2 |
| SPI-6FODA-0 | 3 | - | 1 | - | 2 | 0 | 0 |
| SPI-6FODA-3 | 3 | - | 0.91 | - | 2 | 0.06 | 3 |
| SPI-6FODA-5 | 3 | - | 0.85 | - | 2 | 0.10 | 5 |
| SPI-6FODA-7 | 3 | - | 0.79 | - | 2 | 0.14 | 7 |
| SPI-BFDA-0 | 3 | - | - | 0 | 2 | 0 | 0 |
| SPI-BFDA-1 | 3 | - | - | 0.97 | 2 | 0.02 | 1 |
| SPI-BFDA-3 | 3 | - | - | 0.91 | 2 | 0.06 | 3 |
| SPI-BFDA-5 | 3 | - | - | 0.85 | 2 | 0.10 | 5 |
| SPI-BFDA-7 | 3 | - | - | 0.79 | 2 | 0.14 | 7 |
| Sample | Thickness (μm) | Tensile Strength (MPa) | Young’s Modulus (MPa) | Viscosity (dL g–1) |
|---|---|---|---|---|
| SPI-ODA-0 | 30 | 62.1 | - | - |
| SPI-ODA-1 | 31 | 62.0 | - | - |
| SPI-ODA-2 | 33 | 50.7 | - | - |
| SPI-6FODA-0 | 52 | 71.7 | 1720 | 0.83 |
| SPI-6FODA-3 | 52 | 47.4 | 1326 | 0.69 |
| SPI-6FODA-5 | 55 | 41.7 | 1223 | 0.72 |
| SPI-6FODA-7 | 53 | 32.1 | 1145 | 0.61 |
| SPI-BFDA-0 | 47 | 62.3 | - | 0.54 |
| SPI-BFDA-1 | 46 | 66.4 | - | - |
| SPI-BFDA-3 | 44 | 46.2 | - | 0.46 |
| SPI-BFDA-5 | 47 | 41.2 | - | 0.49 |
| SPI-BFDA-7 | 45 | 38.2 | - | 0.31 |
| Polymer | Degree of Branching | IEC (mmol g−1) | |
|---|---|---|---|
| Theoretical | Experimental | ||
| SPI-ODA-0 | 0% | - | - |
| SPI-ODA-1 | 1% | - | - |
| SPI-ODA-2 | 2% | - | - |
| SPI-6FODA-0 | 0% | 2.01 | 1.98 |
| SPI-6FODA-3 | 3% | 2.01 | 1.91 |
| SPI-6FODA-5 | 5% | 1.99 | 1.89 |
| SPI-6FODA-7 | 7% | 1.97 | 1.90 |
| SPI-BFDA-0 | 0% | 2.38 | 1.98 |
| SPI-BFDA-1 | 1% | 2.36 | 2.08 |
| SPI-BFDA-3 | 3% | 2.33 | 1.82 |
| SPI-BFDA-5 | 5% | 2.31 | 1.82 |
| SPI-BFDA-7 | 7% | 2.29 | 2.04 |
| Membrane | Broken Time | Weight Residue after 24 h | Weight residue after 120 h |
|---|---|---|---|
| SPI-ODA-0 | 140 min | - | - |
| SPI-ODA-1 | 130 min | - | - |
| SPI-ODA-2 | 150 min | - | - |
| SPI-6FODA-0 | >120 h | 100% | 92.8%(Flexible) |
| SPI-6FODA-3 | >120 h | 97.2% | 89.2%(Flexible) |
| SPI-6FODA-5 | >120 h | 100% | 90.3%(Flexible) |
| SPI-6FODA-7 | >120 h | 98.2% | 87.5%(Flexible) |
| SPI-BFDA-0 | 210 min | - | - |
| SPI-BFDA-1 | 290 min | - | - |
| SPI-BFDA-3 | 270 min | - | - |
| SPI-BFDA-5 | 240 min | - | - |
| SPI-BFDA-7 | 260 min | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Chen, J.; Zhang, B.; Wang, L. Effect of Chemical Structure and Degree of Branching on the Stability of Proton Exchange Membranes Based on Sulfonated Polynaphthylimides. Polymers 2020, 12, 652. https://doi.org/10.3390/polym12030652
Gao C, Chen J, Zhang B, Wang L. Effect of Chemical Structure and Degree of Branching on the Stability of Proton Exchange Membranes Based on Sulfonated Polynaphthylimides. Polymers. 2020; 12(3):652. https://doi.org/10.3390/polym12030652
Chicago/Turabian StyleGao, Chunmei, Jiale Chen, Boping Zhang, and Lei Wang. 2020. "Effect of Chemical Structure and Degree of Branching on the Stability of Proton Exchange Membranes Based on Sulfonated Polynaphthylimides" Polymers 12, no. 3: 652. https://doi.org/10.3390/polym12030652
APA StyleGao, C., Chen, J., Zhang, B., & Wang, L. (2020). Effect of Chemical Structure and Degree of Branching on the Stability of Proton Exchange Membranes Based on Sulfonated Polynaphthylimides. Polymers, 12(3), 652. https://doi.org/10.3390/polym12030652