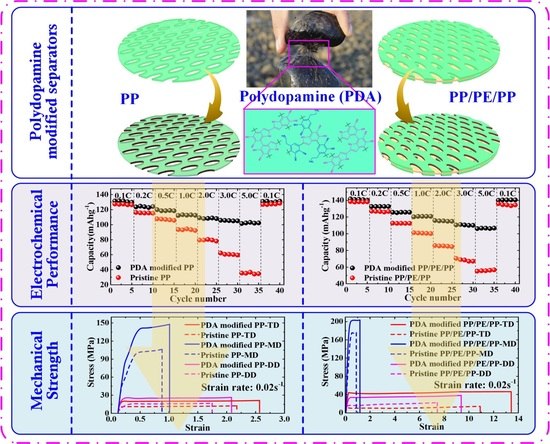
Self-Polymerized Dopamine Nanoparticles Modified Separators for Improving Electrochemical Performance and Enhancing Mechanical Strength of Lithium-Ion Batteries
Abstract
1. Introduction
2. Experimental
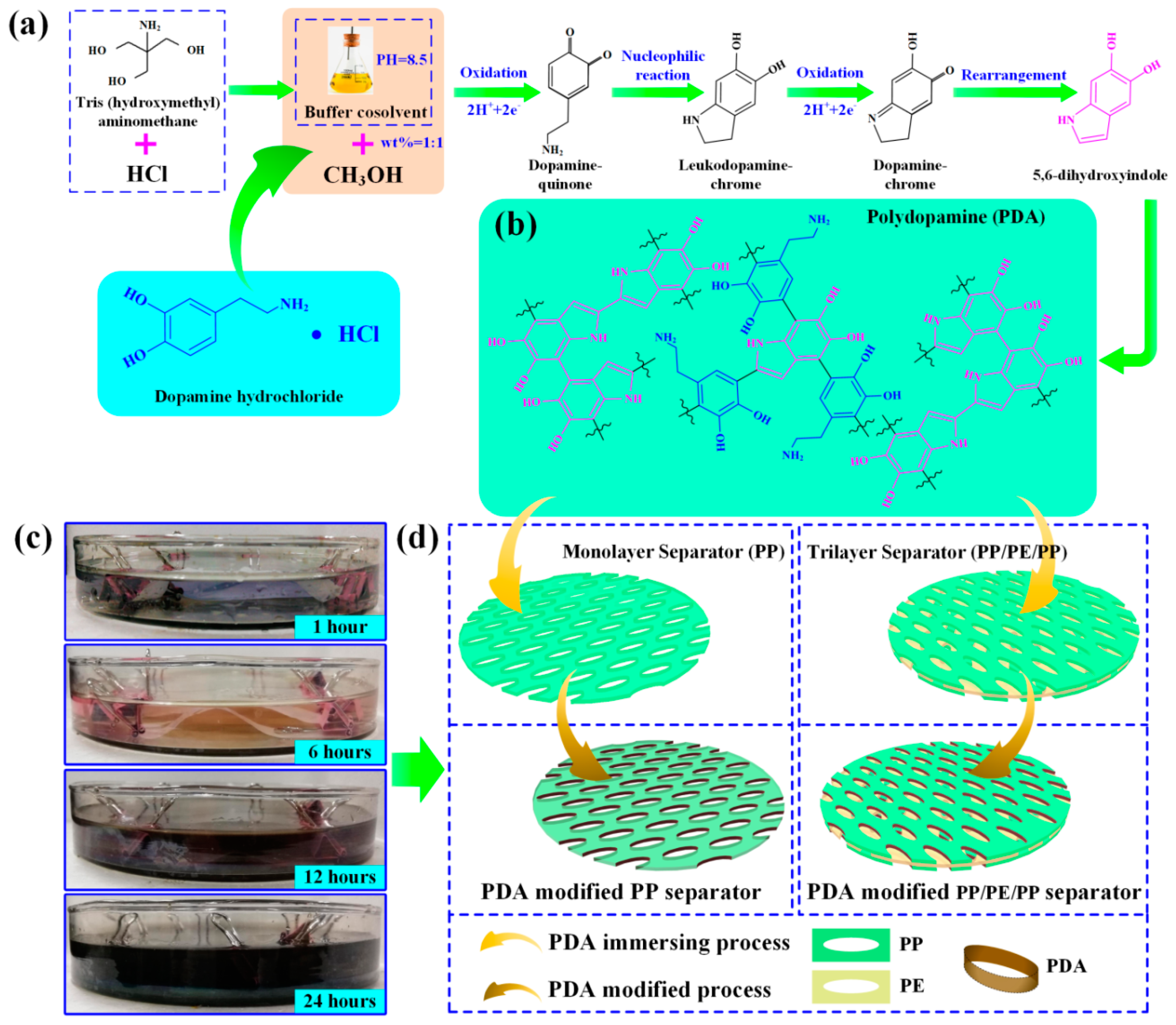
2.1. Polydopamine Modified Separator Preparation
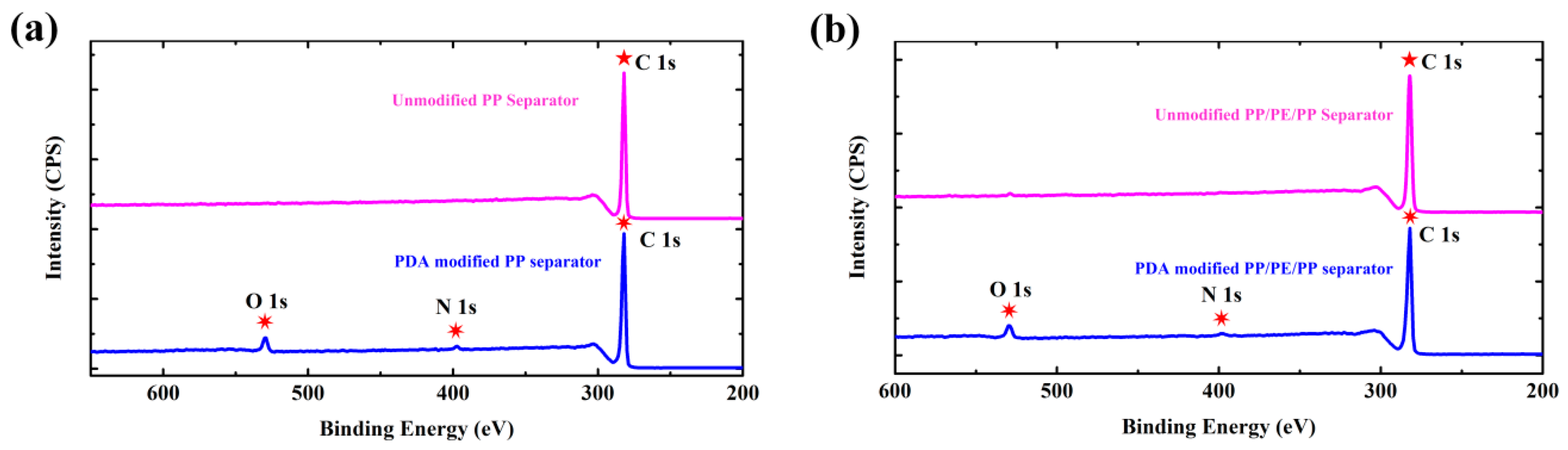
2.2. Materials Characterizations
2.3. Electrochemical Performance Experiments
2.4. Mechanical Strength Experiments
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takada, K. Progress and prospective of solid-state lithium batteries. Acta Mater. 2013, 61, 759–770. [Google Scholar] [CrossRef]
- Xu, J.; Liu, B.H.; Hu, D.Y. State of charge dependent mechanical integrity behavior of 18650 lithium-ion batteries. Sci. Rep. 2016, 6, 21829. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Yin, S.; Xu, J. Integrated computation model of lithium-ion battery subject to nail penetration. Appl. Energy 2016, 183, 278–289. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, N.; Cao, Q.; Jing, B.; Wang, X.; Wang, Q.; Kuang, H. A novel electrospun PVDF/PMMA gel polymer electrolyte with in situ TiO2 for Li-ion batteries. Solid State Ion. 2013, 249, 93–97. [Google Scholar] [CrossRef]
- Liu, B.H.; Zhao, H.; Yu, H.L.; Li, J.; Xu, J. Multiphysics computational framework for cylindrical lithium-ion batteries under mechanical abusive loading. Electrochim. Acta 2017, 256, 172–184. [Google Scholar] [CrossRef]
- Hao, W.Q.; Xie, J.M.; Zhang, X.; Wang, P.; Wang, F.H. Strain rate effect and micro-buckling behavior of anisotropy macromolecular separator for lithium-ion battery. Express Polym. Lett. 2020, 14, 206–219. [Google Scholar] [CrossRef]
- Huang, W.Y.; Liao, Y.H.; Li, G.J.; He, Z.H.; Luo, X.H.; Li, W.S. Investigation on polyethylene supported poly(butyl methacrylate-acrylonitrile-styrene) terpolymer based gel electrolyte reinforced by doping nano-SiO2 for high voltage lithium ion battery. Electrochim. Acta 2017, 251, 145–154. [Google Scholar] [CrossRef]
- Hao, W.Q.; Xie, J.M.; Bo, X.Q.; Wang, F.H. Resistance exterior force property of lithium-ion pouch batteries with different positive materials. Int. J. Energy Res. 2019, 43, 4976–4986. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.B.; Guan, J.; Yin, S. Coupled effect of strain rate and solvent on dynamic mechanical behaviors of separators in lithium ion batteries. Mater. Des. 2016, 95, 319–328. [Google Scholar] [CrossRef]
- Hao, W.Q.; Xie, J.M.; Wang, F.H. The indentation analysis triggering internal short circuit of lithium-ion pouch battery based on shape function theory. Int. J. Energy Res. 2018, 42, 3696–3703. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.K.; Park, J.H. Clay nanosheets in skeletons of controlled phase inversion separators for thermally stable li-ion batteries. Adv. Funct. Mater. 2015, 25, 3399–3404. [Google Scholar] [CrossRef]
- Jung, Y.S.; Cavanagh, A.S.; Gedvilas, L.; Widjonarko, N.E.; Scott, I.D.; Lee, S.H.; Kim, G.H.; George, S.M.; Dillon, A.C. Improved functionality of lithium-ion batteries enabled by atomic layer deposition on the porous microstructure of polymer separators and coating electrodes. Adv. Energy Mater. 2012, 2, 1022–1027. [Google Scholar] [CrossRef]
- Lee, Y.; Ryou, M.H.; Seo, M.; Choi, J.W.; Lee, Y.M. Effect of polydopamine surface coating on polyethylene separators as a function of their porosity for high-power Li-ion batteries. Electrochim. Acta 2013, 113, 433–438. [Google Scholar] [CrossRef]
- Xi, Z.Y.; Xu, Y.Y.; Zhu, L.P.; Wang, Y.; Zhu, B.K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine). J. Membr. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Ma, W.; Zhang, S.J.; Du, H.M.; Mu, Y.H.; Li, G.Y. Multiaxial creep of frozen loess. Mech. Mater. 2016, 95, 172–191. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Ma, W.; Zhang, S.J.; Mu, Y.H.; Li, G.Y. Experimental investigation of the path-dependent strength and deformation behaviours of frozen loess. Eng. Geol. 2020, 265, 105449. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Ma, W.; Zhang, S.J.; Mu, Y.H.; Li, G.Y. Effect of freeze-thaw cycles in mechanical behaviors of frozen loess. Cold Reg. Sci. Technol. 2018, 146, 9–18. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, Y.; Lim, D.Y. Plasma-modified polyethylene membrane as a separator for lithium-ion polymer battery. Electrochim. Acta 2009, 54, 3714–3719. [Google Scholar] [CrossRef]
- Chiappone, A.; Nair, J.R.; Gerbaldi, C.; Bongiovanni, R.; Zeno, E. UV-cured Al2O3-laden cellulose reinforced polymer electrolyte membranes for Li-based batteries. Electrochim. Acta 2015, 153, 97–105. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, Y.M.; Bhattacharya, B.; Nho, Y.C.; Park, J.K. Separator grafted with siloxane by electron beam irradiation for lithium secondary batteries. Electrochim. Acta 2009, 54, 4312–4315. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Ryou, M.; Lee, Y.M.; Park, J.; Choi, J.W. Mussel-inspired polydopamine-treated polyethylene separators for high-power li-ion batteries. Adv. Mater. 2011, 23, 3066–3070. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; You, I.; Cho, W.K.; Shon, H.K.; Lee, T.G.; Choi, I.S.; Karp, J.M.; Lee, H. One-step modification of superhydrophobic surfaces by a mussel-inspired polymer coating. Angew. Chem. Int. Ed. 2010, 49, 9401–9404. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Z.; Yu, L.; Zhang, K.; Na, H.; Ying, S.; Xu, D.; Zhang, G. Polydopamine hydrophilic modification of polypropylene separator for lithium ion battery. J. Appl. Polym. Sci. 2014, 131, 40543. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy. Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Sarada, T.; Sawyer, L.C.; Ostler, M.I. Three dimensional structure of celgard® microporous membranes. J. Membr. Sci. 1983, 15, 97–113. [Google Scholar] [CrossRef]
- Celgard Product Literature. Available online: https://www.celgard.com/literature (accessed on 10 December 2017).
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Yu, J.; Wei, W.; Danner, E.; Israelachvili, J.N.; Waite, J.H. Effects of interfacial redox in mussel adhesive protein films on mica. Adv. Mater. 2011, 23, 2362–2366. [Google Scholar] [CrossRef]
- Yoon, K.R.; Lee, K.A.; Jo, S.; Yook, S.H.; Lee, K.Y.; Kim, I.D.; Kim, J.Y. Mussel-inspired polydopamine-treated reinforced composite membranes with self-supported CeOx radical scavengers for highly stable PEM fuel cells. Adv. Funct. Mater. 2019, 29, 1806929. [Google Scholar] [CrossRef]
- Martinez-Cisneros, C.; Antonelli, C.; Levenfeld, B.; Varez, A.; Sanchez, J.Y. Evaluation of polyolefin-based macroporous separators for high temperature Li-ion batteries. Electrochim. Acta 2016, 216, 68–78. [Google Scholar] [CrossRef]
- Cho, K.; Saheb, D.N.; Choi, J.; Yang, H. Real time in situ X-ray diffraction studies on the melting memory effect in the crystallization of β-isotactic polypropylene. Polymer 2002, 43, 1407–1416. [Google Scholar] [CrossRef]
- Davies, R.J.; Zafeiropoulos, N.E.; Schneider, K.; Roth, S.V.; Burghammer, M.; Riekel, C.; Kotek, J.C.; Stamm, M. The use of synchrotron X-ray scattering coupled with in situ mechanical testing for studying deformation and structural change in isotactic polypropylene. Colloid Polym. Sci. 2004, 282, 854–866. [Google Scholar] [CrossRef]
- Xu, L.; Xu, K.; Chen, D.; Zheng, Q.; Liu, F.; Chen, M. Thermal behavior of isotactic polypropylene in different content of β-nucleating agent. J. Anal. Calorim. 2009, 96, 733–740. [Google Scholar] [CrossRef]
- Bafana, A.P.; Yan, X.R.; Wei, X.; Patel, M.; Guo, Z.H.; Wei, S.Y.; Wujcik, E.K. Polypropylene nanocomposites reinforced with low weight percent graphene nanoplatelets. Compos. Part B Eng. 2017, 109, 101–107. [Google Scholar] [CrossRef]
- Chen, X.D.; Xu, R.J.; Xie, J.Y.; Lin, Y.F.; Lei, C.H.; Li, L.B. The study of room-temperature stretching of annealed polypropylene cast film with row-nucleated crystalline structure. Polymer 2016, 94, 31–42. [Google Scholar]
- Binsbergen, F.L.; De Lange, B.G.M. Morphology of polypropylene crystallized from the melt. Polymer 1968, 9, 23–40. [Google Scholar] [CrossRef]
- Padden, F.J., Jr.; Keith, H.D. Crystallization in thin films of isotactic polypropylene. J. Appl. Phys. 1966, 37, 4013–4020. [Google Scholar] [CrossRef]
- Lovinger, A.J. Microstructure and unit-cell orientation in α-polypropylene. J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 97–110. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, Z.; Feng, C.; Lv, W.; Wei, Z.; Zhang, K.H.L.; Lin, B.; Wu, S.; Lei, T.; Guo, X.; et al. An upgraded lithium ion battery based on a polymeric separator incorporated with anode active materials. Adv. Energy Mater. 2019, 9, 1803627. [Google Scholar] [CrossRef]



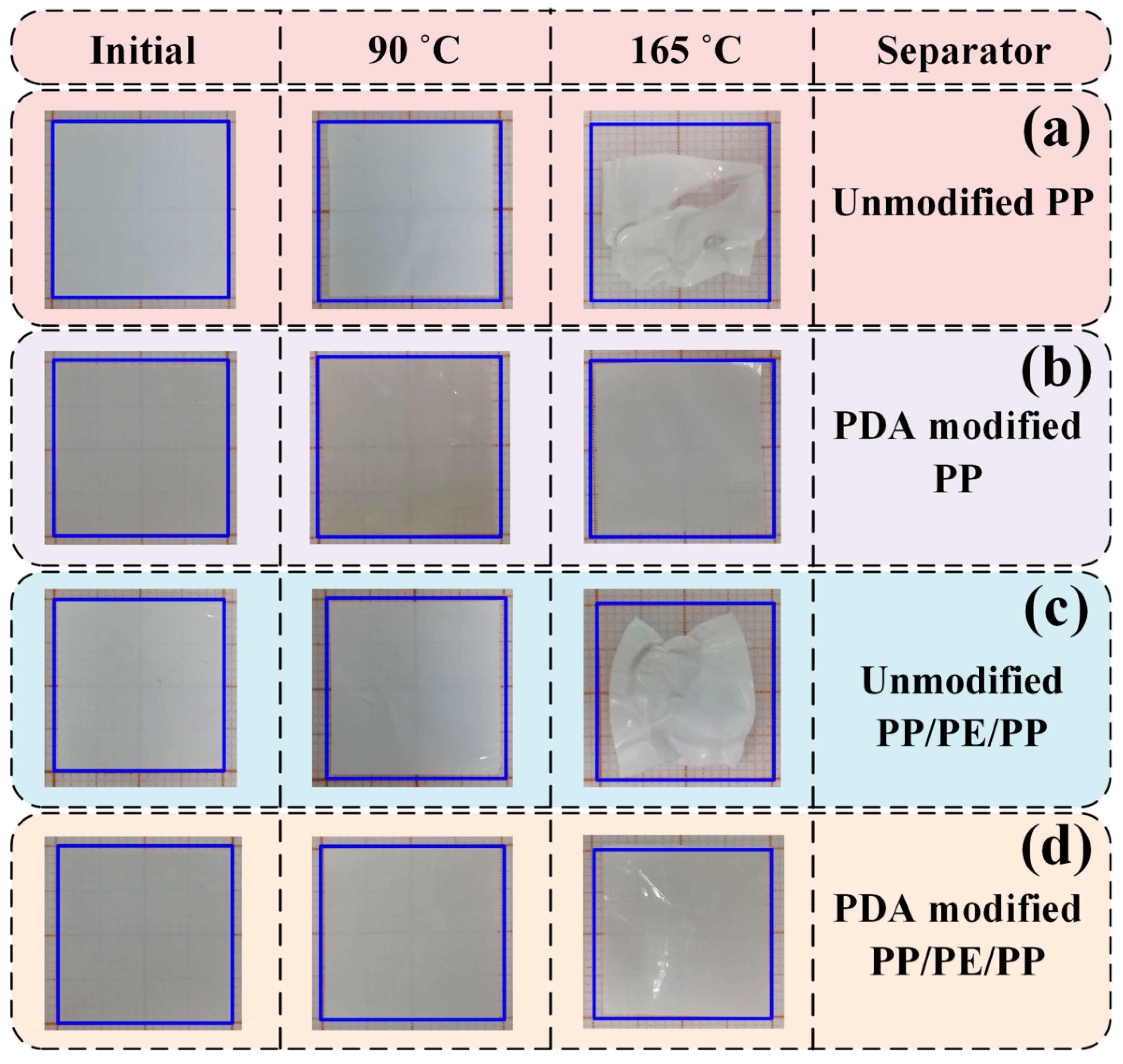

| Separators | Thickness (µm) | Permeability (s) | Porosity (%) | PP Pore Size (µm) | Tensile Strength (MPa) | Puncture Strength (N) | Thermal Stability (Shrinkage) 90°C/1h (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| TD | MD | TD | MD | ||||||
| Single-layer (PP) | 25 | 200 | 55 | 0.064 | 13.24 | 103.46 | >3.283 | 0 | <5 |
| Tri-layer (PP/PE/PP) | 25 | 620 | 39 | 0.028 | 14.71 | 166.71 | >3.724 | 0 | <5 |
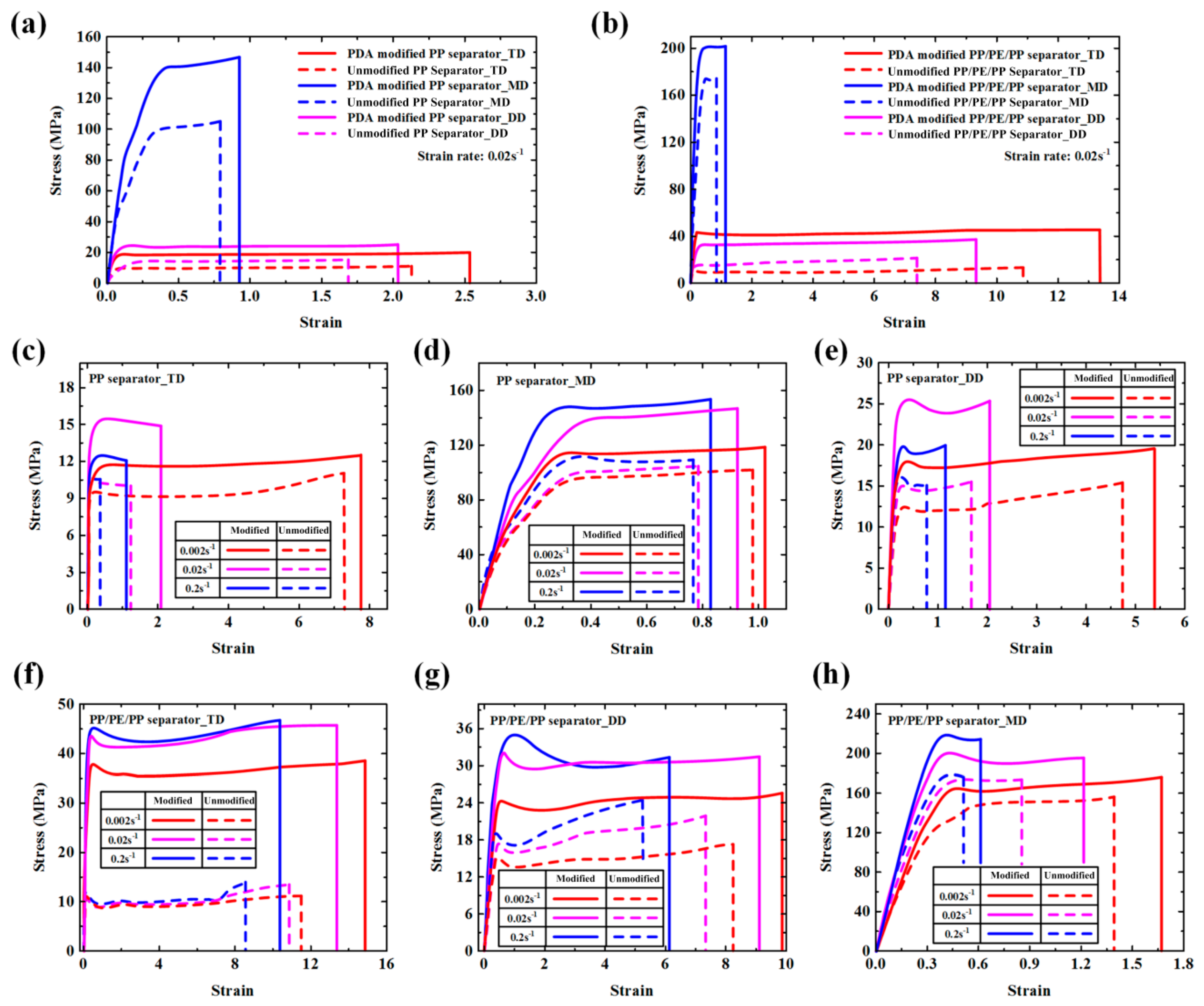
| Material Parameters | Yield Stress (MPa) | Failure Stress (MPa) | Failure Strain | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tension Direction | MD | TD | DD | MD | TD | DD | MD | TD | DD |
| Unmodified PP | 95.92 | 9.55 | 12.42 | 102.21 | 11.08 | 15.42 | 0.98 | 7.24 | 4.78 |
| PDA-modified PP | 114.40 | 11.69 | 17.97 | 118.33 | 12.57 | 19.54 | 1.02 | 7.72 | 5.41 |
| Unmodified PP/PE/PP | 147.69 | 11.22 | 15.17 | 156.38 | 11.22 | 17.52 | 1.40 | 11.47 | 8.24 |
| PDA-modified PP/PE/PP | 165.07 | 37.91 | 24.41 | 175.93 | 38.67 | 25.63 | 1.67 | 14.87 | 9.86 |
| Material Parameters | Yield Stress (MPa) | Failure Stress (MPa) | Failure Strain | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tension Direction | MD | TD | DD | MD | TD | DD | MD | TD | DD |
| Unmodified PP | 100.22 | 9.42 | 14.30 | 105.45 | 10.93 | 15.52 | 0.79 | 2.13 | 1.69 |
| PDA-modified PP | 140.74 | 18.85 | 24.79 | 147.03 | 19.97 | 25.61 | 0.93 | 2.54 | 2.03 |
| Unmodified PP/PE/PP | 174.83 | 10.98 | 15.10 | 174.83 | 13.27 | 21.97 | 0.86 | 10.86 | 7.39 |
| PDA-modified PP/PE/PP | 201.37 | 43.48 | 32.95 | 202.75 | 46.22 | 37.53 | 1.14 | 13.37 | 9.32 |
| Material Parameters | Yield Stress (MPa) | Failure Stress (MPa) | Failure Strain | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tension Direction | MD | TD | DD | MD | TD | DD | MD | TD | DD |
| Unmodified PP | 112.04 | 10.64 | 16.14 | 109.68 | 10.59 | 15.03 | 0.77 | 0.32 | 0.95 |
| PDA-modified PP | 148.21 | 12.50 | 19.87 | 153.71 | 12.06 | 20.00 | 0.83 | 1.29 | 1.30 |
| Unmodified PP/PE/PP | 178.12 | 12.31 | 19.09 | 178.84 | 13.83 | 24.76 | 0.52 | 8.58 | 5.26 |
| PDA-modified PP/PE/PP | 218.82 | 45.21 | 35.13 | 213.94 | 46.84 | 31.47 | 0.62 | 10.36 | 6.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, W.; Kong, D.; Xie, J.; Chen, Y.; Ding, J.; Wang, F.; Xu, T. Self-Polymerized Dopamine Nanoparticles Modified Separators for Improving Electrochemical Performance and Enhancing Mechanical Strength of Lithium-Ion Batteries. Polymers 2020, 12, 648. https://doi.org/10.3390/polym12030648
Hao W, Kong D, Xie J, Chen Y, Ding J, Wang F, Xu T. Self-Polymerized Dopamine Nanoparticles Modified Separators for Improving Electrochemical Performance and Enhancing Mechanical Strength of Lithium-Ion Batteries. Polymers. 2020; 12(3):648. https://doi.org/10.3390/polym12030648
Chicago/Turabian StyleHao, Wenqian, Dechong Kong, Jiamiao Xie, Yaping Chen, Jian Ding, Fenghui Wang, and Tingting Xu. 2020. "Self-Polymerized Dopamine Nanoparticles Modified Separators for Improving Electrochemical Performance and Enhancing Mechanical Strength of Lithium-Ion Batteries" Polymers 12, no. 3: 648. https://doi.org/10.3390/polym12030648
APA StyleHao, W., Kong, D., Xie, J., Chen, Y., Ding, J., Wang, F., & Xu, T. (2020). Self-Polymerized Dopamine Nanoparticles Modified Separators for Improving Electrochemical Performance and Enhancing Mechanical Strength of Lithium-Ion Batteries. Polymers, 12(3), 648. https://doi.org/10.3390/polym12030648