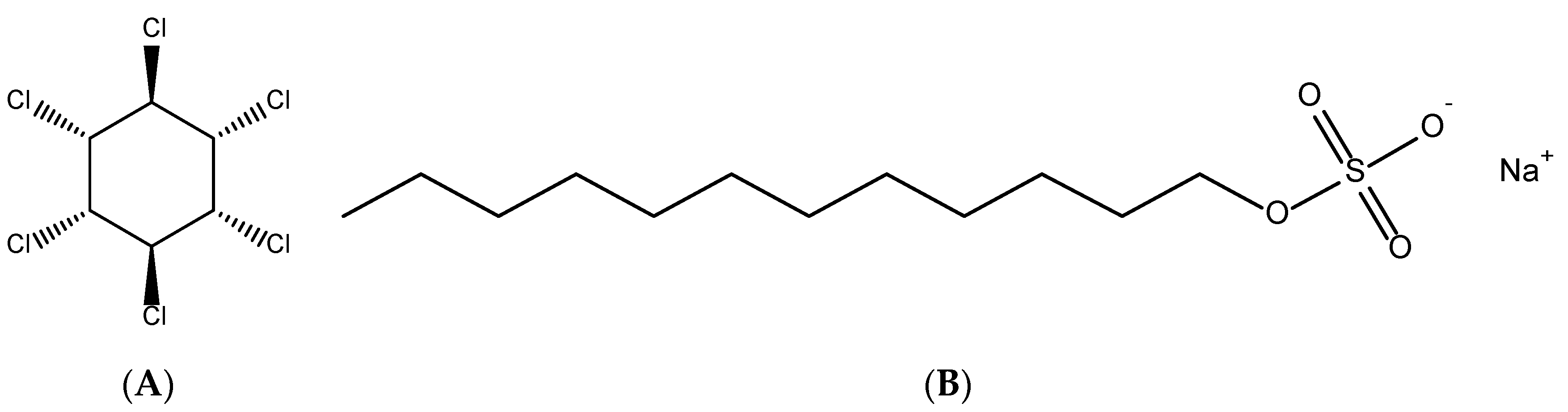
Removal of Lindane from Aqueous Solution Using Aluminum Hydroxide Nanoparticles with Surface Modification by Anionic Surfactant
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Aluminum Hydroxide Nanoparticles
2.3. Characterization Methods
2.4. The Analysis of Lindane
2.5. Adsorption Studies
3. Results and Discussion
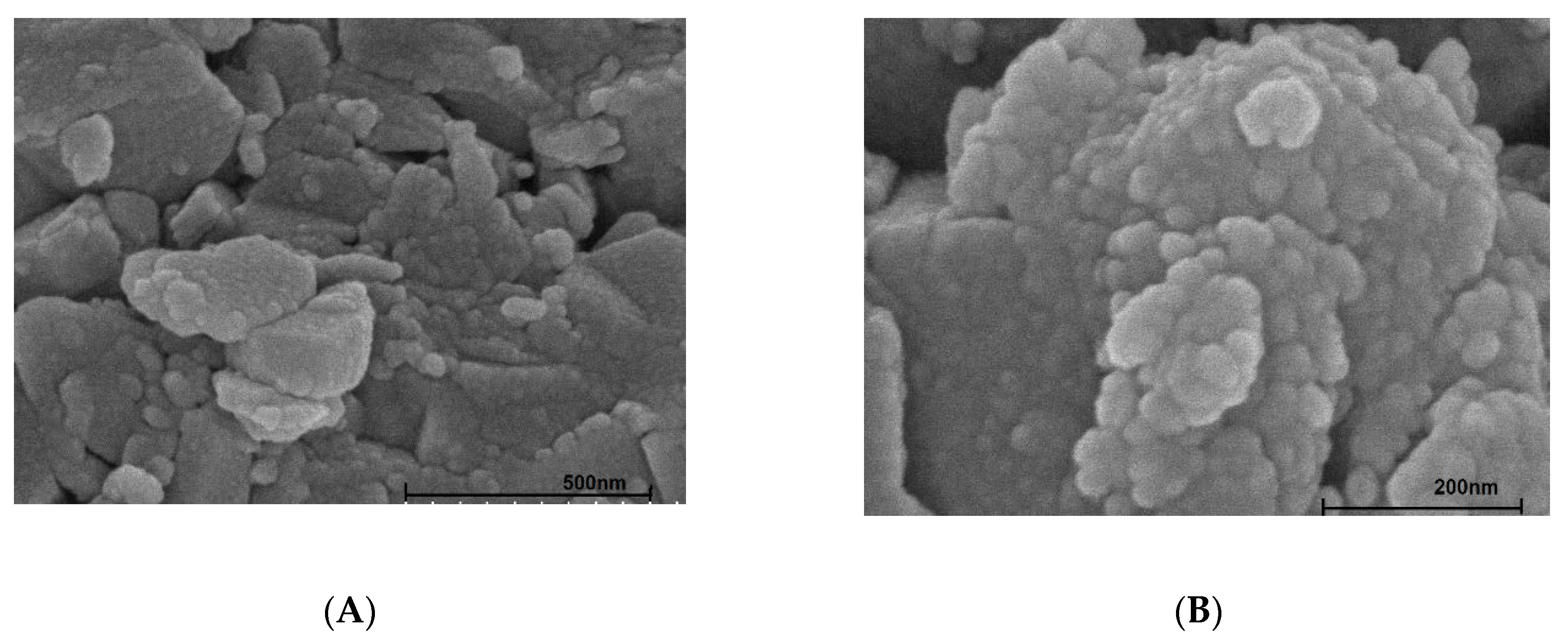
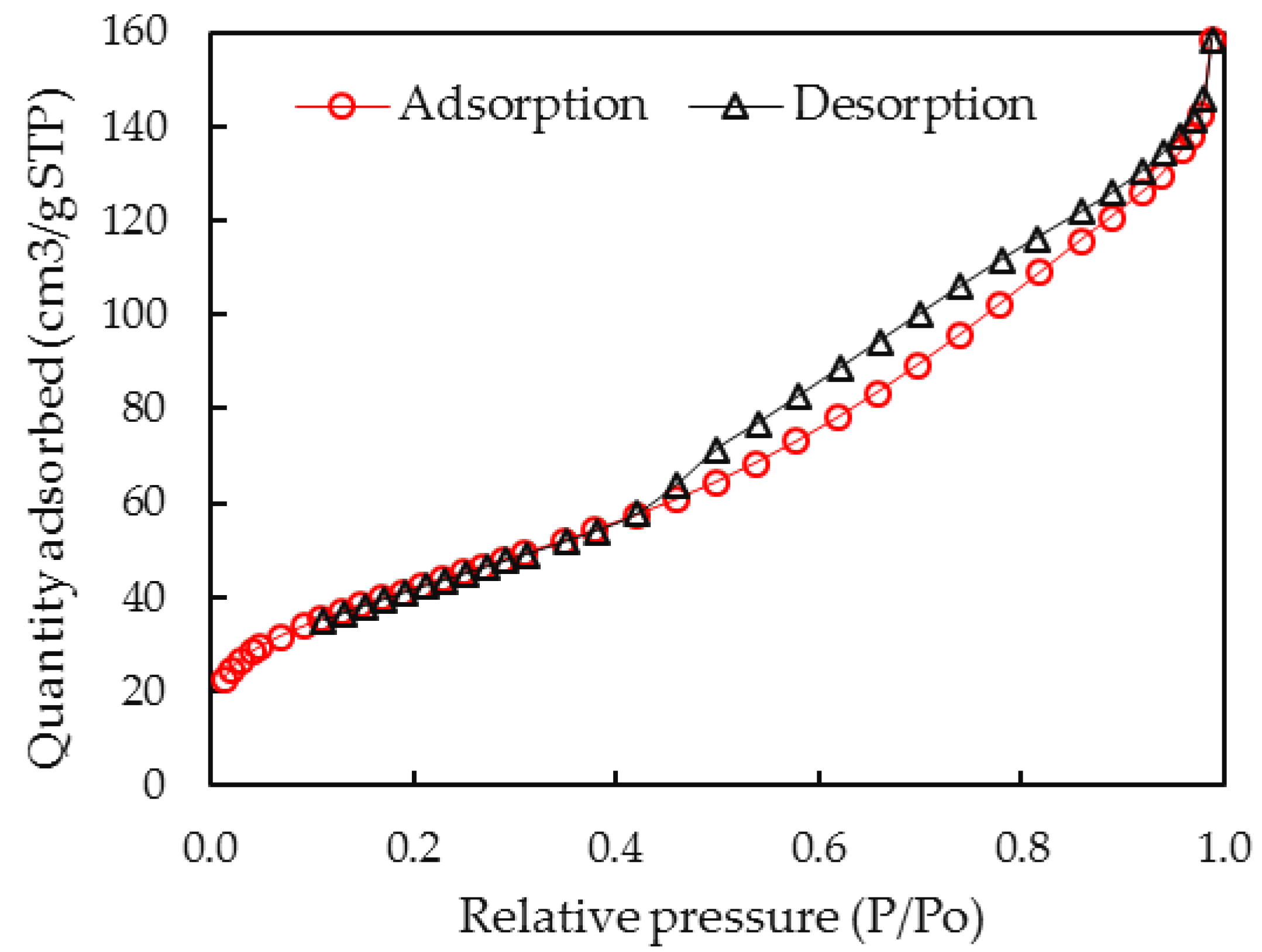
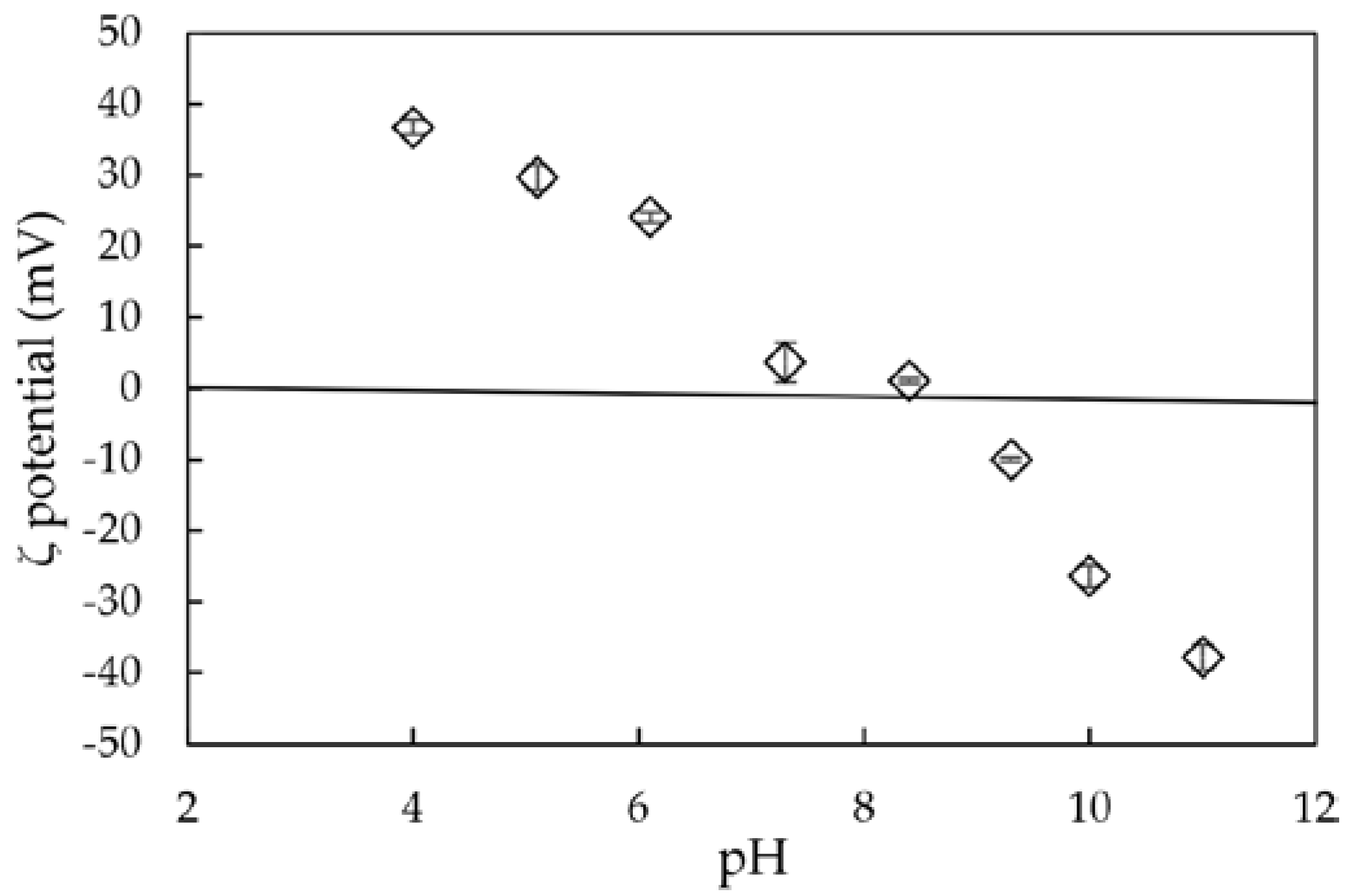
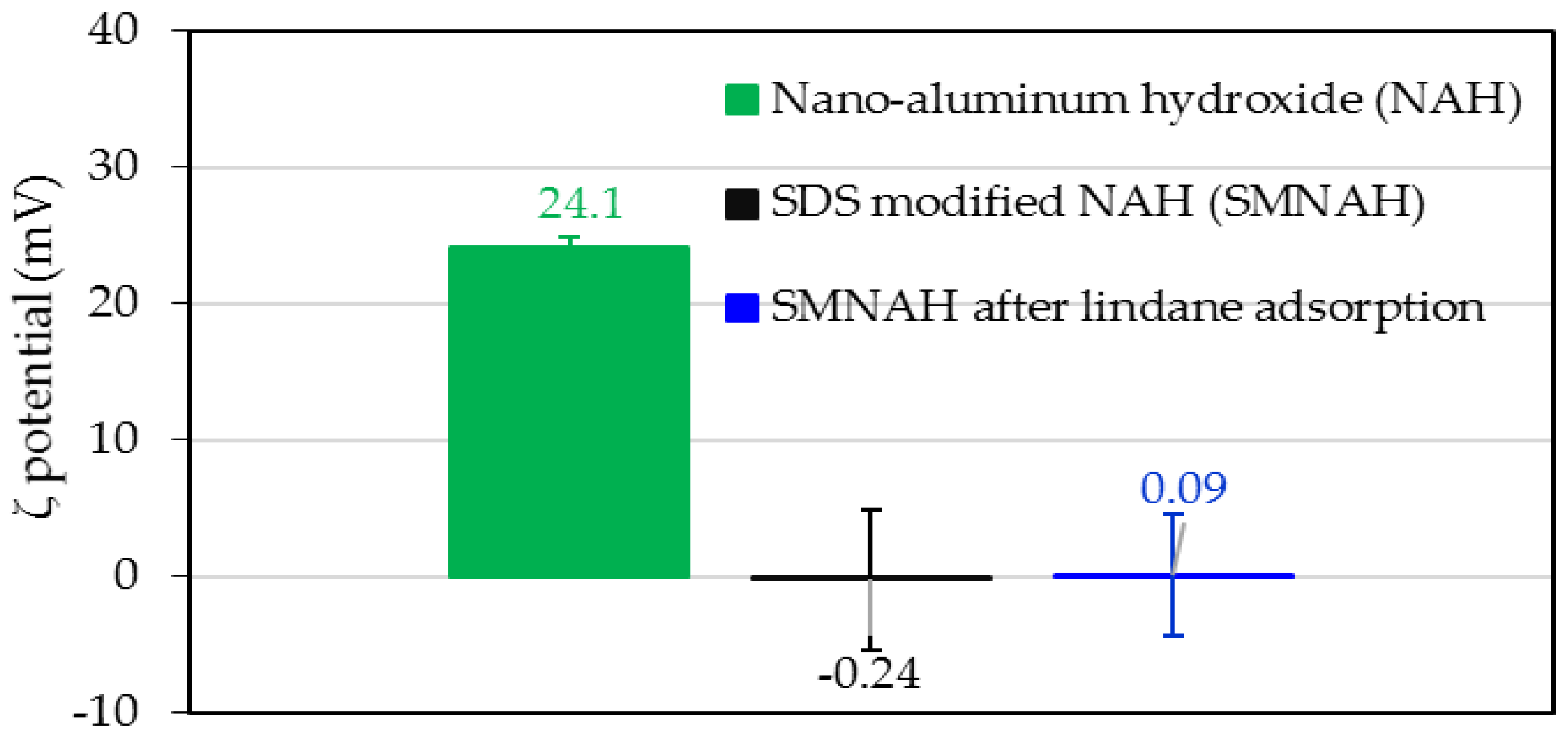
3.1. Characterization of Synthesized Aluminum Hydroxide Nanoparticles
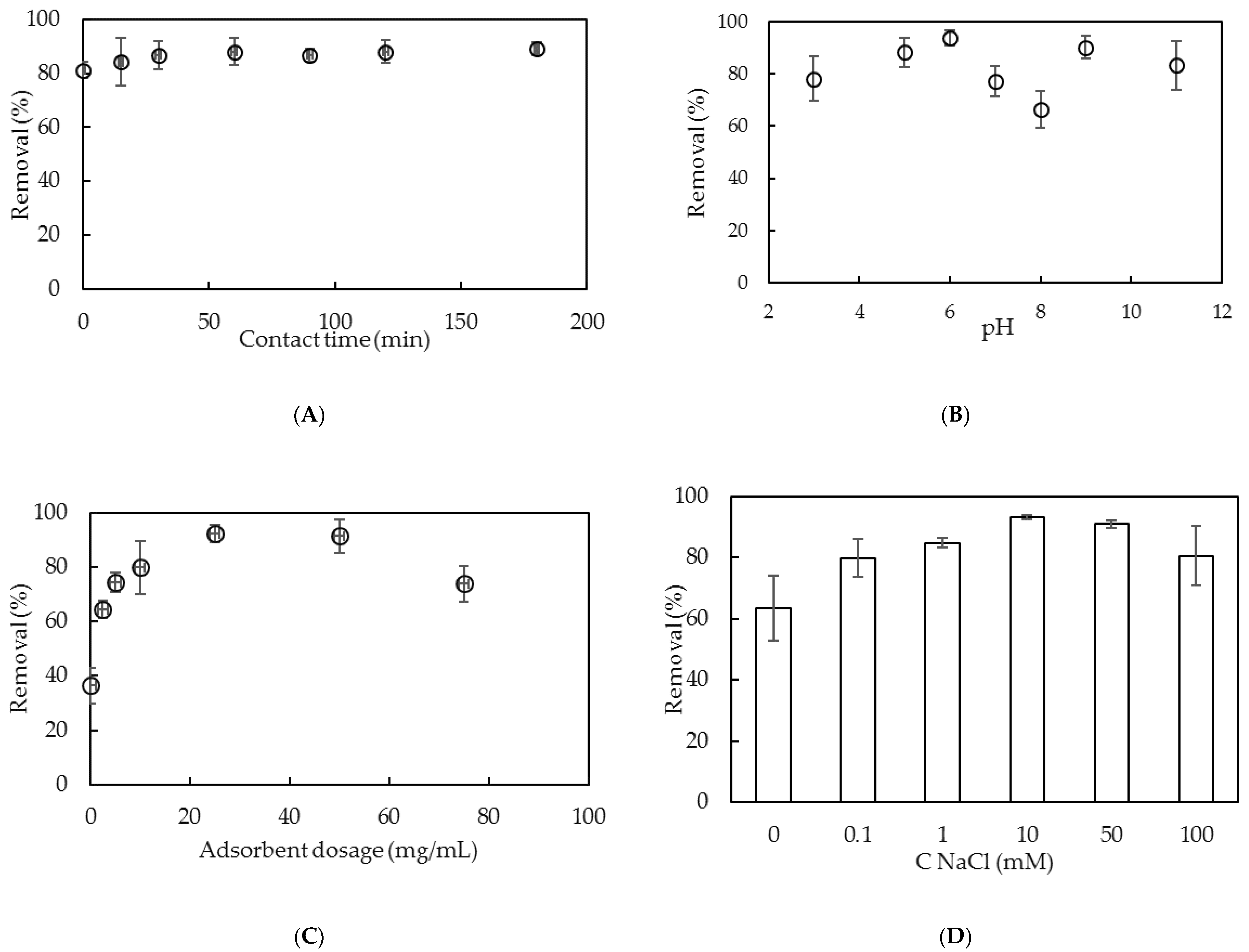
3.2. Comparison of the Removal of Lindane Using Nano-Aluminum Hydroxide without and with SDS
3.3. Adsorptive Removal of Lindane Using Surfactant-Modified Nano-aluminum Hydroxide (SMNAH)
3.4. Adsorption Isotherms of Lindane on SDS-Modified Nano-Aluminum Hydroxide (SMNAH)
3.5. Adsorption Mechanisms of Lindane onto SDS-Modified Nano-Aluminum Hydroxide (SMNAH)
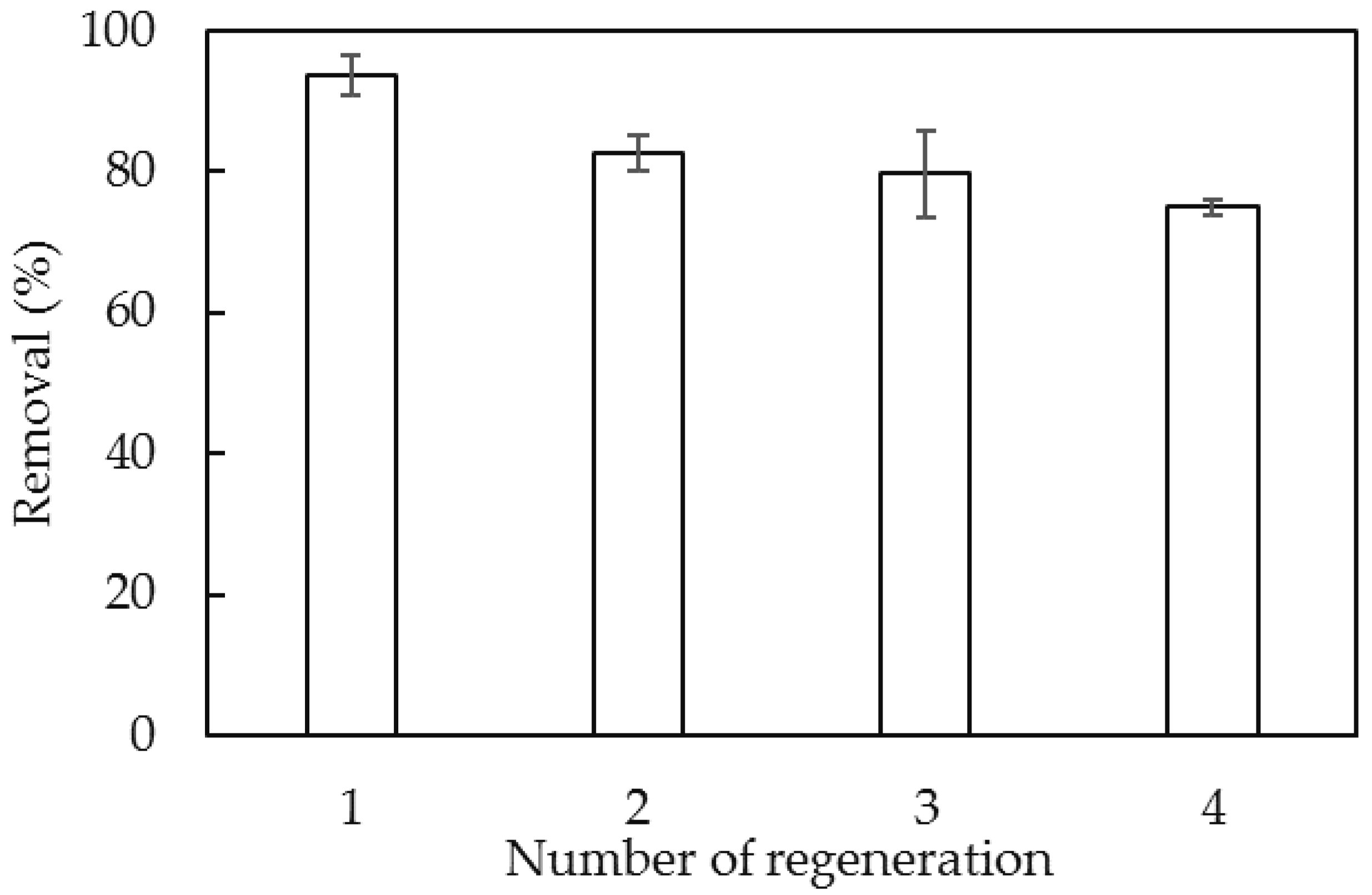
3.6. Comparison of Effectiveness of Surfactant-Modified Nano Aluminum Hydroxide (SMNAH) and Other Adsorbents for Lindane Removal
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gupta, V.K.; Jain, C.K.; Ali, I.; Chandra, S.; Agarwal, S. Removal of lindane and malathion from wastewater using bagasse fly ash—A sugar industry waste. Water Res. 2002, 36, 2483–2490. [Google Scholar] [CrossRef]
- Khan, S.; He, X.; Khan, H.M.; Boccelli, D.; Dionysiou, D.D. Efficient degradation of lindane in aqueous solution by iron (II) and/or UV activated peroxymonosulfate. J. Photochem. Photobiol. A: Chem. 2016, 316, 37–43. [Google Scholar] [CrossRef]
- Khan, S.; He, X.; Khan, J.A.; Khan, H.M.; Boccelli, D.L.; Dionysiou, D.D. Kinetics and mechanism of sulfate radical- and hydroxyl radical-induced degradation of highly chlorinated pesticide lindane in UV/peroxymonosulfate system. Chem. Eng. J. 2017, 318, 135–142. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Bhattacharya, B.D.; Bhattacharya, A.; Chatterjee, M.; Alam, A.; Satpathy, K.K.; Jonathan, M.P. Occurrence, distribution and possible sources of organochlorine pesticide residues in tropical coastal environment of India: An overview. Environ. Int. 2008, 34, 1062–1071. [Google Scholar] [CrossRef]
- Azuma, T.; Otomo, K.; Kunitou, M.; Shimizu, M.; Hosomaru, K.; Mikata, S.; Mino, Y.; Hayashi, T. Removal of pharmaceuticals in water by introduction of ozonated microbubbles. Sep. Purif. Technol. 2019, 212, 483–489. [Google Scholar] [CrossRef]
- Cheng, Y.; He, P.; Dong, F.; Nie, X.; Ding, C.; Wang, S.; Zhang, Y.; Liu, H.; Zhou, S. Polyamine and amidoxime groups modified bifunctional polyacrylonitrile-based ion exchange fibers for highly efficient extraction of U(VI) from real uranium mine water. Chem. Eng. J. 2019, 367, 198–207. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T. Heavy-metal removal from aqueous solution by fungus Mucor rouxii. Water Res. 2003, 37, 4486–4496. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Adsorption characteristics of anionic azo dye onto large α-alumina beads. Colloid Polym. Sci. 2015, 293, 1877–1886. [Google Scholar] [CrossRef]
- Pham, T.D.; Pham, T.T.; Phan, M.N.; Ngo, T.M.V.; Dang, V.D.; Vu, C.M. Adsorption characteristics of anionic surfactant onto laterite soil with differently charged surfaces and application for cationic dye removal. J. Mol. Liq. 2020, 301, 112456. [Google Scholar] [CrossRef]
- Young, E.; Banks, C.J. The Removal of Lindane from Aqueous Solution using a Fungal Biosorbent: The Influence of pH, Temperature, Biomass Concentration, and Culture Age. Environ. Technol. 1998, 19, 619–625. [Google Scholar] [CrossRef]
- Wan, J.; Meng, D.; Long, T.; Ying, R.; Ye, M.; Zhang, S.; Li, Q.; Zhou, Y.; Lin, Y. Simultaneous Removal of Lindane, Lead and Cadmium from Soils by Rhamnolipids Combined with Citric Acid. PLoS ONE 2015, 10, e0129978. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Privitera, V.; Impellizzeri, G. Selective photodegradation of 2,4-D pesticide from water by molecularly imprinted TiO2. J. Photochem. Photobiol. A Chem. 2019, 380, 111872. [Google Scholar] [CrossRef]
- Golshan, M.; Kakavandi, B.; Ahmadi, M.; Azizi, M. Photocatalytic activation of peroxymonosulfate by TiO2 anchored on cupper ferrite (TiO2@CuFe2O4) into 2,4-D degradation: Process feasibility, mechanism and pathway. J. Hazard. Mater. 2018, 359, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B. Chemistry of alumina, reactions in aqueous solution and its application in water treatment. Adv. Colloid Interface Sci. 2004, 110, 19–48. [Google Scholar] [CrossRef]
- Franks, G.V.; Gan, Y. Charging Behavior at the Alumina–Water Interface and Implications for Ceramic Processing. J. Am. Ceram. Soc. 2007, 90, 3373–3388. [Google Scholar] [CrossRef]
- Geuli, O.; Lewinstein, I.; Mandler, D. Composition-Tailoring of ZnO-Hydroxyapatite Nanocomposite as Bioactive and Antibacterial Coating. ACS Appl. Nano Mater. 2019, 2, 2946–2957. [Google Scholar] [CrossRef]
- Zhao, D.; Kim, J.F.; Ignacz, G.; Pogany, P.; Lee, Y.M.; Szekely, G. Bio-Inspired Robust Membranes Nanoengineered from Interpenetrating Polymer Networks of Polybenzimidazole/Polydopamine. ACS Nano 2019, 13, 125–133. [Google Scholar] [CrossRef]
- Fei, F.; Le Phuong, H.A.; Blanford, C.F.; Szekely, G. Tailoring the Performance of Organic Solvent Nanofiltration Membranes with Biophenol Coatings. ACS Appl. Polym. Mater. 2019, 1, 452–460. [Google Scholar] [CrossRef]
- Thakur, S.; Misra, M.; Mohanty, A.K. Sustainable Hydrophobic and Moisture-Resistant Coating Derived from Downstream Corn Oil. ACS Sustain. Chem. Eng. 2019, 7, 8766–8774. [Google Scholar] [CrossRef]
- Adak, A.; Pal, A.; Bandyopadhyay, M. Fixed bed column study for the removal of crystal violet (C. I. Basic Violet 3) dye from aquatic environment by surfactant-modified alumina. Dyes and Pigments 2006, 69, 245–251. [Google Scholar] [CrossRef]
- Adak, A.; Bandyopadhyay, M.; Pal, A. Removal of crystal violet dye from wastewater by surfactant-modified alumina. Sep. Purif. Technol. 2005, 44, 139–144. [Google Scholar] [CrossRef]
- Chu, T.P.M.; Nguyen, N.T.; Vu, T.L.; Dao, T.H.; Dinh, L.C.; Nguyen, H.L.; Hoang, T.H.; Le, T.S.; Pham, T.D. Synthesis, Characterization, and Modification of Alumina Nanoparticles for Cationic Dye Removal. Materials 2019, 12, 450. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.T.; Do, T.P.T.; Hoang, T.S.; Nguyen, N.V.; Pham, H.D.; Nguyen, T.D.; Pham, T.N.M.; Le, T.S.; Pham, T.D. Adsorption of Anionic Surfactants onto Alumina: Characteristics, Mechanisms, and Application for Heavy Metal Removal. Int. J. Polym. Sci. 2018, 2018, 11. [Google Scholar] [CrossRef]
- Pham, T.D.; Do, T.T.; Ha, V.L.; Doan, T.H.Y.; Nguyen, T.A.H.; Mai, T.D.; Kobayashi, M.; Adachi, Y. Adsorptive removal of ammonium ion from aqueous solution using surfactant-modified alumina. Environ. Chem. 2017, 14, 327–337. [Google Scholar] [CrossRef]
- Pham, T.D.; Tran, T.T.; Le, V.A.; Pham, T.T.; Dao, T.H.; Le, T.S. Adsorption characteristics of molecular oxytetracycline onto alumina particles: The role of surface modification with an anionic surfactant. J. Mol. Liq. 2019, 287, 110900. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Piccin, J.S.; Cadaval, T.R.S.A.; de Pinto, L.A.A.; Dotto, G.L. Adsorption Isotherms in Liquid Phase: Experimental, Modeling, and Interpretations. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D.I., Reynel-Ávila, H.E., Eds.; Springer Nature: Cham, Switzerland, 2017; pp. 19–51. [Google Scholar] [CrossRef]
- Zhu, B.-Y.; Gu, T. Surfactant adsorption at solid-liquid interfaces. Adv. Colloid Interface Sci. 1991, 37, 1–32. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Adsorption of anionic surfactant sodium dodecyl sulfate onto alpha alumina with small surface area. Colloid Polym. Sci. 2015, 293, 217–227. [Google Scholar] [CrossRef]
- Pham, T.D.; Nguyen, H.H.; Nguyen, N.V.; Vu, T.T.; Pham, T.N.M.; Doan, T.H.Y.; Nguyen, M.H.; Ngo, T.M.V. Adsorptive Removal of Copper by Using Surfactant Modified Laterite Soil. J. Chem. 2017, 2017, 10. [Google Scholar] [CrossRef]
- Hiemstra, T.; Yong, H.; Van Riemsdijk, W.H. Interfacial Charging Phenomena of Aluminum (Hydr) oxides. Langmuir 1999, 15, 5942–5955. [Google Scholar]
- Delgado, A.V.; González-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena. J. Colloid Interface Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef] [PubMed]
- Salazar, H.; Nunes-Pereira, J.; Correia, D.M.; Cardoso, V.F.; Gonçalves, R.; Martins, P.M.; Ferdov, S.; Martins, M.D.; Botelho, G.; Lanceros-Méndez, S. Poly(vinylidene fluoride-hexafluoropropylene)/bayerite composite membranes for efficient arsenic removal from water. Mater. Chem. Phys. 2016, 183, 430–438. [Google Scholar] [CrossRef]
- Del Nero, M.; Galindo, C.; Barillon, R.; Halter, E.; Madé, B. Surface reactivity of α-Al2O3 and mechanisms of phosphate sorption: In situ ATR-FTIR spectroscopy and ζ potential studies. J. Colloid Interface Sci. 2010, 342, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.K.; Shijina, A.V.; Praveen, A.K. Mesoporous γ-alumina nanoparticles: Synthesis, characterization and dye removal efficiency. Mater. Lett. 2012, 82, 42–44. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Interfacial characterization of α-alumina with small surface area by streaming potential and chromatography. Colloids Surf. A: Physicochem. Eng. Asp. 2013, 436, 148–157. [Google Scholar] [CrossRef]
- Weston, J.S.; Harwell, J.H.; Shiau, B.J.; Kabir, M. Disrupting Admicelle Formation and Preventing Surfactant Adsorption on Metal Oxide Surfaces Using Sacrificial Polyelectrolytes. Langmuir 2014, 30, 6384–6388. [Google Scholar] [CrossRef]
- Mazloomi, F.; Jalali, M. Ammonium removal from aqueous solutions by natural Iranian zeolite in the presence of organic acids, cations and anions. J. Environ. Chem. Eng. 2016, 4, 1664–1673. [Google Scholar] [CrossRef]
- Pham, T.D.; Bui, T.T.; Nguyen, V.T.; Bui, T.K.V.; Tran, T.T.; Phan, Q.C.; Pham, T.D.; Hoang, T.H. Adsorption of Polyelectrolyte onto Nanosilica Synthesized from Rice Husk: Characteristics, Mechanisms, and Application for Antibiotic Removal. Polymers 2018, 10, 220. [Google Scholar] [CrossRef]
- Pham, T.D.; Bui, T.T.; Trang Truong, T.T.; Hoang, T.H.; Le, T.S.; Duong, V.D.; Yamaguchi, A.; Kobayashi, M.; Adachi, Y. Adsorption characteristics of beta-lactam cefixime onto nanosilica fabricated from rice HUSK with surface modification by polyelectrolyte. J. Mol. Liq. 2020, 298, 111981. [Google Scholar] [CrossRef]
- Hind, A.R.; Bhargava, S.K.; McKinnon, A. At the solid/liquid interface: FTIR/ATR—The tool of choice. Adv. Colloid Interface Sci. 2001, 93, 91–114. [Google Scholar] [CrossRef]
- Sperline, R.P.; Song, Y.; Freiser, H. Fourier transform infrared attenuated total reflection spectroscopy linear dichroism study of sodium dodecyl sulfate adsorption at the alumina/water interface using alumina-coated optics. Langmuir 1992, 8, 2183–2191. [Google Scholar] [CrossRef]
- Liyan, S.; Youcai, Z.; Guojian, W.; Bing, L.; Dongjie, N.; Xiaoli, C. Biomimetic fat cell (BFC) preparation and for lindane removal from aqueous solution. J. Hazard. Mater. 2007, 146, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Sprynskyy, M.; Ligor, T.; Buszewski, B. Clinoptilolite in study of lindane and aldrin sorption processes from water solution. J. Hazard. Mater. 2008, 151, 570–577. [Google Scholar] [CrossRef]
- Saez, J.M.; Álvarez, A.; Benimeli, C.S.; Amoroso, M.J. Enhanced lindane removal from soil slurry by immobilized Streptomyces consortium. Int. Biodeterior. Biodegrad. 2014, 93, 63–69. [Google Scholar] [CrossRef]
- Boucher, F.R.; Lee, F.G. Adsorption of lindane and dieldrin pesticides on unconsolidated aquifer sands. Environ. Sci. Technol. 1972, 6, 538–543. [Google Scholar] [CrossRef]
- Ratola, N.; Botelho, C.; Alves, A. Influence of Metals on Lindane Adsorption onto Pine Bark. Water Air Soil Pollut. Focus 2003, 3, 181–188. [Google Scholar] [CrossRef]
- Khobragade, M.U.; Pal, A. Adsorptive removal of Mn(II) from water and wastewater by surfactant-modified alumina. Desalin. Water Treat. 2016, 57, 2775–2786. [Google Scholar] [CrossRef]
- Khobragade, M.U.; Pal, A. Investigation on the adsorption of Mn(II) on surfactant-modified alumina: Batch and column studies. J. Environ. Chem. Eng. 2014, 2, 2295. [Google Scholar] [CrossRef]
- Das, A.K.; Saha, S.; Pal, A.; Maji, S.K. Surfactant-modified alumina: An efficient adsorbent for malachite green removal from water environment. J. Environ. Sci. Health Part A 2009, 44, 896. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Pal, A.; Bandyopadhyay, M. Removal of phenol from water environment by surfactant-modified alumina through adsolubilization. Colloids Surf. A: Physicochem. Eng. Asp. 2006, 277, 63–68. [Google Scholar] [CrossRef]


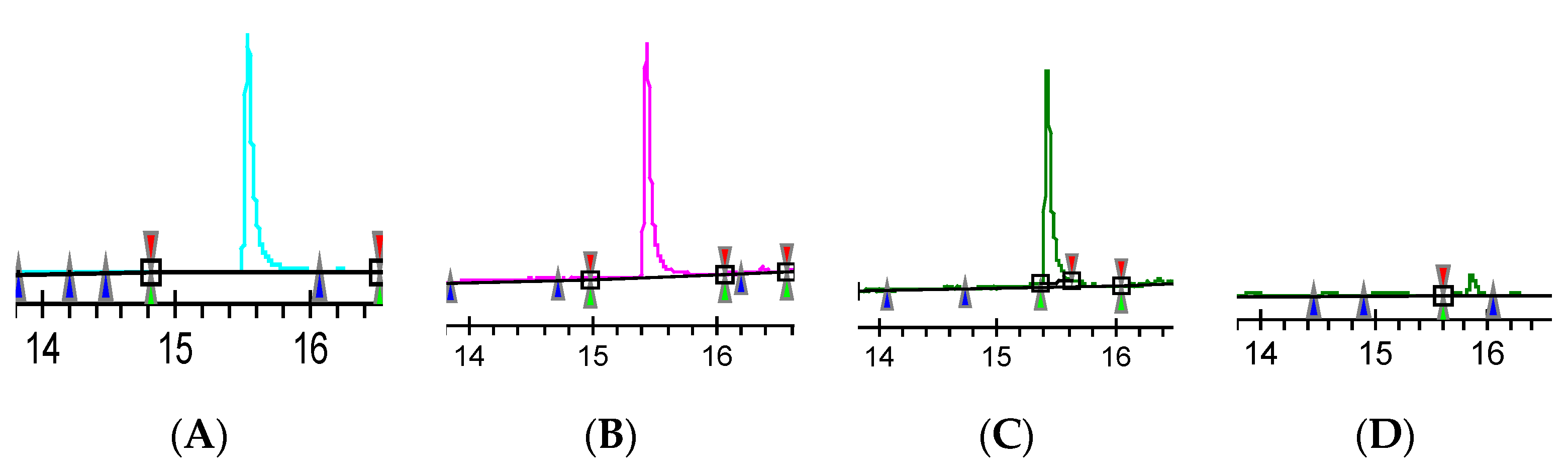


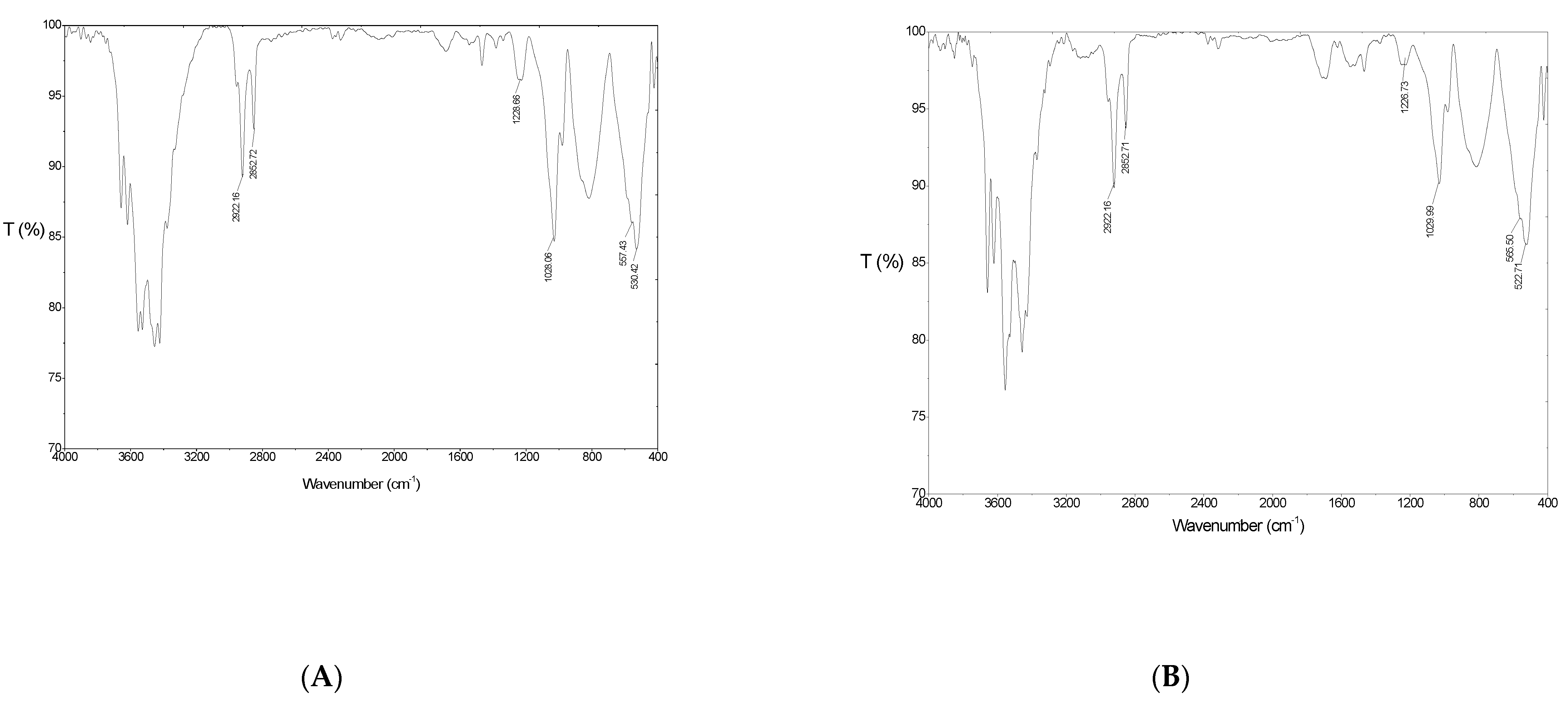

| Parameters | GC-ECD |
|---|---|
| Injector temperature | 250 °C |
| Injection type | Splitless |
| Injection volume | 2 μL |
| Carries gas flow rate | Nitrogen (99.99%), 1.2 mL·min−1 |
| Make-up gas | Nitrogen (99.99%), 25 mL·min−1 |
| Oven temperature | 160 °C (hold 2 min), at 5 °C /min to 280 °C (4 min). Total running time: 18 min. |
| Detector temperature | 300 °C |
| CNaCl (mM) | k1 (103 g/mg) | k2 (103 g/mg)n−1 | n | |
|---|---|---|---|---|
| 10 | 325 | 5.0 | 8.5 | 2.1 |
| 1 | 320 | 5.0 | 8.0 | 2.1 |
| Adsorbent | Adsorption Capacity (µg/g) | Removal Efficiency (%) | References |
|---|---|---|---|
| Bagasse fly ash | 2.51 | NI | [1] |
| Fungal biosorbent | NI | 82.75 | [10] |
| Biomimetic fat cell | NI | 72.03 | [45] |
| Prepolymer | NI | 15.65 | [45] |
| Clinoptilolite rock | 5.6 | 68.0 | [46] |
| Soil slurry | 35.3 | NI | [47] |
| Aquifer sand | NI | 73.2 | [48] |
| Pine bark | 3.14 | 83.51 | [49] |
| Surfactant-modified nano-aluminum hydroxide (SMNAH) | 325.0 | 93.68 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.H.; Nguyen, T.T.L.; Pham, T.D.; Le, T.S. Removal of Lindane from Aqueous Solution Using Aluminum Hydroxide Nanoparticles with Surface Modification by Anionic Surfactant. Polymers 2020, 12, 960. https://doi.org/10.3390/polym12040960
Nguyen TH, Nguyen TTL, Pham TD, Le TS. Removal of Lindane from Aqueous Solution Using Aluminum Hydroxide Nanoparticles with Surface Modification by Anionic Surfactant. Polymers. 2020; 12(4):960. https://doi.org/10.3390/polym12040960
Chicago/Turabian StyleNguyen, Thi Hang, Thi Thuy Linh Nguyen, Tien Duc Pham, and Thanh Son Le. 2020. "Removal of Lindane from Aqueous Solution Using Aluminum Hydroxide Nanoparticles with Surface Modification by Anionic Surfactant" Polymers 12, no. 4: 960. https://doi.org/10.3390/polym12040960
APA StyleNguyen, T. H., Nguyen, T. T. L., Pham, T. D., & Le, T. S. (2020). Removal of Lindane from Aqueous Solution Using Aluminum Hydroxide Nanoparticles with Surface Modification by Anionic Surfactant. Polymers, 12(4), 960. https://doi.org/10.3390/polym12040960





