Coumarins into Polyurethanes for Smart and Functional Materials
Abstract
1. Introduction
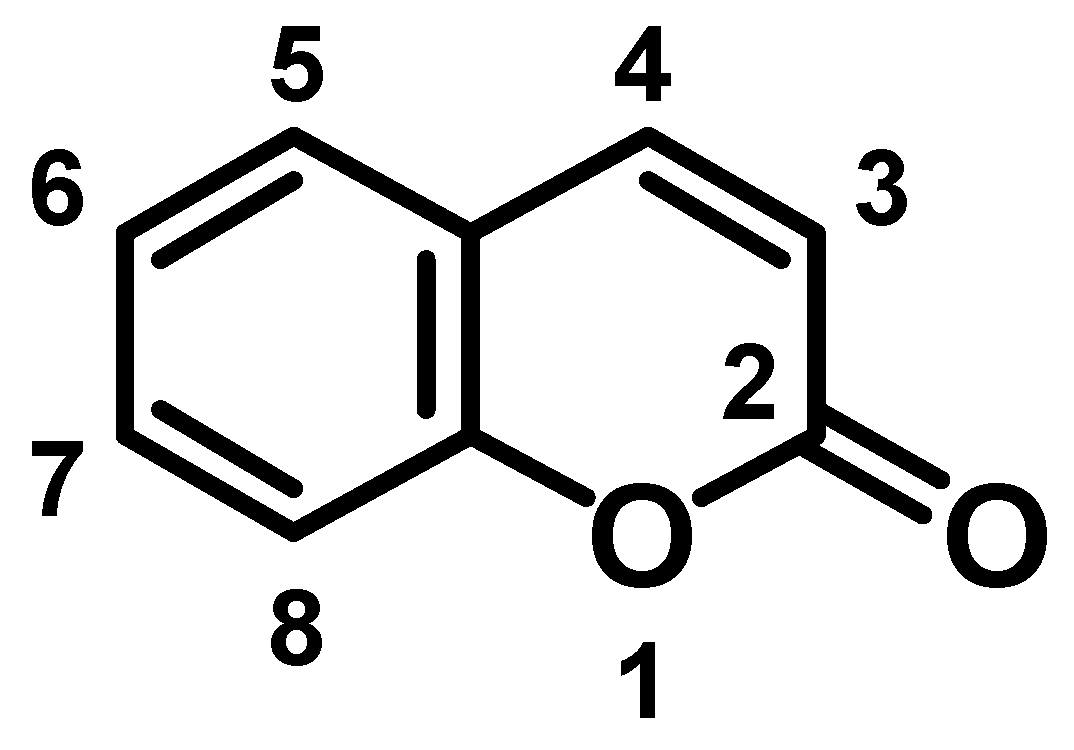
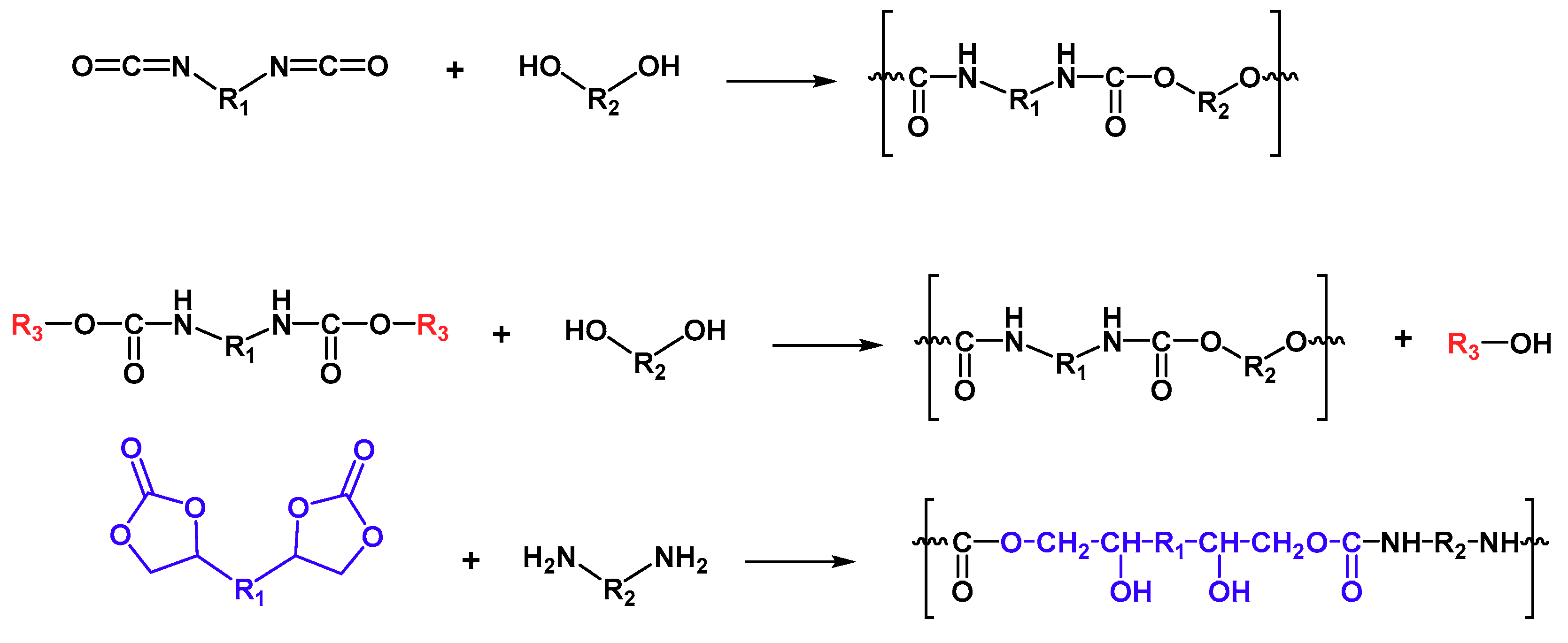
2. Coumarins into Polymers
3. Photo-physics of Coumarins in Polyurethanes
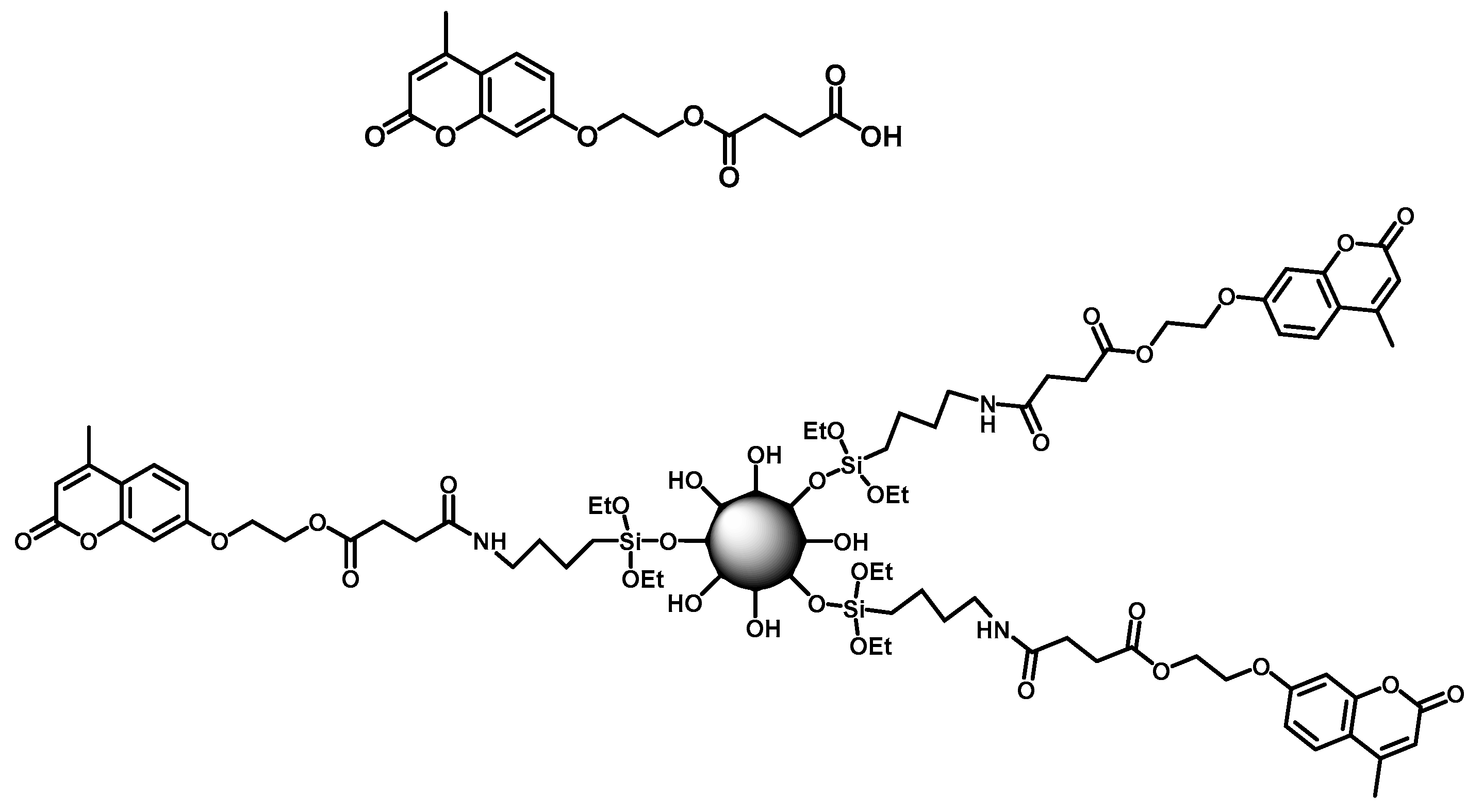
3.1. Effective UV-Light Absorber
3.2. Fluorescent Probes
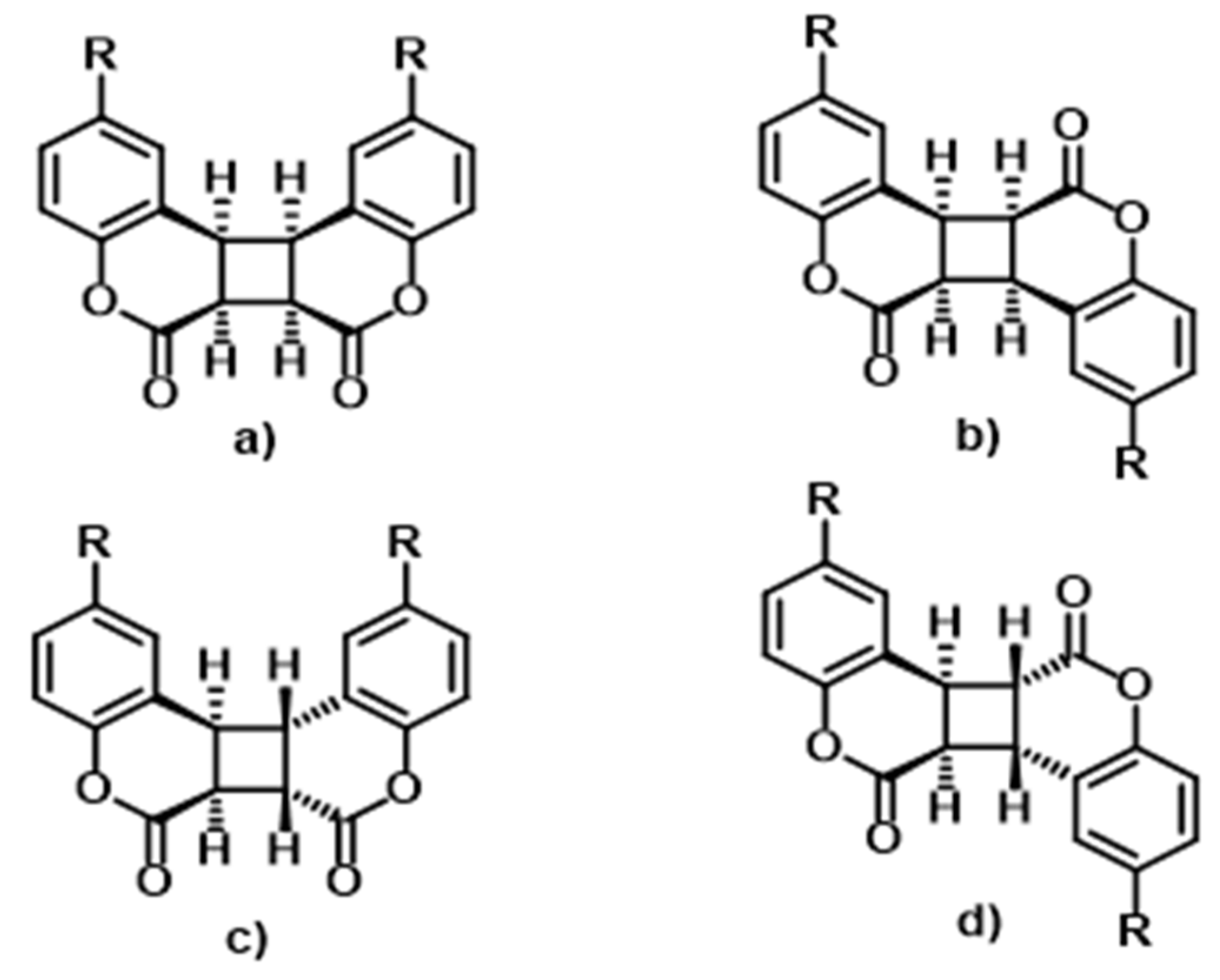
4. Photochemistry of Coumarins in Polyurethanes
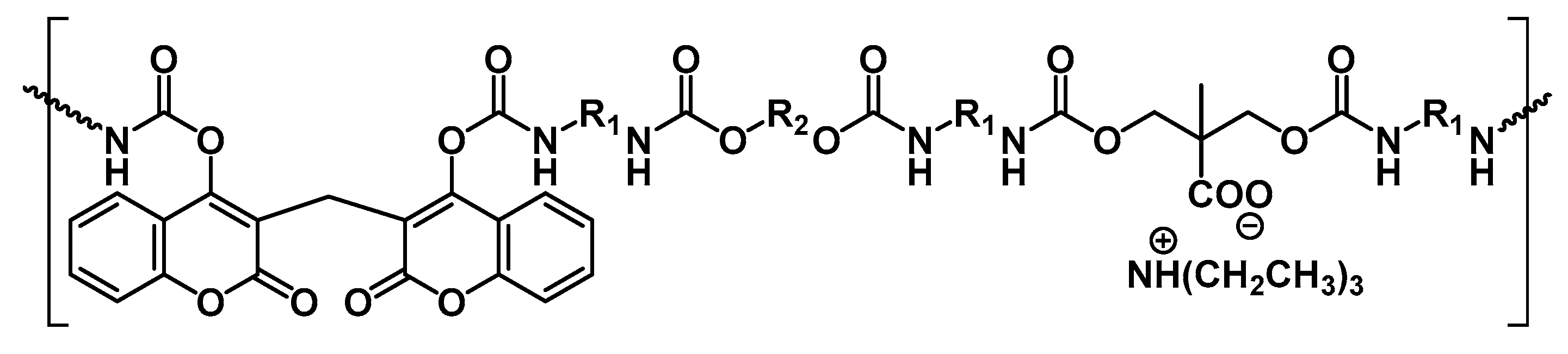
4.1. Recognition Ability
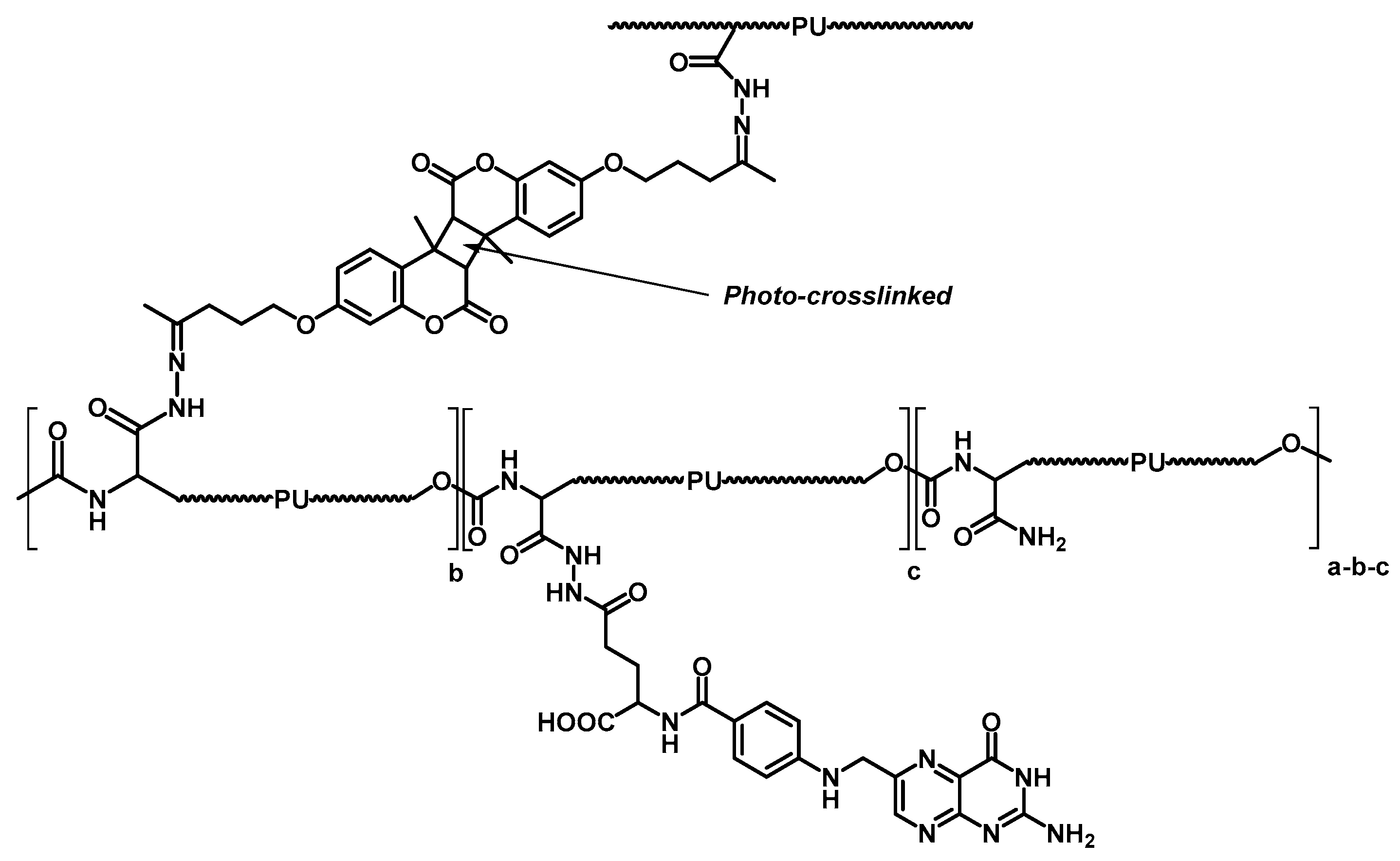
4.2. Controlled Drug Delivery
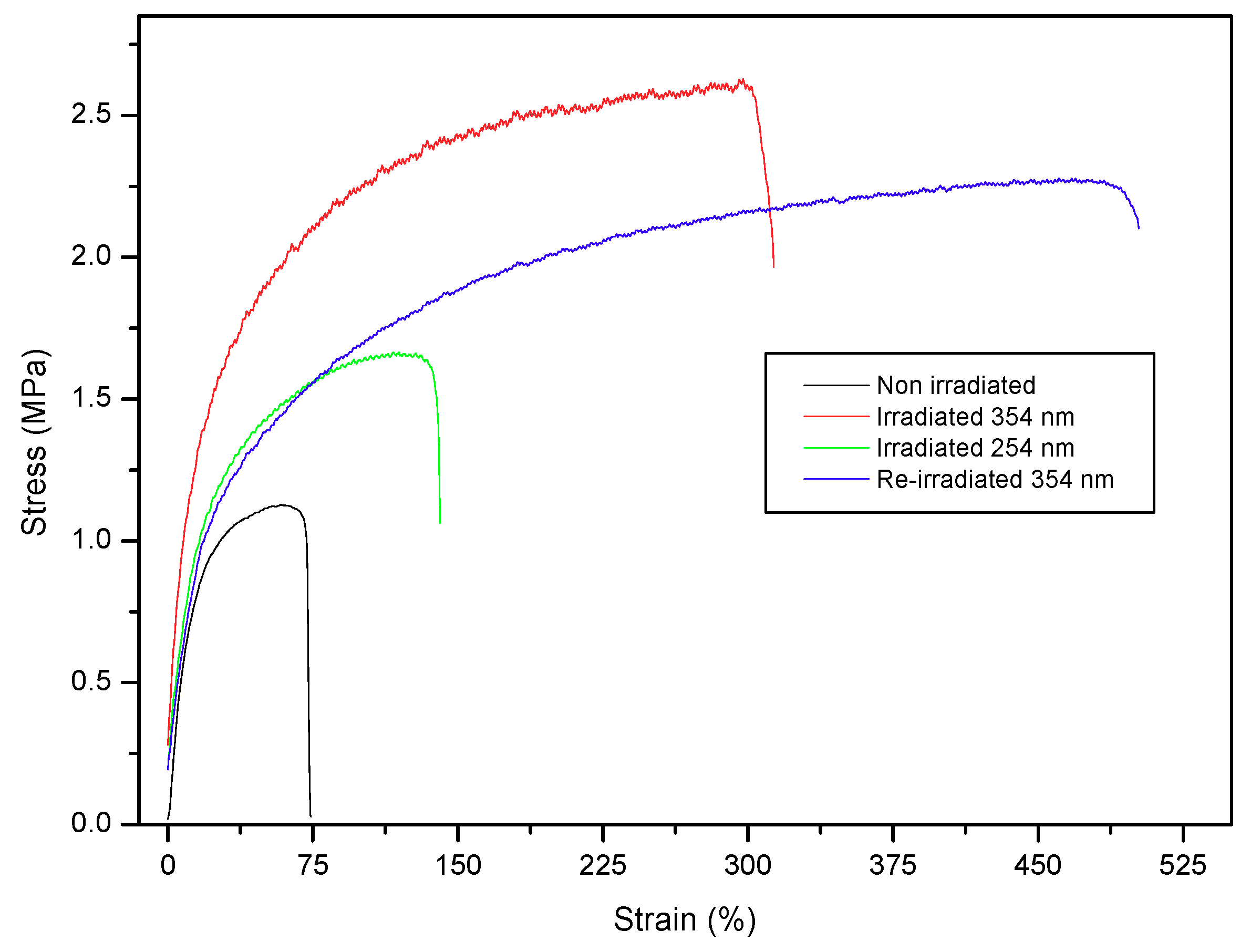
4.3. Enhancement Mechanical Properties by Irradiation
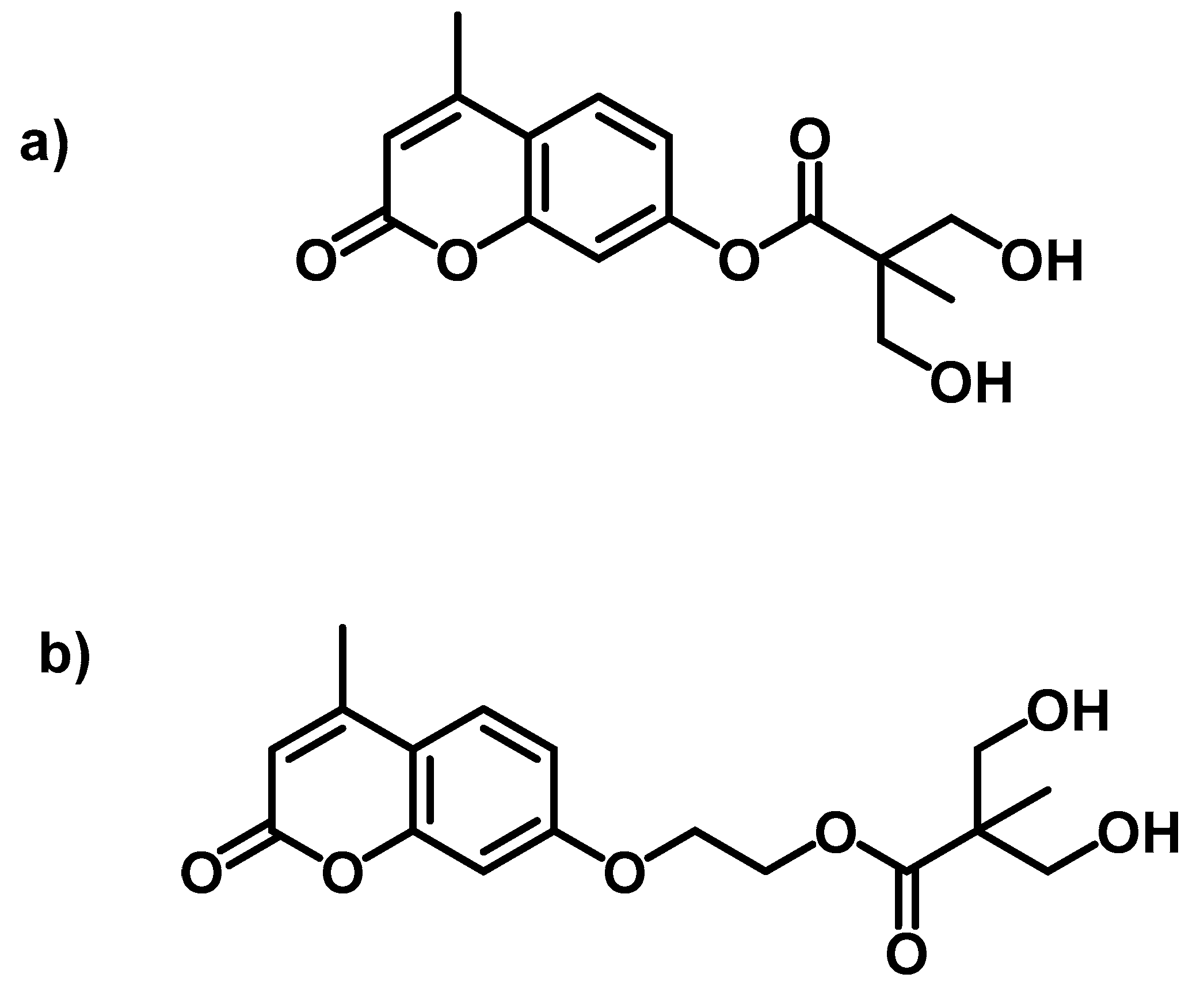
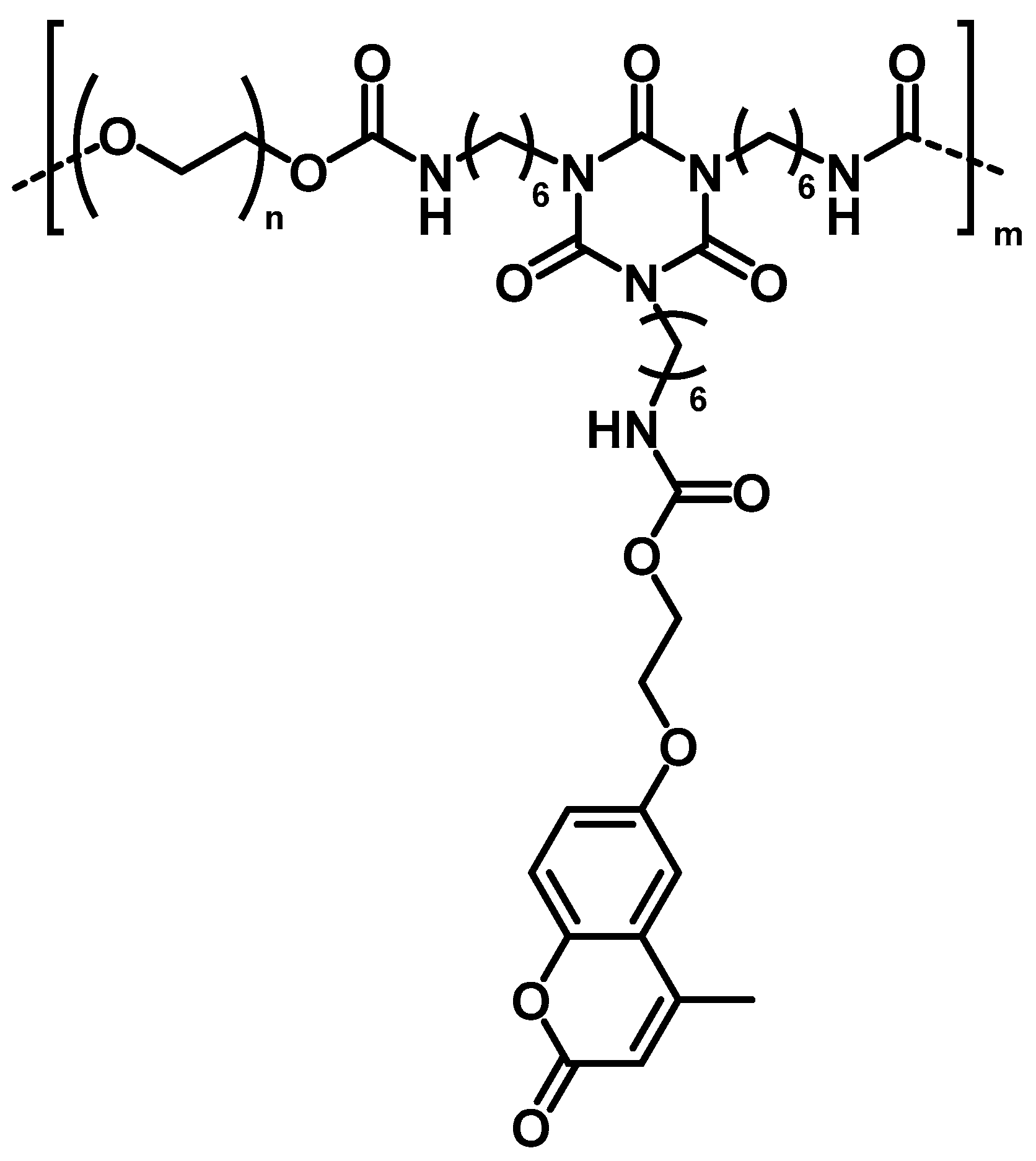
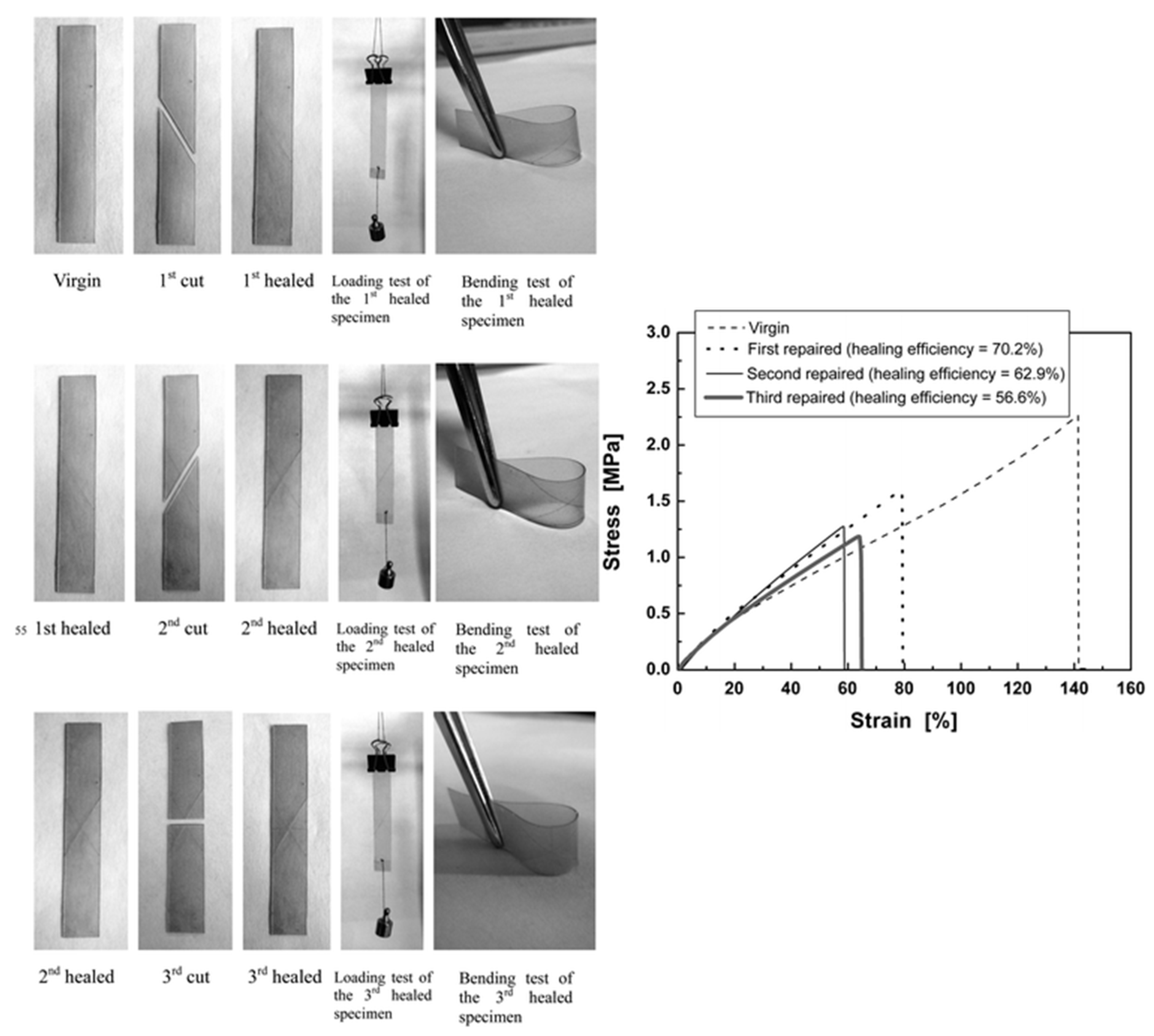
4.4. Self-Healing Ability
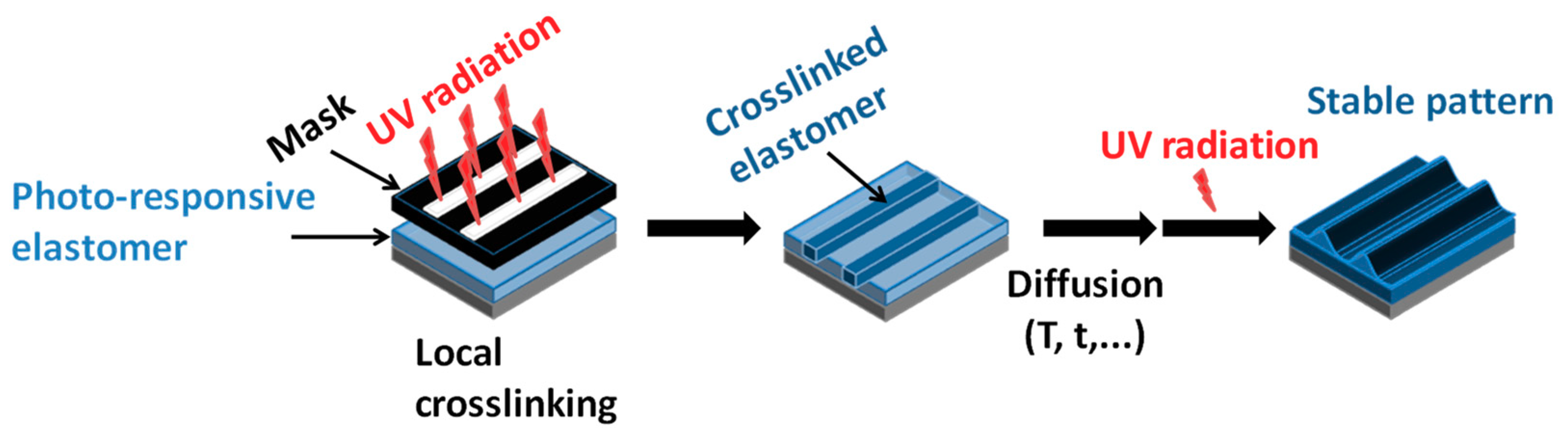
4.5. Shape Memory Polymers
5. Other Properties of Coumarin Additives in Polyurethanes
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Medina, F.G.; Marrero, J.G.; Macías-Alonso, M.; González, M.C.; Córdova-Guerrero, I.; García, A.G.T.; Osegueda-Robles, S. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Coumarin derivatives: An updated patent review (2015–2016). Expert Opin. Ther. Pat. 2017, 27, 1201–1226. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem. Rev. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. Phytochem. Isol. Characterisation Role Hum. Health 2015, 113–140. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Progress in the chemistry of naturally occurring coumarins. In Progress in the Chemistry of Organic Natural Products 106; Springer: Berlin, Germany, 2017; pp. 241–304. [Google Scholar]
- Da Silveira Pinto, L.S.; de Souza, M.V. Sonochemistry as a general procedure for the synthesis of coumarins, including multigram synthesis. Synthesis 2017, 49, 2677–2682. [Google Scholar]
- Harkiss, A.H.; Sutherland, A. Recent advances in the synthesis and application of fluorescent α-amino acids. Org. Biomol. Chem. 2016, 14, 8911–8921. [Google Scholar] [CrossRef]
- Sharma, R.K.; Katiyar, D. Recent advances in transition-metal-catalyzed synthesis of coumarins. Synthesis 2016, 48, 2303–2322. [Google Scholar]
- Valizadeh, H.; Gholipur, H.; Shockravi, A. Microwave assisted synthesis of coumarins via potassium carbonate catalyzed knoevenagel condensation in 1-n-butyl-3-methylimidazolium bromide ionic liquid. J. Heterocycl. Chem. 2007, 44, 867–870. [Google Scholar] [CrossRef]
- Verdía, P.; Santamarta, F.; Tojo, E. Knoevenagel reaction in [MMIm][MSO4]: Synthesis of coumarins. Molecules 2011, 16, 4379–4388. [Google Scholar] [CrossRef] [PubMed]
- Potdar, M.K.; Mohile, S.S.; Salunkhe, M.M. Coumarin syntheses via Pechmann condensation in Lewis acidic chloroaluminate ionic liquid. Tetrahedron Lett. 2001, 42, 9285–9287. [Google Scholar] [CrossRef]
- Murray, R.; Ballantyne, M. Claisen rearrangements—I: Synthesis of the coumarin, pinnarin. Tetrahedron 1970, 26, 4667–4671. [Google Scholar] [CrossRef]
- Van, T.N.; Debenedetti, S.; De Kimpe, N. Synthesis of coumarins by ring-closing metathesis using Grubbs’ catalyst. Tetrahedron Lett. 2003, 44, 4199–4201. [Google Scholar]
- Dighe, N.S.; Pattan, S.R.; Dengale, S.S.; Musmade, D.S.; Shelar, M.; Tambe, V.; Hole, M.B. Synthetic and pharmacological profiles of coumarins: A review. Arch. Appl. Sci. Res. 2010, 2, 65–71. [Google Scholar]
- Bairagi, S.H.; Salaskar, P.P.; Loke, S.D.; Surve, N.N.; Tandel, D.V.; Dusara, M.D. Medicinal significance of coumarins: A review. Int. J. Pharm. Res. 2012, 4, 16–19. [Google Scholar]
- Ghosh, S.; Roy, N.; Singh, T.S.; Chattopadhyay, N. Photophysics of a coumarin based Schiff base in solvents of varying polarities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 252–257. [Google Scholar] [CrossRef]
- Shreykar, M.R.; Sekar, N. Coumarin-rhodamine hybrids–synthesis, photophysical properties, NLO properties and DFT studies. ChemistrySelect 2017, 2, 1464–1478. [Google Scholar] [CrossRef]
- Yamaji, M.; Hakoda, Y.; Okamoto, H.; Tani, F. Photochemical synthesis and photophysical properties of coumarins bearing extended polyaromatic rings studied by emission and transient absorption measurements. Photochem. Photobiol. Sci. 2017, 16, 555–563. [Google Scholar] [CrossRef]
- Gandioso, A.; Bresolí-Obach, R.; Nin-Hill, A.; Bosch, M.; Palau, M.; Galindo, A.; Contreras, S.; Rovira, A.; Rovira, C.; Nonell, S. Redesigning the coumarin scaffold into small bright fluorophores with far-red to near-infrared emission and large Stokes shifts useful for cell imaging. J. Org. Chem. 2018, 83, 1185–1195. [Google Scholar] [CrossRef]
- Taneja, L.; Sharma, A.; Singh, R. Study of photophysical properties of Coumarins: Substituent and concentration dependence. J. Lumin. 1995, 63, 203–214. [Google Scholar] [CrossRef]
- Toseland, C.P. Fluorescent labeling and modification of proteins. J. Chem. Biol. 2013, 6, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zheng, H.; Guo, M.; Du, L.; Liu, G.; Wang, P. Synthesis of polymeric fluorescent brightener based on coumarin and its performances on paper as light stabilizer, fluorescent brightener and surface sizing agent. Appl. Surf. Sci. 2016, 367, 167–173. [Google Scholar] [CrossRef]
- Bakhtiari, G.; Moradi, S.; Soltanali, S. A novel method for the synthesis of coumarin laser dyes derived from 3-(1H-benzoimidazol-2-yl) coumarin-2-one under microwave irradiation. Arab. J. Chem. 2014, 7, 972–975. [Google Scholar] [CrossRef]
- Mishra, V.R.; Sekar, N. Photostability of coumarin laser dyes-a mechanistic study using global and local reactivity descriptors. J. Fluoresc. 2017, 27, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-T.; Yen, C.-K.; Yang, W.-P.; Chen, H.-H.; Liao, C.-H.; Tsai, C.-H.; Chen, C.H. Efficient green coumarin dopants for organic light-emitting devices. Org. Lett. 2004, 6, 1241–1244. [Google Scholar] [CrossRef]
- Jung, H.; Lee, H.; Kang, S.; Shin, D.-H.; Kay, K.-Y.; Park, J. New anthracene derivatives containing coumarin moiety for organic light-emitting diodes. Mol. Cryst. Liq. Cryst. 2017, 654, 90–95. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.J.; Lee, J.; Shin, H.; Kay, K.-Y.; Park, J. New amino methyl coumarin derivative for OLED blue emitter. Mol. Cryst. Liq. Cryst. 2015, 620, 139–146. [Google Scholar] [CrossRef]
- Gautier-Thianche, E.; Sentein, C.; Lorin, A.; Denis, C.; Raimond, P.; Nunzi, J.-M. Effect of coumarin on blue light-emitting diodes based on carbazol polymers. J. Appl. Phys. 1998, 83, 4236–4241. [Google Scholar] [CrossRef]
- Lapina, V.A.; Pavich, T.A.; Pershukevich, P.P.; Trofimov, A.V.; Trofimova, N.N.; Tsaplev, Y.B.; Zak, P.P. Exploring the utility of coumarins-based luminescent spectra converters. J. Phys. Org. Chem. 2017, 30, e3731. [Google Scholar] [CrossRef]
- Tsaplev, Y.; Trofimov, A.; Pershukevich, P.; Pavich, T.; Zak, P.; Trofimova, N.; Lapina, V. Tuning luminescent converters based on coumarins and their photostability. J. Appl. Spectrosc. 2017, 84, 859–865. [Google Scholar] [CrossRef]
- Chang, H.; Shi, M.; Sun, Y.-N.; Jiang, J.-Q. Photo-dimerization characteristics of coumarin pendants within amphiphilic random copolymer micelles. Chin. J. Polym. Sci. 2015, 33, 1086–1095. [Google Scholar] [CrossRef]
- Ciamician, G.; Silber, P. Chemische lichtwirkungen. Ber. Dtsch. Chem. Ges. 1902, 35, 4128–4131. [Google Scholar] [CrossRef]
- Kaur, G.; Johnston, P.; Saito, K. Photo-reversible dimerisation reactions and their applications in polymeric systems. Polym. Chem. 2014, 5, 2171–2186. [Google Scholar] [CrossRef]
- Buckup, T.; Southan, A.; Kim, H.-C.; Hampp, N.; Motzkus, M. Optimisation of two-photon induced cleavage of molecular linker systems for drug delivery. J. Photochem. Photobiol. A Chem. 2010, 210, 188–192. [Google Scholar] [CrossRef]
- Härtner, S.; Kim, H.-C.; Hampp, N. Photodimerized 7-hydroxycoumarin with improved solubility in PMMA: Single-photon and two-photon-induced photocleavage in solution and PMMA films. J. Photochem. Photobiol. A Chem. 2007, 187, 242–246. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, R.T. Photopolymerization of 7, 7′-coumarinyl polymethylene dicarboxylates: Fluorescence and kinetic study. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 2999–3008. [Google Scholar] [CrossRef]
- Krauch, C.H.; Metzner, W.; Schenck, G.O. Photochemische C4-und C3O-Cycloadditionen an Cumaron. Chem. Ber. 1966, 99, 1723–1731. [Google Scholar] [CrossRef]
- Krauch, C.H.; Farid, S.; Schenck, G.O. Photo-C4-Cyclodimerisation von Cumarin. Chem. Ber. 1966, 99, 625–633. [Google Scholar] [CrossRef]
- Lewis, F.D.; Barancyk, S.V. Lewis acid catalysis of photochemical reactions. 8. Photodimerization and cross-cycloaddition of coumarin. J. Am. Chem. Soc. 1989, 111, 8653–8661. [Google Scholar] [CrossRef]
- Morrison, H.; Curtis, H.; McDowell, T. Solvent Effects on the Photodimerization of Coumarin1. J. Am. Chem. Soc. 1966, 88, 5415–5419. [Google Scholar] [CrossRef]
- Kuznetsova, N.Y.A.; Kaliya, O.L. The photochemistry of coumarins. Russ. Chem. Rev. 1992, 61, 683–696. [Google Scholar] [CrossRef]
- Wolff, T.; Görner, H. Photodimerization of coumarin revisited: Effects of solvent polarity on the triplet reactivity and product pattern. Phys. Chem. Chem. Phys. 2004, 6, 368–376. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Ramamurthy, V. Templating photodimerization of coumarins within a water-soluble nano reaction vessel. J. Org. Chem. 2006, 71, 6409–6413. [Google Scholar] [CrossRef]
- Tanaka, K.; Fujiwara, T. Enantioselective [2 + 2] photodimerization reactions of coumarins in solution. Org. Lett. 2005, 7, 1501–1503. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.D. The photochemistry of organic solids. Angew. Chem. Int. Ed. Engl. 1975, 14, 386–393. [Google Scholar] [CrossRef]
- Cohen, M.; Schmidt, G.; Sonntag, F. 384. Topochemistry. Part II. The photochemistry of trans-cinnamic acids. J. Chem. Soc. (Resumed) 1964, 2000–2013. [Google Scholar] [CrossRef]
- Schmidt, G. 385. Topochemistry. Part III. The crystal chemistry of some trans-cinnamic acids. J. Chem. Soc. (Resumed) 1964, 2014–2021. [Google Scholar] [CrossRef]
- Gnanaguru, K.; Ramasubbu, N.; Venkatesan, K.; Ramamurthy, V. A study on the photochemical dimerization of coumarins in the solid state. J. Org. Chem. 1985, 50, 2337–2346. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Venkatesan, K.; Weiss, R.G. Photodimerization of coumarins in solid cyclodextrin inclusion complexes. J. Org. Chem. 1992, 57, 3292–3297. [Google Scholar] [CrossRef]
- Brett, T.J.; Alexander, J.M.; Stezowski, J.J. Chemical insight from crystallographic disorder-structural studies of supramolecular photochemical systems. Part 2. 1 The β-cyclodextrin–4, 7-dimethylcoumarin inclusion complex: A new β-cyclodextrin dimer packing type, unanticipated photoproduct formation, and an examination of guest influence on β-CD dimer packing. J. Chem. Soc. Perkin Trans. 2 2000, 1095–1103. [Google Scholar] [CrossRef]
- Yonezawa, N.; Yoshida, T.; Hasegawa, M. Symmetric and asymmetric photocleavage of the cyclobutane rings in head-to-head coumarin dimers and their lactone-opened derivatives. J. Chem. Soc. Perkin Trans. 1 1983, 1083–1086. [Google Scholar] [CrossRef]
- Yonezawa, N.; Yamashita, T.; Kanoe, T.; Saigo, K.; Hasegawa, M. Application of anisotropic photocleavage of head-to-head type cyclobutane compounds. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 593–598. [Google Scholar] [CrossRef]
- Rabek, J.F.; Scott, G.W. Photochemistry and Photophysics; CRC Press: Boca Raton, FL, USA, 1989; Volume 1. [Google Scholar]
- Jiang, M.; Paul, N.; Bieniek, N.; Buckup, T.; Hampp, N.; Motzkus, M. Photocleavage of coumarin dimers studied by femtosecond UV transient absorption spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 4597–4606. [Google Scholar] [CrossRef] [PubMed]
- Wolff, T.; Görner, H. Photocleavage of dimers of coumarin and 6-alkylcoumarins. J. Photochem. Photobiol. A Chem. 2010, 209, 219–223. [Google Scholar] [CrossRef]
- Schmidt, R.; Geissler, D.; Hagen, V.; Bendig, J. Mechanism of photocleavage of (coumarin-4-yl) methyl esters. J. Phys. Chem. A 2007, 111, 5768–5774. [Google Scholar] [CrossRef] [PubMed]
- Brett, T.J.; Alexander, J.M.; Clark, J.L.; Ross, C.R., II; Harbison, G.S.; Stezowski, J.J. Chemical insight from crystallographic disorder: Structural studies of a supramolecular β-cyclodextrin/coumarin photochemical system. Chem. Commun. 1999, 1275–1276. [Google Scholar] [CrossRef]
- Venkatesan, S.; Ranjithkumar, B.; Rajeshkumar, S.; Basha, K.A. Synthesis, characterization, thermal stability and antibacterial activity of coumarin based methacrylate copolymers. Chin. J. Polym. Sci. 2014, 32, 1373–1380. [Google Scholar] [CrossRef]
- Yamada, A.; Hiruta, Y.; Wang, J.; Ayano, E.; Kanazawa, H. Design of environmentally responsive fluorescent polymer probes for cellular imaging. Biomacromolecules 2015, 16, 2356–2362. [Google Scholar] [CrossRef]
- Maddipatla, M.V.; Wehrung, D.; Tang, C.; Fan, W.; Oyewumi, M.O.; Miyoshi, T.; Joy, A. Photoresponsive coumarin polyesters that exhibit cross-linking and chain scission properties. Macromolecules 2013, 46, 5133–5140. [Google Scholar] [CrossRef]
- Fiore, G.L.; Rowan, S.J.; Weder, C. Optically healable polymers. Chem. Soc. Rev. 2013, 42, 7278–7288. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, D.; Yang, Y.; Huang, Q.; Zhu, S.; Zheng, Z. Self-healing materials for next-generation energy harvesting and storage devices. Adv. Energy Mater. 2017, 7, 1700890. [Google Scholar] [CrossRef]
- Bekas, D.; Tsirka, K.; Baltzis, D.; Paipetis, A. Self-healing materials: A review of advances in materials, evaluation, characterization and monitoring techniques. Compos. Part B Eng. 2016, 87, 92–119. [Google Scholar] [CrossRef]
- Binder, W.H. Self-Healing Polymers: From Principles to Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Hia, I.L.; Vahedi, V.; Pasbakhsh, P. Self-healing polymer composites: Prospects, challenges, and applications. Polym. Rev. 2016, 56, 225–261. [Google Scholar] [CrossRef]
- Wagner, B.D. The use of coumarins as environmentally-sensitive fluorescent probes of heterogeneous inclusion systems. Molecules 2009, 14, 210–237. [Google Scholar] [CrossRef]
- Wong, P.T.; Choi, S.K. Mechanisms of drug release in nanotherapeutic delivery systems. Chem. Rev. 2015, 115, 3388–3432. [Google Scholar] [CrossRef]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases 2012, 7, 9. [Google Scholar] [CrossRef]
- Makhlouf, A.S.H.; Abu-Thabit, N.Y. Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications: Volume 1: Types and Triggers; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Aguilar, M.R.; San Román, J. Smart Polymers and Their Applications; Woodhead Publishing: Cambridge, UK, 2019. [Google Scholar]
- Trenor, S.R.; Shultz, A.R.; Love, B.J.; Long, T.E. Coumarins in polymers: From light harvesting to photo-cross-linkable tissue scaffolds. Chem. Rev. 2004, 104, 3059–3078. [Google Scholar] [CrossRef]
- Teixeira, E.; Lima, J.C.; Parola, A.J.; Branco, P.S. Incorporation of coumarin-based fluorescent monomers into co-oligomeric molecules. Polymers 2018, 10, 396. [Google Scholar] [CrossRef]
- Bisen, R.; Tripathi, J.; Sharma, A.; Khare, A.; Kumar, Y.; Tripathi, S. Optical behaviour of coumarin dye in PVA and PMMA film matrices. Vacuum 2018, 152, 65–69. [Google Scholar] [CrossRef]
- Shi, Y.; Cao, X.; Hu, D.; Gao, H. Highly branched polymers with layered structures that mimic light-harvesting processes. Angew. Chem. 2018, 130, 525–529. [Google Scholar] [CrossRef]
- Ji, W.; Qin, M.; Feng, C. Photoresponsive coumarin-based supramolecular hydrogel for controllable dye release. Macromol. Chem. Phys. 2018, 219, 1700398. [Google Scholar] [CrossRef]
- Karthik, S.; Jana, A.; Selvakumar, M.; Venkatesh, Y.; Paul, A.; Shah, S.S.; Singh, N.P. Coumarin polycaprolactone polymeric nanoparticles: Light and tumor microenvironment activated cocktail drug delivery. J. Mater. Chem. B 2017, 5, 1734–1741. [Google Scholar] [CrossRef]
- Stefanello, T.F.; Couturaud, B.; Szarpak-Jankowska, A.; Fournier, D.; Louage, B.; Garcia, F.P.; Nakamura, C.V.; De Geest, B.G.; Woisel, P.; Van Der Sanden, B. Coumarin-containing thermoresponsive hyaluronic acid-based nanogels as delivery systems for anticancer chemotherapy. Nanoscale 2017, 9, 12150–12162. [Google Scholar] [CrossRef]
- Yang, L.; Tang, H.; Sun, H. Progress in photo-responsive polypeptide derived nano-assemblies. Micromachines 2018, 9, 296. [Google Scholar] [CrossRef]
- Jellali, R.; Alexandre, M.; Jérôme, C. Photosensitive polydimethylsiloxane networks for adjustable-patterned films. Polym. Chem. 2017, 8, 2499–2508. [Google Scholar] [CrossRef]
- Mhiri, C.; Ternane, R.; Hamdi, N.; Baklouti, L. Synthesis of coumarin derivative using polymer supported reagents. Eur. J. Chem. 2018, 9, 89–91. [Google Scholar] [CrossRef]
- Engels, H.W.; Pirkl, H.G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile materials and sustainable problem solvers for today’s challenges. Angew. Chem. Int. Ed. 2013, 52, 9422–9441. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.; Ghazali, S.; Islam, M.; Jeyaratnam, N.; Yuvaraj, A. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons: Hoboken, NJ, USA, 2014; Volume 11. [Google Scholar]
- Maisonneuve, L.; Lamarzelle, O.A.; Rix, E.; Grau, E.; Cramail, H. Isocyanate-free routes to polyurethanes and poly (hydroxy urethane) s. Chem. Rev. 2015, 115, 12407–12439. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.J. Optically-active polyurethanes containing coumarin dimer component: Synthesis, characterization, and chiral recognition ability. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 2699–2707. [Google Scholar] [CrossRef]
- Tathe, A.B.; Sekar, N. NLOphoric red emitting bis coumarins with O-BF 2-O core-synthesis, photophysical properties and DFT studies. J. Fluoresc. 2016, 26, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Bruin, P.; Meeuwsen, E.; Van Andel, M.; Worst, J.; Pennings, A. Autoclavable highly cross-linked polyurethane networks in ophthalmology. Biomaterials 1993, 14, 1089–1097. [Google Scholar] [CrossRef]
- Laube, T.; Apel, H.; Koch, H.-R. Ultraviolet radiation absorption of intraocular lenses. Ophthalmology 2004, 111, 880–885. [Google Scholar] [CrossRef]
- Brockmann, C.; Schulz, M.; Laube, T. Transmittance characteristics of ultraviolet and blue-light-filtering intraocular lenses. J. Cataract Refract. Surg. 2008, 34, 1161–1166. [Google Scholar] [CrossRef]
- Wang, C.; Kuo, Y.; Chao, D. A study of fluorescent-dye polyurethane ionomer 1. Polym. Adv. Technol. 2000, 11, 127–135. [Google Scholar] [CrossRef]
- Honarkar, H. Waterborne polyurethanes: A review. J. Dispers. Sci. Technol. 2018, 39, 507–516. [Google Scholar] [CrossRef]
- Li, M.; Qiang, X.; Xu, W.; Zhang, H. Synthesis, characterization and application of AFC-based waterborne polyurethane. Prog. Org. Coat. 2015, 84, 35–41. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Gonzalez, A.S.; García-Martínez, J.C.; Ramos, M.J.; De Lucas, A.; Rodriguez, J.F. Click-ligation of coumarin to polyether polyols for polyurethane foams. Polym. Int. 2013, 62, 783–790. [Google Scholar] [CrossRef]
- Hanai, T.; Hatano, H. Advances in Liquid Chromatography: 35 Years of Column Liquid Chromatography in Japan; World Scientific: Singapore, 1996; Volume 3. [Google Scholar]
- Naeem, M.; Kim, W.; Cao, J.; Jung, Y.; Yoo, J.-W. Enzyme/pH dual sensitive polymeric nanoparticles for targeted drug delivery to the inflamed colon. Colloids Surf. B Biointerfaces 2014, 123, 271–278. [Google Scholar] [CrossRef]
- Finke, J.H.; Richter, C.; Gothsch, T.; Kwade, A.; Büttgenbach, S.; Müller-Goymann, C.C. Coumarin 6 as a fluorescent model drug: How to identify properties of lipid colloidal drug delivery systems via fluorescence spectroscopy? Eur. J. Lipid Sci. Technol. 2014, 116, 1234–1246. [Google Scholar] [CrossRef]
- Guo, Y.; Zong, S.; Pu, Y.; Xu, B.; Zhang, T.; Wang, B. Advances in pharmaceutical strategies enhancing the efficiencies of oral colon-targeted delivery systems in inflammatory bowel disease. Molecules 2018, 23, 1622. [Google Scholar] [CrossRef] [PubMed]
- Lautenschläger, C.; Schmidt, C.; Fischer, D.; Stallmach, A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2014, 71, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Merlin, D. Nanoparticle-based oral drug delivery systems targeting the colon for treatment of ulcerative colitis. Inflamm. Bowel Dis. 2018, 24, 1401–1415. [Google Scholar] [CrossRef]
- Yamaoka, T.; Makita, Y.; Sasatani, H.; Kim, S.-I.; Kimura, Y. Linear type azo-containing polyurethane as drug-coating material for colon-specific delivery: Its properties, degradation behavior, and utilization for drug formulation. J. Control. Release 2000, 66, 187–197. [Google Scholar] [CrossRef]
- Long, Y.-B.; Gu, W.-X.; Pang, C.; Ma, J.; Gao, H. Construction of coumarin-based cross-linked micelles with pH responsive hydrazone bond and tumor targeting moiety. J. Mater. Chem. B 2016, 4, 1480–1488. [Google Scholar] [CrossRef]
- Jia, F.; Wang, Y.; Wang, H.; Jin, Q.; Cai, T.; Chen, Y.; Ji, J. Light cross-linkable and pH de-cross-linkable drug nanocarriers for intracellular drug delivery. Polym. Chem. 2015, 6, 2069–2075. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, K.H. Synthesis and reversible photocleavage of novel polyurethanes containing coumarin dimer components. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 613–624. [Google Scholar] [CrossRef]
- Aguirresarobe, R.; Irusta, L.; Fernández-Berridi, M. UV-light responsive waterborne polyurethane based on coumarin: Synthesis and kinetics of reversible chain extension. J. Polym. Res. 2014, 21, 505. [Google Scholar] [CrossRef]
- Seoane Rivero, R.; Bilbao Solaguren, P.; Gondra Zubieta, K.; Peponi, L.; Marcos-Fernández, A. Synthesis, kinetics of photo-dimerization/photo-cleavage and physical properties of coumarin-containing branched polyurethanes based on polycaprolactones. Express Polym. Lett. 2016, 10, 84. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, R.; Ren, Y.; Yin, J. Responsive polymer nanoparticles formed by poly (ether amine) containing coumarin units and a poly (ethylene oxide) short chain. Langmuir 2009, 25, 9629–9632. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Rong, M.-Z.; Zhang, M.-Q. Effect of molecular weight of PEG soft segments on photo-stimulated self-healing performance of coumarin functionalized polyurethanes. Chin. J. Polym. Sci. 2014, 32, 1286–1297. [Google Scholar] [CrossRef]
- Seoane Rivero, R.; Bilbao Solaguren, P.; Gondra Zubieta, K.; Gonzalez-Jimenez, A.; Valentin, J.L.; Marcos-Fernandez, A. Synthesis and characterization of a photo-crosslinkable polyurethane based on a coumarin-containing polycaprolactone diol. Eur. Polym. J. 2016, 76, 245–255. [Google Scholar] [CrossRef]
- Salgado, C.; Arrieta, M.P.; Peponi, L.; Fernández-García, M.; López, D. Influence of Poly (ε-caprolactone) molecular weight and coumarin amount on photo-responsive polyurethane properties. Macromol. Mater. Eng. 2017, 302, 1600515. [Google Scholar] [CrossRef]
- Salgado, C.; Arrieta, M.P.; Peponi, L.; Fernández-García, M.; López, D. Silica-nanocomposites of photo-crosslinkable poly (urethane) s based on poly (ε-caprolactone) and coumarin. Eur. Polym. J. 2017, 93, 21–32. [Google Scholar] [CrossRef]
- Salgado, C.; Arrieta, M.P.; Peponi, L.; López, D.; Fernández-García, M. Photo-crosslinkable polyurethanes reinforced with coumarin modified silica nanoparticles for photo-responsive coatings. Prog. Org. Coat. 2018, 123, 63–74. [Google Scholar] [CrossRef]
- Seoane Rivero, R.; Navarro, R.; Bilbao Solaguren, P.; Gondra Zubieta, K.; Cuevas, J.M.; Marcos-Fernández, A. Synthesis and characterization of photo-crosslinkable linear segmented polyurethanes based on coumarin. Eur. Polym. J. 2017, 92, 263–274. [Google Scholar] [CrossRef]
- Song, M.-M.; Wang, Y.-M.; Liang, X.-Y.; Zhang, X.-Q.; Zhang, S.; Li, B.-J. Functional materials with self-healing properties: A review. Soft Matter 2019, 15, 6615–6625. [Google Scholar] [CrossRef]
- Ling, J.; Rong, M.Z.; Zhang, M.Q. Coumarin imparts repeated photochemical remendability to polyurethane. J. Mater. Chem. 2011, 21, 18373–18380. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Li, J.; Ling, L.; Zhang, G.; Sun, R.; Wong, C.-P. UV-triggered self-healing polyurethane with enhanced stretchability and elasticity. Polymer 2019, 172, 187–195. [Google Scholar] [CrossRef]
- Ling, J.; Rong, M.Z.; Zhang, M.Q. Photo-stimulated self-healing polyurethane containing dihydroxyl coumarin derivatives. Polymer 2012, 53, 2691–2698. [Google Scholar] [CrossRef]
- Langeveld, J.; Dixon, J.; Jaworskic, J. Development perspectives of the biobased economy: A review. Crop Sci. 2010, 50. [Google Scholar] [CrossRef]
- Wong, C.S.; Hassan, N.I.; Su’ait, M.S.; Serra, M.A.P.; Gonzalez, J.A.M.; Granda, L.A.; Badri, K.H. Photo-activated self-healing bio-based polyurethanes. Ind. Crop. Prod. 2019, 140, 111613. [Google Scholar] [CrossRef]
- Wang, K.; Jia, Y.-G.; Zhao, C.; Zhu, X. Multiple and two-way reversible shape memory polymers: Design strategies and applications. Prog. Mater. Sci. 2019, 2019, 100572. [Google Scholar] [CrossRef]
- Xie, H.; Yang, K.-K.; Wang, Y.-Z. Photo-cross-linking: A powerful and versatile strategy to develop shape-memory polymers. Prog. Polym. Sci. 2019. [Google Scholar] [CrossRef]
- Xie, H.; Cheng, C.-Y.; Du, L.; Fan, C.-J.; Deng, X.-Y.; Yang, K.-K.; Wang, Y.-Z. A facile strategy to construct PDLLA-PTMEG network with triple-shape effect via photo-cross-linking of anthracene groups. Macromolecules 2016, 49, 3845–3855. [Google Scholar] [CrossRef]
- Xie, H.; Deng, X.Y.; Cheng, C.Y.; Yang, K.K.; Wang, Y.Z. New strategy to access dual-stimuli-responsive triple-shape-memory effect in a non-overlapping pattern. Macromol. Rapid Commun. 2017, 38, 1600664. [Google Scholar] [CrossRef]
- Wu, L.; Jin, C.; Sun, X. Synthesis, properties, and light-induced shape memory effect of multiblock polyesterurethanes containing biodegradable segments and pendant cinnamamide groups. Biomacromolecules 2011, 12, 235–241. [Google Scholar] [CrossRef]
- Wen, Z.-B.; Liu, D.; Li, X.-Y.; Zhu, C.-H.; Shao, R.-F.; Visvanathan, R.; Clark, N.A.; Yang, K.-K.; Wang, Y.-Z. Fabrication of liquid crystalline polyurethane networks with a pendant azobenzene group to access thermal/photoresponsive shape-memory effects. ACS Appl. Mater. Interfaces 2017, 9, 24947–24954. [Google Scholar] [CrossRef]
- Zhao, X.; Dang, Y.; Deng, J.; Zhang, J. Photoinduced shape fixity and thermal-induced shape recovery properties based on polyvinyl alcohol bearing coumarin. Colloid Polym. Sci. 2014, 292, 85–95. [Google Scholar] [CrossRef]
- Defize, T.; Thomassin, J.-M.; Ottevaere, H.; Malherbe, C.D.; Eppe, G.; Jellali, R.; Alexandre, M.L.; Jérôme, C.; Riva, R.L. Photo-cross-linkable coumarin-based Poly (ε-caprolactone) for light-controlled design and reconfiguration of shape-memory polymer networks. Macromolecules 2018, 52, 444–456. [Google Scholar] [CrossRef]
- Nagata, M.; Yamamoto, Y. Photocurable shape-memory copolymers of ε-caprolactone and L-lactide. Macromol. Chem. Phys. 2010, 211, 1826–1835. [Google Scholar] [CrossRef]
- Nagata, M.; Yamamoto, Y. Synthesis and characterization of photocrosslinked poly (ε-caprolactone) s showing shape-memory properties. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2422–2433. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, Z.; Huang, H.; Chen, Y. The design of triple shape memory polymers with stable yet tunable temporary shapes by introducing photo-responsive units into a crystalline domain. Polym. Chem. 2019, 10, 1537–1543. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, H.; Chen, Y. Synthesis of triple shape memory polyurethanes by introducing photo-responsive coumarin units into the crystalline soft segment. Mater. Today Proc. 2019, 16, 1507–1511. [Google Scholar] [CrossRef]
- El-Wahab, H.A.; El-Fattah, M.A.; El-Khalik, N.A.; Nassar, H.S.; Abdelall, M.M. Synthesis and characterization of coumarin thiazole derivative 2-(2-amino-1, 3-thiazol-4-yl)-3H-benzo [f] chromen-3-one with anti-microbial activity and its potential application in antimicrobial polyurethane coating. Prog. Org. Coat. 2014, 77, 1506–1511. [Google Scholar] [CrossRef]
- El-Fattah, M.A.; El-Wahab, H.A.; Bashandy, M.; El-Eisawy, R.; El-hai, F.A.; Saeed, M. Potential application of some coumarin derivatives incorporated thiazole ring as ecofriendly antimicrobial, flame retardant and corrosion inhibitor additives for polyurethane coating. Prog. Org. Coat. 2017, 111, 57–66. [Google Scholar] [CrossRef]
- Kashyap, S.J.; Garg, V.K.; Sharma, P.K.; Kumar, N.; Dudhe, R.; Gupta, J.K. Thiazoles: Having diverse biological activities. Med. Chem. Res. 2012, 21, 2123–2132. [Google Scholar] [CrossRef]
- Singh, L.R.; Avula, S.R.; Raj, S.; Srivastava, A.; Palnati, G.R.; Tripathi, C.; Pasupuleti, M.; Sashidhara, K.V. Coumarin–benzimidazole hybrids as a potent antimicrobial agent: Synthesis and biological elevation. J. Antibiot. 2017, 70, 954–961. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Ali, W.B.; Khadom, A.A. Synthesis and investigations of heterocyclic compounds as corrosion inhibitors for mild steel in hydrochloric acid. Int. J. Ind. Chem. 2019, 10, 159–173. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas, J.M.; Seoane-Rivero, R.; Navarro, R.; Marcos-Fernández, Á. Coumarins into Polyurethanes for Smart and Functional Materials. Polymers 2020, 12, 630. https://doi.org/10.3390/polym12030630
Cuevas JM, Seoane-Rivero R, Navarro R, Marcos-Fernández Á. Coumarins into Polyurethanes for Smart and Functional Materials. Polymers. 2020; 12(3):630. https://doi.org/10.3390/polym12030630
Chicago/Turabian StyleCuevas, José María, Rubén Seoane-Rivero, Rodrigo Navarro, and Ángel Marcos-Fernández. 2020. "Coumarins into Polyurethanes for Smart and Functional Materials" Polymers 12, no. 3: 630. https://doi.org/10.3390/polym12030630
APA StyleCuevas, J. M., Seoane-Rivero, R., Navarro, R., & Marcos-Fernández, Á. (2020). Coumarins into Polyurethanes for Smart and Functional Materials. Polymers, 12(3), 630. https://doi.org/10.3390/polym12030630







