Self-Consistent Mean Field Calculations of Polyelectrolyte-Surfactant Mixtures in Solution and upon Adsorption onto Negatively Charged Surfaces
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
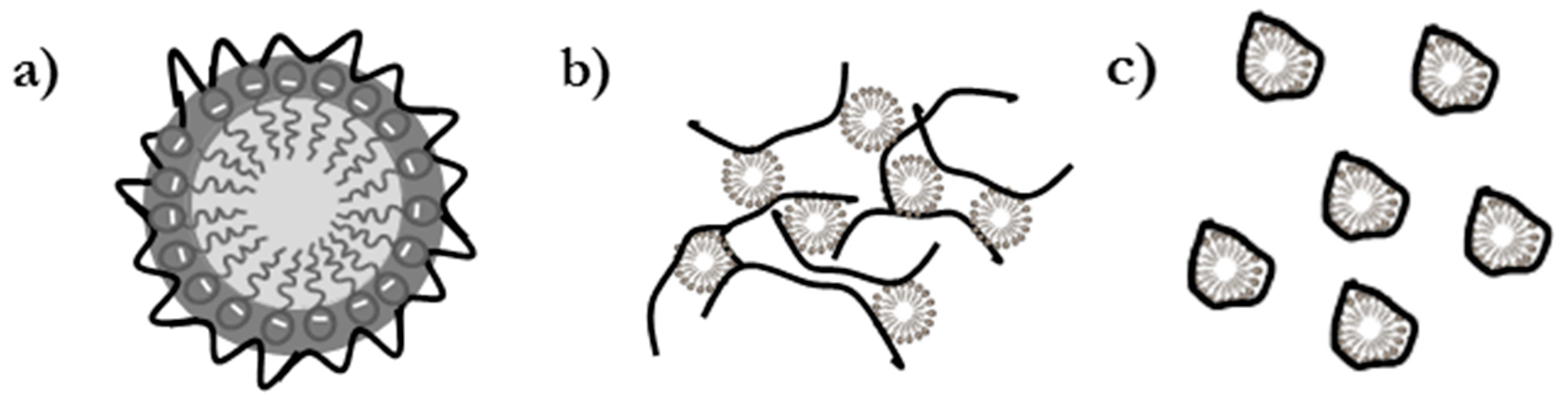
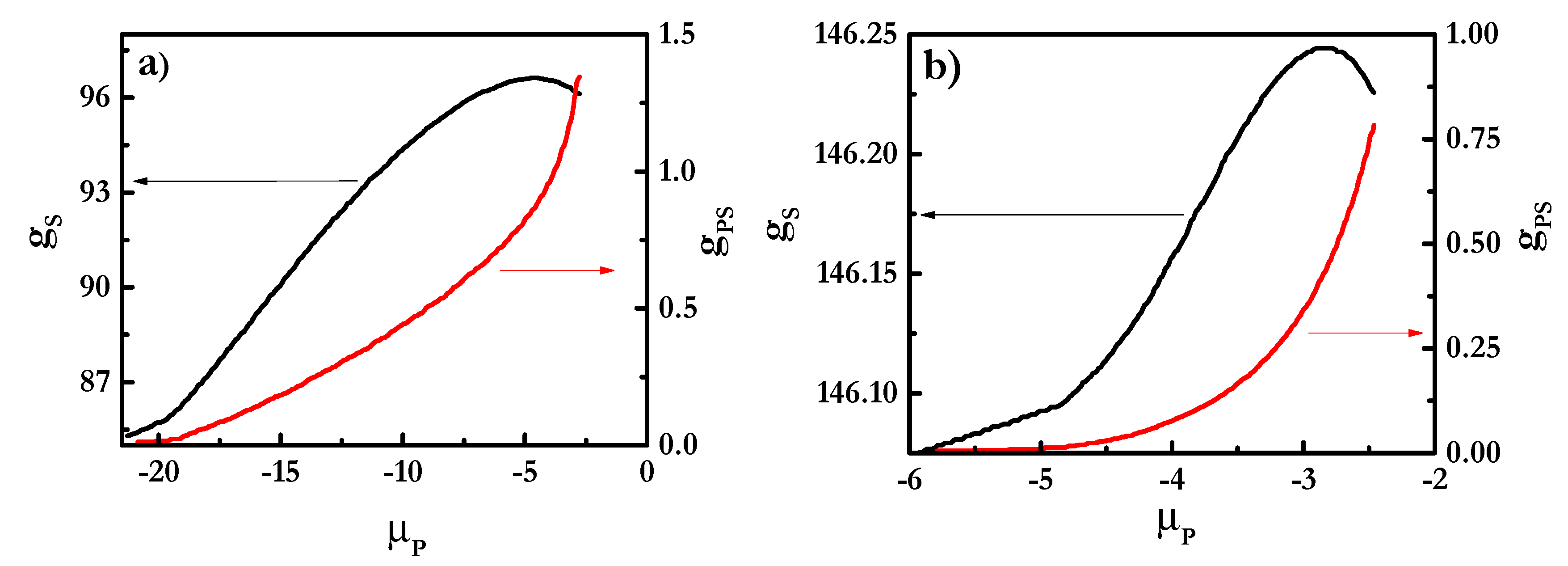
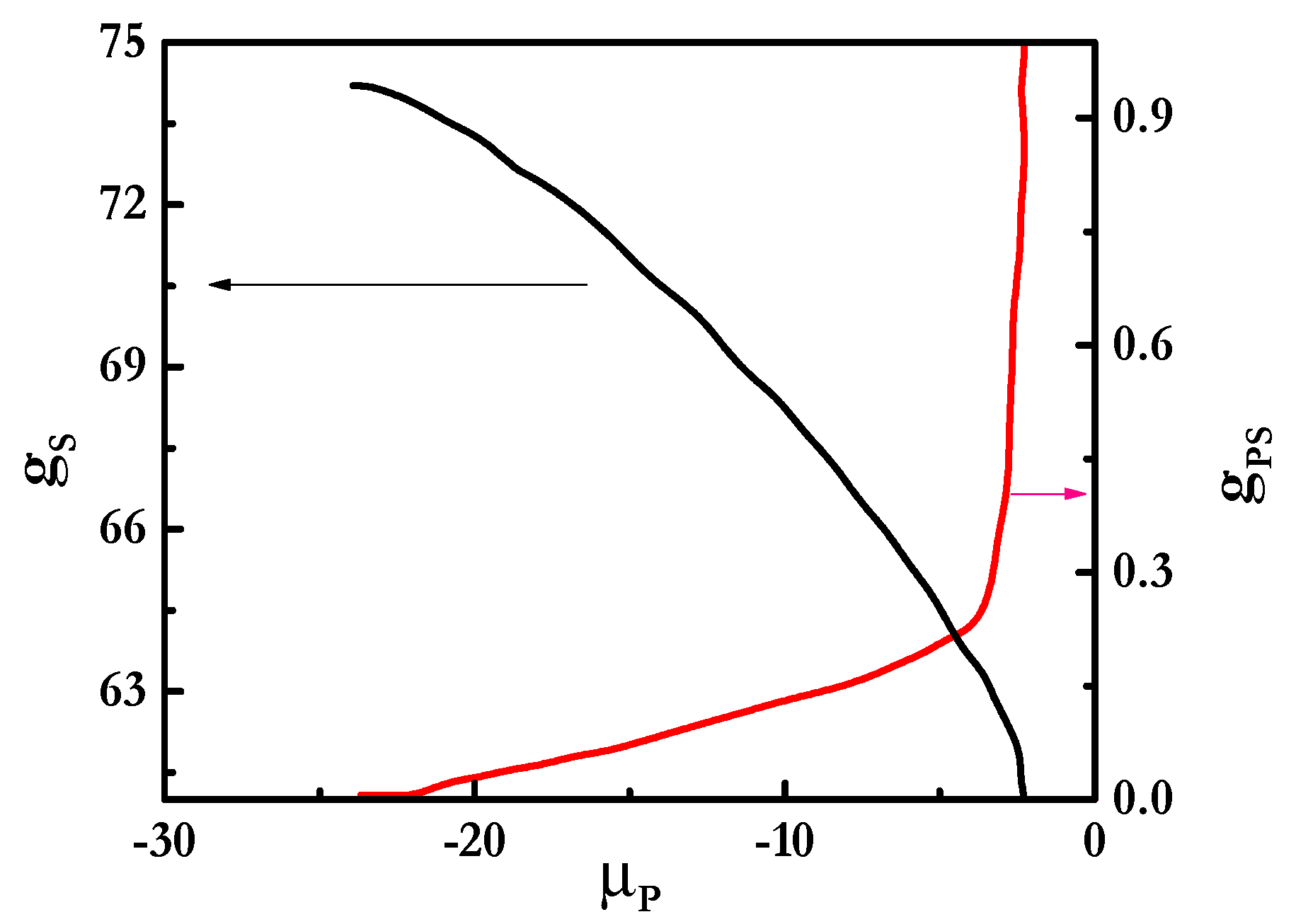
3.1. SCF Calculations of Pseudo-Binary Polyelectrolyte-Surfactant Mixtures in the Bulk
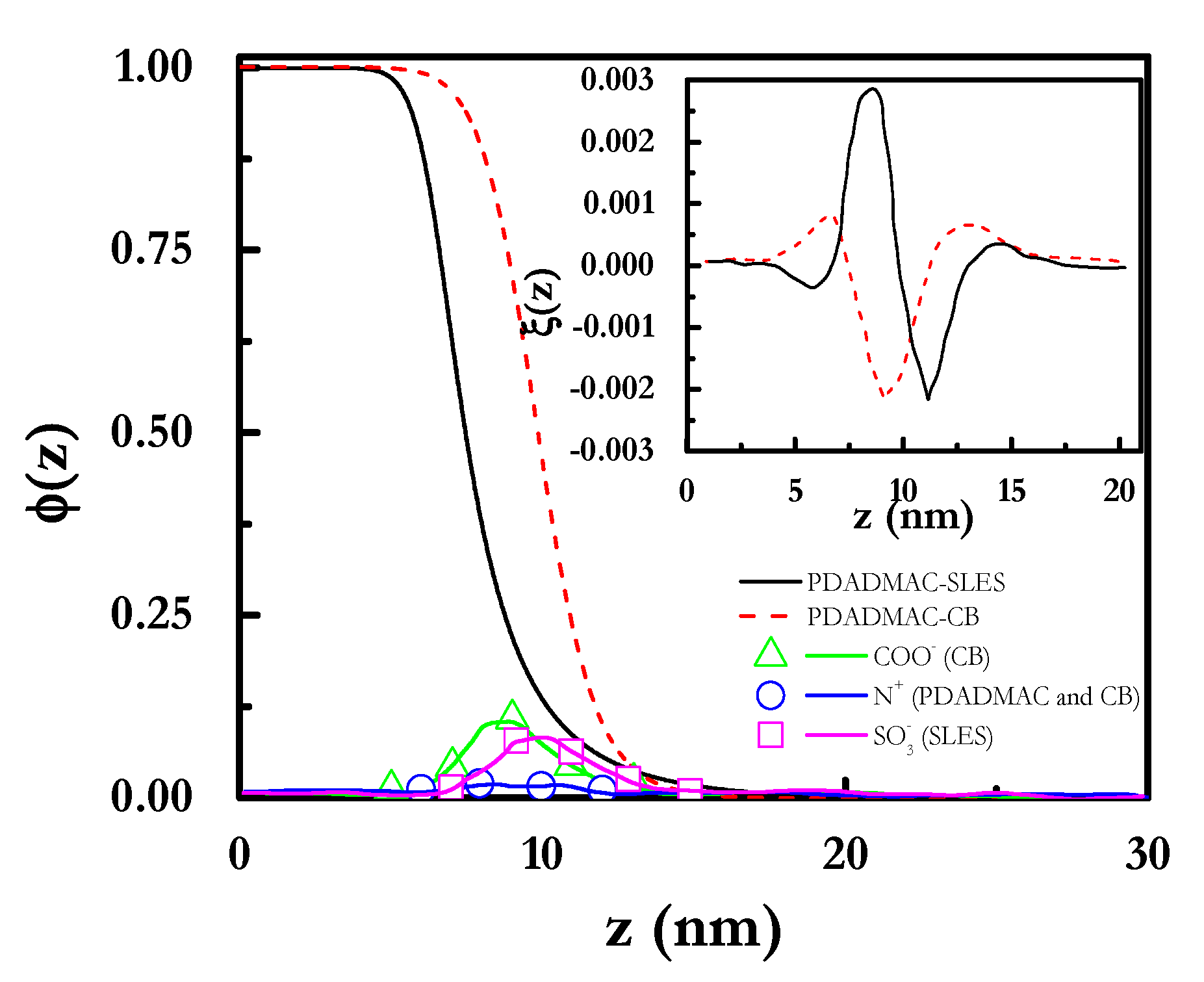
3.2. SCF Calculations of Polyelectrolyte-Surfactant Mixture Adsorption onto a Solid Interface
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guzmán, E.; Llamas, S.; Maestro, A.; Fernández-Peña, L.; Akanno, A.; Miller, R.; Ortega, F.; Rubio, R.G. Polymer–surfactant systems in bulk and at fluid interfaces. Adv. Colloid Interface Sci. 2016, 233, 38–64. [Google Scholar] [CrossRef] [PubMed]
- Llamas, S.; Guzmán, E.; Ortega, F.; Baghdadli, N.; Cazeneuve, C.; Rubio, R.G.; Luengo, G.S. Adsorption of polyelectrolytes and polyelectrolytes-surfactant mixtures at surfaces: A physico-chemical approach to a cosmetic challenge. Adv. Colloid Interface Sci. 2015, 222, 461–487. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.D.; Claesson, P.M.; Langevin, D.; Meszaros, R.; Nylander, T.; Stubenrauch, C.; Titmuss, S.; von Klitzing, R. Complexes of surfactants with oppositely charged polymers at surfaces and in bulk. Adv. Colloid Interface Sci. 2010, 155, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Nylander, T.; Samoshina, Y.; Lindman, B. Formation of polyelectrolyte–surfactant complexes on surfaces. Adv. Colloid Interface Sci. 2006, 123-126, 105–123. [Google Scholar] [CrossRef]
- Varga, I.; Campbell, R.A. General Physical Description of the Behavior of Oppositely Charged Polyelectrolyte/Surfactant Mixtures at the Air/Water Interface. Langmuir 2017, 33, 5915–5924. [Google Scholar] [CrossRef]
- Li, P.; Penfold, J.; Thomas, R.K.; Xu, H. Multilayers formed by polyelectrolyte-surfactant and related mixtures at the air-water interface. Adv. Colloid Interface Sci. 2019, 269, 43–86. [Google Scholar] [CrossRef]
- Thomas, R.K.; Penfold, J. Thermodynamics of the Air–Water Interface of Mixtures of Surfactants with Polyelectrolytes, Oligoelectrolytes, and Multivalent Metal Electrolytes. J. Phys. Chem. B 2018, 122, 12411–12427. [Google Scholar] [CrossRef]
- Chiappisi, L.; Hoffmann, I.; Gradzielski, M. Complexes of oppositely charged polyelectrolytes and surfactants—Recent developments in the field of biologically derived polyelectrolytes. Soft Matter 2013, 9, 3896–3909. [Google Scholar] [CrossRef] [Green Version]
- Kizilay, E.; Dinsmore, A.D.; Hoagland, D.A.; Sun, L.; Dubin, P.L. Evolution of hierarchical structures in polyelectrolyte–micelle coacervates. Soft Matter 2013, 9, 7320–7332. [Google Scholar] [CrossRef]
- Szczepanowicz, K.; Bazylińska, U.; Pietkiewicz, J.; Szyk-Warszyńska, L.; Wilk, K.A.; Warszyński, P. Biocompatible long-sustained release oil-core polyelectrolyte nanocarriers: From controlling physical state and stability to biological impact. Adv. Colloid Interface Sci. 2015, 222, 678–691. [Google Scholar] [CrossRef]
- Lindman, B.; Antunes, F.; Aidarova, S.; Miguel, M.; Nylander, T. Polyelectrolyte-surfactant association—From fundamentals to applications. Colloid J. 2014, 76, 585–594. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, J.H. Hybrid. Enhanced Oil Recovery using Smart Waterflooding; Gulf Professional Publishing: Houston, TX, USA, 2019. [Google Scholar]
- Aguilar, Z.P. Nanomaterials for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Bernardes, J.S.; Piculell, L.; Loh, W. Self-Assembly of Polyion-Surfactant Ion Complex Salts in Mixtures with Water and n-Alcohols. J. Phys. Chem. B 2011, 115, 9050–9058. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, S.; Gustavsson, C.; Gudmundsson, C.; Linse, P.; Piculell, L. When Do Water-Insoluble Polyion-Surfactant Ion Complex Salts "Redissolve" by Added Excess Surfactant? Langmuir 2011, 27, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Janiak, J.; Piculell, L.; Olofsson, G.; Schillen, K. The aqueous phase behavior of polyion-surfactant ion complex salts mixed with nonionic surfactants. Phys. Chem. Chem. Phys. 2011, 13, 3126–3138. [Google Scholar] [CrossRef]
- Svensson, A.; Norrman, J.; Piculell, L. Phase behavior of polyion-surfactant ion complex salts: Effects of surfactant chain length and polyion length. J. Phys. Chem. B 2006, 110, 10332–10340. [Google Scholar] [CrossRef] [PubMed]
- Bali, K.; Varga, Z.; Kardos, A.; Meszaros, R. Impact of local inhomogeneities on the complexation between poly (diallyldimethylammoniumchloride) and sodium dodecyl sulfate. Colloids Surf. A 2019, 574, 21–28. [Google Scholar] [CrossRef]
- Bali, K.; Varga, Z.; Kardos, A.; Varga, I.; Gilanyi, T.; Domjan, A.; Wacha, A.; Bota, A.; Mihaly, J.; Meszaros, R. Effect of Dilution on the Nonequilibrium Polyelectrolyte/Surfactant Association. Langmuir 2018, 34, 14652–14660. [Google Scholar] [CrossRef]
- Bodnar, K.; Fegyver, E.; Nagy, M.; Meszaros, R. Impact of Polyelectrolyte Chemistry on the Thermodynamic Stability of Oppositely Charged Macromolecule/Surfactant Mixtures. Langmuir 2016, 32, 1259–1268. [Google Scholar] [CrossRef]
- Plazzotta, B.; Fegyver, E.; Meszaros, R.; Pedersen, J.S. Anisometric Polyelectrolyte/Mixed Surfactant Nanoassemblies Formed by the Association of Poly (diallyldimethylammonium chloride) with Sodium Dodecyl Sulfate and Dodecyl Maltoside. Langmuir 2015, 31, 7242–7250. [Google Scholar] [CrossRef]
- Naderi, A.; Claesson, P.M.; Bergstrom, M.; Dedinaite, A. Trapped non-equilibrium states in aqueous solutions of oppositely charged polyelectrolytes and surfactants: Effects of mixing protocol and salt concentration. Colloids Surf. A Physicochem. Eng. Asp. 2005, 253, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Naderi, A.; Claesson, P.M. Association between poly (vinylamine) and sodium dodecyl sulfate: Effects of mixing protocol, blending procedure, and salt concentration. J. Dispers. Sci. Technol. 2005, 26, 329–340. [Google Scholar] [CrossRef]
- Ferreira, G.A.; Piculell, L.; Loh, W. Addition of n-Alcohols Induces a Variety of Liquid-Crystalline Structures in Surfactant-Rich Cores of Dispersed Block Copolymer/Surfactant Nanoparticles. ACS Omega 2016, 1, 1104–1113. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, G.A.; Piculell, L.; Loh, W. Hydration-Dependent Hierarchical Structures in Block Copolymer−Surfactant Complex Salts. Macromolecules 2018, 51, 9915–9924. [Google Scholar] [CrossRef]
- Santore, M.; Dias, R.D.; Piculell, L. Editorial Overview—Polynucleotides. Curr. Opin. Colloid Interface Sci. 2016, 26, A1. [Google Scholar] [CrossRef]
- Nizri, G.; Lagerge, S.; Kamyshny, A.; Major, D.T.; Magdassi, S. Polymer-surfactant interactions: Binding mechanism of sodium dodecyl sulfate to poly (diallyldimethylammonium chloride). J. Colloid Interface Sci. 2008, 320, 74–81. [Google Scholar] [CrossRef]
- Nizri, G.; Magdassi, S.; Schmidt, J.; Cohen, Y.; Talmon, Y. Microstructural characterization of micro- and nanoparticles formed by polymer-surfactant interactions. Langmuir 2004, 20, 4380–4385. [Google Scholar] [CrossRef]
- Mezei, A.; Meszaros, R. Novel method for the estimation of the binding isotherms of ionic surfactants on oppositely charged polyelectrolytes. Langmuir 2006, 22, 7148–7151. [Google Scholar] [CrossRef]
- Amalia, M.; Pojjak, K.; Robert, M. Nonequilibrium features of the association between poly (vinylamine) and sodium dodecyl sulfate: The validity of the colloid dispersion concept. J. Phys. Chem. B 2008, 112, 9693–9699. [Google Scholar] [CrossRef]
- Mezei, A.; Meszaros, R.; Varga, I.; Gilanyi, T. Effect of mixing on the formation of complexes of hyperbranched cationic polyelectrolytes and anionic surfactants. Langmuir 2007, 23, 4237–4247. [Google Scholar] [CrossRef]
- Llamas, S.; Guzman, E.; Akanno, A.; Fernandez-Pena, L.; Ortega, F.; Campbell, R.A.; Miller, R.; Rubio, R.G. Study of the Liquid/Vapor Interfacial Properties of Concentrated Polyelectrolyte-Surfactant Mixtures Using Surface Tensiometry and Neutron Reflectometry: Equilibrium, Adsorption Kinetics, and Dilational Rheology. J. Phys. Chem. C 2018, 122, 4419–4427. [Google Scholar] [CrossRef]
- Llamas, S.; Fernandez-Pena, L.; Akanno, A.; Guzman, E.; Ortega, V.; Ortega, F.; Csaky, A.G.; Campbell, R.A.; Rubio, R.G. Towards understanding the behavior of polyelectrolyte-surfactant mixtures at the water/vapor interface closer to technologically-relevant conditions. Phys. Chem. Chem. Phys. 2018, 20, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Arteta, M.Y.; Angus-Smyth, A.; Nylander, T.; Noskov, B.A.; Varga, I. Direct Impact of Nonequilibrium Aggregates on the Structure and Morphology of Pdadmac/SDS Layers at the Air/Water Interface. Langmuir 2014, 30, 8664–8674. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Kardos, A.; Mezei, A.; Campbell, R.A.; Varga, I. Effects of Ionic Strength on the Surface Tension and Nonequilibrium Interfacial Characteristics of Poly(sodium styrenesulfonate)/Dodecyltrimethylammonium Bromide Mixtures. Langmuir 2014, 30, 4970–4979. [Google Scholar] [CrossRef]
- Angus-Smyth, A.; Bain, C.D.; Varga, I.; Campbell, R.A. Effects of bulk aggregation on PEI-SDS monolayers at the dynamic air-liquid interface: Depletion due to precipitation versus enrichment by a convection/spreading mechanism. Soft Matter 2013, 9, 6103–6117. [Google Scholar] [CrossRef]
- Campbell, R.A.; Arteta, M.Y.; Angus-Smyth, A.; Nylander, T.; Varga, I. Effects of Bulk Colloidal Stability on Adsorption Layers of Poly(diallyldimethylammonium Chloride)/Sodium Dodecyl Sulfate at the Air-Water Interface Studied by Neutron Reflectometry. J. Phys. Chem. B 2011, 115, 15202–15213. [Google Scholar] [CrossRef]
- Fernández-Peña, L.; Guzmán, E.; Leonforte, F.; Serrano-Pueyo, A.; Regulski, K.; Tournier-Couturier, L.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Effect of molecular structure of eco-friendly glycolipid biosurfactants on the adsorption of hair-care conditioning polymers. Colloids Surf. B 2020, 185, 110578. [Google Scholar] [CrossRef]
- Guzmán, E.; Llamas, S.; Fernández-Peña, L.; Léonforte, F.; Baghdadli, N.; Cazeneuve, C.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Effect of a natural amphoteric surfactant in the bulk and adsorption behavior of polyelectrolyte-surfactant mixtures. Colloids Surf. A 2019, 124178. [Google Scholar] [CrossRef]
- Fleer, G.J.; Skvortsov, A.M. Reconciling lattice and continuum models for polymers at interfaces. J. Chem. Phys. 2012, 136, 134707. [Google Scholar] [CrossRef]
- Fleer, G.J. Polymers at interfaces and in colloidal dispersions. Adv. Colloid Interface Sci. 2010, 159, 99–116. [Google Scholar] [CrossRef]
- Fleer, G.J.; Leermakers, F.A.M. Statistical thermodynamics of polymer layers. Curr. Opin. Colloid Interface Sci. 1997, 2, 308–314. [Google Scholar] [CrossRef]
- Fleer, G.; Cohen-Stuart, M.A.; Scheutjens, J.; Crosgrove, T.; Vincent, B. Polymer at Interfaces; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Israels, R.; Scheutjens, J.; Fleer, G.J. Adsorption of Ionic Block-Copolymers-Self-Consistent-Field Analysis and Scaling Predictions. Macromolecules 1993, 26, 5405–5413. [Google Scholar] [CrossRef]
- Evers, O.A.; Scheutjens, J.M.H.M.; Fleer, G.J. Statistical thermodynamics of block copolymer adsorption. Part 2.—Effect of chain composition on the adsorbed amount and layer thickness. J. Chem. Soc. Farady Trans. 1990, 86, 1333–1340. [Google Scholar] [CrossRef]
- Scheujtens, J.M.H.M.; Fleer, G.J. Statistical theory of the adsorption of interacting chain molecules. 1. Partition function, segment density distribution, and adsorption isotherms. J. Phys. Chem. 1979, 83, 1619–1635. [Google Scholar] [CrossRef]
- Llamas, S.; Guzmán, E.; Baghdadli, N.; Ortega, F.; Cazeneuve, C.; Rubio, R.G.; Luengo, G.S. Adsorption of poly(diallyldimethylammonium chloride)—Sodium methyl-cocoyl-taurate complexes onto solid surfaces. Colloids Surf. A 2016, 505, 150–157. [Google Scholar] [CrossRef]
- Scheutjens, J.M.H.M.; Fleer, G.J. Statistical theory of the adsorption of interacting chain molecules. 2. Train, loop, and tail size distribution. J. Phys. Chem. 1980, 84, 178–190. [Google Scholar] [CrossRef]
- Li, F.; Schellekens, M.; de Bont, J.; Peters, R.; Overbeek, A.; Leermakers, F.A.M.; Tuinier, R. Self-Assembled Structures of PMAA–PMMA Block Copolymers: Synthesis, Characterization, and Self-Consistent Field Computations. Macromolecules 2015, 48, 1194–1203. [Google Scholar] [CrossRef]
- Leermakers, F.A.M.; Scheutjens, J.M.H.M. Statistical thermodynamics of association colloids. 2. Lipid vesicles. J. Phys. Chem. 1989, 93, 7417–7426. [Google Scholar] [CrossRef]
- Postmus, B.R.; Leermakers, F.A.M.; Cohen Stuart, M.A. Self-Consistent Field Modeling of Poly (ethylene oxide) Adsorption onto Silica: The Multiple Roles of Electrolytes. Langmuir 2008, 24, 1930–1942. [Google Scholar] [CrossRef]
- Postmus, B.R.; Leermakers, F.A.M.; Cohen Stuart, M.A. Self-Consistent Field Modeling of Adsorption from Polymer/Surfactant Mixtures. Langmuir 2008, 24, 6712–6720. [Google Scholar] [CrossRef]
- Li, F.; Tuinier, R.; van Casteren, I.; Tennebroek, R.; Overbeek, A.; Leermakers, F.A.M. Self-Organization of Polyurethane Pre-Polymers as Studied by Self-Consistent Field Theory. Macromol. Theory Simul. 2016, 25, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Postmus, B.R.; Leermakers, F.A.M.; Stuart, M.A.C. Self-Consistent Field Modeling of Non-ionic Surfactants at the Silica−Water Interface: Incorporating Molecular Detail. Langmuir 2008, 24, 3960–3969. [Google Scholar] [CrossRef]
- Leermakers, F.A.M.; Scheutjens, J.M.H.M. Statistical thermodynamics of association colloids: V. critical micelle concentration, micellar size and shape. J. Colloid Interface Sci. 1990, 136, 231–241. [Google Scholar] [CrossRef]
- Banerjee, S.; Cazeneuve, C.; Baghdadli, N.; Ringeissen, S.; Leermakers, F.A.M.; Luengo, G.S. Surfactant-polymer interactions: Molecular architecture does matter. Soft Matter 2015, 11, 2504–2511. [Google Scholar] [CrossRef]
- Banerjee, S.; Cazeneuve, C.; Baghdadli, N.; Ringeissen, S.; Lénforte, F.; Leermakers, F.A.M.; Luengo, G.S. Modeling of Polyelectrolyte Adsorption from Micellar Solutions onto Biomimetic Substrates. J. Phys. Chem. B 2017, 121, 8638–8651. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Bleuel, M.; Prabhu, V.M. Lower Critical Solution Temperature in Polyelectrolyte Complex Coacervates. ACS Macro Lett. 2019, 8, 289–293. [Google Scholar] [CrossRef]
- Edwards, S. The Statistical Mechanics of Polymers with Excluded Volume. Proc. Phys. Soc. Lond. 1965, 85, 613–624. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Hill, T. An. Introduction in Statistical Thermodynamics; Wesley Publisher: London, UK, 1960. [Google Scholar]
- Khan, N.; Brettmann, B. Intermolecular Interactions in Polyelectrolyte and Surfactant Complexes in Solution. Polymers 2018, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Szilagyi, I.; Trefalt, G.; Tiraferri, A.; Maroni, P.; Borkovec, M. Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 2014, 10, 2479–2502. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, I.; Heunemann, P.; Prévost, S.; Schweins, R.; Wagner, N.J.; Gradzielski, M. Self-Aggregation of Mixtures of Oppositely Charged Polyelectrolytes and Surfactants Studied by Rheology, Dynamic Light Scattering and Small-Angle Neutron Scattering. Langmuir 2011, 27, 4386–4396. [Google Scholar] [CrossRef]
- Hoffmann, I.; Prévost, S.; Medebach, M.; Rogers, S.; Wagner, N.J.; Gradzielski, M. Control of Rheological Behaviour with Oppositely Charged Polyelectrolyte Surfactant Mixtures. Tenside Surfactants Deterg. 2011, 48, 488–494. [Google Scholar] [CrossRef]
- Akanno, A.; Guzmán, E.; Fernández-Peña, L.; Ortega, F.; Rubio, R.G. Surfactant-Like Behavior for the Adsorption of Mixtures of a Polycation and Two Different Zwitterionic Surfactants at the Water/Vapor Interface. Molecules 2019, 24, 3442. [Google Scholar] [CrossRef] [Green Version]
- Akanno, A.; Guzmán, E.; Fernández-Peña, L.; Llamas, S.; Ortega, F.; Rubio, R.G. Equilibration of a Polycation–Anionic Surfactant Mixture at the Water/Vapor Interface. Langmuir 2018, 34, 7455–7464. [Google Scholar] [CrossRef]
- Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Llamas, S.; Ortega, F.; Rubio, R.G. Two Different Scenarios for the Equilibration of Polycation—Anionic Solutions at Water–Vapor Interfaces. Coatings 2019, 9, 438. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.H.; Lv, W.J.; Zhao, S.L.; Shang, Y.Z.; Peng, C.J.; Wang, H.L.; Liu, H.L. Effects of the hydrophilicity or hydrophobicity of the neutral block on the structural formation of a block polyelectrolyte/surfactant complex: A molecular dynamics simulation study. Comput. Cond. Matter 2015, 2, 16–24. [Google Scholar] [CrossRef] [Green Version]


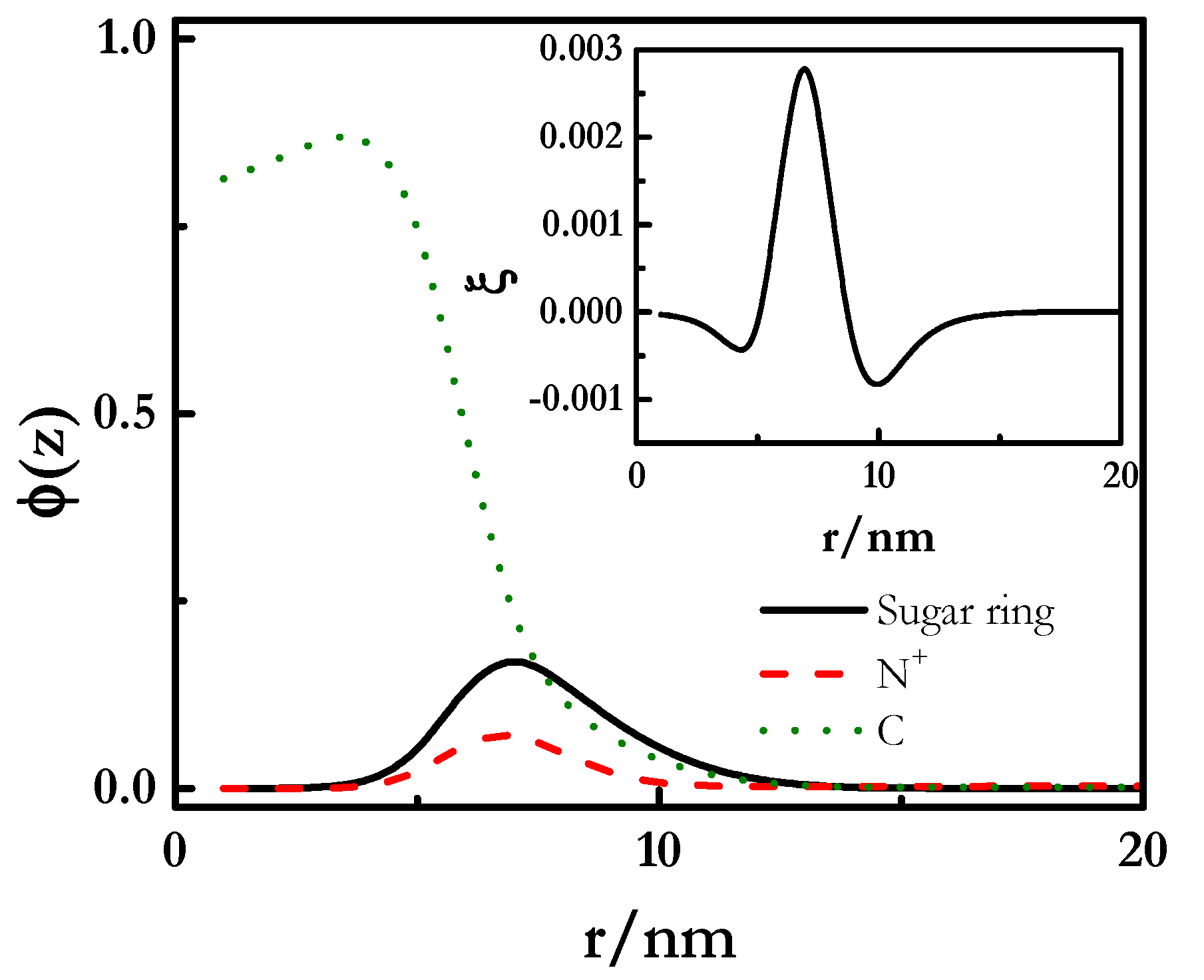

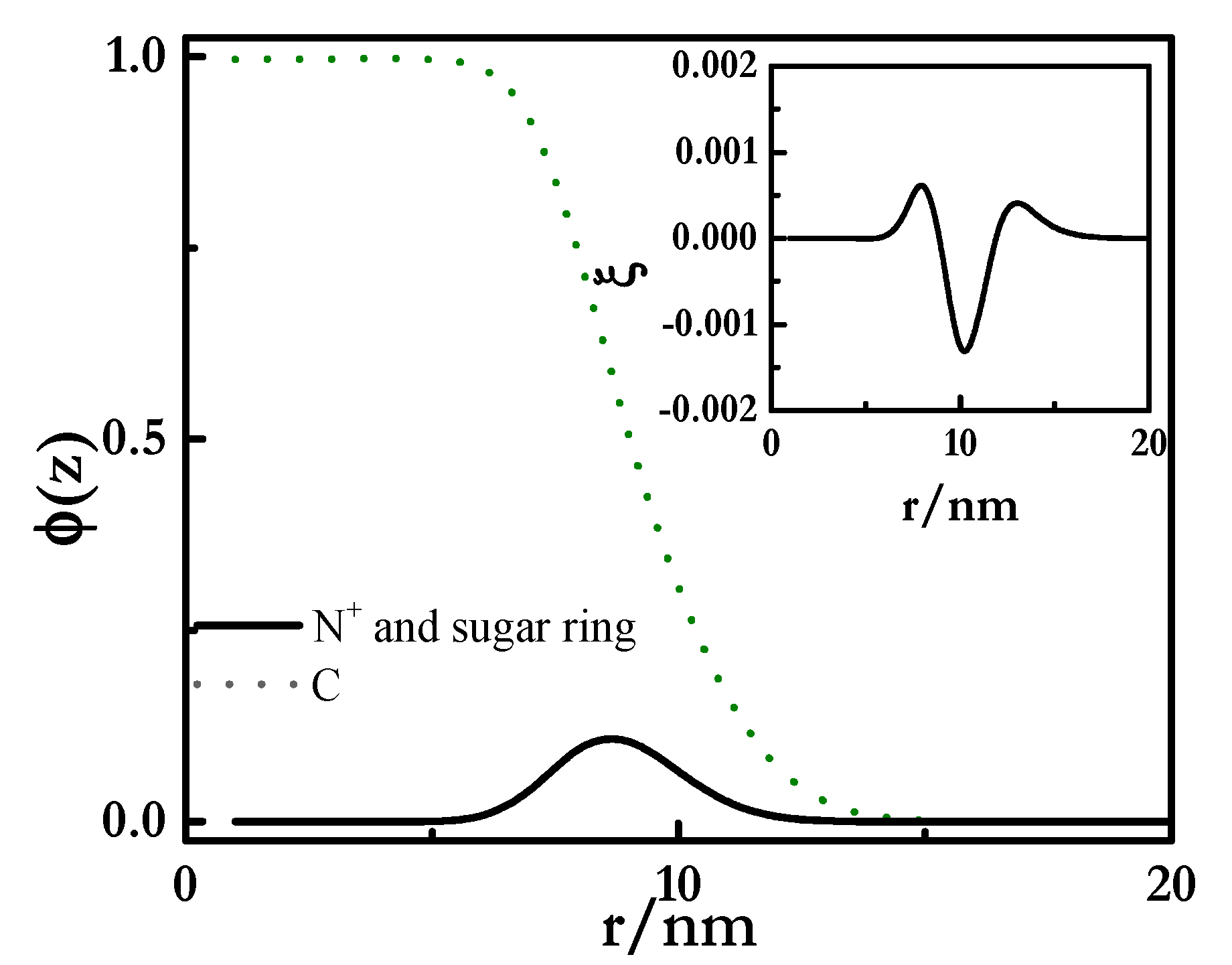
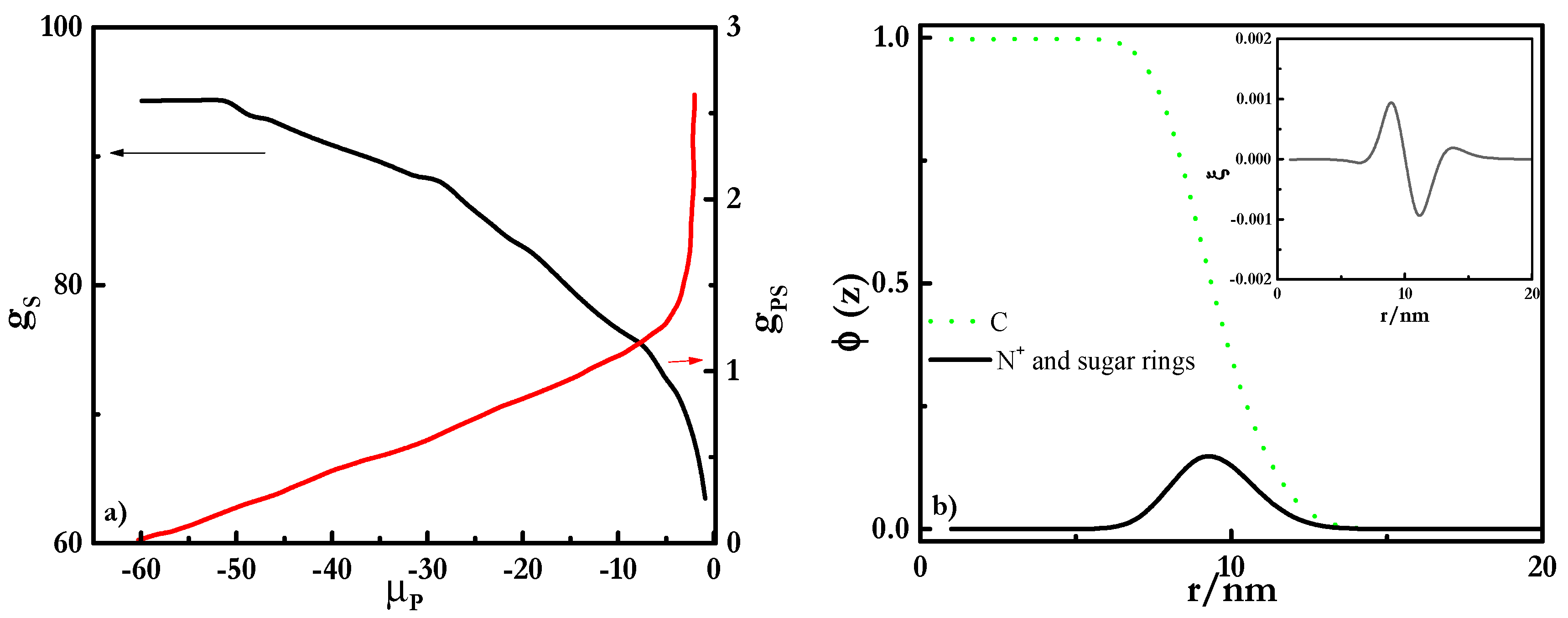
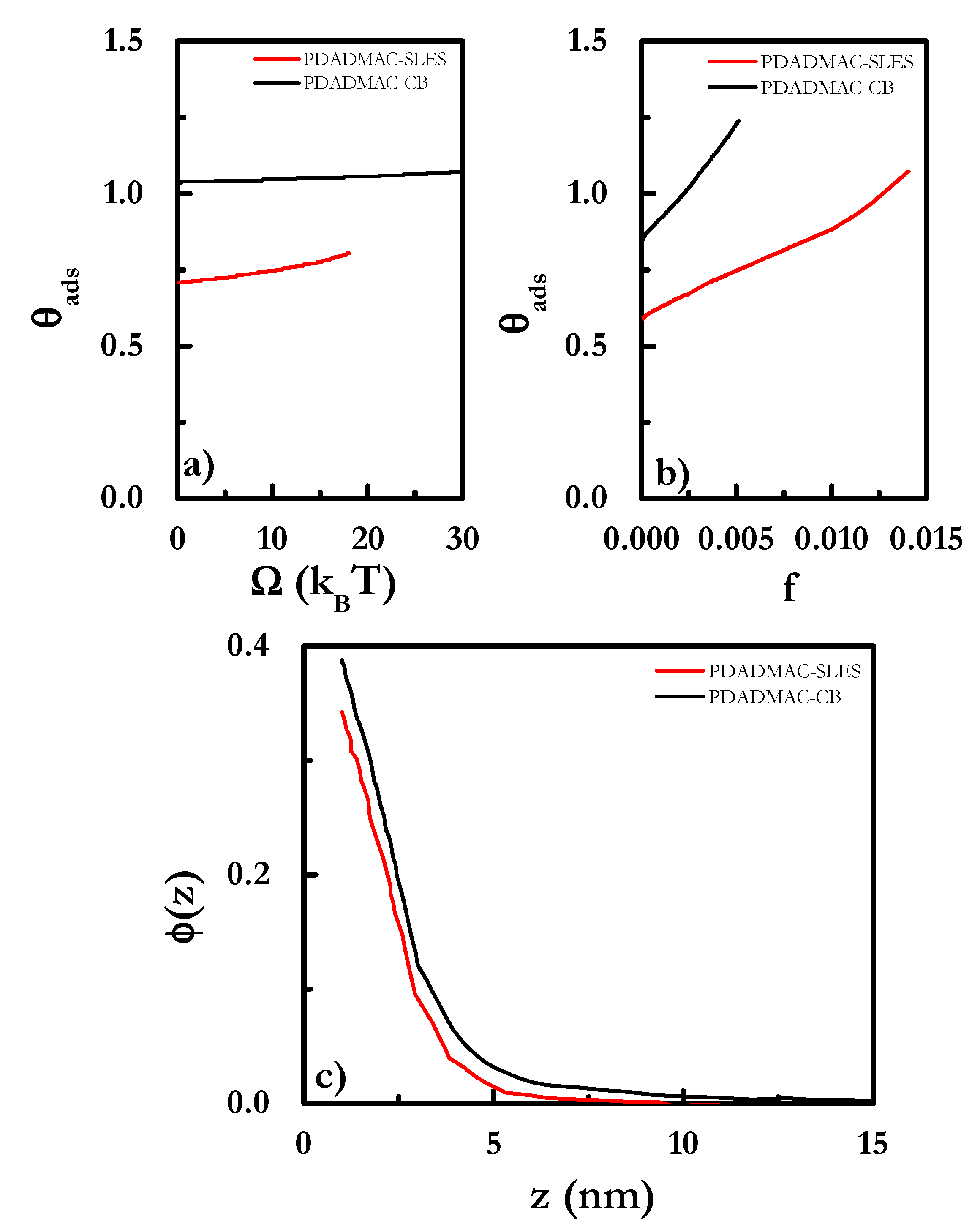
| χ | w | C | O | S | N | K | Cl | OH | X | Si | ε | ν |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| w | 0 | 1.6 | −0.6 | 0 | 0.5 | 0 | 0 | −0.6 | 0 | 1 | 80 | 0 |
| C | 1.6 | 0 | 1.6 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 |
| O | −0.6 | 1.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.5 | 0 |
| S | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.4 | −0.2 |
| N | 0.5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0.2 |
| K | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6.1 | 1 |
| Cl | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6.1 | −1 |
| OH | −0.6 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.5 | 0 |
| X | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.5 | −0.2 |
| Si | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | −0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán, E.; Fernández-Peña, L.; S. Luengo, G.; Rubio, A.M.; Rey, A.; Léonforte, F. Self-Consistent Mean Field Calculations of Polyelectrolyte-Surfactant Mixtures in Solution and upon Adsorption onto Negatively Charged Surfaces. Polymers 2020, 12, 624. https://doi.org/10.3390/polym12030624
Guzmán E, Fernández-Peña L, S. Luengo G, Rubio AM, Rey A, Léonforte F. Self-Consistent Mean Field Calculations of Polyelectrolyte-Surfactant Mixtures in Solution and upon Adsorption onto Negatively Charged Surfaces. Polymers. 2020; 12(3):624. https://doi.org/10.3390/polym12030624
Chicago/Turabian StyleGuzmán, Eduardo, Laura Fernández-Peña, Gustavo S. Luengo, Ana María Rubio, Antonio Rey, and Fabien Léonforte. 2020. "Self-Consistent Mean Field Calculations of Polyelectrolyte-Surfactant Mixtures in Solution and upon Adsorption onto Negatively Charged Surfaces" Polymers 12, no. 3: 624. https://doi.org/10.3390/polym12030624
APA StyleGuzmán, E., Fernández-Peña, L., S. Luengo, G., Rubio, A. M., Rey, A., & Léonforte, F. (2020). Self-Consistent Mean Field Calculations of Polyelectrolyte-Surfactant Mixtures in Solution and upon Adsorption onto Negatively Charged Surfaces. Polymers, 12(3), 624. https://doi.org/10.3390/polym12030624








