Gas Transport Phenomena and Polymer Dynamics in PHB/PLA Blend Films as Potential Packaging Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biopolymers Film Preparation
2.3. Scanning Electron Microscopy
2.4. Differential Scanning Calorimetry (DSC) Measurements
2.5. ESR Characterization
2.6. Barrier Properties Evaluation
2.7. Thickness Determination
2.8. Colour Characterization
3. Results and Discussion
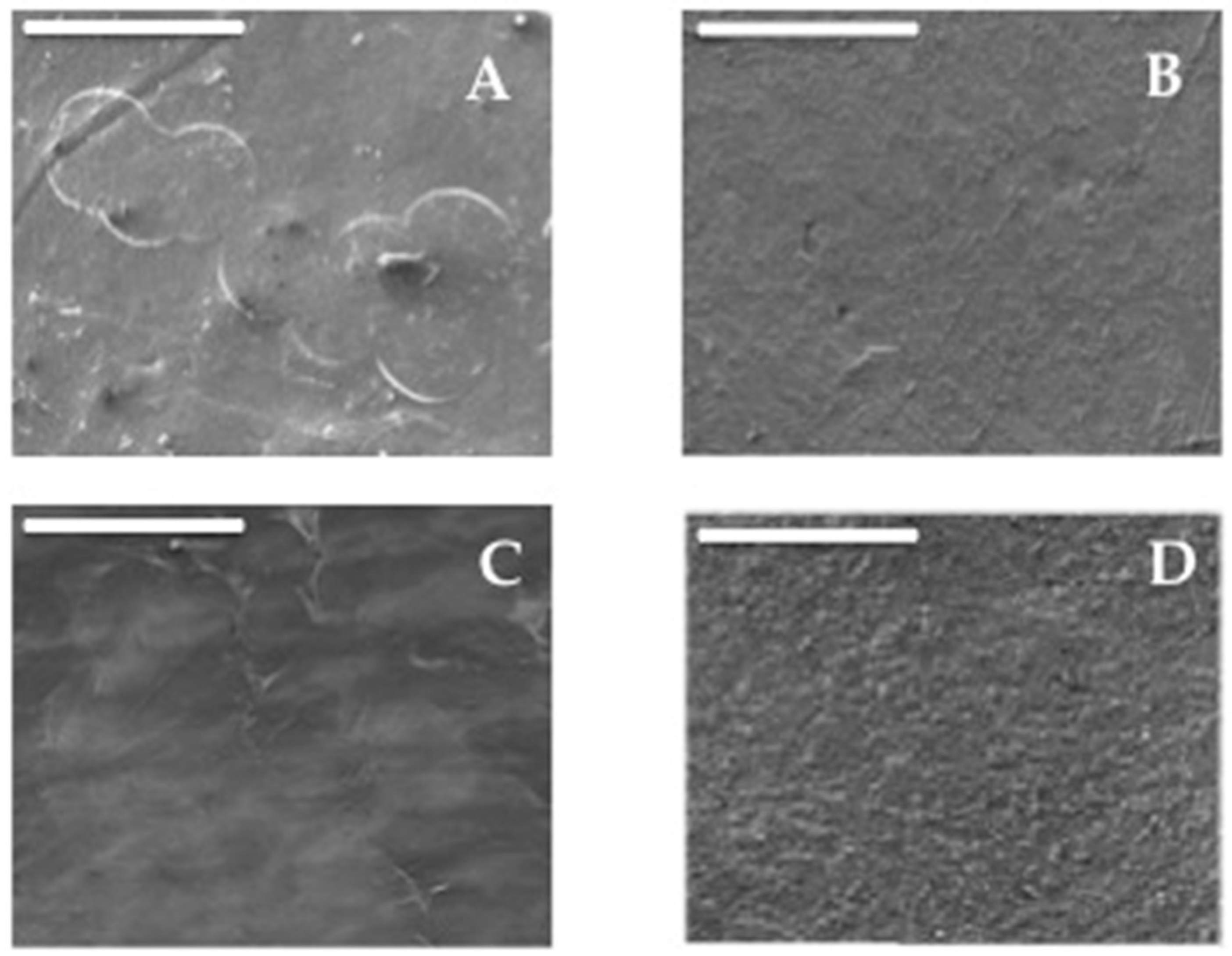
3.1. Scanning Electron Microscopy
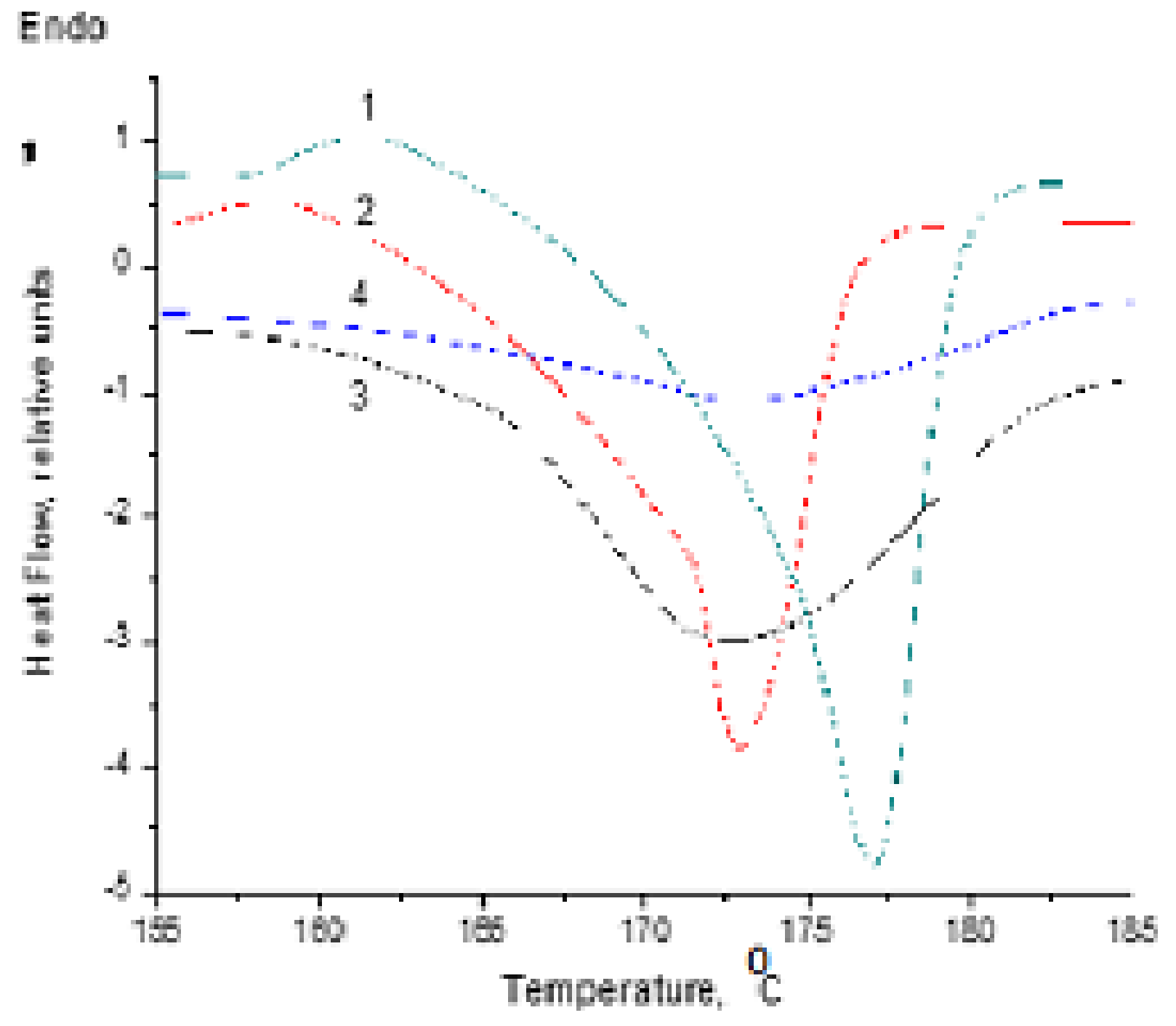
3.2. Thermal Characterization
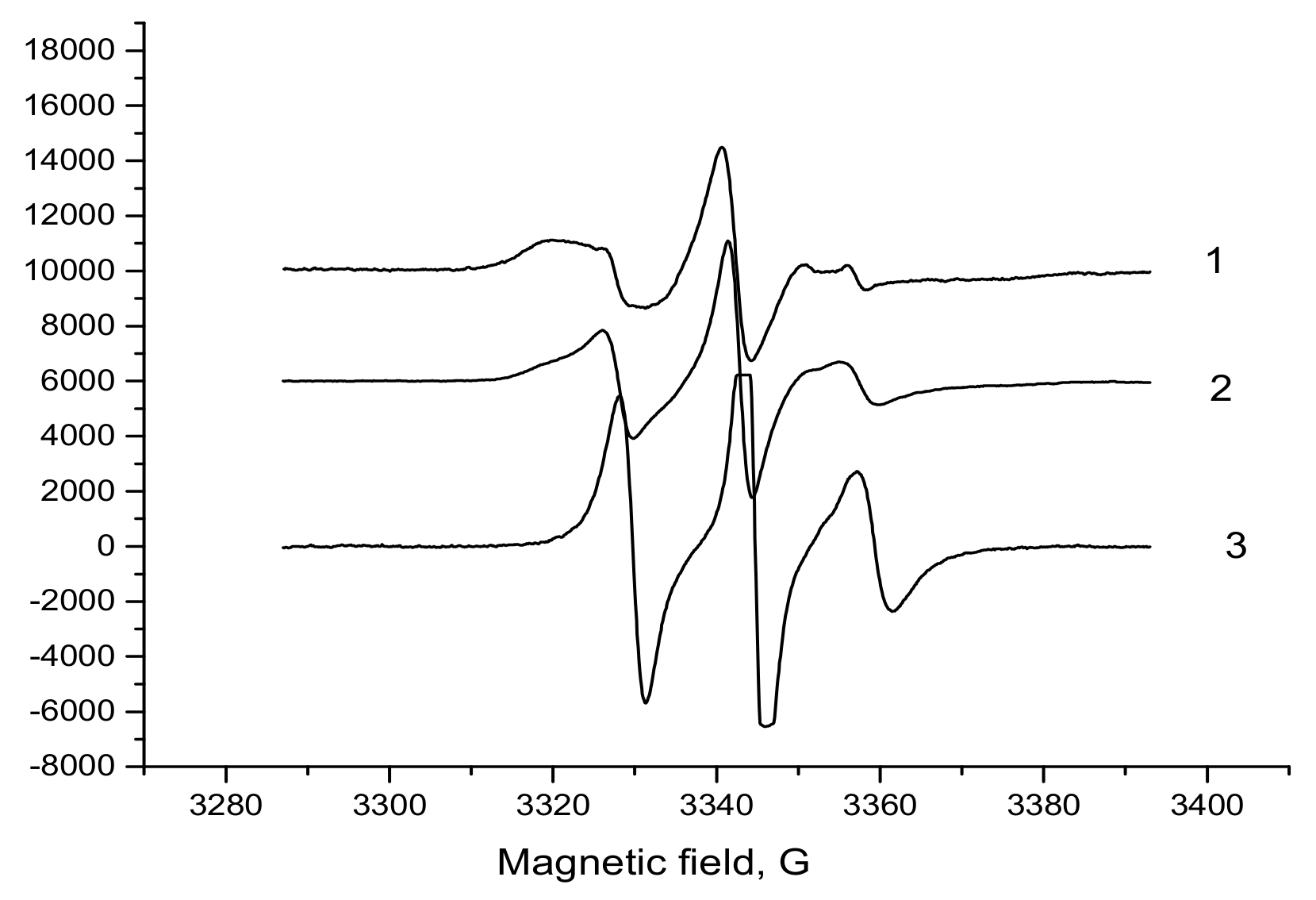
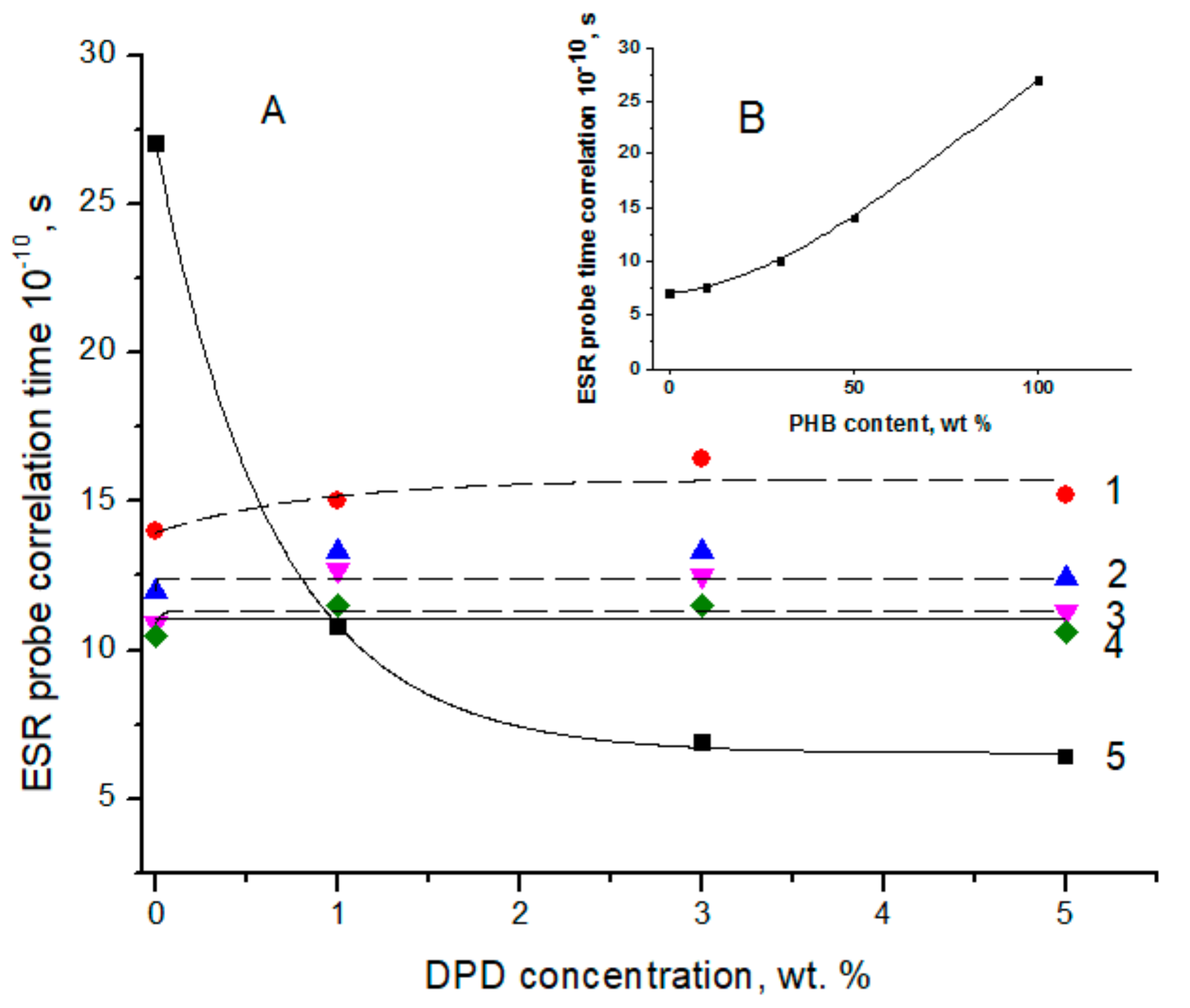
3.3. ESR Spectral Characterization of PHB/PLA Films
3.4. Gas permeability Characteristics
3.4.1. Isothermal Permeability
3.4.2. Temperature Dependency of Gas Permeability
3.5. Color Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sonchaeng, U.; Iñiguez-Franco, F.; Auras, R.; Selke, S.; Rubino, M.; Lim, L.-T. Poly(lactic acid) mass transfer properties. Prog. Polym. Sci. 2018, 86, 85–121. [Google Scholar] [CrossRef]
- Pankova, Y.; Shchegolikhin, A.; Iordanskii, A.; Zhulkina, A.; Ol’Khov, A.; Zaikov, G. The characterization of novel biodegradable blends based on polyhydroxybutyrate: The role of water transport. J. Mol. Liq. 2010, 156, 65–69. [Google Scholar] [CrossRef]
- Gårdebjer, S.; Larsson, M.; Gebäck, T.; Skepö, M.; Larsson, A. An overview of the transport of liquid molecules through structured polymer films, barriers and composites—Experiments correlated to structure-based simulations. Adv. Colloid Interface Sci. 2018, 256, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Larrañaga, A.; Pompanon, F.; Gruffat, N.; Palomares, T.; Alonso-Varona, A.; Varga, A.L.; Yagüe, M.F.; Biggs, M.; Sarasua, J.-R. Effects of isothermal crystallization on the mechanical properties of a elastomeric medium chain length polyhydroxyalkanoate. Eur. Polym. J. 2016, 85, 401–410. [Google Scholar] [CrossRef]
- Dinjaski, N.; Prieto, M.A. Smart polyhydroxyalkanoate nanobeads by protein based functionalization. Nanomed. Nanotechn. Biol. Med. 2015, 11, 885–899. [Google Scholar] [CrossRef]
- Han, J.; Xiong, L.; Jiang, X.; Yuan, X.; Zhao, Y.; Yang, D. Bio-functional electrospun nanomaterials: From topology design to biological applications. Prog. Polym. Sci. 2019, 91, 1–28. [Google Scholar] [CrossRef]
- Laycock, B.; Nikolic, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.E.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, A.; Ospina, A.A.; Rodríguez-Vélez, P.; Arana-Florez, R. What is the new about food packaging material? A bibliometric review during 1996–2016. Trends Food Sci. Technol. 2019, 85, 252–261. [Google Scholar] [CrossRef]
- Bradney, L.; Wijesekara, H.; Palansooriya, K.N.; Obadamudalige, N.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Kim, K.-H.; Kirkham, M. Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ. Int. 2019, 131, 104937. [Google Scholar] [CrossRef] [PubMed]
- Raddadi, N.; Fava, F. Biodegradation of oil-based plastics in the environment: Existing knowledge and needs of research and innovation. Sci. Total Environ. 2019, 679, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Fabra, M.J.; Sánchez, G.; López-Rubio, A.; Lagarón, J.M. Microbiological and ageing performance of polyhydroxyalkanoate-based multilayer structures of interest in food packaging. LWT 2014, 59, 760–767. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Rodriguez, B.; Castro-Mayorga, J.L.; Reis, M.A.; Sammon, C.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Preparation and Characterization of Electrospun Food Biopackaging Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Derived From Fruit Pulp Biowaste. Front. Sustain. Food Syst. 2018, 2, 38. [Google Scholar] [CrossRef]
- Ragaert, P.; Buntinx, M.; Maes, C.; Vanheusden, C.; Peeters, R.; Wang, S.; D’Hooge, D.R.; Cardon, L. Polyhydroxyalkanoates for Food Packaging Applications. In Reference Module in Food Science; Elsevier BV: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Sanchez-Garcia, M.D.; Gimenez, E.; Lagaron, J.M. Morphology and barrier properties of nanobiocomposites of PHB and layered silicates. J. Appl. Polym. Sci. 2008, 108, 2787–2801. [Google Scholar] [CrossRef]
- Miguel, O.; Fernández-Berridi, M.J.; Iruin, J. Survey on transport properties of liquids, vapors, and gases in biodegradable poly(3-hydroxybutyrate) (PHB). J. Appl. Polym. Sci. 1997, 64, 1849–1859. [Google Scholar] [CrossRef]
- Miguel, O.; Iruin, J.J. Water transport properties in poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biopolymers. J. Appl. Polym. Sci. 1999, 73, 455–468. [Google Scholar] [CrossRef]
- Wolf, C.; Angellier-Coussy, H.; Gontard, N.; Doghieri, F.; Guillard, V. How the shape of fillers affects the barrier properties of polymer/non-porous particles nanocomposites: A review. J. Membr. Sci. 2018, 556, 393–418. [Google Scholar] [CrossRef]
- Figoli, F.G.A.A.; Briceno, K.; Marino, T.; De Gisi, S.; Christensen, K.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018, 564, 562–586. [Google Scholar]
- Sanchez-Garcia, M.D.; López-Rubio, A.; Lagaron, J.M. Natural micro and nanobiocomposites with enhanced barrier properties and novel functionalities for food biopackaging applications. Trends Food Sci. Technol. 2010, 21, 528–536. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Courgneau, C.; Vitrac, O.; Ducruet, V.; Riquet, A.-M. Local demixion in plasticized polylactide probed by electron spin resonance. J. Magn. Reson. 2013, 233, 37–48. [Google Scholar] [CrossRef]
- Bor, Y.; Alin, J.; Hakkarainen, M. Electrospray Ionization-Mass Spectrometry Analysis Reveals Migration of Cyclic Lactide Oligomers from Polylactide Packaging in Contact with Ethanolic Food Simulant. Packag. Technol. Sci. 2012, 25, 427–433. [Google Scholar] [CrossRef]
- Yu, I.; Ebrahimi, T.; Hatzikiriakos, S.G.; Mehrkhodavandi, P. Star-shaped PHB–PLA block copolymers: Immortal polymerization with dinuclear indium catalysts. Dalton Trans. 2015, 44, 14248–14254. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.C.C.; Muiruri, J.; Tan, B.H.; Thitsartarn, W.; Kong, J.; Zhang, X.; Li, Z.; He, C. Biodegradable PHB-Rubber Copolymer Toughened PLA Green Composites with Ultrahigh Extensibility. ACS Sustain. Chem. Eng. 2018, 6, 15517–15527. [Google Scholar] [CrossRef]
- Simmons, H.; Tiwary, P.; Colwell, J.E.; Kontopoulou, M. Improvements in the crystallinity and mechanical properties of PLA by nucleation and annealing. Polym. Degrad. Stab. 2019, 166, 248–257. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López-Martínez, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Wang, Y. Development of PLA-PHB-based biodegradable active packaging and its application to salmon. Packag. Technol. Sci. 2018, 31, 739–746. [Google Scholar] [CrossRef]
- Siracusa, V.; Rosa, M.D.; Iordanskii, A. Performance of Poly(lactic acid) Surface Modified Films for Food Packaging Application. Materials 2017, 10, 850. [Google Scholar] [CrossRef]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Permeability in Clay/Polyesters Nano-Biocomposites. In Green Energy and Technology; Springer Science and Business Media LLC: Amsterdam, The Netherlands, 2012; pp. 237–264. [Google Scholar]
- Vieira, M.G.A.; Da Silva, M.A.; Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Lopez, J.; López, D.; Kenny, J.M.; Peponi, L. Effect of chitosan and catechin addition on the structural, thermal, mechanical and disintegration properties of plasticized electrospun PLA-PHB biocomposites. Polym. Degrad. Stab. 2016, 132, 145–156. [Google Scholar] [CrossRef]
- Mlalila, N.; Hilonga, A.; Swai, H.; Devlieghere, F.; Ragaert, P. Antimicrobial packaging based on starch, poly(3-hydroxybutyrate) and poly(lactic-co-glycolide) materials and application challenges. Trends Food Sci. Technol. 2018, 74, 1–11. [Google Scholar] [CrossRef]
- Zembouai, I.; Bruzaud, S.; Kaci, M.; Benhamida, A.; Corre, Y.M.; Grohens, Y.; Taguet, A.; Lopez-Cuesta, J.-M. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/polylactide blends: Thermal stability, flammability and thermo-mechanical behavior. J. Polym. Environ. 2014, 22, 131–139. [Google Scholar] [CrossRef]
- D’Amico, D.; Montes, M.I.; Manfredi, L.B.; Cyras, V.P. Fully bio-based and biodegradable polylactic acid/poly(3-hydroxybutirate) blends: Use of a common plasticizer as performance improvement strategy. Polym. Test. 2016, 49, 22–28. [Google Scholar] [CrossRef]
- Bartczak, Z.; Galeski, A.; Kowalczuk, M.; Sobota, M.; Malinowski, R. Tough blends of poly(lactide) and amorphous poly([R,S]-3-hydroxy butyrate)—Morphology and properties. Eur. Polym. J. 2013, 49, 3630–3641. [Google Scholar] [CrossRef]
- Li, L.; Huang, W.; Wang, B.; Wei, W.; Gu, Q.; Chen, P. Properties and structure of polylactide/poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PLA/PHBV) blend fibers. Polymer 2015, 68, 183–194. [Google Scholar] [CrossRef]
- Dawidziuk, K.A. Peroxide-initiated Modification of Polylactic acid (PLA) and Poly(3-hydroxyalkanoates) (PHAs) in the Presence of Allylic and Acrylic Coagents. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2018. [Google Scholar]
- Dawin, T.P.; Ahmadi, Z.; Taromi, F.A. Biocompatible PLA/PHB coatings obtained from controlled solid state polymerization. Prog. Org. Coat. 2019, 132, 41–49. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Aldureid, A.; Cabedo, L.; Lagaron, J.M. Reactive Melt Mixing of Poly(3-Hydroxybutyrate)/Rice Husk Flour Composites with Purified Biosustainably Produced Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate). Materials 2019, 12, 2152–2173. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López-Martínez, J.; López, D.; Kenny, J.M.; Peponi, L. Development of flexible materials based on plasticized electrospun PLA–PHB blends: Structural, thermal, mechanical and disintegration properties. Eur. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Hoppe, M.; de Voogt, P.; Franz, R. Identification and quantification of oligomers as potential migrants in plastics food contact materials with a focus in polycondensates—A review. Trends Food Sci. Technol. 2016, 50, 118–130. [Google Scholar] [CrossRef]
- Rahman, M.; Brazel, C.S. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, C.; Deng, X. Miscibility, crystallization and morphology of poly(β-hydroxybutyrate) / poly(d,l-lactide) blends. Polymer 1996, 37, 235–241. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N. Blending polylactic acid with polyhydroxybutyrate: The effect on thermal, mechanical, and biodegradation properties. Adv. Polym. Technol. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López-Martínez, J.; Kenny, J.M. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Shogren, R. Water vapor permeability of biodegradable polymers. J. Polym. Environ. 1997, 5, 91–95. [Google Scholar] [CrossRef]
- Razavi, S.M.; Dadbin, S.; Frounchi, M. Oxygen barrier properties of PLA-PVAcAl blends as biodegradable films. Special Issue: Biopolymers and Renewably Sourced Polymers. J. Appl. Polym. Sci. 2012, 125, E20–E26. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, Q.-Y.; Yang, H.-T.; Wang, J.-J.; Li, X.-B. Understanding the oxygen barrier property of highly transparent poly(lactic acid)/benzoxazine composite film by analyzing the UV-shielding performance. J. Appl. Polym. Sci. 2019, 136, 47510. [Google Scholar] [CrossRef]
- Siracusa, V.; Ingrao, C.; Karpova, S.G.; Olkhov, A.A.; Iordanskii, A. Gas transport and characterization of poly(3 hydroxybutyrate) films. Eur. Polym. J. 2017, 91, 149–161. [Google Scholar] [CrossRef]
- Budil, D.; Lee, S.; Saxena, S.; Freed, J.H. Nonlinear-Least-Squares Analysis of Slow-Motion EPR Spectra in One and Two Dimensions Using a Modified Levenberg–Marquardt Algorithm. J. Magn. Reson. Ser. A 1996, 120, 155–189. [Google Scholar] [CrossRef]
- Timofeev, V.P.; Misharin, A.Y.; Tkachev, Y.V. Simulation of EPR spectra of the radical TEMPO in water-lipid systems in different microwave ranges. Biophysics 2011, 56, 407–417. [Google Scholar] [CrossRef]
- Buchachenko, A.L.; Vasserman, A.M. Stable Radicals; Khimiya: Moscow, Russia, 1973. [Google Scholar]
- Siracusa, V.; Ingrao, C. Correlation amongst gas barrier behaviour, temperature and thickness in BOPP films for food packaging usage: A lab-scale testing experience. Polym. Test. 2017, 59, 277–289. [Google Scholar] [CrossRef]
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables: A Review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I.; Romani, S.; Tylewicz, U.; Rocculi, P.; Rosa, M.D. Poly(lactic acid)-modified films for food packaging application: Physical, mechanical, and barrier behavior. J. Appl. Polym. Sci. 2012, 125, e390–e401. [Google Scholar] [CrossRef]
- Mrkić, S.; Galić, K.; Ivankovic, M.; Hamin, S.; Ciković, N. Gas transport and thermal characterization of mono- and di-polyethylene films used for food packaging. J. Appl. Polym. Sci. 2005, 99, 1590–1599. [Google Scholar] [CrossRef]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Syahidad, K.; Rosnah, S.; Noranizan, M.A.; Zaulia, O.; Anvarjon, A. Quality changes of fresh cut cantaloupe (Cucumis melo L. var Reticulatus cv. Glamour) in different types of polypropylene packaging. Int. Food Res. J. 2015, 22, 753–760. [Google Scholar]
- Genovese, L.; Soccio, M.; Gigli, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. Gas permeability, mechanical behaviour and compostability of fully-aliphatic bio-based multiblock poly(ester urethane)s. RSC Adv. 2016, 6, 55331–55342. [Google Scholar] [CrossRef]
- Righetti, M.C.; Gazzano, M.; Di Lorenzo, M.L.; Androsch, R. Enthalpy of melting of α- and β-crystals of poly(L-lactic acid. Eur. Polym. J. 2015, 70, 215–220. [Google Scholar] [CrossRef]
- Lin, K.-W.; Lan, C.-H.; Sun, Y.-M. Poly[(R)3-hydroxybutyrate] (PHB)/poly(L-lactic acid) (PLLA) blends with poly(PHB/PLLA urethane) as a compatibilizer. Polym. Degr. Stab. 2016, 134, 30–40. [Google Scholar] [CrossRef]
- Cavalcante, M.P.; Toledo, A.L.M.M.; Rodrigues, E.; Neto, R.C.; Tavares, M.I. Correlation between traditional techniques and TD-NMR to determine the morphology of PHB/PCL blends. Polym. Test. 2017, 58, 159–165. [Google Scholar] [CrossRef]
- Qiu, Z.; Yang, W.; Ikehara, T.; Nishi, T. Miscibility and crystallization behavior of biodegradable blends of two aliphatic polyesters. Poly(3-hydroxybutyrate-co-hydroxyvalerate) and poly(ε-caprolactone. Polymer 2005, 46, 11814–11819. [Google Scholar] [CrossRef]
- Lovera, D.; Marquez, L.; Balsamo, V.; Taddei, A.; Castelli, C.; Müller, A.J. Crystallization, Morphology, and Enzymatic Degradation of Polyhydroxybutyrate/Polycaprolactone (PHB/PCL) Blends. Macromol. Chem. Phys. 2007, 208, 924–937. [Google Scholar] [CrossRef]
- Veksli, Z.; Andreis, M.; Rakvin, B. ESR spectroscopy for the study of polymer heterogeneity. Prog. Polym. Sci. 2000, 25, 949–986. [Google Scholar] [CrossRef]
- Kanemoto, K.; Mizutani, N.; Muramatsu, K.; Hashimoto, H.; Baba, M.; Yamauchi, J. ESR investigations on doped conjugated polymers diluted in a solid matrix. Chem. Phys. Lett. 2010, 494, 41–44. [Google Scholar] [CrossRef]
- Bartoš, J.; Švajdlenková, H. On the mutual relationships between spin probe mobility, free volume and relaxation dynamics in organic glass-formers: Glycerol. Chem. Phys. Lett. 2017, 670, 58–63. [Google Scholar] [CrossRef]
- Cao, K.; Liu, Y.; Olkhov, A.; Siracusa, V.; Iordanskii, A.L. PLLA-PHB fiber membranes obtained by solvent-free electrospinning for short-time drug delivery. Drug Deliv. Transl. Res. 2017, 8, 291–302. [Google Scholar] [CrossRef]
- Baudet, C.; Grandidier, J.-C.; Cangémi, L. A two-phase model for the diffuson-mechanical behaviour of semicrystalline polymers in gaseous environment. Int. J. Sol. Struc. 2009, 46, 1389–1401. [Google Scholar] [CrossRef][Green Version]
- Shimada, S. ESR studies on molecular motion and chemical reactions in solid polymers in relation to structure. Prog. Polym. Sci. 1992, 17, 1045–1106. [Google Scholar] [CrossRef]
- Ma, Q.; Pyda, M.; Mao, B.; Cebe, P. Relationship between the rigid amorphous phase and mesophase in electrospun fibers. Polymer 2013, 54, 2544–2554. [Google Scholar] [CrossRef]
- Iordanskii, A.; Karpova, S.; Olkhov, A.; Borovikov, P.; Kildeeva, N.; Liu, Y. Structure-morphology impact upon segmental dynamics and diffusion in the biodegradable ultrafine fibers of polyhydroxybutyrate-polylactide blends. Eur. Polym. J. 2019, 117, 208–216. [Google Scholar] [CrossRef]
- Švajdlenková, H.; Arrese-Igor, S.; Nógellová, Z.; Alegria, A.; Bartos, J. Molecular dynamic heterogeneity in relation to free volume and relaxation dynamics in organic glass-formers: Oligomeric cis-1,4-poly(isoprene. Phys. Chem. Chem. Phys. 2017, 19, 15215–15226. [Google Scholar] [CrossRef] [PubMed]
- Barashkova, I.I.; Bermeshev, M.V.; Wasserman, A.M.; Yampolskii, Y.P. Mobility of the spin probe TEMPO in glassy metathesis polymers with substituents containing flexible Si-O-Si groups. Polym. Sci. Ser. A 2015, 57, 279–284. [Google Scholar] [CrossRef]
- Kurzbach, D.; Junk, M.J.N.; Hinderberger, D. Nanoscale Inhomogeneities in Thermoresponsive Polymers. Macromol. Rap. Com. 2013, 34, 119–134. [Google Scholar] [CrossRef]
- Kusumoto, N.; Sano, S.; Zaitsu, N.; Motozato, Y. Molecular motions and segmental size of vulcanized natural and acrylonitrile-butadiene rubbers by the spin-probe method. Polymer 1976, 17, 448–454. [Google Scholar] [CrossRef]
- Bullock, A.T.; Cameron, G.G.; Miles, I.S. Segment size in synthetic polymers by the spin-probe method. Polymer 1982, 23, 1536–1539. [Google Scholar] [CrossRef]
- Bueche, F. Viscosity, Self-diffusion, and Allied Effects in Solid Polymers. J. Chem. Phys. 1952, 20, 1959. [Google Scholar] [CrossRef]
- Siracusa, V.; Genovese, L.; Ingrao, C.; Munari, A.; Lotti, N. Barrier Properties of Poly(Propylene Cyclohexanedicarboxylate) Random Eco-Friendly Copolyesters. Polymer 2018, 10, 502. [Google Scholar] [CrossRef]
- Hannesschläger, C.; Horner, A.; Pohl, P. Intrinsic Membrane Permeability to Small Molecules. Chem. Rev. 2019, 119, 5922–5953. [Google Scholar] [CrossRef] [PubMed]
- Craster, B.; Jones, T.G. Permeation of a Range of Species through Polymer Layers under Varying Conditions of Temperature and Pressure: In Situ Measurement Methods. Polymer 2019, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Aitken, C.L.; Koros, W.J.; Paul, D.R. Effect of structural symmetry on gas transport properties of polysulfones. Macromolecules 1992, 25, 3424–3434. [Google Scholar] [CrossRef]
- Costello, L.M.; Koros, W.J. Temperature dependence of gas sorption and transport properties in polymers: Measurement and applications. Ind. Eng. Chem. Res. 1992, 31, 2708–2714. [Google Scholar] [CrossRef]
- Matteucci, S.; Yampolskii, Y.; Freeman, B.D.; Pinnau, I. Transport of Gases and Vapors in Glassy and Rubbery Polymers; Wiley: Hoboken, NJ, USA, 2006; pp. 1–47. [Google Scholar]
- De Oliveira, M.J. Equilibrium Thermodynamics; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Lock, S.; Keong, L.K.; Mash’Al, A.-A.B.; Shariff, A.M.; Yeong, Y.F.; Mei, I.L.S.; Ahmad, F. Elucidation on the Effect of Operating Temperature to the Transport Properties of Polymeric Membrane Using Molecular Simulation Tool. In E-Business and Telecommunication Networks; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017; Volume 752, pp. 456–471. [Google Scholar]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]

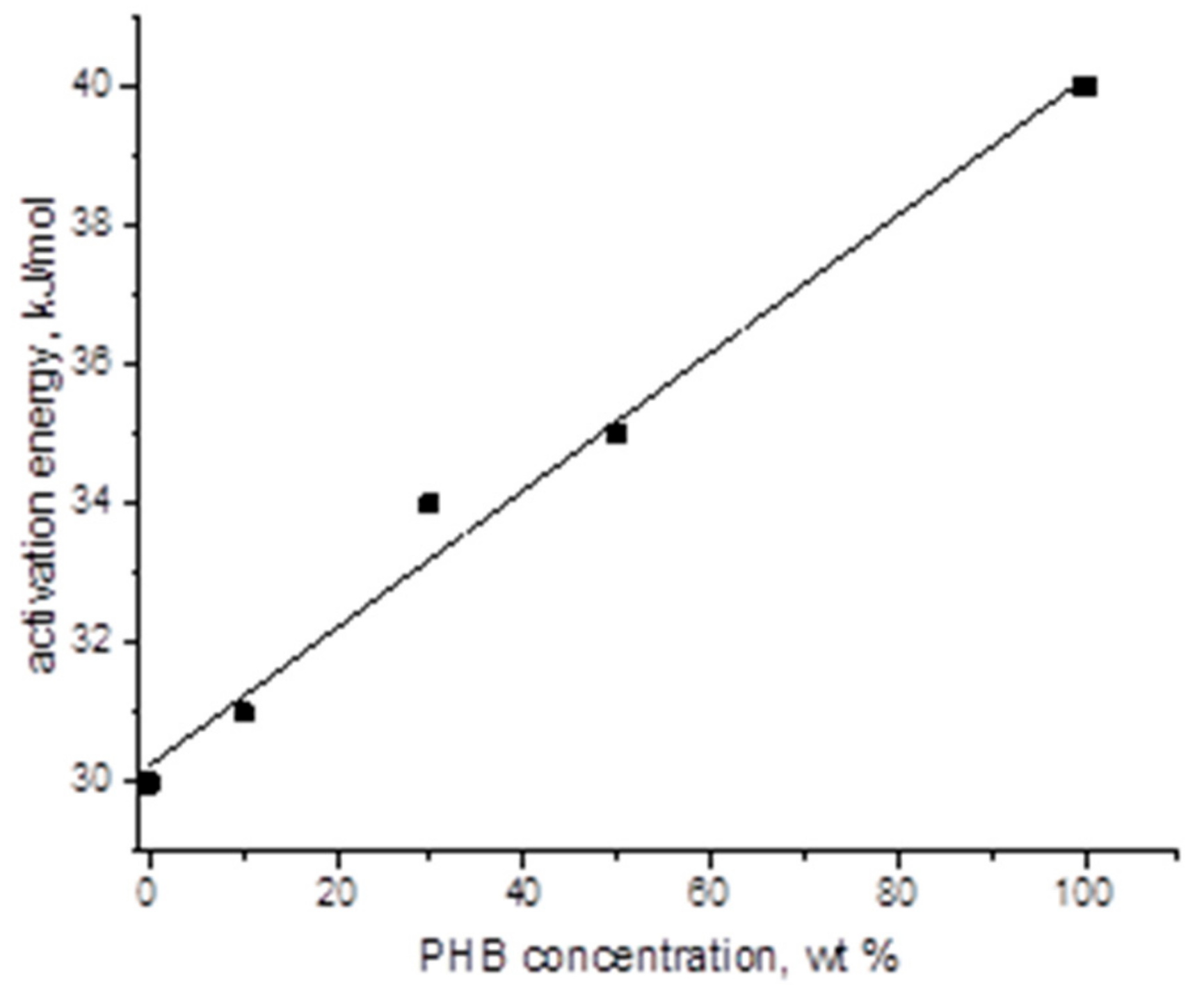
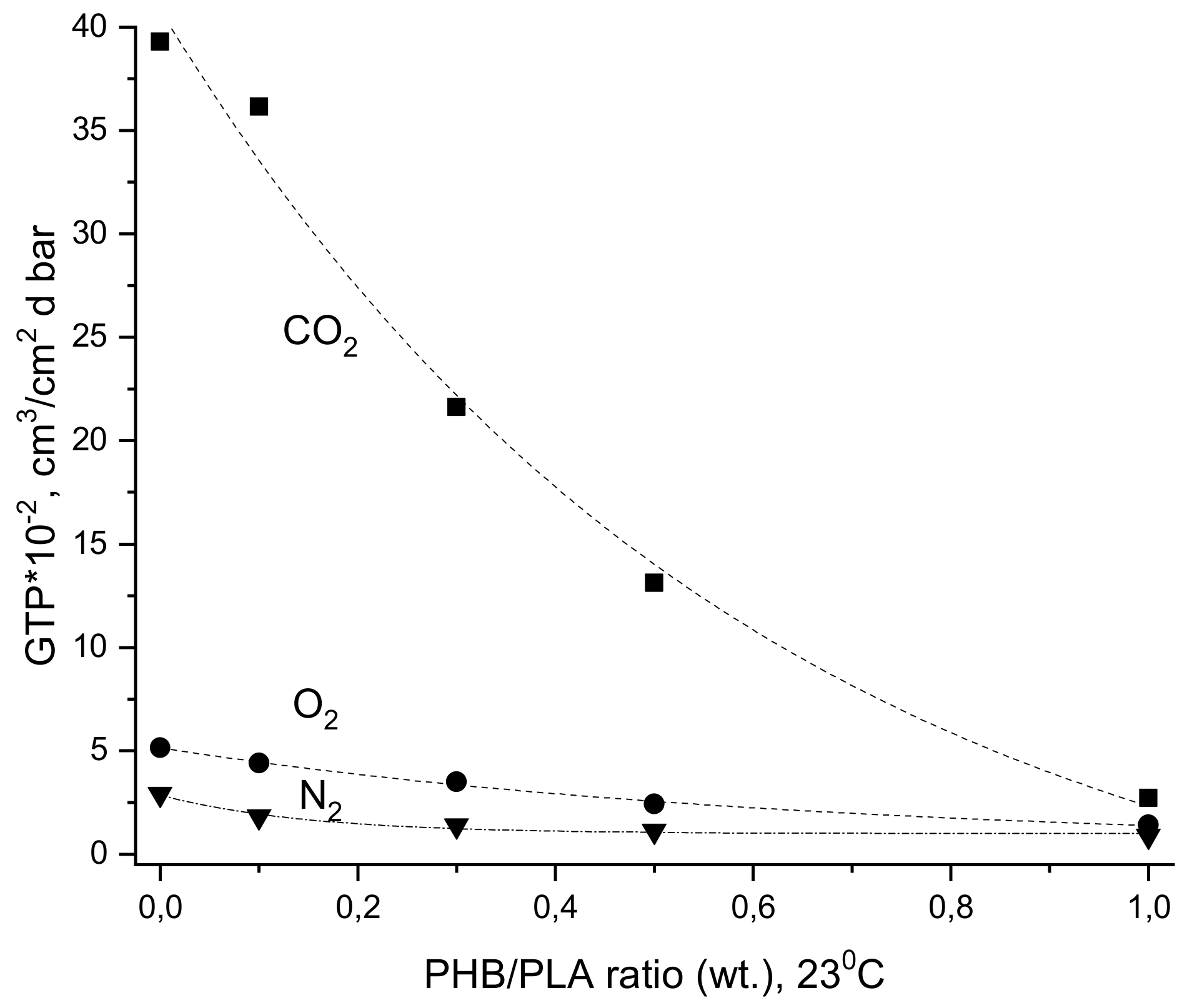

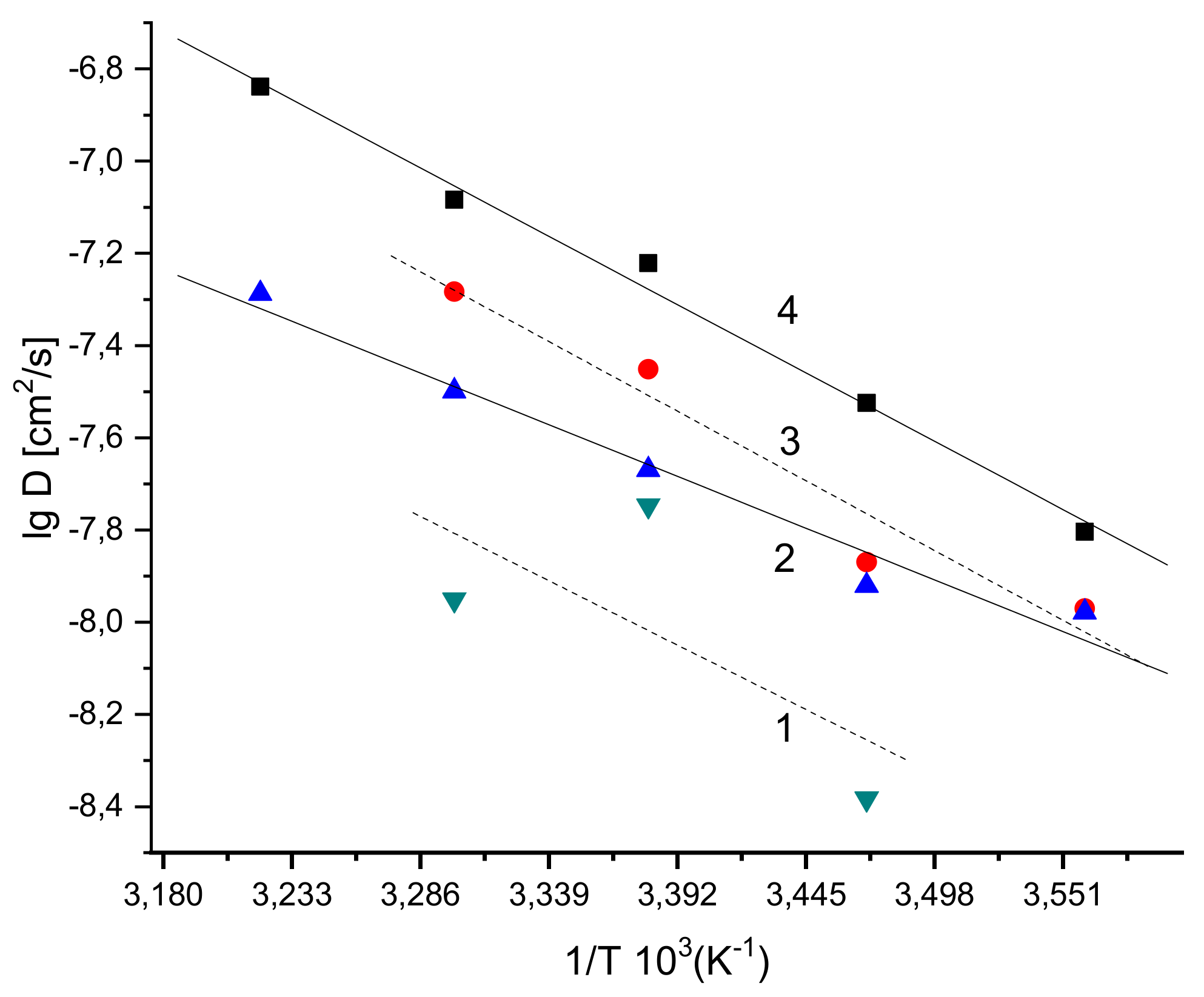
| Sample PHB/PLA (w/w) | Tg (°C) | Tcc (°C) | Tm (°C) | ΔHcc (J/g) | ΔHm (J/g) | Χc* (%) |
|---|---|---|---|---|---|---|
| 1/0 | - | 177 | 93.0 | 64 | ||
| 3/7 | 40.0 | 69.9 | 173 | 2.3 | 30.7 | 50 |
| 5/5 | 39.5 | 67.8 | 172 | 2.6 | 38.2 | 49 |
| 0/1 | 43.5 | - | 172.5 | - | 54.8 | 51 |
| PHB/PLA sample (w/w) | EP (kJ/mol) | ED (kJ/mol) | ΔHS (KJ/mol) |
|---|---|---|---|
| 1/0 | 22.8 ± 4.4 | 50.6 ± 4.5 | −27.8 ± 4.5 |
| 3/7 | 41.4 ± 2.4 | 54.4 ± 4.9 | −13.1 ± 3.5 |
| 5/5 | 32.5 ± 1.5 | 40.6 ± 4.2 | −8.1 ± 3.8 |
| 0/1 | 24.9 ± 4.5 | 53.6 ± 3.0 | −28.7 ± 3.7 |
| PHB/PLA Sample | L* | a* | b* | ΔE | C* | hab |
|---|---|---|---|---|---|---|
| White standard | 67.16 ± 0.02 | −0.69 ± 0.01 | 1.25 ± 0.01 | - | 0.93 | 138 |
| 1/0 (PHB) | 34.49 ± 0.67 | −0.24 ± 0.03 | −1.69 ± 0.13 | 32.70 | 1.71 | 98 |
| 1/9 | 35.60 ± 0.01 | −0.43 ± 0.01 | −1.10 ± 0.01 | 31.61 | 1.18 | 111 |
| 3/7 | 35.02 ± 0.04 | −0.36 ± 0.01 | −1.96 ± 0.01 | 32.24 | 1.99 | 100 |
| 5/5 | 34.31 ± 0.02 | −0.34 ± 0.02 | −1.77 ± 0.01 | 32.94 | 1.80 | 101 |
| 0/1 (PLA) | 33.66 ± 0.31 | −0.67 ± 0.01 | −3.14 ± 0.01 | 33.71 | 3.21 | 102 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siracusa, V.; Karpova, S.; Olkhov, A.; Zhulkina, A.; Kosenko, R.; Iordanskii, A. Gas Transport Phenomena and Polymer Dynamics in PHB/PLA Blend Films as Potential Packaging Materials. Polymers 2020, 12, 647. https://doi.org/10.3390/polym12030647
Siracusa V, Karpova S, Olkhov A, Zhulkina A, Kosenko R, Iordanskii A. Gas Transport Phenomena and Polymer Dynamics in PHB/PLA Blend Films as Potential Packaging Materials. Polymers. 2020; 12(3):647. https://doi.org/10.3390/polym12030647
Chicago/Turabian StyleSiracusa, Valentina, Svetlana Karpova, Anatoliy Olkhov, Anna Zhulkina, Regina Kosenko, and Alexey Iordanskii. 2020. "Gas Transport Phenomena and Polymer Dynamics in PHB/PLA Blend Films as Potential Packaging Materials" Polymers 12, no. 3: 647. https://doi.org/10.3390/polym12030647
APA StyleSiracusa, V., Karpova, S., Olkhov, A., Zhulkina, A., Kosenko, R., & Iordanskii, A. (2020). Gas Transport Phenomena and Polymer Dynamics in PHB/PLA Blend Films as Potential Packaging Materials. Polymers, 12(3), 647. https://doi.org/10.3390/polym12030647








