The Mechanical Properties, Secondary Structure, and Osteogenic Activity of Photopolymerized Fibroin
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Isolation of Fb
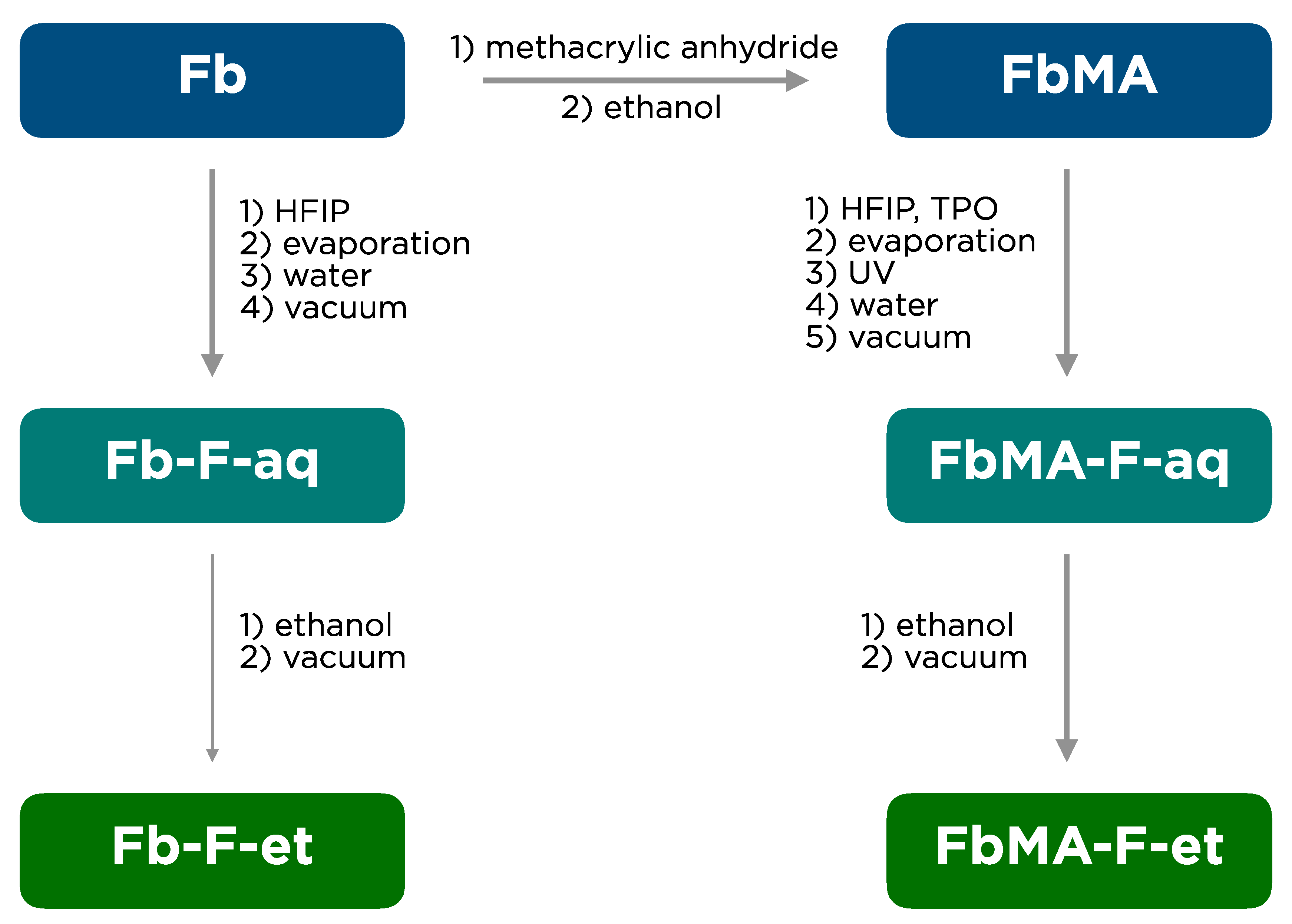
2.3. Synthesis of FbMA
2.4. Preparation of Fb-HFIP Solution
2.5. Preparation of FbMA-HFIP Solution
2.6. Film Fabrication
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Mechanical Tests
2.9. Atomic Force Microscopy (AFM)
2.10. Culturing Human MG63 Osteoblast-Like Cells on Fb Substrates
2.11. Cell Viability Tests
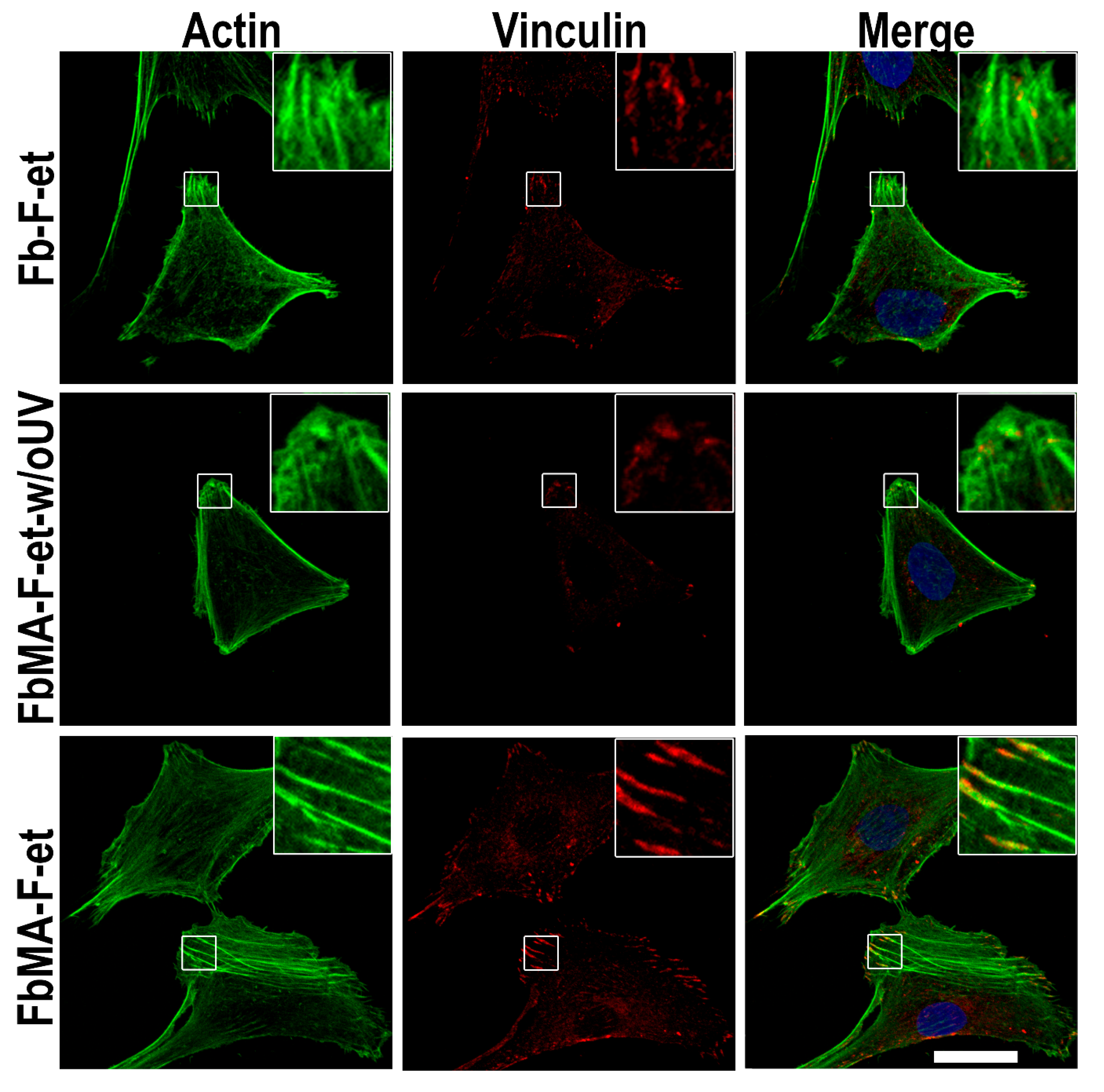
2.12. Cytoskeleton Morphology
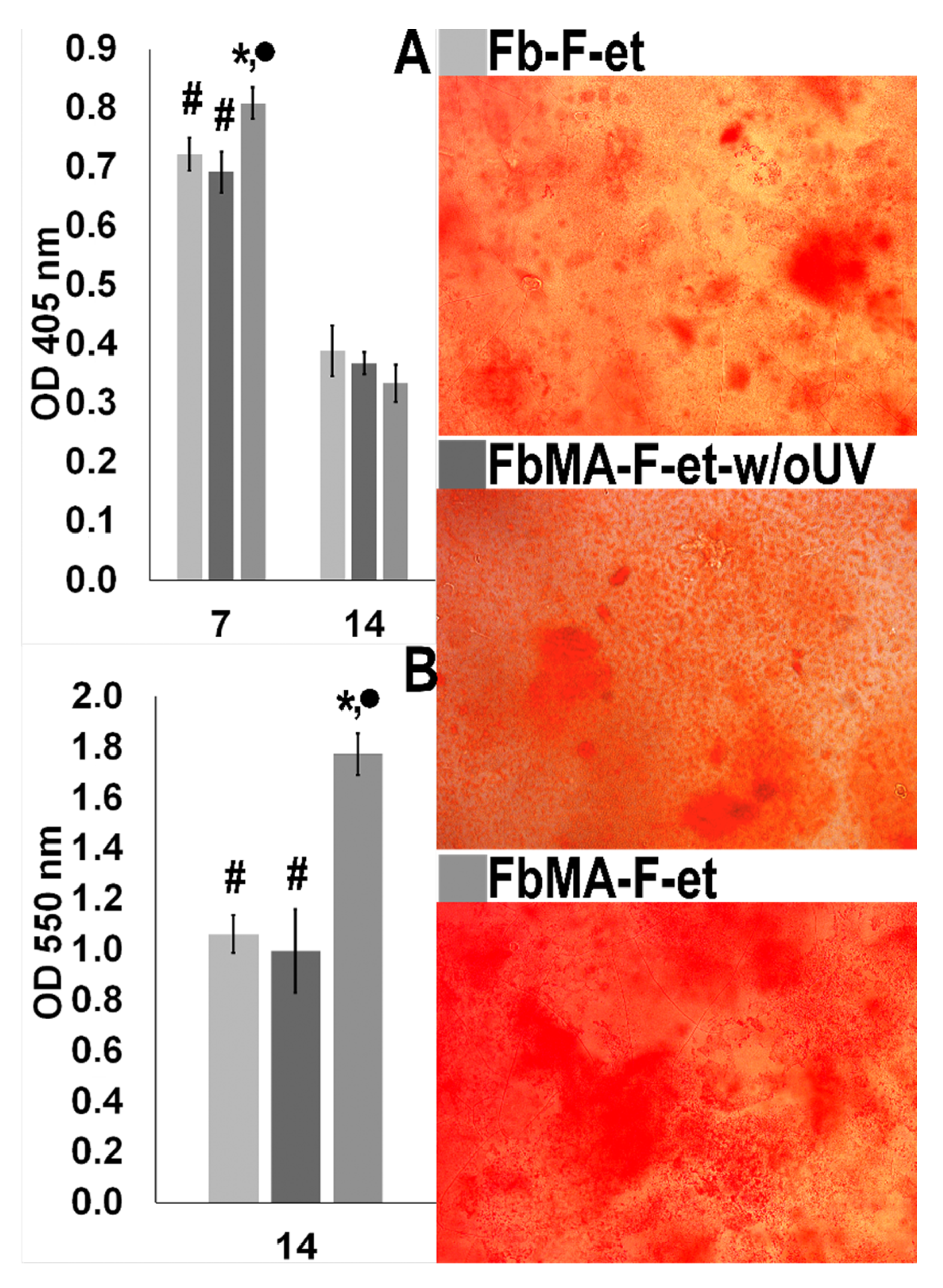
2.13. Activity of Alkaline Phosphatase (ALP)
2.14. Deposition of Calcium Phosphate by MG63 Cells on Fb Scaffolds
3. Results
3.1. Film Fabrication
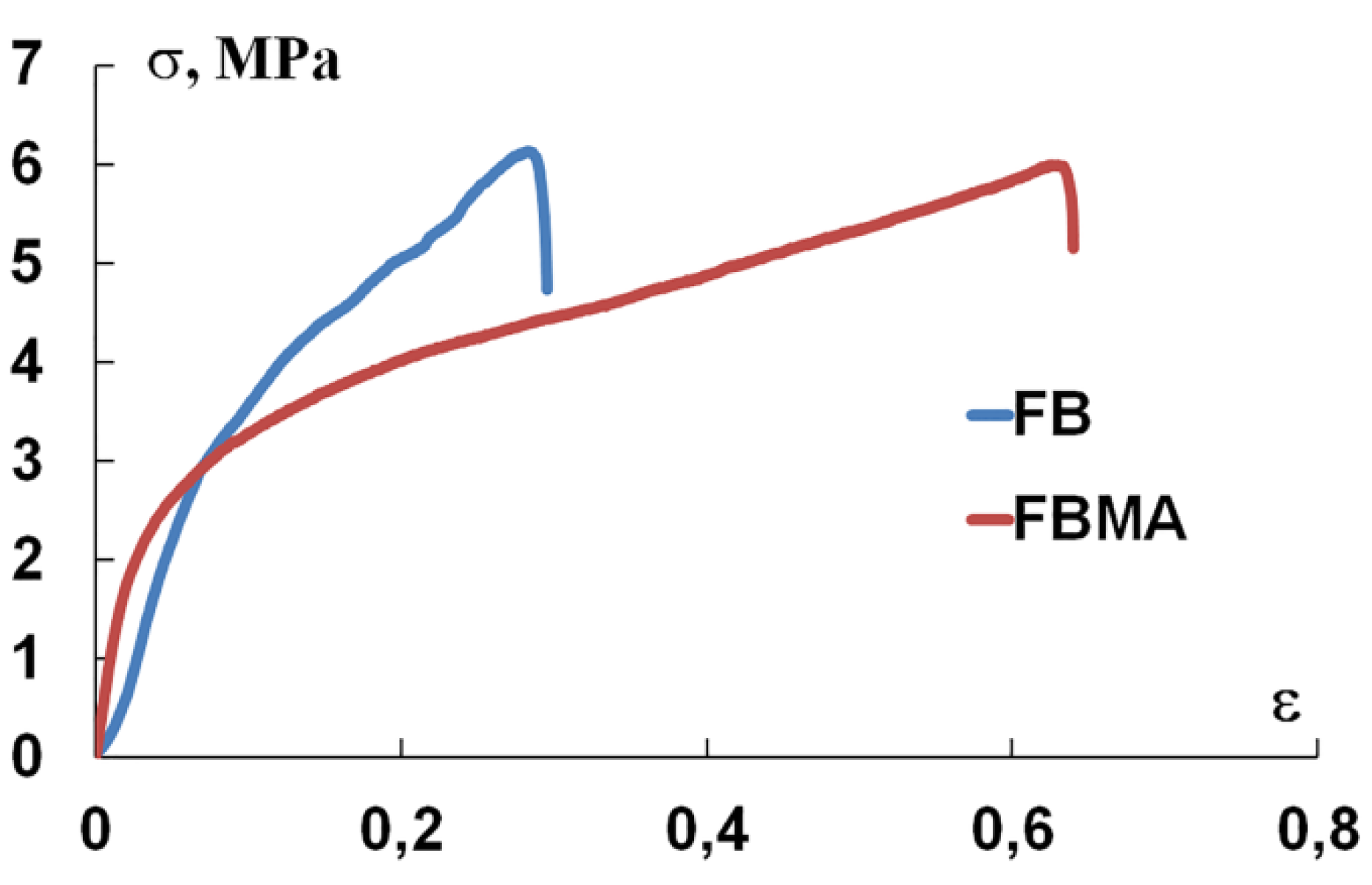
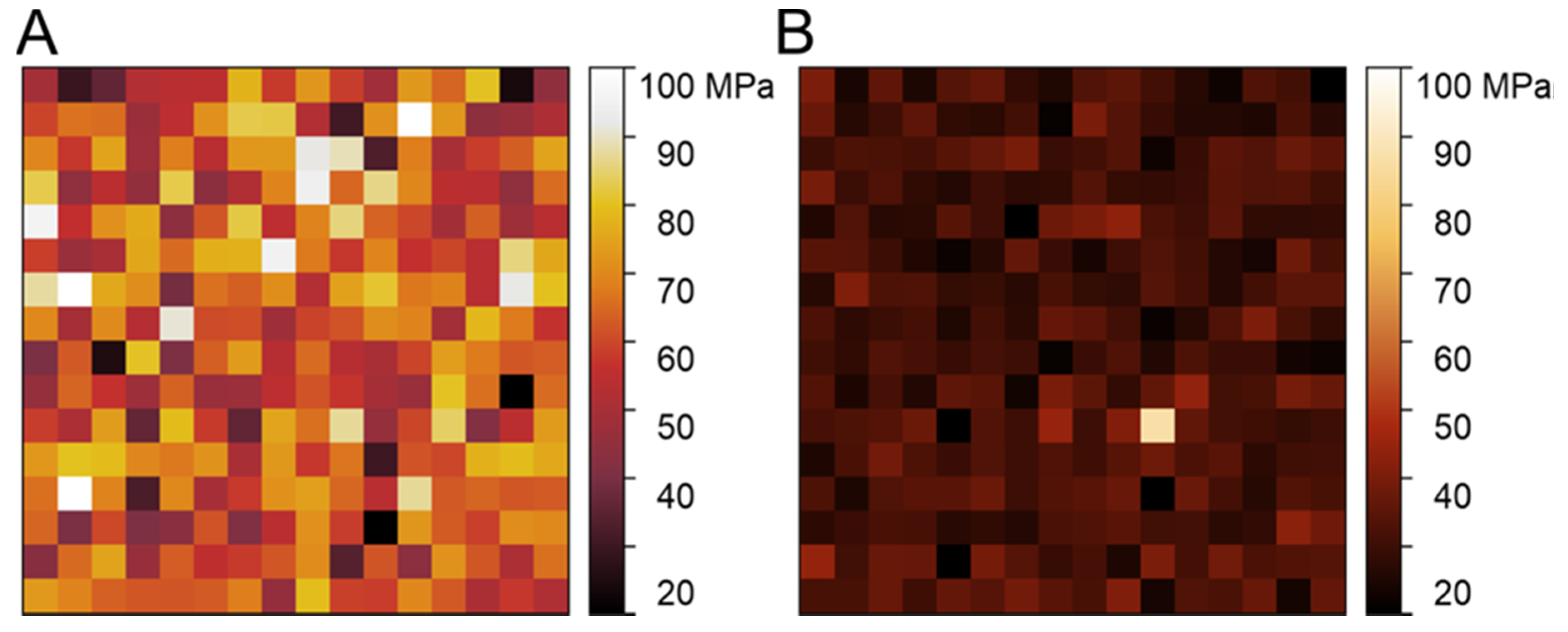
3.2. Mechanical Properties
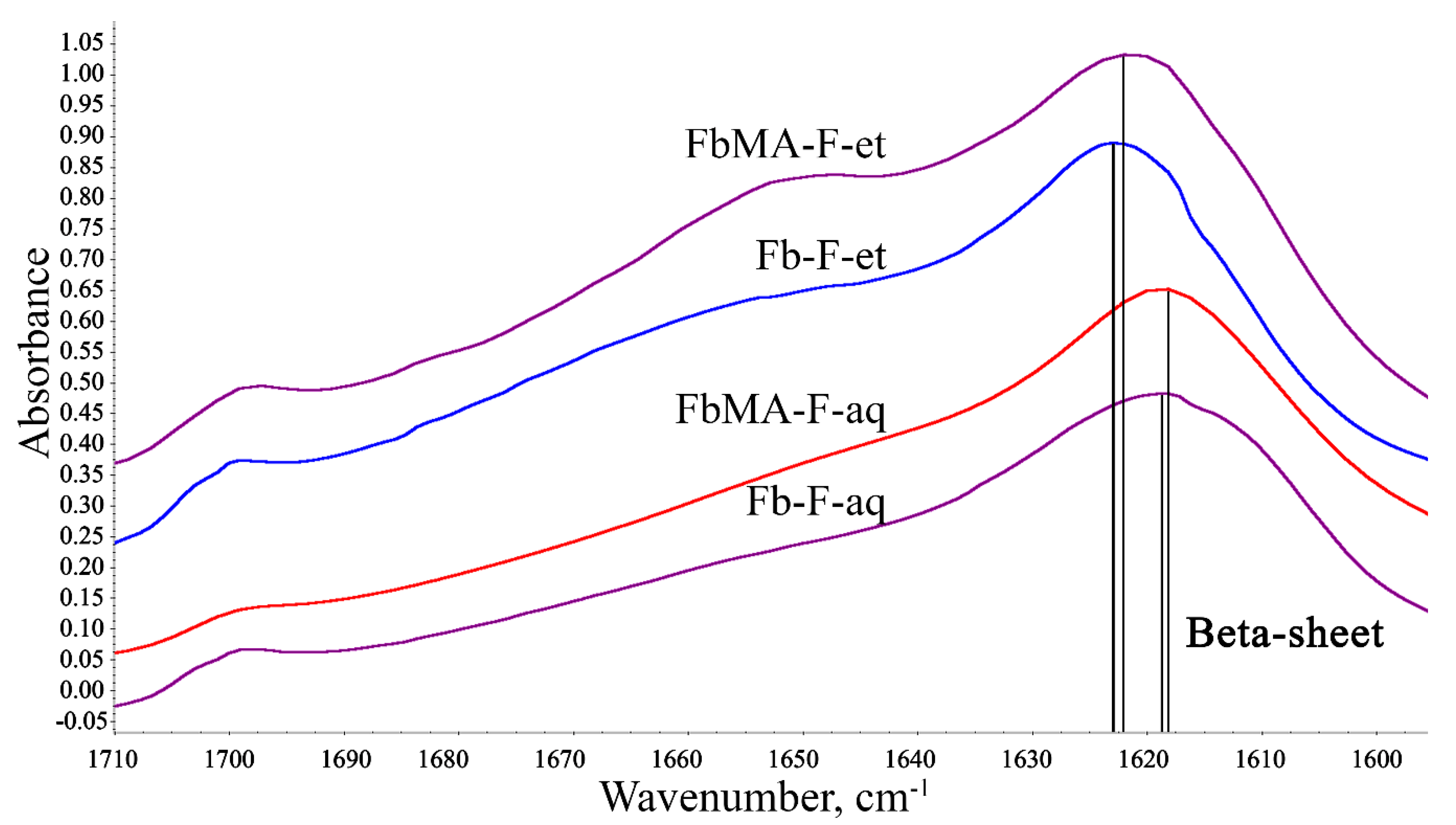
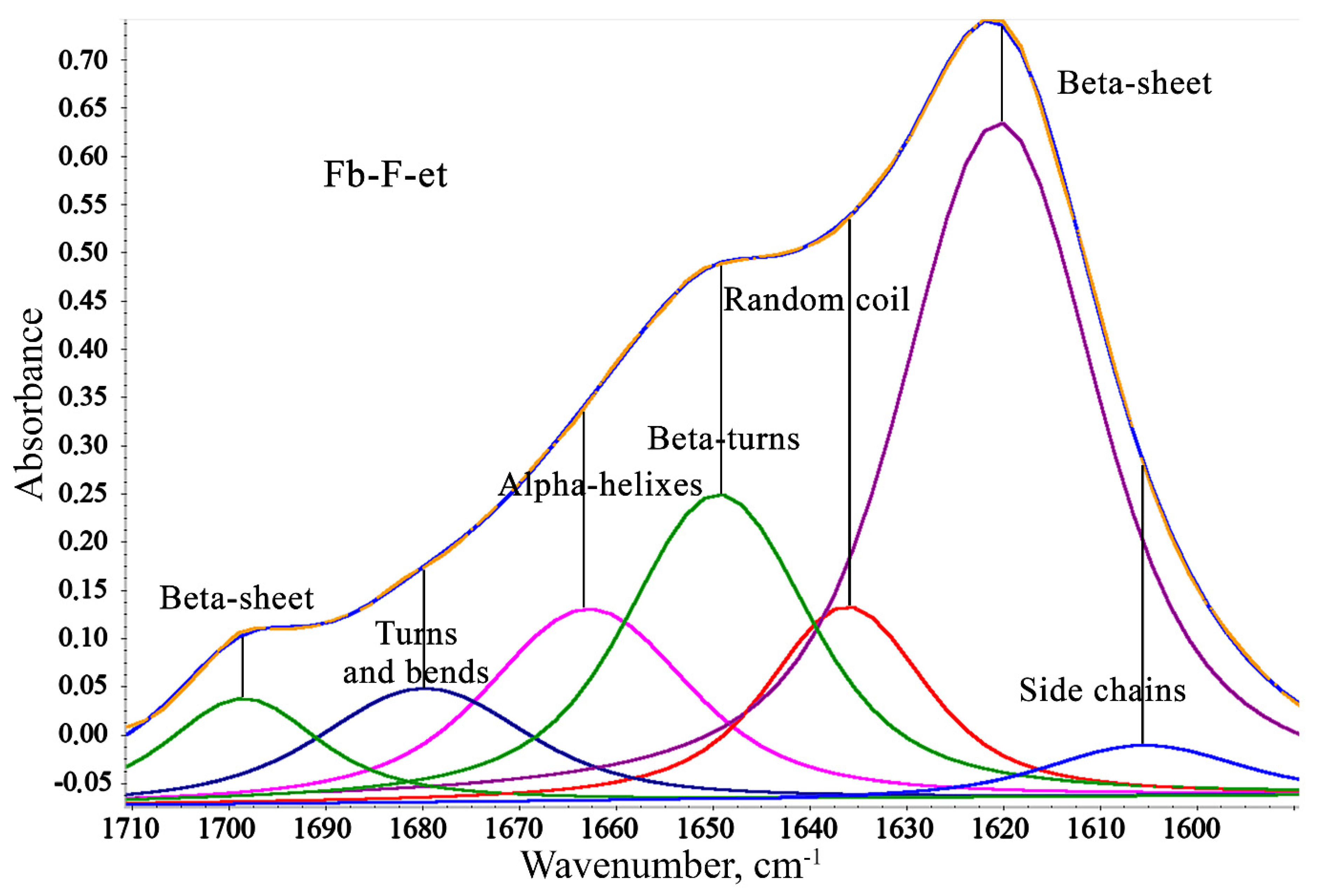
3.3. Secondary Structure
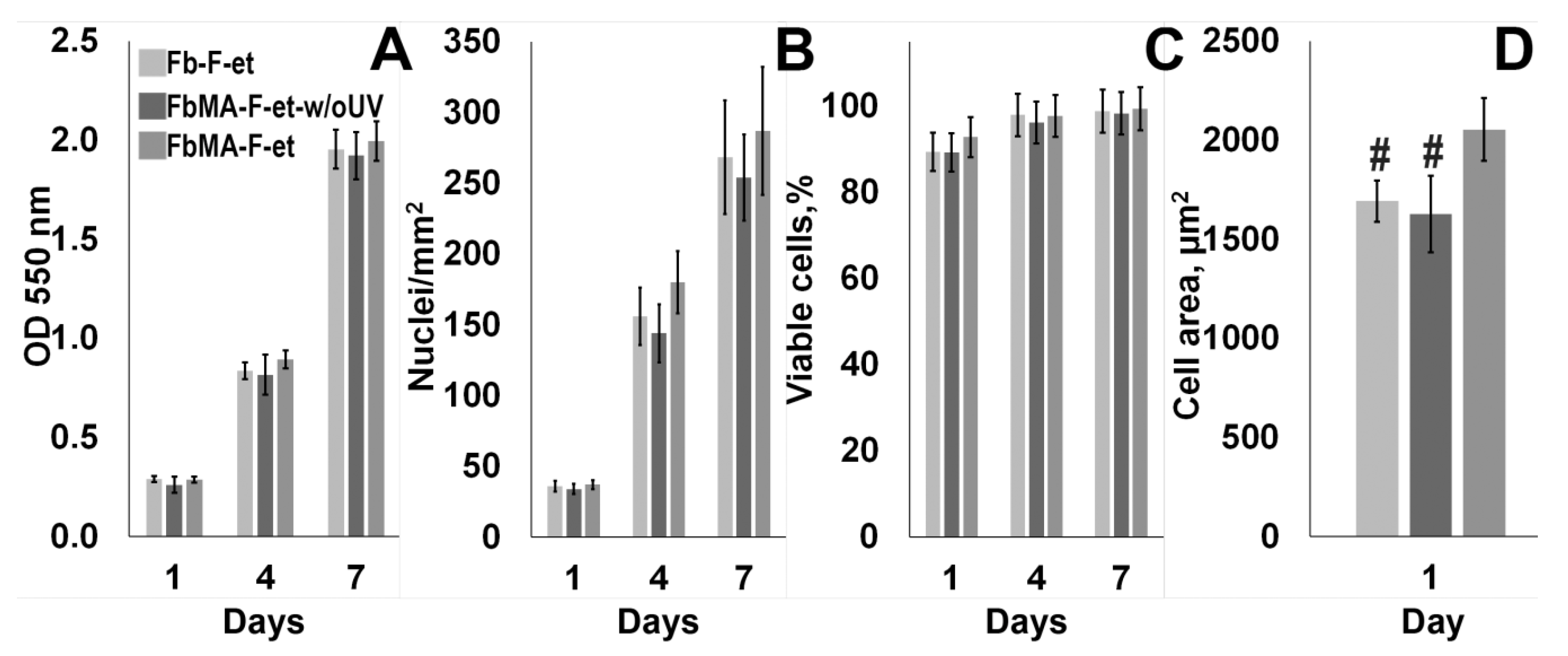
3.4. Biological Properties
4. Discussion
4.1. Film Fabrication
4.2. Mechanical Properties
4.3. Secondary Structure
4.4. Biological Properties
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tee, S.Y.; Bausch, A.R.; Janmey, P.A. The mechanical cell. Curr. Biol. 2009, 19, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Human, I.; Lamellae, B.; Dry, U. Nanoindentation Discriminates the Elastic Properties of. Bone 2002, 30, 178–184. [Google Scholar]
- Yusupov, V.I.; Khmelenin, D.N.; Koroleva, A.; Volkov, V.V.; Asadchikov, V.E. Digging deeper: Structural background of PEGylated fi brin gels in cell migration and lumenogenesis. RSC Adv. 2020, 10, 4190–4200. [Google Scholar]
- Bonartsev, A.P.; Bonartseva, G.A.; Reshetov, I.V.; Kirpichnikov, M.P.; Shaitan, K.V. Application of polyhydroxyalkanoates in medicine and the biological activity of natural poly(3-hydroxybutyrate). Acta Nat. 2019, 11, 4–16. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.; Le, T.; Le, Q. Van Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef]
- Hixon, K.R.; Eberlin, C.T.; Kadakia, P.U.; McBride-Gagyi, S.H.; Jain, E.; Sell, S.A. A comparison of cryogel scaffolds to identify an appropriate structure for promoting bone regeneration. Biomed. Phys. Eng. Express 2016, 2, 035014. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.-W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Correia, C.; Bhumiratana, S.; Yan, L.-P.; Oliveira, A.L.; Gimble, J.M.; Rockwood, D.; Kaplan, D.L.; Sousa, R.A.; Reis, R.L.; Vunjak-Novakovic, G. Development of silk-based scaffolds for tissue engineering of bone from human adipose-derived stem cells. Acta Biomater. 2012, 8, 2483–2492. [Google Scholar] [CrossRef]
- Mandal, B.B.; Grinberg, A.; Seok Gil, E.; Panilaitis, B.; Kaplan, D.L. High-strength silk protein scaffolds for bone repair. Proc. Natl. Acad. Sci. USA 2012, 109, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Gosline, J.M.; Guerette, P.A.; Ortlepp, C.S.; Savage, K.N. The mechanical design of spider silks: From fibroin sequence to mechanical function. J. Exp. Biol. 1999, 202, 3295–3303. [Google Scholar] [PubMed]
- Bai, S.; Han, H.; Huang, X.; Xu, W.; Kaplan, D.L.; Zhu, H.; Lu, Q. Silk scaffolds with tunable mechanical capability for cell differentiation. Acta Biomater. 2015, 20, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Z.; Confalomieri, F.; Medina, N.; Zivanovic, Y.; Esuault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine organization of Bombyx mori fibroin heavy chain gene. Nucl. Acid Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef]
- Xu, G.; Gong, L.; Yang, Z.; Liu, X.Y. What makes spider silk fibers so strong? From molecular-crystallite network to hierarchical network structures. Soft Matter 2014, 10, 2116–2123. [Google Scholar] [CrossRef]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef]
- Maziz, A.; Leprette, O.; Boyer, L.; Blatché, C.; Bergaud, C. Tuning the properties of silk fibroin biomaterial via chemical cross-linking. Biomed. Phys. Eng. Express 2018, 4, 065012. [Google Scholar] [CrossRef]
- Bessonov, I.V.; Rochev, Y.A.; Arkhipova, A.Y.; Kopitsyna, M.N.; Bagrov, D.V.; Karpushkin, E.A.; Bibikova, T.N.; Moysenovich, A.M.; Soldatenko, A.S.; Nikishin, I.I.; et al. Fabrication of hydrogel scaffolds via photocrosslinking of methacrylated silk fibroin. Biomed. Mater. 2019, 14, 034102. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Bagrov, D.V.; Dubrovin, E.V.; Shaitan, K.V.; Yaminskii, I.V. Atomic force microscopy of animal cells: Advances and prospects. Biophysics 2011, 56, 257–267. [Google Scholar] [CrossRef]
- Kim, D.H.; Wirtz, D. Focal adhesion size uniquely predicts cell migration. FASEB J. 2013, 27, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shmelev, K.; Sun, L.; Gil, E.S.; Park, S.H.; Cebe, P.; Kaplan, D.L. Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules 2011, 12, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef]
- Payne, K.J.; Veis, A. Fourier transform ir spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef]
- Meinel, L.; Hofmann, S.; Karageorgiou, V.; Kirker-head, C.; Mccool, J.; Gronowicz, G.; Zichner, L.; Langer, R.; Novakovic, G.V.; Kaplan, D.L. The inflammatory responses to silk films in vitro and in vivo. Biomaterials 2005, 26, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Meinel, L.; Hofmann, S.; Malhotra, A.; Volloch, V.; Kaplan, D. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J. Biomed. Mater. Res. Part A 2004, 71, 528–537. [Google Scholar] [CrossRef]
- Mitropoulos, A.; Burpo, F.; Nguyen, C.; Nagelli, E.; Ryu, M.; Wang, J.; Sims, R.; Woronowicz, K.; Wickiser, J. Noble Metal Composite Porous Silk Fibroin Aerogel Fibers. Materials 2019, 12, 894. [Google Scholar] [CrossRef] [PubMed]
- Ronca, A.; Maiullari, F.; Milan, M.; Pace, V.; Gloria, A.; Rizzi, R.; De Santis, R.; Ambrosio, L. Surface functionalization of acrylic based photocrosslinkable resin for 3D printing applications. Bioact. Mater. 2017, 2, 131–137. [Google Scholar] [CrossRef]
- Keddie, J.L.; Jones, R.A.L.; Cory, R.A. Size-dependent depression of the glass transition temperature in polymer films. EPL 1994, 27, 59–64. [Google Scholar] [CrossRef]
- Guo, Q. Polymer Morphology: Principles, Characterization, and Processing; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-1-118-45215-8. [Google Scholar]
- Hu, X.; Kaplan, D.; Cebe, P. Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- McGill, M.; Holland, G.P.; Kaplan, D.L. Experimental Methods for Characterizing the Secondary Structure and Thermal Properties of Silk Proteins. Macromol. Rapid Commun. 2019, 40, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.-J.; Park, J.; Joo Kim, H.; Wada, M.; Kaplan, D.L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials 2005, 26, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hotz, B.; Ling, S.; Guo, J.; Haas, D.S.; Marelli, B.; Omenetto, F.; Lin, S.J.; Kaplan, D.L. Regenerated silk materials for functionalized silk orthopedic devices by mimicking natural processing. Biomaterials 2016, 110, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef]
- Thai, T.H.; Nuntanaranont, T.; Kamolmatyakul, S.; Meesane, J. In Vivo evaluation of modified silk fibroin scaffolds with a mimicked microenvironment of fibronectin/decellularized pulp tissue for maxillofacial surgery. Biomed. Mater. 2017, 13, 015009. [Google Scholar] [CrossRef]
- Perrone, G.S.; Leisk, G.G.; Lo, T.J.; Moreau, J.E.; Haas, D.S.; Papenburg, B.J.; Golden, E.B.; Partlow, B.P.; Fox, S.E.; Ibrahim, A.M.S.; et al. The use of silk-based devices for fracture fixation. Nat. Commun. 2014, 5, 3385. [Google Scholar] [CrossRef]
- Midha, S.; Murab, S.; Ghosh, S. Osteogenic signaling on silk-based matrices. Biomaterials 2016, 97, 133–153. [Google Scholar] [CrossRef]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.I.L.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1–14. [Google Scholar]
- Hytönen, V.P.; Wehrle-Haller, B. Protein conformation as a regulator of cell-matrix adhesion. Phys. Chem. Chem. Phys. 2014, 16, 6342–6357. [Google Scholar] [CrossRef]
- Pacifici, A.; Laino, L.; Gargari, M.; Guzzo, F.; Luz, A.V.; Polimeni, A.; Pacifici, L. Decellularized hydrogels in bone tissue engineering: A topical review. Int. J. Med. Sci. 2018, 15, 492–497. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, D.; Zhou, C.; Yuan, Q.; Ye, L.; Zhou, X. Substrate elasticity regulates adipose-derived stromal cell differentiation towards osteogenesis and adipogenesis through β-catenin transduction. Acta Biomater. 2018, 79, 83–95. [Google Scholar] [CrossRef] [PubMed]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Sonowal, H.; Kumar, A.; Bhattacharyya, J.; Gogoi, P.; Jaganathan, B. Inhibition of actin polymerization decreases osteogeneic differentiation of mesenchymal stem cells through p38 MAPK pathway. J. Biomed. Sci. 2013, 20, 71. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, W.H.; Auernheimer, V.; Thievessen, I.; Fabry, B. Vinculin, cell mechanics and tumour cell invasion. Cell Biol. Int. 2013, 37, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Bays, J.L.; DeMali, K.A. Vinculin in cell-cell and cell-matrix adhesions. Cell. Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Jannatbabaei, A.; Tafazzoli-Shadpour, M.; Seyedjafari, E.; Fatouraee, N. Cytoskeletal remodeling induced by substrate rigidity regulates rheological behaviors in endothelial cells. J. Biomed. Mater. Res. Part A 2019, 107, 71–80. [Google Scholar] [CrossRef]
- Matsuoka, F.; Takeuchi, I.; Agata, H.; Kagami, H.; Shiono, H.; Kiyota, Y.; Honda, H.; Kato, R. Morphology-Based Prediction of Osteogenic Differentiation Potential of Human Mesenchymal Stem Cells. PLoS ONE 2013, 8, e55082. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials 2004, 25, 5947–5954. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc. Natl. Acad. Sci. USA 2005, 102, 5953–5957. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.; Migliaresi, C.; Lloyd, A.W.; Denyer, S.P.; Santin, M. Serum protein absorption on silk fibroin fibers and films: Surface opsonization and binding strength. J. Bioact. Compat. Polym. 2002, 17, 23–35. [Google Scholar] [CrossRef]
| Parameter | Conditional Yield Strength σT, MPa | Tensile Strength σp, MPa | EM, MPa | Conditional Elongation, εT at σT | Elongation, εp at σp | |
|---|---|---|---|---|---|---|
| Sample | ||||||
| FbMA-F-et | 2.8 ± 0.5 | 5.8 ± 0.5 | 130 ± 1 | 0.4 ± 0.1 | 0.61 ± 0.12 | |
| Fb-F-et | 3.5 ± 0.4 | 6.9 ± 0.8 | 64 ± 6 | 0.9 ± 0.2 | 0.43 ± 0.14 | |
| Secondary Structure | Wavenumber, cm−1 | FbMA-F-aq | Fb-F-aq | FbMA-F-et | Fb-F-et |
|---|---|---|---|---|---|
| Side chains | 1590–1605 | 10.8 | 6.5 | 2.8 | 3.2 |
| Beta-sheets | 1610–1635, 1695–1710 | 41.5 | 46.9 | 49.8 | 49.0 |
| Random coils | 1635–1645 | 10.8 | 16.0 | 9.8 | 9.9 |
| Beta-turns | 1647–1654 | 12.3 | 15.1 | 18.2 | 18.1 |
| Alpha-helixes | 1658–1664 | 13.8 | 9.4 | 11.9 | 12.3 |
| Bends and turns | 1666–1695 | 10.8 | 6.2 | 7.4 | 7.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessonov, I.; Moysenovich, A.; Arkhipova, A.; Ezernitskaya, M.; Efremov, Y.; Solodilov, V.; Timashev, P.; Shaytan, K.; Shtil, A.; Moisenovich, M. The Mechanical Properties, Secondary Structure, and Osteogenic Activity of Photopolymerized Fibroin. Polymers 2020, 12, 646. https://doi.org/10.3390/polym12030646
Bessonov I, Moysenovich A, Arkhipova A, Ezernitskaya M, Efremov Y, Solodilov V, Timashev P, Shaytan K, Shtil A, Moisenovich M. The Mechanical Properties, Secondary Structure, and Osteogenic Activity of Photopolymerized Fibroin. Polymers. 2020; 12(3):646. https://doi.org/10.3390/polym12030646
Chicago/Turabian StyleBessonov, Ivan, Anastasia Moysenovich, Anastasia Arkhipova, Mariam Ezernitskaya, Yuri Efremov, Vitaliy Solodilov, Peter Timashev, Konstantin Shaytan, Alexander Shtil, and Mikhail Moisenovich. 2020. "The Mechanical Properties, Secondary Structure, and Osteogenic Activity of Photopolymerized Fibroin" Polymers 12, no. 3: 646. https://doi.org/10.3390/polym12030646
APA StyleBessonov, I., Moysenovich, A., Arkhipova, A., Ezernitskaya, M., Efremov, Y., Solodilov, V., Timashev, P., Shaytan, K., Shtil, A., & Moisenovich, M. (2020). The Mechanical Properties, Secondary Structure, and Osteogenic Activity of Photopolymerized Fibroin. Polymers, 12(3), 646. https://doi.org/10.3390/polym12030646





